Abstract
Background
While treatment for atopic rhinitis is aimed mostly to relieve symptoms, only allergen‐specific immunotherapy (AIT) is targeted to modify the natural history of allergic diseases. This results in sustained clinical tolerance, even when treatment has stopped. The immunomodulatory effects of AIT are attributed mainly to increased regulatory T‐cell function and increased allergen‐specific IgG4, yet little is known about the effect on the memory B‐cell compartment.
Objective
We aimed to examine the effects of AIT on the IgE‐ and IgG subclass‐expressing memory B cells.
Methods
We recruited 29 patients with atopic seasonal rhinoconjunctivitis and performed a longitudinal analysis of the peripheral immune compartment before, during, and after sublingual immunotherapy (SLIT) for allergy to temperate grass pollen, predominantly to ryegrass pollen (RGP; Lolium perenne). Using flow cytometry on peripheral blood mononuclear cells and serum immunoassays, we analyzed the effects of a 4 months preseasonal treatment regimen comprising two or three courses in consecutive years on circulating IgE+ and IgG+ memory B cells and allergen‐specific Ig levels.
Results
SLIT increased RGP‐specific serum IgG2 and IgG4, as well as the frequencies of IgG2 + and IgG4 + memory B cells, whereas no effect was observed on the IgE+ memory B‐cell compartment. Furthermore, SLIT enhanced proportions of regulatory T cells specific to RGP. These changes were associated with clinical improvement.
Conclusion
Our data provide evidence for immunological effects of SLIT on B‐cell memory. Skewing responses toward IgG2 and IgG4 subclasses might be a mechanism to suppress IgE‐mediated allergic responses.
Keywords: B cells, flow cytometry, IgE, immunotherapy and tolerance induction, Rhinitis
This study examines the effect of ryegrass pollen AIT on B‐cell responses in a population of 29 patients with allergic rhinitis. Successful immunotherapy for ryegrass pollen allergy increases allergen‐specific IgG2 and IgG4 serum levels, and proportions of IgG2‐ and IgG4‐expressing memory B cells. Skewing toward the anti‐inflammatory IgG2 and IgG4 subclasses might be a mechanism to suppress IgE‐mediated allergic responses.

Abbreviations
- AIT
allergen‐specific immunotherapy
- FeNO
fractional exhaled nitric oxide
- IL
interleukin
- RGP
ryegrass pollen
- SCIT
subcutaneous immunotherapy
- SHM
somatic hypermutation
- SLIT
sublingual immunotherapy
- SPT
skin prick test
- Th1/2
T helper 1/2
- Treg
regulatory T cell
- VAS
visual analog score
1. INTRODUCTION
Rhinoconjunctivitis and other IgE‐mediated allergies are an increasing disease burden globally.1 Most therapies for allergies are directed at relieving symptoms, but allergen‐specific immunotherapy (AIT) is the only current therapy that modifies the natural course of allergic diseases. Subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) are both proven effective treatments for grass pollen‐induced rhinoconjunctivitis.2, 3, 4 The therapeutic effect is maintained beyond the conclusion of treatment.5, 6, 7 In patients with allergic rhinitis, AIT can prevent the onset of new sensitizations8 and decrease the likelihood of developing asthma.9 The immunomodulatory properties of AIT affect local and systemic immune responses, with an impact on the number and function of mast cells, basophils, antigen‐presenting cells, T cells, and B cells.10, 11
Allergic patients manifest sensitization by means of allergen‐specific IgE bound to effector cells, particularly mast cells and basophils.12 The underlying mechanism is thought to be a shifted T‐cell balance toward a T helper 2 (Th2) phenotype, and these cells produce interleukin (IL)‐4 and IL‐13 that direct allergen‐specific B cells to produce IgE.13 Furthermore, Th2 cells produce IL‐5 which promotes the involvement of eosinophils in the pathogenesis of allergic diseases.14 In contrast, Th1 responses are promoted by IFN‐γ and skew away from a Th2 phenotype.15 Effective immunotherapy has been shown to reverse the Th2 dominance and to result in anergy of allergen‐specific T cells,16, 17 induction of regulatory T cells (Treg),18, 19, 20, 21 and production of blocking antibodies of the IgG and IgA isotypes.22, 23 Specifically, TGF‐β and IL‐10 produced by Treg are pivotal for the successful immune deviation in AIT.24, 25
The tolerogenic functions of IL‐10 are extensive, but mainly encompass the inhibition of mast cell activity,26 suppression of IL‐5 production by Th2 cells,27 and cell death induction in eosinophils.28 Furthermore, IL‐10 in combination with IL‐4 and IL‐13 directs B‐cell immunoglobulin class switching to IgG4 instead of IgE.29 Indeed, one of the known effects of AIT is an increase in allergen‐specific serum IgG4 and an increased serum IgG4/IgE antibody ratio that is associated with clinical efficacy.30
SCIT and SLIT have distinct immunomodulatory capabilities that appear related to the different routes of administration. Sublingual administration results in fewer systemic adverse effects, but some studies indicate diminished clinical and immunological efficacy compared with subcutaneous administration.2, 31 SLIT results in increased numbers of FoxP3+ Treg both in the oral epithelium and in the peripheral blood.23, 32 Further systemic alterations are more diverse. Some studies report an initial increase in allergen‐specific IgE serum levels, followed by a decrease after 1 month.33 Furthermore, allergen‐specific IgG2, IgG4, and IgA serum levels are reported to increase in as little as 1 day after the start of therapy.33, 34, 35 However, other studies detected no systemic alterations with regard to allergen‐specific lymphoproliferation, cytokine secretion, or Ig serum levels.36, 37
IgG2 and IgG4 heavy chain constant regions are encoded by genes in the IGH locus. Ig class switching to IgG2 and IgG4 frequently occurs indirectly following a switch from IgM to the more proximal IgG3 and IgG1 genes rather than directly from IgM to IgG2 or IgG4.38 Given the higher loads of somatic hypermutation (SHM) in variable regions of IgG2 and IgG4 transcripts, it has been suggested that B cells expressing these transcripts have spent more time in the germinal center response.39 In addition, the majority of IgG2‐ and IgG4‐expressing B cells co‐express CD27, and their frequencies increase with age.40, 41 Hence, it appears that these Ig class switches occur following repeated exposure to the same antigen.
Since AIT has been shown to have long‐lasting beneficial effects, it is important to determine whether this is the result of changes in immunological memory. We here address this question in our cohort of patients with moderate‐to‐severe seasonal allergic rhinitis, studied longitudinally before, during, and after SLIT for grass pollen allergy.42 As published previously,42, 43 SLIT in our cohort resulted in allergic rhinitis symptom relief and conferred significant protection from epidemic thunderstorm asthma, making this an ideal cohort to examine the effects of a 4‐month treatment regimen and the subsequent effects of two further courses of treatment over 3 years on circulating IgE+‐ and IgG subclass‐expressing memory B cells and allergen‐specific Ig levels.
2. METHODS
2.1. Study design
Using an open‐label longitudinal design (ClinicalTrials.gov identifier: NCT02014623), 29 participants were recruited for treatment with a commercial 5‐grass pollen SLIT tablet (Oralair®; Stallergenes) using a 4‐month (May‐September) regimen completed prior to the Australian pollen season, for 3 consecutive years (2014‐2016; subject numbers at each time point shown in Figure 1A). Treatment with Oralair® involved dissolution under the tongue (at least 2 minutes) followed by swallowing the residue. The treatment regimen comprised the following: day 1—1 tablet 100 IR (index of reactivity); day 2—2 tablets 100 IR; and day 3 to day 120—1 daily tablet 300 IR. Blood samples were collected immediately before initial treatment (May 2014) and after the first 4 months of treatment (September 2014), followed by annual collections in May 2015 and May 2016 (prior to commencement of 2nd and 3rd courses of SLIT), and May 2017 (Figure 1A).
Figure 1.
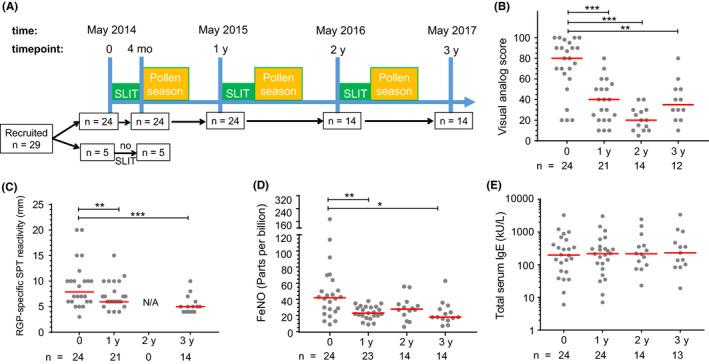
Study design and clinical parameters of allergic rhinitis decreased after SLIT. A, Timeline of SLIT for grass pollen allergy between May 2014 and 2017. Time points indicate blood sampling. B, Allergic rhinitis symptoms by visual analog scale measured during peak pollen season. C, Wheal diameter (in mm) from skin prick test (SPT) with RGP. D, Fractional exhaled nitric oxide (FeNO) measured immediately prior to starting SLIT. E, Total IgE in serum. Each dot represents one individual; red lines indicate median values. Statistical analysis was performed between baseline and each follow‐up time point to assess changes induced by SLIT using the Wilcoxon signed‐rank test; *P < .05, **P < .01, and ***P < .001
2.2. Participant characteristics
Participants were recruited from The Alfred Hospital Allergy Clinic, Melbourne, Victoria, Australia. All had well‐characterized moderate‐to‐severe seasonal allergic rhinitis (plus or minus asthma) due to RGP allergy with positive serum RGP‐specific IgE (≥0.35 kU/L; ImmunoCAP, Phadia). Exclusion criteria were a co‐existing immunodeficiency, previous immunotherapy within the last 5 years, ongoing immunotherapy with other allergens, and treatment with continuous oral corticosteroids and/or β‐blockers. The use of usual medications for allergic rhinitis was permitted, including antihistamines and topical corticosteroids. Alfred Hospital Research and Ethics Committee approval and written informed consent from each participant were obtained prior to inclusion (project number 514/13). Twenty‐nine participants (12 males) were recruited for treatment, with a mean age of 35 years (range 18‐59 year) and mean serum RGP‐specific IgE of 52 kU/L. Five withdrew after the first (baseline) time point (n = 4 tongue swelling, upset stomach; n = 1 failed to attend) leaving 24 participants who commenced SLIT (Table S1). At subsequent time points, three participants failed to attend after 4 months of treatment and a further seven participants were excluded after 1 year (n = 2 opted to receive SCIT, n = 2 opted to receive sublingual drops, and n = 3 withdrew). Blood samples for serum and flow cytometric analysis were obtained from n = 24 patients in May 2014 prior to starting SLIT, n = 24 in September 2014, n = 21 in May 2015, n = 14 in May 2016, and n = 14 in May 2017. Details on sample numbers for each analysis are included in Table S2. A further 5 RGP‐allergic subjects who did not receive SLIT (ie, received usual medication alone) were included as untreated patients at baseline and 4 months.
2.3. Clinical parameters of allergic rhinitis
Allergic rhinitis symptoms during the peak RGP season were recorded by the participants using a visual analog score (VAS; scale, 0‐100). Fractional exhaled nitric oxide (FeNO, in parts per billion [ppb]; HypoAirFeNO) was measured according to the manufacturer's instructions (NIOX, Uppsala, Sweden). FeNO was measured immediately before the start of SLIT therapy outside of the grass pollen season to minimize effects of daily fluctuations in pollen levels.
2.4. Quantification of serum total IgE and allergen‐specific IgE, IgG2, and IgG4
Serum total IgE, RGP‐specific‐IgE, and ‐IgG4 levels were measured by ImmunoCAP. Serum RGP‐specific IgG2 antibodies were measured by in‐house ELISA, as described previously.44 Briefly, ELISA plate wells were coated with an aqueous RGP extract (Stallergenes Greer), blocked with 2% bovine serum albumin in PBS (Sigma‐Aldrich), and incubated with serial dilutions of serum samples. Separate wells were coated with serial dilutions of purified human IgG2 (Sigma‐Aldrich, #I5404) to generate a standard curve for quantification of IgG2 in serum samples. Bound IgG2 was detected using biotinylated anti‐hIgG2 (clone HP6002; Thermo Scientific) followed by Pierce High Sensitivity Streptavidin‐HRP (Thermo Scientific). ELISA was developed using TMB (Thermo Scientific), and the reaction stopped with 1 mol/L HCl. Absorbance (OD 450 nm) was measured using a FLUOstar Optima plate reader (BMG Labtech).
2.5. In vitro RGP stimulation of PBMC, Treg staining, and measurement of cytokines
PBMC were isolated by Ficoll‐paque density centrifugation. Fresh PBMC were used for in vitro culture, and the remaining cells stored in liquid nitrogen. PBMC were labeled with CFSE (0.5 µmol/L CFSE/107 PBMC; Molecular Probes) and cultured with an aqueous RGP extract (50 μg/mL; Stallergenes Greer) or tetanus toxoid (20 Lfu/mL; Statens Serum Institut, Copenhagen, Denmark). On day 7, cells were stained with CD4‐PE Cy7, CD25‐PE (both from BD Biosciences), FoxP3‐APC (eBioscience), and aqua live/dead dye (Life Technologies). The Treg gating strategy is shown in Figure S1. Data were acquired using an LSR‐II flow cytometer (BD Biosciences).
The levels of IFN‐γ, IL‐5, IL‐10, and IL‐13 in 7‐day culture supernatants were determined using a Luminex human premixed multi‐analyte kit (R&D Systems Inc) according to the manufacturer's instructions. Due to changes in IL‐5 production observed after 3 years of SLIT, IL‐13 was also assessed at the same time point to further investigate Th2 cytokine production. Tetanus toxoid was included as a control antigen to determine RGP specificity. “No antigen” values were subtracted from test values.
2.6. B‐cell subset analysis by flow cytometry
One million thawed PBMC were incubated with 11‐color antibody cocktails against B‐cell markers for 15 minutes at room temperature in 100 µL total volume (Table S3). Flow cytometric analyses were performed on a 4‐laser LSRFortessa (BD Biosciences), and data were analyzed using FACSDiva V8.0 (BD Biosciences). B‐cell subsets were defined as described previously.41, 45, 46 Briefly, within the CD19+ B‐cell population, the proportions were determined of plasmablasts (CD27+CD38high), transitional (CD27−CD38high), naive mature (CD27−IgM+IgD+), natural effector memory B cells (CD27+IgM+IgD+), and IgM‐only memory B cells (CD27+IgM+IgD–). Furthermore, we analyzed Ig switched CD27−CD38dim and CD27+CD38dim memory B cells expressing IgA, IgE, IgG, or each of the 4 IgG subclasses.
2.7. Molecular analysis of Ig gene rearrangements
RNA was isolated from PBMC from a limited cohort of 5 subjects treated with SLIT (Table EI; patient no. 1, 11, 13, 15, and 19) with a GenElute mammalian RNA kit (Sigma‐Aldrich) and reverse transcribed to cDNA with random primers (Invitrogen Life Technologies, Waltham, MA). Rearranged IgG transcripts were amplified in a multiplex PCR approach using 4 different IGHV family leader forward primers in combination with an IGHG‐consensus reverse primer.47 PCR products were cloned into a pGEMT easy vector (Promega), amplified by colony PCR, and sequenced by the Micromon facility of Monash University on an Applied Biosystems 3730s DNA Analyzer (Thermo Scientific). Obtained sequences were analyzed using the IMGT database (http://www.imgt.org) to assign the IGHV, IGHD, and IGHJ gene alleles and to identify SHM. For each unique clone, the position and frequency of mutations were determined within the entire IGHV gene (FR1‐CDR1‐FR2‐CDR2‐FR3). SHM was determined as variations on the best‐matched V‐gene and represented as the percentage of mutations of the total sequenced V‐gene nucleotides. The IgG subclasses were determined using the IGH reference sequence (NG_001019).
2.8. Statistical analysis
Differences in symptom scores, serum Ig values, cytokines, and B‐ and T‐cell subsets before, during, and after treatment were analyzed with the Wilcoxon signed‐rank test. All analyses were two‐tailed, and differences were considered statistically significant if P‐values were <.05. Due to missing values at year 1, 2, and 3 measures, it was not possible to use repeated measures ANOVA. Therefore, we performed pairwise analysis between time point 0 and each follow‐up sample. Differences in IgG subclass usage of unique IGH transcripts were statistically analyzed with the chi‐squared test. Statistical analysis was performed using GraphPad Prism software, version 7.01 (GraphPad Software).
3. RESULTS
3.1. SLIT reduces symptoms of allergic rhinitis
To study the clinical effects of SLIT, we assessed the severity of symptoms for allergic rhinitis using a VAS. Before the start of treatment, participants reported a median VAS of 80 mm for the 2013 pollen season (Figure 1B). In the first pollen season after commencing SLIT, participants experienced fewer symptoms (median VAS 40 mm, P < .001), and these remained low for the second and third seasons following repeat SLIT courses (median VAS 20 mm at 2 years, P < .001; median VAS 35 mm at 3 years, P < .01), confirming sustained clinical efficacy.
The level of RGP sensitization was monitored by skin prick tests (SPT) with wheal diameters (mm) positively correlating with symptoms of allergic disease.48 SLIT significantly decreased SPT wheal diameter in response to SPT with RGP extract within 1 year of commencing therapy (Figure 1C). Wheal size remained low on retesting after the third year of SLIT. In addition, the severity of airway inflammation and bronchial hyperreactivity was assessed by measurement of FeNO.49, 50 SLIT significantly decreased FeNO from baseline at 1, 2, and 3 years after commencing SLIT (Figure 1D). The significant decreases in SPT wheal diameter and FeNO were consistent with decreased symptoms of allergic rhinitis 1 year after commencing treatment as well as after the second and third successive years of SLIT. The reduction in VAS and FeNO after SLIT did not correspond with any changes in total serum IgE levels (Figure 1E).
3.2. Induction of RGP‐specific Treg and IL‐10 production following SLIT
Successful AIT has been associated with proliferation of allergen‐specific Treg.50 To assess Treg proliferation in response to allergen, we stimulated PBMC with RGP and measured the proliferation of activated Treg (CD4+CD25+FoxP3+) using CFSE. RGP‐induced proliferation of Treg was enhanced by SLIT after 4 months and remained raised throughout subsequent years (Figure 2A).
Figure 2.

Sublingual immunotherapy (SLIT) alters in vitro Treg proliferation and cytokine production in response to RGP. PBMC stimulated with RGP were assessed for A, Treg proliferation and production of B, IL‐10, C, IFN‐γ, and D, IL‐5 were determined for all patients included at t = 0, 1, 2, 3 y. E. Paired analysis of IL‐5 and F. IL‐13 at t = 0 and t = 3 y. The lower limit of detection of IL‐10 levels (0.5 pg/mL) in panel B is depicted by a dashed line and datapoints representing undetectable levels are placed below it. Statistical analysis was performed between baseline and each follow‐up time point to assess changes induced by SLIT using the Wilcoxon signed‐rank test; *P < .05, **P < .01, ***P < .001 and ****P < .0001
Given the role of cytokines in skewing T‐cell responses, we quantified IL‐5, IL‐10, IL‐13, and IFN‐γ production from RGP‐stimulated PBMC. After 3 years of SLIT, T cells produced significantly more IL‐10 and IFN‐γ and significantly less IL‐5 and IL‐13 (Figure 2B‐E). SLIT did not alter the PBMC cytokine response to tetanus toxoid (Figure S2). Taken together, the data from our study suggest SLIT enhances Treg response to allergen within 4 months without impacting the Treg response to other antigens. Furthermore, SLIT may alter the Th1/Th2 cytokine profile away from pro‐allergic Th2 cytokines toward a regulatory and Th1‐biased response.
3.3. Increased IgG4 serum levels and IgG4 + memory B‐cell frequencies after 4 months SLIT
To study the short‐term effects of SLIT on the immune system, we analyzed serum Ig levels and B‐cell subsets before and directly after the first 4 months of therapy. The gating strategies for flow cytometric detection of memory B cells expressing the four IgG subclasses are shown in Figure 3A. We observed that after 4 months of immunotherapy, RGP‐specific serum IgG2 increased from a median of 2.46 to 5.08 μg/mL (Figure 3B). Furthermore, all participants showed an increase in RGP‐specific serum IgG4 from a median of 0.37 μg/mL pretreatment to 1.16 μg/mL post‐treatment after 4 months of SLIT. This was accompanied by a significant increase in the frequency of IgG+ memory B cells (CD19+CD38dim) expressing IgG4 (Figure 3C). The increase in the IgG4 + memory B‐cell frequencies was not directly correlated with the increase in RGP‐specific serum IgG4 (p > .05). SLIT did not change IgE+ memory B‐cell frequencies (Figure 3D). However, the increase in IgG4 + memory B cells resulted in a significantly higher IgG4 +/IgE+ memory B‐cell ratio following 4 months of treatment (Figure 3E). The frequencies of all other B‐cell subsets, including transitional, naive mature, memory, and plasmablasts, remained unchanged after 4 months of SLIT (Figure S3). Thus, 4 months SLIT quite specifically affected allergen‐specific IgG4 serum levels and the frequencies of IgG4‐expressing memory B cells.
Figure 3.
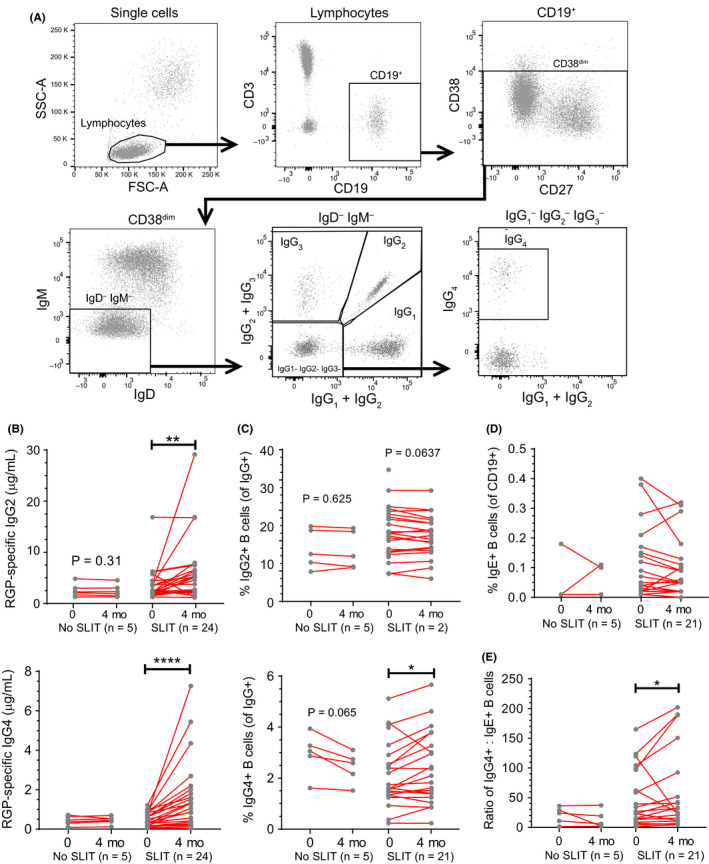
RGP‐specific IgG4 and IgG4 +:IgE+ memory B‐cell ratio increased after a 4‐mo course of SLIT. A, Gating strategy for Ig isotype and IgG subclass‐expressing memory B cells by flow cytometry. B, RGP‐specific IgG2 and IgG4 in sera and C, proportion of IgG2 + and IgG4 + memory B cells as a percentage of IgG+ population after 4 mo of SLIT. D, Proportion of IgE+ memory B cells as a percentage of total CD19+ B cells after 4 mo of SLIT. E, Ratio of IgG4 + to IgE+ B‐cell percentages after 4 mo of SLIT (from C and D). Each dot represents one individual; red lines indicate median values. Statistical analysis was performed between baseline and each follow‐up time point to assess changes induced by SLIT using the Wilcoxon signed‐rank test. *P < .05 and ****P < .0001
3.4. SLIT has persistent long‐term effects on IgG2 and IgG4 memory B cells
In addition to short‐term effects of SLIT, we studied the longer‐term effects of SLIT, that is, 1, 2, and 3 years after the start of the first treatment course. SLIT did not significantly alter serum RGP‐specific IgE levels (Figure 4A). RGP‐specific IgG2 levels increased after a total of 3 courses of SLIT (Figure 4A). RGP‐specific IgG4 increased after each consecutive course of treatment at 1, 2, and 3 years (Figure 4A). Similar to RGP‐specific IgE and IgG2 antibodies, frequencies of IgE+ memory B cells were unchanged by SLIT, while IgG2 + memory B cells were significantly increased 3 years after commencing SLIT (Figure 4B). Frequencies of IgG4 + memory B cells were increased 2 years after commencing SLIT.
Figure 4.
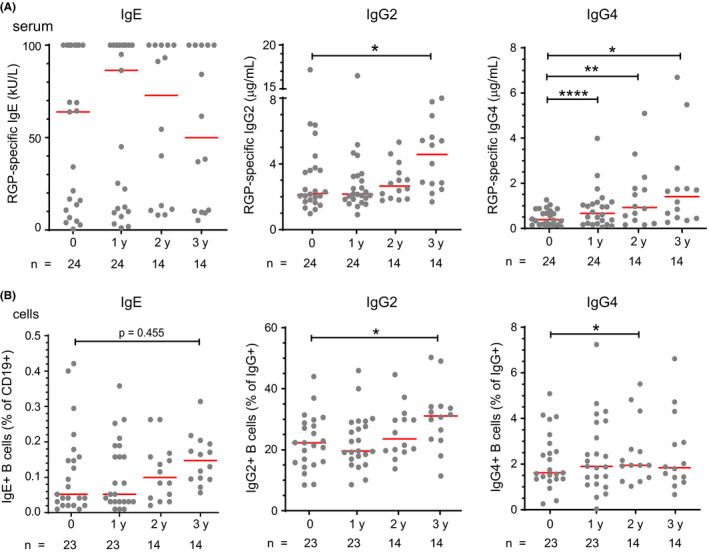
Persistent increase in IgG4 and late rise in IgG2 after three 4‐mo SLIT courses. A, RGP‐specific IgE, IgG2 and IgG4 in serum. B, Proportions of IgE+, IgG2 +, and IgG4 + memory B cells in peripheral blood. IgE+ memory B cells presented as a percentage of CD19+ B cells. IgG2 + and IgG4 + memory B cells presented as a percentage of IgG+ memory B cells. Baseline data for IgE+ and IgG4+ B cells are the same as those in Figure 3C and D. Statistical analysis was performed between baseline and each follow‐up time point to assess changes induced by SLIT using the Wilcoxon signed‐rank test; *P < .05 and **P < .01
3.5. Molecular analysis of Ig gene rearrangements
Given that SLIT increased IgG2 and IgG4 antibodies and memory B‐cell proportions, we investigated whether these changes were reflected in the proportions of unique IgG transcripts from blood B cells for a subgroup of 5 participants with an increased percentage of IgG2 + memory B cells after SLIT. Median frequencies of somatic hypermutations did not differ between the proximal IgG1 and the distal IgG2 subclasses (Figure 5A and B) nor were significantly different after 3 years SLIT. However, after 3 years SLIT the relative usage of the IgG2 and IgG4 subclasses were significantly increased at the expense of IgG1 (Figure 5C). Taken together, these data demonstrate that repeat courses of SLIT for grass pollen allergy induce allergen‐specific IgG2 and IgG4 responses, evidenced by an increase in IgG2 + and IgG4 + B‐cell proportions and skewing toward unique IgG2 and IgG4 transcripts.
Figure 5.
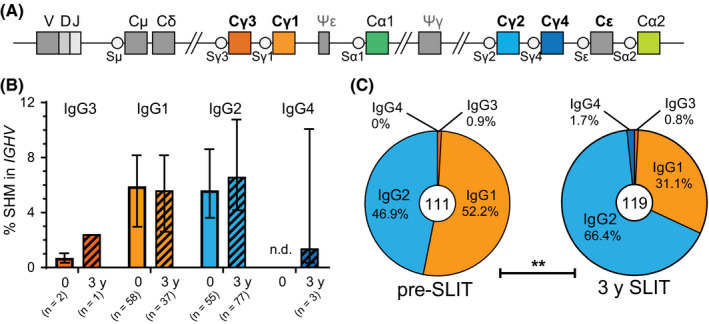
Sublingual immunotherapy (SLIT) increases frequency of unique IgG2 transcripts. A, Schematics of the human IGH locus depicting the positioning of the constant gene regions relative to the rearranged VDJ exon. B, Somatic hypermutation frequencies of unique IgG transcripts obtained from 5 patients before (2014) and after SLIT (2017) and grouped per IgG subclass. C, Relative isotype distribution of unique IgG transcripts. Central number indicates total unique IgG sequences identified. Significance was determined by chi‐squared test; **P = .0011
4. DISCUSSION
We here report that SLIT for grass pollen allergy not only has long‐term beneficial clinical effects, but also results in sustained systemic effects on the immune system. SLIT induced a rapid and prolonged increase in RGP‐specific serum IgG4 accompanied by an increase in the frequency of peripheral blood IgG4 + memory B cells. Furthermore, repeat courses of SLIT resulted in a similar increase in RGP‐specific IgG2 in serum corresponding with increased frequency of IgG2 + memory B cells in the blood.
Currently, grass pollen SLIT is recommended as a preseasonal and co‐seasonal course starting 4 months prior to the hay fever season, confirmed by meta‐analyses as clinically effective.2 Yet, long‐term treatment regimens are costly and discourage treatment adherence.51 As patients are exposed to grass pollens during the spring season, we reasoned that a 4 months preseasonal treatment regimen would avoid the risk of adding to excessive and unpredictable allergen loads during the Melbourne Spring. Based on our analysis of symptom scores, this approach is highly effective.42, 43 Prolonged treatment (duration > 12 months) is known to have beneficial effects on symptom and medication scores.52 The fact that some immunological effects are delayed, only occurring after the second or third treatment year as observed for serum RGP‐specific IgG2 levels, or continuing to rise after consecutive treatment as for serum RGP‐specific IgG4 levels, supports these premises.
In particular, we observed a marked increase in RGP‐specific IgG. Previously, allergen‐specific immunotherapy, either SCIT or SLIT, has already been demonstrated to result in increased allergen‐specific IgG4 serum levels.53, 54 Increased allergen‐specific IgG4 has been postulated as one of the explanations for the beneficial effects of immunotherapy and has been observed as a natural effect in beekeepers exposed to bee venom for prolonged periods,55 yet the exact desensitizing effect of specific IgG4 in immunotherapy remains unclear. Allergen‐specific IgG4 can competitively inhibit IgE from binding to allergens and may subsequently reduce allergic responses by preventing FcεR‐mediated activation of granulocytes.56 Furthermore, IgG4 antibody has been proposed to inhibit inflammatory responses by preventing C1q complement activation and binding to the inhibitory receptor FcγRIIb (CD32b).57, 58
SLIT also increased RGP‐specific IgG2 after three consecutive courses of SLIT. This suggests repeated or high‐dose exposure to RGP from SLIT is required to enhance RGP‐specific IgG2 beyond that which is generated from annual RGP exposure during the pollen season. Furthermore, sublingual administration of RGP may have preferentially induced an IgG2 response not seen from environmental exposure through the airway.
The immune mechanisms by which allergen‐specific IgG2 may contribute to the benefits of immunotherapy remain unclear. IgG2 has been shown to inhibit histamine release from basophils by activating FcγRIIb and may reduce allergic symptoms by this mechanism.59 In a similar manner to IgG4, IgG2 may also bind allergen and prevent effector cell degranulation by masking IgE epitopes.
The source of increased serum IgG2 and IgG4 levels is IgG2‐ and IgG4‐producing plasma cells, respectively. As the majority of serum IgG is produced by bone marrow residing plasma cells, we were unable to assess these. However, we did assess the more immature plasmablasts in blood, finding that their frequencies were not affected by SLIT. Further characterization of Ig isotypes and IgG subclasses was not possible due to our approach for membrane staining, since the majority of plasmablasts (especially those producing IgG) lack surface Ig expression. Hence, we focused on the analysis of memory B cells, which are abundant in the blood due to their circulatory nature, and their capacity to quickly differentiate into plasma cells in subsequent antigen responses.60
We observed that SLIT drives increased frequencies of IgG2 + and IgG4 + memory B cells, whereas there was no effect on frequencies of IgE+ memory B‐cell subsets. The latter observation can explain the absence of a decline in IgE serum levels as also demonstrated by others.52, 61, 62 Since we observed the increase in IgG4 + memory B cells after 4 months of treatment with SLIT, and before the pollen season, this effect can be directly attributed to the treatment with Oralair®. Our observation that frequencies of IgG4 + memory B cells remain increased for at least 3 years can be an explanation for the long‐lasting effects attributed to immunotherapy.5, 6, 7
Allergen‐specific IgG2 and IgG4 appear to be robust markers of repeated allergen exposure. Increases in allergen‐specific IgG2 and IgG4 alongside increased frequency of IgG2 + and IgG4 + memory B cells may arise from class switching of allergen‐specific IgG1 + memory B cells upon repeated exposure to allergen. Sequential Ig class switching can only occur 5’–3’ along the IGH locus, which is arranged in the following order: IgG3 > IgG1 > IgG2 > IgG4 > IgE > IgA (Figure 5A). As such, IgG1 + B cells may switch to IgG2 or IgG4 but not vice versa. In a study of allergen‐specific antibodies in children from birth to 10 years old, IgE responses to aeroallergens were typically preceded by IgG.63 Allergen‐specific IgG+ memory B cells may therefore provide a reservoir for switching to IgE that can be induced by repeated exposure to the allergen and subsequently cause allergic sensitization. A similar pathway may give rise to allergen‐specific IgG2 and IgG4, whereby allergen exposure promotes class switching of allergen‐specific IgG1 + memory B cells to IgG2 and IgG4. Switching to IgG2 and IgG4 may help explain why many allergies subside with age, perhaps due to repeated allergen exposure throughout childhood. However, it remains to be determined whether allergen‐specific IgG2 and IgG4 are indispensable for generating clinical benefit by SLIT in lieu of T cell–mediated tolerance.
In line with previous reports, we observed that proliferation of Treg from patients after SLIT was increased in response to in vitro stimulation with RGP. Previous studies have observed new generation of allergen‐specific Treg, as well as clonal expansion of allergen‐specific Treg in response to AIT.56, 64 Furthermore, we observed that SLIT increased IL‐10 production from RGP‐stimulated PBMC, whereas IL‐13 was diminished. The cytokines IL‐4 and IL‐13 promote Ig class switch recombination (CSR) to IgE, and these are predominantly produced by Th2 cells. In addition to Th2 cytokines, CSR to IgG4 is also regulated by IL‐10 which is predominantly secreted by Treg. Our data are consistent with SLIT‐induced proliferation of allergen‐specific Treg, whereby increased IL‐10 production induces Ig class switching of allergen‐specific B cells to IgG4. B regulatory cells (Breg) and monocyte‐derived macrophages may also be an alternative source of IL‐10 in our in vitro assay, further enhancing IgG4 class switching in response to RGP.65, 66
In conclusion, our data provide evidence for long‐lasting effects of allergen SLIT on the memory compartment of the immune system. Increased Treg frequencies and increased IL‐10 production were associated with increased frequency of IgG4 + memory B cells and a beneficial shift in the IgG4 +/IgE+ memory B‐cell ratio, reflecting the increased IgG4/IgE antibody fraction in serum and resultant clinically favorable outcome. Moreover, to our knowledge, our study is the first to demonstrate increases in memory B cells expressing IgG2 or IgG4 following allergen immunotherapy. As IgG2 and IgG4 have anti‐inflammatory properties and are induced following repeated antigen‐exposure,41 this B‐cell memory compartment is a potential mechanism by which allergen immunotherapy modifies the natural course of disease. In future studies, it would therefore be of interest to examine the functional properties of allergen‐specific B cells, as well as the effector functions of allergen‐specific IgG subclasses.67
CONFLICT OF INTEREST
All authors declare that no conflict of interest exists.
Supporting information
ACKNOWLEDGMENTS
The authors gratefully acknowledge Ms Kirsten Deckert for collection of clinical data and blood samples from patients, and Ms Tracy Phan for technical assistance. We are also grateful to the AMREP flow cytometry team for technical assistance. The studies were financially supported by a grant from the Num Pon Soon Charitable Trust, grant S698 from the Sophia Children's Hospital Fund (SKF) and an NHMRC Senior Research Fellowship GNT1117687 to MCvZ.
Heeringa JJ, McKenzie CI, Varese N, et al. Induction of IgG2 and IgG4 B‐cell memory following sublingual immunotherapy for ryegrass pollen allergy. Allergy. 2020;75:1121–1132. 10.1111/all.14073
Jorn J. Heeringa and Craig I. McKenzie equal contribution.
REFERENCES
- 1. Björkstén B, Clayton T, Ellwood P, Stewart A, Strachan D, Group IPIS . Worldwide time trends for symptoms of rhinitis and conjunctivitis: phase III of the international study of asthma and allergies in childhood. Pediatr Allergy Immunol. 2008;19(2):110‐124. [DOI] [PubMed] [Google Scholar]
- 2. Di Bona D, Plaia A, Leto‐Barone MS, La Piana S, Di Lorenzo G. Efficacy of grass pollen allergen sublingual immunotherapy tablets for seasonal allergic rhinoconjunctivitis: a systematic review and meta‐analysis. JAMA Intern Med. 2015;175(8):1301‐1309. [DOI] [PubMed] [Google Scholar]
- 3. Di Bona D, Plaia A, Scafidi V, Leto‐Barone MS, Di Lorenzo G. Efficacy of sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a systematic review and meta‐analysis. J Allergy Clin Immunol. 2010;126(3):558‐566. [DOI] [PubMed] [Google Scholar]
- 4. Devillier P, Molimard M, Ansolabehere X, et al. Immunotherapy with grass pollen tablets reduces medication dispensing for allergic rhinitis and asthma: A retrospective database study in France. Allergy. 2019;74(7):1317‐1326. [DOI] [PubMed] [Google Scholar]
- 5. Durham SR, Walker SM, Varga EM, et al. Long‐term clinical efficacy of grass‐pollen immunotherapy. N Engl J Med. 1999;341(7):468‐475. [DOI] [PubMed] [Google Scholar]
- 6. Durham SR, Emminger W, Kapp A, et al. Long‐term clinical efficacy in grass pollen‐induced rhinoconjunctivitis after treatment with SQ‐standardized grass allergy immunotherapy tablet. J Allergy Clin Immunol. 2010;125(1):131‐138. [DOI] [PubMed] [Google Scholar]
- 7. Durham SR, Emminger W, Kapp A, et al. SQ‐standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129(3):717‐725. [DOI] [PubMed] [Google Scholar]
- 8. Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six‐year follow‐up study. Clin Exp Allergy. 2001;31(9):1392‐1397. [DOI] [PubMed] [Google Scholar]
- 9. Moller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT‐study). J Allergy Clin Immunol. 2002;109(2):251‐256. [DOI] [PubMed] [Google Scholar]
- 10. Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen‐specific immunotherapy. Nat Rev Immunol. 2006;6(10):761‐771. [DOI] [PubMed] [Google Scholar]
- 11. Akdis CA, Akdis M. Advances in allergen immunotherapy: aiming for complete tolerance to allergens. Sci Transl Med. 2015;7(280):280ps6‐280ps6. [DOI] [PubMed] [Google Scholar]
- 12. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S73‐S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class‐switch recombination. Nat Rev Immunol. 2003;3(9):721‐732. [DOI] [PubMed] [Google Scholar]
- 14. Takatsu K, Kouro T, Nagai Y. Interleukin 5 in the link between the innate and acquired immune response. Adv Immunol. 2009;101:191‐236. [DOI] [PubMed] [Google Scholar]
- 15. Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol. 2004;113(3):395‐400. [DOI] [PubMed] [Google Scholar]
- 16. Ebner C, Siemann U, Bohle B, et al. Immunological changes during specific immunotherapy of grass pollen allergy: reduced lymphoproliferative responses to allergen and shift from TH2 to TH1 in T‐cell clones specific for Phl p 1, a major grass pollen allergen. Clin Exp Allergy. 1997;27(9):1007‐1015. [DOI] [PubMed] [Google Scholar]
- 17. Gardner LM, O'Hehir RE, Rolland JM. High dose allergen stimulation of T cells from house dust mite‐allergic subjects induces expansion of IFN‐gamma+ T Cells, apoptosis of CD4+IL‐4+ T cells and T cell anergy. Int Arch Allergy Immunol. 2004;133(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 18. Francis JN, Till SJ, Durham SR. Induction of IL‐10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003;111(6):1255‐1261. [DOI] [PubMed] [Google Scholar]
- 19. Mobs C, Slotosch C, Loffler H, Jakob T, Hertl M, Pfutzner W. Birch pollen immunotherapy leads to differential induction of regulatory T cells and delayed helper T cell immune deviation. J Immunol. 2010;184(4):2194‐2203. [DOI] [PubMed] [Google Scholar]
- 20. Varona R, Ramos T, Escribese MM, et al. Persistent regulatory T‐cell response 2 years after 3 years of grass tablet SLIT: Links to reduced eosinophil counts, sIgE levels, and clinical benefit. Allergy. 2019;74(2):349‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gardner LM, Thien FC, Douglass JA, Rolland JM, O'Hehir RE. Induction of T 'regulatory' cells by standardized house dust mite immunotherapy: an increase in CD4+ CD25+ interleukin‐10+ T cells expressing peripheral tissue trafficking markers. Clin Exp Allergy. 2004;34(8):1209‐1219. [DOI] [PubMed] [Google Scholar]
- 22. Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen‐IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003;112(5):915‐922. [DOI] [PubMed] [Google Scholar]
- 23. Scadding GW, Shamji MH, Jacobson MR, et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3‐expressing cells and elevated allergen‐specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E‐facilitated allergen binding to B cells. Clin Exp Allergy. 2010;40(4):598‐606. [DOI] [PubMed] [Google Scholar]
- 24. O'Hehir RE, Gardner LM, de Leon MP, et al. House dust mite sublingual immunotherapy: the role for transforming growth factor‐beta and functional regulatory T cells. Am J Respir Crit Care Med. 2009;180(10):936‐947. [DOI] [PubMed] [Google Scholar]
- 25. Akdis CA, Akdis M. Mechanisms of allergen‐specific immunotherapy and immune tolerance to allergens. World Allergy Organ J. 2015;8(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Royer B, Varadaradjalou S, Saas P, Guillosson JJ, Kantelip JP, Arock M. Inhibition of IgE‐induced activation of human mast cells by IL‐10. Clin Exp Allergy. 2001;31(5):694‐704. [DOI] [PubMed] [Google Scholar]
- 27. Schandene L, Alonso‐Vega C, Willems F, et al. B7/CD28‐dependent IL‐5 production by human resting T cells is inhibited by IL‐10. J Immunol. 1994;152(9):4368‐4374. [PubMed] [Google Scholar]
- 28. Ohkawara Y, Lim KG, Xing Z, et al. CD40 expression by human peripheral blood eosinophils. J Clin Invest. 1996;97(7):1761‐1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL‐10. J Immunol. 1998;160(7):3555‐3561. [PubMed] [Google Scholar]
- 30. Santos AF, James LK, Bahnson HT, et al. IgG4 inhibits peanut‐induced basophil and mast cell activation in peanut‐tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015;135(5):1249‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Di Bona D, Plaia A, Leto‐Barone MS, La Piana S, Di Lorenzo G. Efficacy of subcutaneous and sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a meta‐analysis‐based comparison. J Allergy Clin Immunol. 2012;130(5):1097‐1107. [DOI] [PubMed] [Google Scholar]
- 32. Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn‐Schmid B, Ebner C. Sublingual immunotherapy induces IL‐10‐producing T regulatory cells, allergen‐specific T‐cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120(3):707‐713. [DOI] [PubMed] [Google Scholar]
- 33. Suarez‐Fueyo A, Ramos T, Galan A, et al. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T‐cell generation. J Allergy Clin Immunol. 2014;133(1):130‐138. [DOI] [PubMed] [Google Scholar]
- 34. Bahceciler NN, Arikan C, Taylor A, et al. Impact of sublingual immunotherapy on specific antibody levels in asthmatic children allergic to house dust mites. Int Arch Allergy Immunol. 2005;136(3):287‐294. [DOI] [PubMed] [Google Scholar]
- 35. Sugimoto M, Kamemura N, Nagao M, et al. Differential response in allergen‐specific IgE, IgGs, and IgA levels for predicting outcome of oral immunotherapy. Pediatr Allergy Immunol. 2016;27(3):276‐282. [DOI] [PubMed] [Google Scholar]
- 36. Rolinck‐Werninghaus C, Kopp M, Liebke C, Lange J, Wahn U, Niggemann B. Lack of detectable alterations in immune responses during sublingual immunotherapy in children with seasonal allergic rhinoconjunctivitis to grass pollen. Int Arch Allergy Immunol. 2005;136(2):134‐141. [DOI] [PubMed] [Google Scholar]
- 37. Dehlink E, Eiwegger T, Gerstmayr M, et al. Absence of systemic immunologic changes during dose build‐up phase and early maintenance period in effective specific sublingual immunotherapy in children. Clin Exp Allergy. 2006;36(1):32‐39. [DOI] [PubMed] [Google Scholar]
- 38. Berkowska MA, Driessen G, Bikos V, et al. Human memory B cells originate from three distinct germinal center‐dependent and ‐independent maturation pathways. Blood. 2011;118(8):2150‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jackson K, Wang Y, Collins AM. Human immunoglobulin classes and subclasses show variability in VDJ gene mutation levels. Immunol Cell Biol. 2014;92(8):729‐733. [DOI] [PubMed] [Google Scholar]
- 40. van Zelm MC. B cells take their time: sequential IgG class switching over the course of an immune response? Immunol Cell Biol. 2014;92(8):645‐646. [DOI] [PubMed] [Google Scholar]
- 41. de Jong BG, IJspeert H, Marques L, et al. Human IgG2‐ and IgG4‐expressing memory B cells display enhanced molecular and phenotypic signs of maturity and accumulate with age. Immunol Cell Biol. 2017;95(9):744‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O'Hehir RE, Varese NP, Deckert K, et al. Epidemic thunderstorm asthma protection with five‐grass pollen tablet sublingual immunotherapy: a clinical trial. Am J Respir Crit Care Med. 2018;198(1):126‐128. [DOI] [PubMed] [Google Scholar]
- 43. O'Hehir R, Heeringa JJ, Deckert K, Rolland JM, van Zelm MC, Hew M. Preseasonal grass pollen SLIT in at risk individuals confers protection from epidemic thunderstorm asthma [abstract]. Allergy. 2017;72(Suppl S103):759. [Google Scholar]
- 44. Davies JM, Bright ML, Rolland JM, O'Hehir RE. Bahia grass pollen specific IgE is common in seasonal rhinitis patients but has limited cross‐reactivity with Ryegrass. Allergy. 2005;60(2):251‐255. [DOI] [PubMed] [Google Scholar]
- 45. Berkowska MA, Heeringa JJ, Hajdarbegovic E, et al. Human IgE+ B cells are derived from T cell‐dependent and T cell‐independent pathways. J Allergy Clin Immunol. 2014;134(3):688‐697. [DOI] [PubMed] [Google Scholar]
- 46. Heeringa JJ, Karim AF, van Laar J, et al. Expansion of blood IgG4(+) B, TH2, and regulatory T cells in patients with IgG4‐related disease. J Allergy Clin Immunol. 2018;141(5): 1831–1843. [DOI] [PubMed] [Google Scholar]
- 47. Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT‐PCR and expression vector cloning. J Immunol Methods. 2008;329(1–2):112‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haahtela T, Burbach GJ, Bachert C, et al. Clinical relevance is associated with allergen‐specific wheal size in skin prick testing. Clin Exp Allergy. 2013;44(3):407‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ciprandi G, Tosca MA, Capasso M. Exhaled nitric oxide in children with allergic rhinitis and/or asthma: a relationship with bronchial hyperreactivity. J Asthma. 2010;47(10):1142‐1147. [DOI] [PubMed] [Google Scholar]
- 50. Verini M, Consilvio NP, Di Pillo S, et al. FeNO as a marker of airways inflammation: the possible implications in childhood asthma management. J Allergy. 2010;2010:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lin SY, Erekosima N, Kim JM, et al. Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. JAMA. 2013;309(12):1278‐1288. [DOI] [PubMed] [Google Scholar]
- 52. Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta‐analysis. Allergy. 2005;60(1):4‐12. [DOI] [PubMed] [Google Scholar]
- 53. Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen‐specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116(3):608‐613. [DOI] [PubMed] [Google Scholar]
- 54. Muller U, Helbling A, Bischof M. Predictive value of venom‐specific IgE, IgG and IgG subclass antibodies in patients on immunotherapy with honey bee venom. Allergy. 1989;44(6):412‐418. [DOI] [PubMed] [Google Scholar]
- 55. Aalberse RC, van der Gaag R, van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4‐restricted response. J Immunol. 1983;130(2):722‐726. [PubMed] [Google Scholar]
- 56. Wachholz PA, Durham SR. Mechanisms of immunotherapy: IgG revisited. Curr Opin Allergy Clin Immunol. 2004;4(4):313‐318. [DOI] [PubMed] [Google Scholar]
- 57. Anderson CL, Abraham GN. Characterization of the Fc receptor for IgG on a human macrophage cell line, U937. J Immunol. 1980;125(6):2735‐2741. [PubMed] [Google Scholar]
- 58. Lilienthal G‐M, Rahmöller J, Petry J, Bartsch YC, Leliavski A, Ehlers M. Potential of murine IgG1 and human IgG4 to inhibit the classical complement and Fcγ receptor activation pathways. Front Immunol. 2018;9:958‐958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. MacGlashan D Jr, Hamilton RG. Parameters determining the efficacy of CD32 to inhibit activation of FcERI in human basophils. J Allergy Clin Immunol. 2016;137(4):1256‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tarlinton D, Good‐Jacobson K. Diversity among memory B cells: origin, consequences, and utility. Science. 2013;341(6151):1205‐1211. [DOI] [PubMed] [Google Scholar]
- 61. Vourdas D, Syrigou E, Potamianou P, et al. Double‐blind, placebo‐controlled evaluation of sublingual immunotherapy with standardized olive pollen extract in pediatric patients with allergic rhinoconjunctivitis and mild asthma due to olive pollen sensitization. Allergy. 1998;53(7):662‐672. [DOI] [PubMed] [Google Scholar]
- 62. Lima MT, Wilson D, Pitkin L, et al. Grass pollen sublingual immunotherapy for seasonal rhinoconjunctivitis: a randomized controlled trial. Clin Exp Allergy. 2002;32(4):507‐514. [DOI] [PubMed] [Google Scholar]
- 63. Huang X, Tsilochristou O, Perna S, et al. Evolution of the IgE and IgG repertoire to a comprehensive array of allergen molecules in the first decade of life. Allergy. 2018;73(2):421‐430. [DOI] [PubMed] [Google Scholar]
- 64. Till SJ, Francis JN, Nouri‐Aria K, Durham SR. Mechanisms of immunotherapy. J Allergy Clin Immunol. 2004;113(6):1025‐1034. [DOI] [PubMed] [Google Scholar]
- 65. van de Veen W. The role of regulatory B cells in allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2017;17(6):447‐452. [DOI] [PubMed] [Google Scholar]
- 66. Bianchini R, Roth‐Walter F, Ohradanova‐Repic A, et al. IgG4 drives M2a macrophages to a regulatory M2b‐like phenotype: potential implication in immune tolerance. Allergy. 2019;74(3):483‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van Zelm MC, McKenzie CI, Varese N, Rolland JM, O'Hehir RE. Recent developments and highlights in immune monitoring of allergen immunotherapy. Allergy. 2019. 10.1111/all.14078. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


