Abstract
Aims
Early childhood anaemia, usually attributed to iron deficiency, is associated with persistent detrimental effects on child development. This study investigates the association of anaemia between age six and 23 months with indicators of childhood development at school‐age among children of remote Aboriginal and Torres Strait Islander communities of Far North Queensland.
Methods
The triennial Australian Early Development Census (AEDC) encompasses five domains of early childhood development—physical health and wellbeing, social competence, emotional maturity, language and cognitive skills (school‐based), communication skills and general knowledge. AEDC 2012 and 2015 assessments were linked with health information for children and their mothers from remote Aboriginal and Torres Strait Islander communities of Far North Queensland.
Results
AEDC assessments were available for 250 children who had measurements of haemoglobin recorded at age 6 to 23 months. More children who had had early childhood anaemia (n = 66/143, 46.2%, [37.9%, 54.4%]) were developmentally vulnerable on two or more domains compared to those who had not been anaemic (n = 25/107, 23.4% [15.2%, 31.5%], P < .001). Multivariable analysis confirmed that early childhood anaemia more than doubled the risk of developmental vulnerability (OR 2.2 [1.1, 4.3] P = .020) at school age.
Conclusions
Early childhood anaemia is a risk factor for developmental vulnerability at school‐age in this setting. Interventions combining nutrition promotion and multi‐micronutrient food fortification, are effective in prevention of early childhood anaemia. Such interventions could also improve early childhood development and subsequent educational achievement.
1. INTRODUCTION
Good nutrition in the first thousand days of life—from conception, through pregnancy to around age 2 years—supports the rapid neurodevelopment of early life that provides the scaffolding for subsequent child development.1 Neurodevelopment is “the dynamic interrelationship between environment, genes and the brain whereby the brain develops across time to establish sensory, motor, cognitive, socioemotional, cultural and behavioral adaptive functions.”2 Iron is required for specific neurodevelopment processes before birth and during the first years of life, including neuronal proliferation and growth of axons and dendrites, synapse formation and myelination.1, 3 Iron deficiency during critical phases of neurodevelopment is associated with persistent deficits in cognitive and behavioural performance.4, 5 Anaemia is a late stage of iron deficiency; the detrimental effects of iron deficiency impact on the developing brain before anaemia develops.5, 6
Anaemia—defined as low haemoglobin—among Aboriginal and Torres Strait Islander women in pregnancy and their children in early life, is prevalent in Far North Queensland and elsewhere in remote northern Australia.7, 8, 9, 10, 11 There are various causes of anaemia including nutrition‐related causes (deficiency of iron and/or folate and/or vitamin B12), chronic infections and genetic conditions.12
Anaemia in the first thousand days is usually caused by iron deficiency due to high iron requirements for the rapidly increasing blood volume and tissue growth of pregnancy and early childhood.1, 5
The Australian Early Development Census (AEDC) is a national census of early childhood development, conducted every 3 years since 2009.13 Each child in the first year of full time school is assessed by his/her teacher on five domains of early childhood development; physical health and wellbeing, social competence, emotional maturity, language and cognitive skills (school‐based), communication skills and general knowledge.13 These AEDC domains are predictive of outcomes in health, well‐being and academic success in later life.13 In Australia, AEDC results have been shown to predict subsequent National Assessment Program – Literacy and Numeracy (NAPLAN) scores for numeracy and reading.14
The 2015 AEDC census with 302 003 child participants found that 22.0% were developmentally vulnerable—scoring below the tenth centile—on one or more domains (DV1) and 11.1% developmentally vulnerable on two or more domains (DV2). More boys were developmentally vulnerable than girls.13 Studies in South Australia and the Northern Territory identified perinatal factors associated with developmental vulnerability at school entry; smoking in pregnancy; anaemia of mothers; low birth weight; prematurity among Aboriginal and non‐Aboriginal children.15, 16
Here, we report on the association of early childhood anaemia, defined as a child ever having anaemia between age 6 and 23 months, with AEDC assessment results among Aboriginal and Torres Strait Islander children of remote Far North Queensland.
2. METHODS
This retrospective cohort study used linked information for two cohorts of children and their mothers—the Cape York cohort and the 2009 to 2010 cohort. The Cape York cohort included children born between 2006 and 2008, participants in a previous unpublished study of child growth in remote Cape York communities. The 2009 to 2010 cohort included children born to Aboriginal and/or Torres Strait Islander mothers in 2009 or 2010 in Far North Queensland. Only the first child born to each mother between 2006 and 2010 was included to ensure independence of events for statistical analysis (Figure 1).
Figure 1.
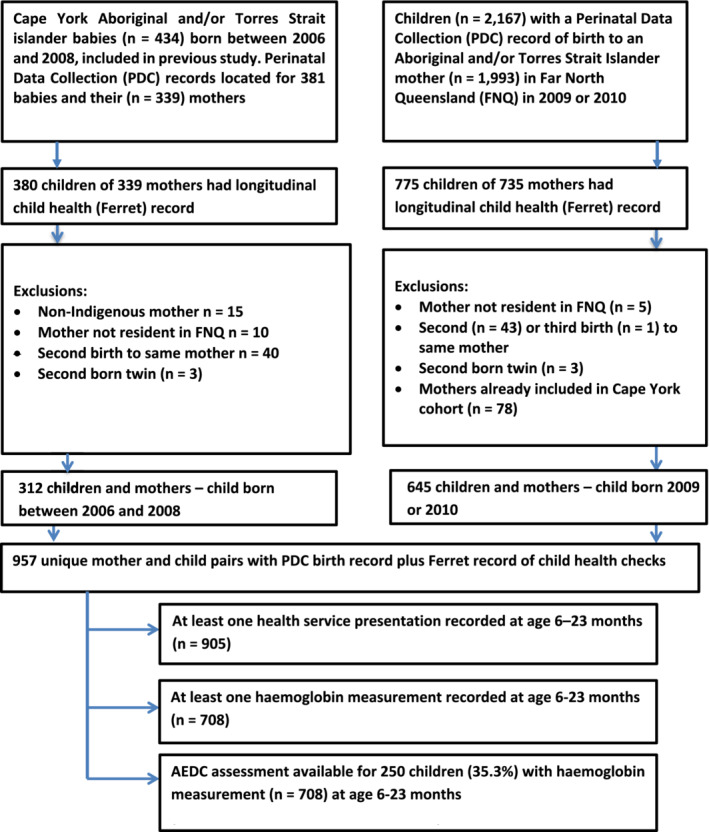
Flow diagram—association of early childhood anaemia and child development at school age among two cohorts of Aboriginal and Torres Strait Islander children and their mothers in Far North Queensland; data available and exclusions
Information was sourced from three Queensland Health data collections; (a) Queensland Pathology Services data collection (Auslab) (b) Queensland Perinatal Data Collection (PDC) (c) the Queensland Health community health electronic record system—Ferret—used in Far North Queensland remote Aboriginal and Torres Strait Islander communities (Map 1). Information recorded on Ferret includes results of routine child haemoglobin measurements from age six months. Health information for individual children and their mothers was linked to the child's assessment from AEDC 2012 or AEDC 2015. The final linked de‐identified dataset was provided to researchers in May 2017. A full description of this linkage process is described elsewhere.17 Table S1 provides more information on these data collections.
Map 1.
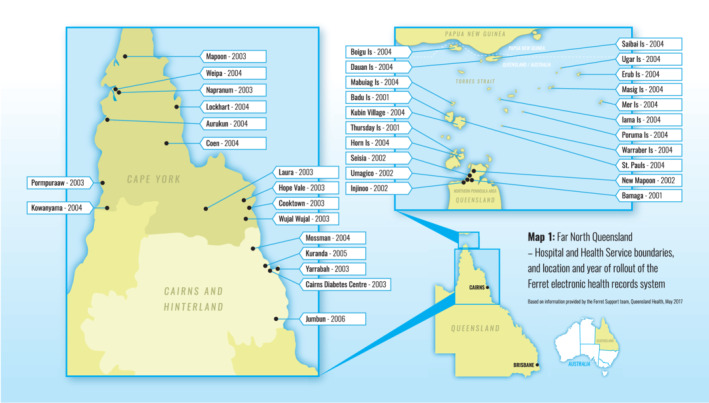
Far North Queensland—location and year of rollout of the Ferret electronic record system. Based on information provided by the Queensland Health Ferret Support Team, May 2017. Reproduced with permission of the Australian New Zealand Journal of Public Health
Ethics approval, granted by Queensland Health Far North Queensland Human Research Ethics Committee (HREC/15/QCH/50‐980) in June 2015. In February 2016, approval under the Queensland Public Health Act 2005 was granted by the Director‐General of Queensland Health. In September 2016, approval was granted by the Australian Government Department of Education and Training Australian to access the Australian Early Development Census data collection. The complete linked de‐identified data were provided to the research group in May 2017.
2.1. Study variables and definitions
Information on the outcome variables—developmental vulnerability—was sourced from the AEDC data collection.13 AEDC has developed categories of developmental status (on track, developmentally at risk, developmentally vulnerable) based on the first AEDC in 2009. Children scoring below the AEDC 2009 tenth centile for each domain are categorised as developmentally vulnerable for that domain. Children with scores below the tenth centile for one or more of the five AEDC domains are categorised as DV1. Children with scores below the tenth centile for two or more domains are categorised as DV2.13 (Table S2).
Information on the characteristics of children and mothers were sourced from the health service data collections. Anaemia in early childhood was defined using the Queensland Health age‐specific criteria; at least one haemoglobin level recorded below 105 g/L from 6 to 11 months, and/or below 110 g/L from 12 to 23 months.18
Some characteristics are as recorded on health service data collections: mother's usual residence, ethnicity, parity, smoking in pregnancy; child's sex, gestational age at birth, birth weight, method of birth. Criteria to define derived variables including maternal age, body mass index (BMI), anaemia before and during pregnancy, insufficient red cell folate levels (folate status less than optimal for women of reproductive age to prevent neural tube defects) 19, 20 and prematurity and birth weight category of the children are detailed in Table S2. Birth weight z‐scores for babies with gestational age of 33 weeks or more, were calculated using the INTERGROWTH‐21ST Neonatal Size Calculator.21, 22
Australian Bureau of Statistics ranks Statistical Local Areas by deciles of relative socio‐economic advantage and disadvantage.23 A ranking of “1” indicates greatest relative disadvantage while a ranking of “10” indicates greatest relative advantage. Mothers were allocated a Socio‐Economic Index for Areas (SEIFA 2011) rank according to usual place of residence.
2.2. Statistical analysis
Analysis was conducted using Stata version 13 (StataCorp, Lakeway Drive, College Station, Texas). Categorical variables were described using absolute and relative frequencies. The distributions of numerical variables were assessed; symmetrically distributed numerical characteristics were described using mean values and SDs; numerical values with a skewed distribution were described using median and inter‐quartile ranges (IQR).
The main outcome variables for bivariate analysis were; (a) developmentally vulnerable for one or more of the five AEDC domains—DV1; and (b) developmentally vulnerable for two or more of the five AEDC domains—DV2. The main outcome variables were presented with 95% confidence interval (95% CI).
The following characteristics of children and their mothers were considered during bi‐ and multivariable analyses: child sex; birthing method (non‐instrumental vaginal, instrumental vaginal, caesarean section); gestational age at birth; premature or not; birth weight; feeding method to age 4 months (only breast milk, only infant formula, both breast milk and formula); child had anaemia at age 6 to 23 months or not; ethnicity of mother (Aboriginal, Torres Strait Islander, both); region of residence of mother; SEIFA category for residence of mother; maternal age; BMI category of mother (underweight, normal weight, overweight, obese); categories of parity (0‐2, ≥3); smoking during pregnancy; diabetes during pregnancy; mother had insufficient red cell folate (RCF) level before or during pregnancy; mother anaemic both before and during pregnancy.
Developmental vulnerability was compared by these characteristics of the children and their mothers, using bivariate logistic regression analyses. In addition, the association of developmental vulnerability for each domain was assessed for; mother anaemic both before and during pregnancy; smoking during pregnancy; early childhood anaemia, using Pearson's χ² tests.
Multivariable logistic regression analyses were conducted to identify independent risk factors for children considered developmentally vulnerable on two or more domains (DV2) for the complete case analysis. Backward and forward stepwise modelling procedures were initially conducted to establish a basic multivariable model. Characteristics that were not part of the basic model were assessed for potential confounding effects. A confounder was assumed to be a variable that changed estimates of characteristics in the basic model by 10% or more.24
Univariate multiple imputation was conducted using Stata's MI commands for missing values for one characteristic: mother anaemic both before and during pregnancy (n = 29). Other characteristics with missing values, including feeding method, parity, BMI of mother, diabetes in pregnancy, and insufficient RCF levels of mother were not imputed because these variables had shown no statistically significant influence on the main outcome variables during bi‐ and multivariable analyses.
The occurrence of missing values of mother anaemic both before and during pregnancy (n = 29) was found unrelated to DV2 using Pearson's χ² test. The pattern of missing values was assessed and judged to be “missing at random”.25 Logistic regression was used for imputation. Imputation models were based on variables with nil missing data; birth weight of baby; smoking during pregnancy; early childhood anaemia; sex of baby; gestational age of baby; AEDC developmental assessment; birthing method; ethnicity of mother, age of mother, SEIFA index; residential region; and cohort. Twenty imputed data sets were created. Multivariable logistic regression analyses were conducted to identify independent risk factors for DV2 for imputed data.
Results of multivariable models for complete case and imputed data analyses are presented as odds ratios (OR) and 95% confidence intervals. P values of less than .05 were considered statistically significant. The authors used the STROBE checklist for cohort studies to guide the preparation of this paper.26
3. RESULTS
Ferret longitudinal health records were available for 1155 children of whom 957 were the first child born to his/her mother in the cohort years (Figure 1). Of these 957 children, 708 children had a record of haemoglobin measurement at age 6 to 23 months. Just over one‐third (35.3%, n = 250) of the 708 children also had a record of AEDC assessment. More than half (58.0%; 95% CI 51.6%, 64.2%) of these 250 children were assessed as DV1 and approximately one third (36.4%; 95% CI 30.4%, 42.7%) as DV2 (Table 1).
Table 1.
Developmental vulnerability (in lower 10th percentile of AEDC assessment score at first year of full‐time school) in one or more domains (DV1) and in two or more domains (DV2) by characteristics of the child—bivariate analysis (logistic regression)
| Part A | |||||
|---|---|---|---|---|---|
| Characteristics of children | Number | AEDC—DV1 (Y) n = 145 (58.0%) | P‐values (DV1 logistic regression) | AEDC—DV2 (Y)n = 91 (36.4%) | P values (DV2 logistic regression) |
| Sex n = 250 | |||||
| Boys | 132 | 88 (66.7%) [58.5%, 74.8%] | P = .004* | 62 (47.0%) [38.3%, 55.6%] | P < .001* |
| Girls | 118 | 57 (48.3%) [39.2%, 57.5%] | 29 (24.6%) [16.7%, 32.5%] | ||
| Birth weight category n = 250 | |||||
| Low birth weighta | 31 | 19 (61.3%) [43.1%, 79.5%] | P = .713 | n = <15 | P = .401 |
| Normal birth weight | 199 | 115 (57.8%)[50.9%, 64.7%] | Base | 68 (34.2%) [27.5%, 40.8%] | Base |
| Macrosmic (>4000 g)a | 20 | n ≤ 15 | P = .810 | n ≤ 15 | P = .164 |
| Premature birth n = 250 | |||||
| Child—premature birtha | 32 | 20 (62.5%) [44.8%, 80.2%] | P = .581 | n ≤ 15 | P = .356 |
| Child—full term birth | 218 | 125 (57.3%) [50.7%, 64.0%] | 77 (35.3%) [28.9%, 41.7%] | ||
| Birth method n = 250 | |||||
| Vaginal | 172 | 99 (57.6%)[50.1%, 65.0%] | Base | 67 (39.0%) [32.0%, 46.3%] | Base |
| Vaginal instrumentala | n ≤ 15 | n ≤ 15 | P = .845 | n ≤ 15 | P = .186 |
| Caesarian | 67 | 40 (59.7%) [47.6%, 71.8%] | P = .763 | 22 (32.8%) [21.3%, 44.4%] | P = .380 |
| Feeding method—birth to age 4 months n = 189 | |||||
| Breast milk only | 83 | 48 (57.8%) [47.0%, 68.7%] | Base | 34 (41.0%) [30.2%, 51.8%] | Base |
| Infant formula only | 25 | 17 (68.0%) [48.3%, 87.7%] | P = .365 | n ≤ 15 | P = .657 |
| Both breast milk and infant formula | 81 | 48 (59.3%) [48.3%, 70.2%] | P = .853 | 30 (37.0%) [26.3%, 47.8%] | P = .606 |
| Early childhood anaemia (age 6–23 months) n = 250 | |||||
| Yes | 143 | 92 (64.3%) [56.4%, 72.3%] | P = .019* | 66 (46.2%) [37.9%, 54.4%] | P < .001* |
| No | 107 | 53 (49.5%) [39.9%, 59.2%) | 25 (23.4%) [15.2%, 31.5%] | ||
| Part B | ||||||
|---|---|---|---|---|---|---|
| Characteristics of children | DV1 = No n = 105 | DV1 = Yes n = 145 | P‐values (logistic regression) | DV2 = No n = 159 | DV2 = Yes n = 91 | P‐values (logistic regression) |
| Birth weight g mean (SD) [95% CI] | 3234 (654) [3108, 3361] | 3161 (615) [3060, 3262] | P = .365 | 3181 (614) [3085, 3277] | 3210 (664) [3072, 3348] | P = .725 |
| Z‐score birthweight adjusted for sex and gestational age mean (SD) [95%CI] {n} | +0.236 (1.1) [+0.2, +0.45] {n = 102} | +0.236 (1.2) [−2.4, +2.9] {n = 143} | P = .500 | +0.13 (1.1) (−0.043, +0.30] {n = 156} | +0.27 (1.2) [+0.03, +0.52) {n = 89} | P = .381 |
| Gestational age at birth weeks median (IQR) [95%CI] | 39 (38,40) [39,40] | 39 (37,40) [29, 38] | P = .166 | 39 (38,40) [39, 39.4] | 39 (37,40) [38,39] | P = .140 |
Note: Categorical variables are shown as absolute and relative frequencies (percentages) with [95% confidence intervals]—child sex, method of birth, birth weight category, premature birth, feeding method, early childhood anaemia. Symmetrically distributed numerical variables are shown as mean (SD) and [95% confidence intervals]—birth weight, z‐score birthweight. Numerical values with a skewed distribution are shown as median and inter‐quartile range (IQR) and [95% confidence intervals] ‐ gestational age at birth.
AEDC require that results relating to cell sizes of 15 or less are not provided to protect confidentiality.
P <.05.
Bivariate analysis showed developmental vulnerability was significantly more prevalent among children who had had early childhood anaemia (DV2 n = 66/143, 46.2% [95% CI 37.9%, 54.4%]) compared to those who had not been anaemic (DV2 n = 25/107, 23.4% [95% CI 15.2%, 31.5%] P < .001). Developmental vulnerability was significantly more prevalent among boys compared to girls; children of mothers who were anaemic both before and during pregnancy; children of mothers who smoked in pregnancy. These effects were seen for both the DV1 and the DV2 categories (Tables 1 and 2). Children of Torres Strait Islander mothers were significantly less likely to be categorised as either DV1 or DV2 compared to the children of other Indigenous mothers (Table 2).
Table 2.
Developmental vulnerability (in lower 10th percentile of AEDC assessment score at first year of full‐time school) in one or more domains (DV1) and in two or more domains (DV2) by characteristics of the mothers—bivariate analysis (logistic regression)
| Part A | |||||
|---|---|---|---|---|---|
| Characteristics of mothers n = 250 unless otherwise specified | Number with that characteristic | AEDC—DV1 (Y) n = 145 (58.0%) | P‐values (DV1—logistic regression) | AEDC—DV2 (Y) n = 91 (36.4%) | P‐values (DV2—logistic regression) |
| Ethnicity of mothera | |||||
| Aboriginal | 132 | 91 (68.9%) [60.9%, 76.9%] | Base | 62 (47.0%) [38.3%, 55.6%] | Base |
| Torres Strait Islander | 97 | 42 (43.3%) [33.3%, 53.3%] | P < .001* | 25 (25.8%) [16.9%, 34.6%] | P = .001* |
| Aboriginal and Torres Strait Islander | 21 | n ≤ 15 | P = .288 | n ≤ 15 | P = .023* |
| Mother's region of residencea | |||||
| Cairns and Hinterland | 19 | n ≤ 15 | P = .218 | n ≤ 15 | P = .135 |
| Cape York | 134 | 90 (67.2%) [59.1%, 75.2%] | Base | 60 (44.8%) [36.2%, 53.3%] | Base |
| Torres Strait | 97 | 45 (46.4%) [36.3%, 56.5%] | P = .002* | 26 (26.8%) [17.8%, 35.8%] | P = .006* |
| Mother's age when child born—quartiles | |||||
| Teenager—younger than 20 years | 52 | 33 (63.5%) [49.9%, 77.0%] | P = .125 | 17 (32.7%) [19.5%, 45.9%] | P = .824 |
| 20‐23 years | 65 | 32 (49.2%) [36.7%, 61.7%] | Base | 20 (30.8%) [19.2%, 42.9%] | Base |
| 24‐30 years | 69 | 39 (56.5%) [44.5%, 68.5%] | P = .398 | 25 (36.2%) [24.6%, 47.9%] | P = .504 |
| >30 years | 64 | 41 (64.1%) [52.0%, 76.1%] | P = .908 | 29 (45.3%) [32.8%, 57.8%] | P = .090 |
| SEIFA—mother's usual localitya | |||||
| Mother lives in SEIFA 1 locality | 219 | 128 (58.5%) [51.9%, 65.0%] | P = .703 | 80 (36.5%) [30.1%, 43.0%] | P = .910 |
| Mother does NOT live in SEIFA 1 locality | 31 | 17 (54.8%)[36.3%, 73.4%] | n ≤ 15 | ||
| Mother's parity—cohort pregnancy n = 173 | |||||
| Parity 0‐2 | 92 | 58 (63.0%) [53.0%, 73.1%] | P = .859 | 38 (41.3%) [31.1%, 51.6%] | P = .810 |
| Parity 3 or more | 81 | 50 (61.7%) [50.9%, 72.5%] | 32 (39.5%) [28.6%, 50.4%] | ||
| Mother's body mass index n = 161 | |||||
| Under or healthy weight | 57 | 36 (63.2%) [50.2%, 76.1%] | Base | 23 (40.4%) [27.2%, 53.5%] | Base |
| Overweight | 42 | 23 (54.8%) [39.1%, 70.5%] | P = .401 | n = <15 [16.4, 45.5%] | P = .338 |
| Obese | 62 | 30 (48.4%) [35.6%, 61.2%] | P = .107 | 21 (33.9%) [21.8%, 46.0%] | P = .465 |
| Smoking in pregnancy | |||||
| Yes | 153 | 101 (66.0%) [58.4%, 73.6%] | P = .001* | 65 (42.5%) [34.7%, 50.4%] | P = .013* |
| No | 97 | 44 (45.4%) [35.3%, 55.4%] | 26 (26.8%) [17.8%, 35.8%] | ||
| Diabetes in pregnancy (gestational diabetes OR pre‐existing diabetes) n = 168 | |||||
| Yes | 41 | 24 (58.5%)[42.8%, 66.2%] | P = .905 | 16 (39.0%)[23.4%, 54.6%] | P = .746 |
| No | 127 | 73 (57.5%)[48.8%, 66.2%] | 46 (36.2%)[27.7%, 44.7%] | ||
| Anaemia before and during pregnancy n = 221 | |||||
| Yes | 72 | 53 (73.6%) [63.2%, 84.0%] | P = .004* | 35 (48.6%) [36.8%, 60.4%] | P = .019* |
| No | 149 | 79 (53.0%) [44.9%, 61.1%] | 48 (32.2%) [24.6%, 39.8%] | ||
| Red cell folate level insufficient before OR during pregnancy n = 61a | |||||
| Yes | 46 | 25 (54.4%) [39.4%, 69.3%] | P = .405 | n = <15 | P = .358 |
| No | n ≤ 15 | n ≤ 15 | n ≤ 15 | ||
| Part B | ||||||
|---|---|---|---|---|---|---|
| Characteristics of mothers | DV1 = No n = 105 | DV1 = Yes n = 145 | P‐values (logistic regression) | DV2 = No n = 159 | DV2 = Yes n = 91 | P‐values (logistic regression) |
| Mothers' age in years at baby's birth median (IQR) [95%CI] | 24 (20.5, 29.5) [22, 25] | 24 (20,31) [23, 26] | P = .570 | 23 (20,29) [22, 24] | 26 (21,32) [23, 28.4] | P = .101 |
Note: Categorical variables are shown as absolute and relative frequencies (percentages) with [95% confidence intervals]—mother's ethnicity, region of residence, age group, SEIFA, parity, Body Mass Index, smoking in pregnancy, diabetes in pregnancy, anaemia before and during pregnancy, folate levels insufficient. Numerical values with a skewed distribution are shown as median and inter‐quartile range (IQR) and [95%CI]—mother's age in years at birth of baby.
AEDC require that results relating to cell sizes of 15 or less are not provided to protect confidentiality.
P <.05.
Early childhood anaemia was significantly associated with developmental vulnerability for four out of five domains of early childhood development: physical health and wellbeing, social competence, language and cognitive skills (school‐based), communication skills and general knowledge. Smoking in pregnancy was significantly associated with developmental vulnerability on three domains: physical health and wellbeing, social competence, communication skills and general knowledge. Anaemia of mothers before and during pregnancy was significantly associated with developmental vulnerability on two domains: physical health and wellbeing, communication skills and general knowledge (Table S3).
Multivariable complete case analyses found that children who had had early childhood anaemia (OR 2.2 [95% CI 1.1, 4.3] P = .020) were at significantly higher risk of developmental vulnerability compared to children who had not been anaemic, as were boys compared to girls (OR 2.8 [95%CI 1.5, 5.3] P = .001). Characteristics of mothers significantly associated with developmental vulnerability in their children included smoking in pregnancy (OR 2.0 [95% CI 1.02, 3.9] P = .045) and anaemia both before and during pregnancy (OR 2.1 [95% CI 1.1, 4.1] P = .021). Children of Aboriginal mothers were at higher risk of developmental vulnerability (OR 2.5 [95% CI 1.3, 5.0] P = .009) compared to children of Torres Strait Islander mothers or both Aboriginal and Torres Strait Islander mothers. The results of analysis following multiple imputation of missing values (n = 29) for mothers who were anaemic both before and during pregnancy were consistent with the results of complete case analysis (Table 3).
Table 3.
Risk factors for child (n = 250) developmental vulnerability (in lower 10th percentile of AEDC assessment score) in two or more domains (DV2): multi‐variable analyses—complete case analyses and analysis with imputed data
| Complete case analysis 1 N = 221 | Analysis with imputed dataa N = 250 | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | DV2 (yes) 83 (37.6%) | DV2 (No)138 (62.4%) | Odds‐ratio (95% CI) | P‐value | DV2 (yes) 91 (35.4%) | DV2 (no) 159 (63.6%) | Odds‐ratio (95% CI) | P‐value |
| Child anaemic when aged between 6 and 23 months | ||||||||
| No | 24 (28.9%) | 72 (52.2%) | 1 | 25 (27.5%) | 82 (51.6%) | 1 | ||
| Yes | 59 (71.1%) | 66 (47.8%) | 2.2 (1.1, 4.3) | P = 0.020 | 66 (72.5%) | 77 (48.4%) | 2.2 (1.2, 4.1) | P = .016 |
| Mother anaemic before and during pregnancy | ||||||||
| No | 48 (57.8%) | 101 (73.2%) | 1 | 53 (58.2%) | 117 (73.6%) | 1 | ||
| Yes | 35 (42.2%) | 37 (26.8%) | 2.1 (1.1, 4.1) | P = 0.021 | 38 (41.8%) | 42 (26.4%) | 2.1 (1.1, 4.1) | P = .027 |
| Mother smoked during pregnancy | ||||||||
| No | 25 (30.1%) | 60 (43.5%) | 1 | 26 (28.6%) | 71 (44.7%) | 1 | ||
| Yes | 58 (69.9%) | 78 (56.5%) | 2.0 (1.02, 3.9) | P = .045 | 65 (71.4%) | 88 (55.4%) | 2.2 (1.2, 4.1) | P = .016 |
| Sex of child | ||||||||
| Female | 26 (31.3%) | 76 (55.1%) | 1 | 29 (31.9%) | 89 (56.0%) | 1 | ||
| Male | 57 (68.7%) | 62 (44.9%) | 2.8 (1.5, 5.3) | P = .001 | 62 (68.1%) | 70 (44.0%) | 2.8 (1.6, 5.1) | P = .001 |
| Ethnicity of mother | ||||||||
| Torres Strait Islander | 24 (28.9%) | 67 (48.6%) | 1 | 25 (27.5%) | 70 (44.0%) | 1 | ||
| Aboriginal | 55 (66.3%) | 59 (42.8%) | 2.5 (1.3, 5.0) | P = .009 | 62 (68.1%) | 72 (45.3%) | 2.4 (1.3, 4.7) | P = .008 |
| Botha | n ≤ 15 | n ≤ 15 | 1.0 (0.3, 3.8) | P = .990 | n ≤ 15 | n ≤ 15 | 0.7 (0.2, 2.6) | P = .623 |
| Age of mother at birth of child | ||||||||
| ≤30 years | 55 (66.3%) | 106 (76.8%) | 1 | 62 (68.1%) | 124 (78.0%) | 1 | ||
| >30 years | 28 (33.7%) | 32 (23.2%) | 2.1 (1.04, 4.2) | P = .039 | 29 (31.9%) | 35 (22.0%) | 2.0 (1.03, 4.0) | P = .041 |
Note: The following characteristics of children and their mothers were considered during multivariable analyses: sex; birthing method (non‐instrumental vaginal, instrumental vaginal, caesarean section); gestational age at birth; premature or not; birth weight; feeding method to age 4 months (only breast milk, only infant formula, both breast milk and formula); ethnicity of mother (Aboriginal, Torres Strait Islander, both); region of residence of mother; SEIFA category for residence of mother; maternal age; BMI category of mother (underweight, normal weight, overweight, obese); categories of parity (0–2, ≥3); smoking during pregnancy; diabetes during pregnancy; mother had insufficient red cell folate (RCF) level before or during pregnancy; mother anaemic both before and during pregnancy. Models include all variables shown in Table 3 adjusted for the confounding effect of birth method (no missing values imputed for birth methods). Imputed data are averages of 20 imputations.
Abbreviation: 95% CI, 95% confidence interval.
AEDC require that results relating to cell sizes of 15 or less are not provided to protect confidentiality.
4. DISCUSSION
To the best of our knowledge, this is the first study to demonstrate the association of early childhood anaemia with developmental vulnerability at school age in Australia. Children who had anaemia between age six and 23 months had more than twice the risk of developmental vulnerability at school age compared to those who had not been anaemic. Early childhood anaemia was associated with developmental vulnerability in four of the five domains of early childhood development. Anaemia of mothers also doubled the risk of developmental vulnerability of their children at school age. These findings are consistent with the persistent detrimental effects of iron deficiency during critical phases of neurological development before birth and in early childhood.1
One limitation of this study is the small numbers of participants. However, the number of children with a Ferret record available (n = 1155) is close to census population information (n = 1147) for the remote communities where the Ferret system was used (Table S4). While the proportion of children with a haemoglobin measured (74.0%) is lower than expected, this is similar (85.0% and 72.1%) to two reports from elsewhere in northern Australia.7, 11 The proportion of children with a haemoglobin measurement for whom AEDC assessment results were available—250/708 (35.3%)—is consistent with the triennial schedule of the AEDC. Our findings in respect of child's sex, anaemia of mothers and smoking in pregnancy concur with the findings of much larger studies in South Australia and the Northern Territory.15, 16 Nevertheless, additional studies would be of value to determine whether early childhood anaemia is associated with developmental vulnerability in other similar settings.
Another limitation is the absence of information on the causes of the early childhood anaemia among these children. Iron deficiency is the usual cause of anaemia in early life.5, 12 Two Northern Territory studies found iron deficiency was the main cause of anaemia in children there; among 74 pre‐school aged children with anaemia 62 (84%) were iron deficient; among 66 school‐aged anaemic children, 55 (83%) responded to iron therapy.27, 28 Comparable studies are needed for Far North Queensland.
The information on child development used here, is derived from the triennial nation‐wide AEDC. Initially developed in Canada and adapted subsequently for use in Australia and elsewhere, concerns have been raised about the appropriateness of the AEDC instrument in Australian Aboriginal and Torres Strait Islander settings.29, 30 Research undertaken in 2008 to 2009 in urban, regional and remote Western Australia provided the basis of an adaption of the AEDC methodology so that teachers are supported by Indigenous cultural consultants during the assessment of childhood development of Aboriginal and Torres Strait Islander children.29 One strength of the AEDC information is that the information provided encompasses all children in Australia from diverse cultural and linguistic groups including Aboriginal and Torres Strait Islander children.13
Our findings show a higher risk of developmental vulnerability among children of Aboriginal mothers compared to children of Torres Strait Islander mothers. This may reflect different historical experiences as Aboriginal people of mainland Queensland were subject to extensive forced removals compared to the Torres Strait.31 Loss of access to nutrient‐dense traditional food was only one of many grim consequences of this policy.31
In settings where the prevalence of early childhood anaemia exceeds 20%, such as Far North Queensland and elsewhere in northern Australia,7, 10 the World Health Organization recommends interventions that combine nutrition promotion with provision of multi‐micronutrient preparations that include iron, for fortification of complementary food.12, 32 These interventions have been shown to be safe and effective.32 Improvements in haemoglobin levels resulting from such interventions protect against early childhood anaemia, and are also protective against the development deficits associated with early childhood anaemia.33 Potential mechanisms for this improvement include increased oxygen delivery to tissues and brain facilitating increased interaction of the child with his/her environment.33 Other possible mechanisms are improvements in iron availability for myelination, synaptogenesis and neurotransmission.33 One such intervention has been successfully piloted in six remote communities across northern Australia.34 Operational experience of implementation of these nutrition promotion and anaemia prevention interventions in 65 low‐ and middle‐income countries has been recently compiled to assist program managers and researchers manage the challenges entailed in implementation at scale.35, 36
Interventions to prevent anaemia in early childhood will be strengthened by improved food security, and by prevention and treatment anaemia in pregnancy.8, 37 Evaluation is essential, in particular to assess if prevention of maternal and early childhood anaemia translates into improved developmental indicators for children at school‐age. 1, 33
The Australian government is committed to Closing the Gap between Aboriginal and Torres Strait Islander peoples and other Australians.38 Anaemia prevention and better nutrition provide an opportunity to improve early childhood development, educational attainment and contribute to Closing the Gap between Aboriginal and Torres Strait Islander people and other Australians.
CONFLICT OF INTEREST
The authors affirm that they have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
D.L. conceived the research, obtained the necessary approvals to secure the data required, conducted preliminary statistical analysis and prepared the first draft of this manuscript. F.T. assisted with data management and preparation, and contributed to statistical analysis. P.B. contributed to study design and guided, supervised and contributed to statistical analysis. R.M. and M.M. contributed to study design, and manuscript development and preparation.
D.L. was supported by a National Health and Medical Research Council post‐graduate scholarship APP1092732. The funding body had no role in this research and the researchers were independent from the funder. Other agencies provided non‐financial support to this research. We would like to acknowledge and thank the Aboriginal and Torres Strait Islander leaders of the key community‐controlled health service organisations in Far North Queensland who considered and endorsed the research proposal. In addition, we acknowledge and thank the Data Custodians for the Queensland Health data collections and the Data Custodian for The Australian Government Department of Education and Training Australian Early Development Census data collection, their research and data management staff for their assistance and support for this work. These agencies extracted, linked and de‐identified the information used in this research and provided the linked de‐identified dataset to the research team. Other than this, these agencies had no role in the research.
This research uses data from the Australian Early Development Census (AEDC). The AEDC is funded by the Australian Government Department of Education and Training. The findings and views reported are those of the authors and should not be attributed to the Department or the Australian Government.
Supporting information
Table S1 Data collections—Information sources accessed to investigate the relationship between early childhood anaemia and developmental indicators at school entry age
Table S2 Definitions of variables used to describe characteristics of Aboriginal and Torres Strait Islander children (born between 2006 and 2010) and their mothers in Far North Queensland
Table S3 Proportion of children with characteristic categorised as developmentally vulnerable (in lower 10th percentile of AEDC assessment score at first year of full‐time school) for each of the five domains of early childhood development by anaemia and smoking status.
Table S4 Early childhood anaemia more than doubles the risk of developmental vulnerability at school‐age among Aboriginal and Torres Strait Islander children of remote Far North Queensland; comparisons of study participant numbers with census information.
Leonard D, Buettner P, Thompson F, Makrides M, McDermott R. Early childhood anaemia more than doubles the risk of developmental vulnerability at school‐age among Aboriginal and Torres Strait Islander children of remote Far North Queensland: Findings of a retrospective cohort study. Nutrition & Dietetics. 2020;77:298–309. 10.1111/1747-0080.12602
Funding information National Health and Medical Research Council, Grant/Award Number: APP1092732
REFERENCES
- 1. Georgieff MK, Ramel SE, Cusick SE. Nutritional influences on brain development. Acta Paediatr. 2018;107(8):1310‐1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhutta ZA, Guerrant RL, Nelson CA 3rd. Neurodevelopment, Nutrition, and Inflammation: The Evolving Global Child Health Landscape. Pediatrics. 2017;139(Suppl 1):S12‐S22. [DOI] [PubMed] [Google Scholar]
- 3. Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev. 2014;72(4):267‐284. [DOI] [PubMed] [Google Scholar]
- 4. Larson LM, Phiri KS, Iron PSR. Cognitive Development: What Is the Evidence? Ann Nutr Metab. 2017;71(Suppl 3):25‐38. [DOI] [PubMed] [Google Scholar]
- 5. Domellöf M. Iron requirements in infancy. Ann Nutr Metab. 2011;59(1):59‐63. [DOI] [PubMed] [Google Scholar]
- 6. Georgieff MK. Iron assessment to protect the developing brain. Am J Clin Nutr. 2017;106:1588S‐1593S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aquino D, Leonard D, Hadgraft N, Marley J. High prevalence of early onset anaemia amongst Aboriginal and Torres Strait Islander infants in remote northern Australia. Australian J Rur Health. 2018;26:245‐250. [Google Scholar]
- 8. Leonard D, Buettner P, Thompson F, Makrides M, McDermott R. Anaemia in pregnancy among Aboriginal and Torres Strait Islander women of Far North Queensland: a retrospective cohort study. Nutrition and Dietetics. 2018;75:457‐467. [DOI] [PubMed] [Google Scholar]
- 9. Bar‐Zeev S, Barclay L, Kruske S, Kildea S. Factors affecting the quality of antenatal care provided to remote dwelling Aboriginal women in northern Australia. Midwifery. 2014;30(3):289‐296. [DOI] [PubMed] [Google Scholar]
- 10. Leonard D, Buettner P, Thompson F, Makrides M, McDermott R. Anaemia in early childhood among Aboriginal and Torres Strait Islander children of Far North Queensland: A retrospective cohort study. ANZJPH. 2019;43(4):319‐327. [DOI] [PubMed] [Google Scholar]
- 11. Bar‐Zeev SJ, Kruske SG, Barclay LM, Bar‐Zeev N, Kildea SV. Adherence to management guidelines for growth faltering and anaemia in remote dwelling Australian Aboriginal infants and barriers to health service delivery. BMC Health Serv Res. 2013;13:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization . Nutritional Anaemias: Tools for Effective Prevention and Control Geneva. Geneva: World Health Organization; 2017. [Google Scholar]
- 13. Australian Early Development Census . Australian Early Development Census National Report 2015. Canberra: Australian Government Department of Education; 2016. Accessed December 13, 2018. https://www.aedc.gov.au/resources/detail/about-the-aedc-data-collection. [Google Scholar]
- 14. Brinkman S, Gregory T, Harris J, Hart B, Blackmore S, Janus M. Associations between the Early Development Instrument at Age 5 and Reading and Numeracy Skills at Ages 8, 10 and 12: a Prospective Linked Data Study. Child Ind Res. 2013;6:695‐708. [Google Scholar]
- 15. Guthridge S, Lia L, Silburn S, Lia SQ, McKenzie J, Lynch J. Early influences on developmental outcomes among children, at age 5, in Australia's Northern Territory. Early Child Res Q. 2016;35:124‐134. [Google Scholar]
- 16. Chittleborough CR, Searlea AK, Smithers LG, Brinkmana S, Lynch JW. How well can poor child development be predicted from early life characteristics? A whole‐of‐population data linkage study. Early Child Res Q. 2016;35:19‐30. [Google Scholar]
- 17. Leonard D, Buettner P, Thompson F, Makrides M, McDermott R. Linking ‘data silos’ to investiate anaemia among Aboriginal and Torres Strait Islander mothers and children in Far North Queenland. ANZJPH. 2018;42(5):256‐262. [DOI] [PubMed] [Google Scholar]
- 18. Queensland Health . Primary Clinical Care Manual Paediatrics. 9th ed. Brisbane: Queensland Government; 2017. https://www.publications.qld.gov.au/ [Google Scholar]
- 19. World Health Organization . Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 20. Rogers LM, Cordero AM, Pfeiffer CM, et al. Global folate status in women of reproductive age: a systematic review with emphasis on methodological issues. Ann N Y Acad Sci. 2018;1431(1):35‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Villar J, Ismail LC, Victora CG, et al. International standards for newborn weight, length and head cicumference by gestational age and sex: the Newborn Cross‐Sectional Study of the INTERGROWTH‐21st Project. Lancet. 2014;384:857‐868. [DOI] [PubMed] [Google Scholar]
- 22. Intergrowth‐21st Network . (INTERGROWTH‐21ST version 1.3.5); 2014. https://intergrowth21.tghn.org/newborn-size-birth/#c4 Accessed April 2018.
- 23. Australian Bureau of Statistics. Australian Census of Population and Housing . Socio‐Economic Indexes for Areas (SEIFA). Canberra. 2011;2033.0.55.001:2013. [Google Scholar]
- 24. Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic research. Principles and quantitative methods. New York, New York: Van Nostrand Reinhold; 1982. [Google Scholar]
- 25. Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. STROBE . Strengthening the reporting of observational studies in epidemiology—checklist. https://www.strobe-statement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_cohort.pdf2007. Accessed June 2019.
- 27. Kruske SG, Ruben AR, BD R. An iron treatment trial in an Aboriginal community: improving non‐adherence. J Paediatr Child Health. 1999;35:153‐158. [DOI] [PubMed] [Google Scholar]
- 28. Udovicich C, Perera K, Leahy C. Anaemia in school‐aged children in an Australian Indigenous community. Aust J Rural Health. 2017;25(5):285‐289. [DOI] [PubMed] [Google Scholar]
- 29. Silburn S, Brinkman S, Ferguson‐Hill S, Styles I, Walker R, Shepherd C. The Australian Early Development Index (AEDI) Indigenous Adaptation Study. Perth: Menzies School of Health Research; 2009. http://ccde.menzies.edu.au/sites/default/files/resources/AEDI_Indigenous_Adaptation_Study_Report_Nov_2009.pdf. [Google Scholar]
- 30. Janus M, Reid‐Westoby, C . Monitoring the Development of all Children: the Early Development Instrument. Hamilton, ON: McMaster University; 2016. https://bernardvanleer.org/app/uploads/2016/07/Early-Childhood-Matters-2016_7-1.pdf [Google Scholar]
- 31. Australian Law Reform Commission . Pathways to Justice—An Inquiry into the Incarceration Rate of Aboriginal and Torres Strait Islander Peoples. Sydney: Australian Law Reform Commission; 2017. http://apo.org.au/system/files/138661/apo-nid138661-685316.pdf [Google Scholar]
- 32. Dewey KG, Yang ZY, Boy E. Systematic review and meta‐analysis of home fortification of complementary foods. Matern Child Nutr. 2009;5(4):283‐321. [Google Scholar]
- 33. Larson LM, Kubes JN, Ramirez‐Luzuriaga MJ, Khishen S, A HS, Prado EL. Effects of increased hemoglobin on child growth, development, and disease: a systematic review and meta‐analysis. Ann N Y Acad Sci. 2019;1450:83‐104. [DOI] [PubMed] [Google Scholar]
- 34. Aquino D, Marley J, Senior K et al. The Early Childhood Nutrition and Anaemia Prevention Project—Summary Report. https://healthinfonet.ecu.edu.au/key‐resources/publications/25699/?title=Early%20Childhood%20Nutrition%20and%20Anaemia%20Prevention%20Project%20%3A%20summary%20report%3A%20The%20Fred%20Hollows%20Foundation 2013.
- 35. Nyhus Dhillon C, Sarkar D, Klemm RD, et al. Executive summary for the micronutrient powders consultation: lessons learned for operational guidance. Matern Child Nutr. 2017;13(Suppl 1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pelletier D, DePee S. Micronutrient powder programs: New findings and future directions for implementation science. Matern Child Nutr. 2019;15(S5):e12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moradi S, Arghavani H, Issah A, Mohammadi H, Mirzaei K. Food insecurity and anaemia risk: a systematic review and meta‐analysis. Public Health Nutr. 2018;18:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Department of the Prime Minister and Cabinet . Closing the Gap Report 2019. https://ctgreport.niaa.gov.au/sites/default/files/ctg-report-20193872.pdf?a=1:%202019
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Data collections—Information sources accessed to investigate the relationship between early childhood anaemia and developmental indicators at school entry age
Table S2 Definitions of variables used to describe characteristics of Aboriginal and Torres Strait Islander children (born between 2006 and 2010) and their mothers in Far North Queensland
Table S3 Proportion of children with characteristic categorised as developmentally vulnerable (in lower 10th percentile of AEDC assessment score at first year of full‐time school) for each of the five domains of early childhood development by anaemia and smoking status.
Table S4 Early childhood anaemia more than doubles the risk of developmental vulnerability at school‐age among Aboriginal and Torres Strait Islander children of remote Far North Queensland; comparisons of study participant numbers with census information.


