Abstract
Background
Hidradenitis suppurativa (HS) is a chronic, inflammatory, skin condition associated with many comorbidities and often has a substantial impact on patients’ lives.
Objectives
To evaluate symptom burden and health‐related quality of life (HRQoL) at baseline in patients with HS in an observational, real‐world, clinical setting using several tools including a validated HS‐specific instrument.
Methods
This study evaluated HRQoL data from the international UNITE HS disease registry. Administration of patient‐reported outcome (PRO) instruments and collection of data were executed per local regulations. All data were assessed using descriptive statistical methods.
Results
PRO data from 529 adults and 65 adolescents were evaluated. Most adults (64.5%) and adolescents (73.8%) were classified as Hurley Stage II with substantial disease burden at baseline. HS had a large effect (mean DLQI = 12.6) and moderate effect (mean CDLQI = 6.9) on the lives of adults and adolescents, respectively. Approximately 58% of adults and 41% of adolescents had anxiety scores beyond the normal range; 30% of adults and 16% of adolescents exhibited symptoms of depression. Based on HSSA and HSIA scores, approximately 30% of adults reported a substantial burden of multiple HS clinical symptoms and more than 45% reported a significant emotional impact of HS that adversely affected their intimate relationships. Only 60% of adults were employed and of those, 64% reported at least some degree of impairment while working because of HS.
Conclusions
Based on PROs collected from patients enrolled in the UNITE registry, a real‐world, clinical setting, HS has a significant negative impact on the everyday lives of patients affected by this disease.
Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease of hair follicles characterized by recurrent painful, inflamed nodules and/or abscesses occurring predominantly in the axillary, inguinal and anogenital regions.1, 2, 3, 4 These characteristic skin lesions may rupture, forming draining fistulas and ultimately lead to scarring. In addition to pain, patients with HS often experience itching, burning, hyperhidrosis and draining sinus tracts with malodorous discharge.3, 5 These physical symptoms can result in psychological symptoms including feelings of embarrassment, stigmatization and depression.6, 7
Medical treatments for HS include corticosteroids, retinoids, topical antibacterial agents, antibiotics, immunosuppressants and biologics;5, 8, 9, 10, 11 however, the multifaceted nature of HS can make clinical management challenging. Various physical and psychological symptoms have been reported to have a substantial negative impact on patients’ health‐related quality of life (HRQoL);6, 7, 12, 13, 14, 15 however, current literature on the patient‐centred burden of this disease remains limited, particularly in general practice outside of clinical trials. Today, given the increasing emphasis on patient‐oriented care, a thorough assessment of the patient‐reported impact of HS on HRQoL is vital to understanding and optimizing treatment.
To address this gap, we provide a comprehensive assessment of the impact of HS on HRQoL in patients in a real‐world, clinical setting. Our evaluation included several patient‐reported outcome (PRO) measures: the Dermatology Life Quality Index (DLQI) and Children's Dermatology Life Quality Index (CDLQI) to assess dermatology‐specific quality of life; the Hospital Anxiety and Depression Scale (HADS) questionnaire16 to assess anxiety and depression levels; the Patient's Global Assessment of Hidradenitis Suppurativa (HS‐PtGA) to assess patient‐reported disease control; two new questionnaires, the Hidradenitis Suppurativa Symptom Assessment (HSSA)17 and Hidradenitis Suppurativa Impact Assessment (HSIA),17 to assess HS‐specific signs, symptoms and impacts; and the Work Productivity and Activity Impairment: Specific Health Problem (WPAI:SHP) questionnaire18 to assess the effect of HS on productivity. These questionnaires were used to evaluate symptom burden and HRQoL in patients enrolled in the HS disease registry (UNITE), a geographically diverse, prospective, observational registry. The results from this registry may help identify target goals for HS treatment in clinical practice and provide information essential to the development of HS treatment guidelines.
Patients and methods
Study design
This study is an observational study evaluating baseline HRQoL data from the UNITE registry. Baseline HRQoL questionnaires are administered and collected at the time of enrolment per local regulations. Patient treatment is consistent with routine clinical practice at each site, and the use of specific medication(s) or treatment procedure(s) is not mandated by the registry protocol. As this is an observational registry, there were no predefined hypotheses regarding the magnitude of any resulting differences, and all data were assessed using standard statistical methods for observational studies.
Study population
Adults (≥18 years of age) and adolescents (12–<18 years of age) with active HS (defined by the presence of inflammatory HS lesions) were eligible to enrol in the UNITE registry. To minimize outcomes measuring bias, enrolment aimed for a balanced proportion of adults in Hurley Stages I, II and III, representing diverse levels of disease severity; this criterion did not apply to adolescents. Patients with previous or current participation in an originator‐adalimumab clinical study or registry were excluded. The UNITE registry enrolled patients from 73 sites in 12 countries (Australia, Canada, Czech Republic, France, Germany, Greece, Hungary, Italy, Netherlands, Spain, Switzerland, United States). Patients and/or their legal guardians voluntarily signed an informed consent, approved by an Institutional Review Board or Ethics Committee as applicable, according to local law.
Patient‐reported outcomes
The DLQI19 and CDLQI20 are 10‐item questionnaires that measure how skin diseases affect different aspects of quality of life, such as symptoms and feelings, daily activities, leisure, work or school, personal relationships and treatment. The maximum score is 30 with 0 indicating no impact and 30 indicating the highest impact on the patient's quality of life.
The HADS16 is a 14‐item questionnaire used to assess anxiety and depression levels. For HADS, a score of 0–7 is considered normal, 8–10 experiencing some symptoms and 11–21 a probable presence of anxiety/depression.
The Patient's Global Assessment of HS (HS‐PtGA) is a 1‐item questionnaire assessing current disease control on a scale of 0 (complete disease control), 1 (good disease control), 2 (limited disease control) and 3 (uncontrolled disease).
The HSSA17 is a 9‐item questionnaire evaluating the primary symptoms of HS during the 24 hours before completing the questionnaire. Patients provide an assessment of their symptoms on an 11‐point numeric rating scale (NRS), where 0 indicates no symptoms and 10 indicates extreme symptoms. The following symptoms are included: pain, tenderness, hot skin, drainage, swollenness, bad smell, redness, hard skin and itchiness.
The HSIA17 is an 18‐item questionnaire assessing the impact of HS on the patient's quality of life. Various aspects are assessed including the effect of HS on mobility (ability to sit, walk, exercise, move arms) and emotions (sad, worried, self‐conscious, bothered by HS, embarrassed), being bothered by their scars, the ability and/or desire to have sex, satisfaction with their treatment, ability to perform household activities, clothing discomfort and desire to be around others. Patients were asked to rate the impact of their symptoms during the 7 days before completing the questionnaire based on an 11‐point NRS, where 0 indicates no impact and 10 indicates severe impact of HS on quality of life. In addition to an overall score, Mobility and Emotional Scale scores are calculated.
The HSSA and HSIA are recently developed PRO measures. The content and reliability of these questionnaires have been validated.17 At this time, there are no formal interpretations of severity strata for HSSA/HSIA scores published in the literature. Other PROs that are based on a 0‐10 scale have used scores ≥ 7 to indicate severity.21, 22 Therefore, in the present study, item scores ≥ 7 were regarded as being substantially burdensome to the patient.
The WPAI: Specific Health Problems questionnaire18 is a 6‐item questionnaire assessing the following 4 domains: absenteeism (work time missed), presenteeism (reduced on‐the‐job effectiveness), overall work impairment and daily activity impairment. The estimated per cent reduction (0–100%) in productivity was derived from responses to questions; higher scores indicate a greater reduction in work productivity or ability to perform daily activities.
Adults completed the DLQI, HADS, HSSA and HSIA, and adolescents completed the CDLQI and HADS questionnaires. For the WPAI questionnaire, absenteeism, presenteeism and overall work impairment were completed by employed adults and the activity impairment portion was completed by all adults as well as adolescents.
Statistical analysis
Data were summarized using descriptive statistics: means and standard deviations (SD) for continuous variables, and frequency counts and percentages for categorical variables. For the WPAI outcomes of absenteeism, presenteeism and overall work impairment, scores were calculated for employed patients and for the subpopulation of employed patients with absenteeism > 0, presenteeism > 0 and overall work impairment > 0. Only patients who completed the respective questionnaires were included in analyses. All analyses were performed in SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
Between 29 October 2013 and 29 December 2015, a total of 529 adults and 65 adolescents enrolled in the UNITE registry. The mean (±SD) age of adults was 38.6 ± 12.6 years and 68.6% were female; mean age of adolescents was 15.6 ± 1.4 years and 78.5% were female. At baseline, most adults (65.4%) and adolescents (73.8%) were at Hurley Stage II. The mean number of flares experienced over the 6‐month period prior to baseline was 5.8 ± 9.5 and 4.6 ± 5.6 flares for adults and adolescents, respectively.
Baseline PRO scores are provided in Table 1. On average, HS had a large effect on adults according to the DLQI (mean = 12.6 ± 8.0). More than 50% of adult patients expressed some burden for each item of the DLQI (Fig. S1). Patients were especially affected (rated as ‘a lot’ or ‘very much’) by the dermatological symptoms of the disease (59.2%), how the disease influenced the clothes they wore (56.3%) and the embarrassment and self‐consciousness experienced (53.5%). On average, HS had a moderate effect on the lives of adolescents according to the CDLQI (6.9 ± 6.2). More than 20% of adolescents indicated that they were especially affected (rated as ‘a lot’ or ‘very much’) by the dermatological symptoms of the disease, the embarrassment and self‐consciousness experienced, playing or doing hobbies, how the disease influenced the clothes they wore, their avoidance of swimming or other sports and during their school work or holiday time (Fig. S2). As shown in Table 1, mean DLQI/CDLQI scores increased with increasing disease severity for both adults (8.8, 11.6 and 15.7 for Hurley Stage I, II and III, respectively) and adolescents (5.5, 6.6 and 12.8 for Hurley Stage I, II and III, respectively).
Table 1.
Baseline patient‐reported outcomes in adults and adolescents with HS: overall and stratified by Hurley Stage
| Measures | Overall | Hurley Stage I | Hurley Stage II | Hurley Stage III | ||||
|---|---|---|---|---|---|---|---|---|
| n | Value | n | Value | n | Value | n | Value | |
| Adults | N = 529 | N = 28 | N = 346 | N = 155 | ||||
| DLQI total score (0–30), mean ± SD | 505 | 12.6 ± 8.0 | 28 | 8.8 ± 7.3 | 330 | 11.6 ± 7.5 | 147 | 15.7 ± 8.2 |
| HADS – depression category, n (%) | ||||||||
| Normal (0–7) | 508 | 354 (69.7) | 28 | 23 (82.1) | 332 | 237 (71.4) | 148 | 94 (63.5) |
| Experiencing some symptoms (8–10) | 508 | 56 (11.0) | 28 | 1 (3.6) | 332 | 38 (11.4) | 148 | 17 (11.5) |
| Probable presence (11–21) | 508 | 98 (19.3) | 28 | 4 (14.3) | 332 | 57 (17.2) | 148 | 37 (25.0) |
| HADS – anxiety category, n (%) | ||||||||
| Normal (0–7) | 508 | 216 (42.5) | 28 | 13 (46.4) | 332 | 141 (42.5) | 148 | 62 (41.9) |
| Experiencing some symptoms (8–10) | 508 | 131 (25.8) | 28 | 7 (25.0) | 332 | 86 (25.9) | 148 | 38 (25.7) |
| Probable presence (11–21) | 508 | 161 (31.7) | 28 | 8 (28.6) | 332 | 105 (31.6) | 148 | 48 (32.4) |
| HADS, mean ± SD | ||||||||
| Depression scale (0–21) | 508 | 5.8 ± 4.4 | 28 | 5.0 ± 4.6 | 332 | 5.6 ± 4.4 | 148 | 6.4 ± 4.4 |
| Anxiety scale (0–21) | 508 | 8.6 ± 4.5 | 28 | 8.3 ± 4.6 | 332 | 8.7 ± 4.6 | 148 | 8.5 ± 4.3 |
| HS‐PtGA (0–3), mean ± SD | 514 | 1.9 ± 0.9 | 27 | 1.9 ± 0.9 | 335 | 1.9 ± 0.9 | 152 | 2.0 ± 0.8 |
| HSSA, mean ± SD | 521 | 4.8 ± 2.8 | 28 | 4.0 ± 3.1 | 340 | 4.4 ± 2.8 | 153 | 5.7 ± 2.6 |
| HSIA, mean ± SD | ||||||||
| Overall score (0–10) | 521 | 4.9 ± 2.7 | 28 | 4.1 ± 2.9 | 340 | 4.6 ± 2.7 | 153 | 5.6 ± 2.6 |
| Mobility score (0–10) | 521 | 3.7 ± 2.9 | 28 | 2.9 ± 2.8 | 340 | 3.3 ± 2.7 | 153 | 4.8 ± 2.9 |
| Emotional score (0–10) | 521 | 5.7 ± 3.4 | 28 | 5.0 ± 3.7 | 340 | 5.5 ± 3.4 | 153 | 6.2 ± 3.2 |
| WPAI, mean ± SD | ||||||||
| Absenteeism (0–100%) | 304 | 9.9 ± 24.0 | 16 | 2.7 ± 7.7 | 205 | 7.9 ± 21.2 | 83 | 16.0 ± 30.8 |
| Presenteeism (0–100%) | 298 | 29.8 ± 30.0 | 16 | 32.5 ± 34.5 | 202 | 26.7 ± 28.6 | 80 | 37.0 ± 31.8 |
| Overall work impairment (0–100%) | 303 | 35.3 ± 33.3 | 16 | 33.4 ± 35.5 | 204 | 31.5 ± 32.0 | 83 | 45.2 ± 34.3 |
| Activity impairment (0–100%) | 515 | 41.1 ± 33.2 | 28 | 32.9 ± 34.0 | 336 | 36.9 ± 32.8 | 151 | 52.1 ± 31.7 |
| WPAI subpopulation,a mean ± SD | ||||||||
| Absenteeism (0–100%) | 89 | 33.7 ± 34.3 | 3 | 14.5 ± 13.6 | 48 | 33.8 ± 32.4 | 38 | 35.0 ± 37.7 |
| Presenteeism (0–100%) | 203 | 43.7 ± 26.7 | 11 | 47.3 ± 32.0 | 130 | 41.5 ± 25.5 | 62 | 47.7 ± 28.0 |
| Overall work impairment (0–100%) | 219 | 48.9 ± 29.5 | 11 | 48.6 ± 32.7 | 140 | 45.8 ± 28.9 | 68 | 55.2 ± 29.7 |
| Adolescents | N = 65 | N = 12 | N = 48 | N = 5 | ||||
|---|---|---|---|---|---|---|---|---|
| CDLQI total score (0–30), mean ± SD | 50 | 6.9 ± 6.2 | 11 | 5.5 ± 6.2 | 35 | 6.6 ± 6.0 | 4 | 12.8 ± 6.2 |
| HADS – depression category, n (%) | ||||||||
| Normal (0–7) | 63 | 53 (84.1) | 12 | 11 (91.7) | 46 | 39 (84.8) | 5 | 3 (60.0) |
| Experiencing some symptoms (8–10) | 63 | 7 (11.1) | 12 | 1 (8.3) | 46 | 5 (10.9) | 5 | 1 (20.0) |
| Probable presence (11–21) | 63 | 3 (4.8) | 12 | 0 | 46 | 2 (4.3) | 5 | 1 (20.0) |
| HADS – anxiety category, n (%) | ||||||||
| Normal (0–7) | 63 | 37 (58.7) | 12 | 10 (83.3) | 46 | 26 (56.5) | 5 | 1 (20.0) |
| Experiencing some symptoms (8–10) | 63 | 14 (22.2) | 12 | 2 (16.7) | 46 | 10 (21.7) | 5 | 2 (40.0) |
| Probable presence (11–21) | 63 | 12 (19.0) | 12 | 0 | 46 | 10 (21.7) | 5 | 2 (40.0) |
| HADS, mean ± SD | ||||||||
| Depression scale (0–21) | 63 | 3.7 ± 3.4 | 12 | 2.0 ± 2.3 | 46 | 3.9 ± 3.4 | 5 | 6.1 ± 4.5 |
| Anxiety scale (0–21) | 63 | 6.6 ± 4.6 | 12 | 4.5 ± 3.0 | 46 | 6.8 ± 4.9 | 5 | 9.6 ± 2.1 |
| HS‐PtGA (0–3), mean ± SD | 62 | 1.5 ± 0.8 | 12 | 1.4 ± 0.8 | 45 | 1.5 ± 0.8 | 5 | 2.2 ± 0.8 |
| WPAI – activity impairment (0–100%), mean ± SD | 13 | 42.3 ± 36.1 | 1 | 30.0 | 9 | 46.7 ± 32.4 | 3 | 33.3 ± 57.7 |
CDLQI, Children's Dermatology Quality of Life; DLQI, Dermatology Quality of Life; HADS, Hospital Anxiety and Depression Scale; HS, hidradenitis suppurativa; HSIA, Hidradenitis Suppurativa Impact Assessment; HSSA, Hidradenitis Suppurativa Symptom Assessment; HS‐PtGA, Patient's Global Assessment of Hidradenitis Suppurativa; SD, standard deviation; WPAI, Work Productivity and Activity Impairment.
Subpopulation of employed patients with absenteeism > 0, presenteeism > 0 and overall work impairment > 0 for each outcome, respectively.
For adults, HADS anxiety scores indicated that 25.8% exhibited some symptoms and 31.7% the probable presence of anxiety; for adolescents, 22.2% had scores indicating the presence of some symptoms and 19.0% the probable presence of anxiety. HADS depression scores indicated that 11.0% of adults had some symptoms and 19.3% had probable presence of depression. For adolescents, 11.1% had scores indicating the presence of some symptoms and 4.8% the probable presence of depression.
Based on the HS‐PtGA scores, only 4.5% of adults and 8.1% of adolescents indicated that their disease was completely controlled; 67.9% and 46.8% of adults and adolescents, respectively, reported that their disease control was limited or uncontrolled.
Adults reported on the severity of multiple HS‐related symptoms as assessed by the HSSA (Fig. 1). On a scale from 0–10, approximately 30% of adults rated each symptom with a score ≥ 7, indicating that these symptoms were substantially burdensome. The symptoms receiving the highest mean scores were tenderness (6.0 ± 3.4), redness (5.5 ± 3.3), swollenness (5.3 ± 3.5) and itchiness (5.1 ± 3.5). The mean total HSSA score was 4.8 ± 2.8.
Figure 1.
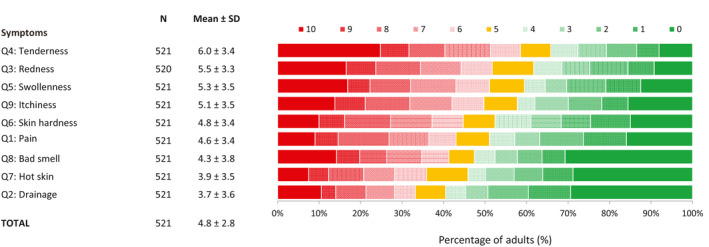
Percentage of adults reporting the severity of their HS symptoms based on the HSSA. Patients rated their symptoms on an 11‐point NRS with 1 indicating no symptoms and 10 extreme symptoms. HS, hidradenitis suppurativa; HSSA, Hidradenitis Suppurativa Symptom Assessment; NRS, numeric rating scale; Q, question; SD, standard deviation.
Adults reported multiple impacts of HS as assessed by the HSIA (Fig. 2). Adults were most impacted by aspects relating to sexual intimacy (desire to have sex, ability to have sex), their scars and items within the Emotional Scale (bothered by HS, self‐conscious, worried, embarrassed, sad), with more than 45% of adults rating each of these items with a score ≥ 7. On average, adults rated the emotional impact of HS higher than the mobility impact (5.7 ± 3.4 vs. 3.7 ± 2.9, respectively). The overall mean impact score for adults was 4.9 ± 2.7. On average, adults spent 33 ± 17 hours/week at work or school and missed approximately 4 ± 12 hours/week from work or school because of their HS.
Figure 2.
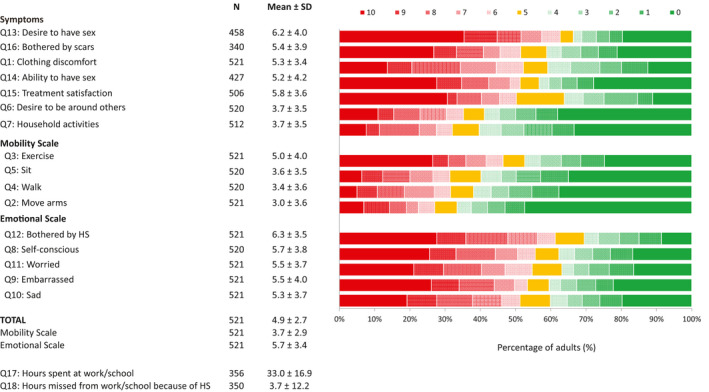
Percentage of adults reporting how HS affected various aspects of their quality of life based on HSIA. Patients rated the impact of their symptoms on their quality of life on an 11‐point NRS with 0 indicating no impact and 10 great impact of HS on quality of life. HS, hidradenitis suppurativa; HSIA, Hidradenitis Suppurativa Impact Assessment; NRS, numeric rating scale; Q, question; SD, standard deviation.
For WPAI, 60% (319/529) of adults reported that they were employed. Some degree of absenteeism was reported by 27.9% (89/319) of employed adults, of which an average of 33.7 ± 34.3% of work time was missed due to HS. Presenteeism issues were reported by 63.6% (203/319) of employed adults, of which an average of 43.7 ± 26.7% impairment while working was because of HS. On average, overall work impairment among employed adults was 48.9 ± 29.5%. Some degree of activity impairment due to HS was reported by 74.7% (395/529) of adults, with an average overall activity impairment of 41.1 ± 33.2%.
Discussion
The UNITE registry is an observational study designed to collect a spectrum of HRQoL assessments and disease characteristics to examine the impact of HS on patients in a real‐world, clinical setting. This analysis provides a comprehensive assessment of the impact of HS on the lives of adults and adolescents at enrolment of the UNITE registry. Patients reported substantial disease burden and a large effect of HS on HRQoL as assessed by the DLQI/CDLQI, with greater effects as disease severity increased. In addition, HADS scores demonstrated that HS substantially affects the psychological well‐being of patients with more than half of adults exhibiting symptoms or probable presence of anxiety and approximately a third exhibiting some symptoms or probable presence of depression. Most patients reported that they did not believe their disease was under control. Patients were burdened by multiple symptoms which ultimately had a significant emotional impact and adversely affected their intimate relationships. HS can also affect the financial well‐being of patients. Although the mean age of adults in this study was approximately 40 years, only 60% reported being employed. Of those who were employed, high degrees of absenteeism and presenteeism (measure of reduced on‐the‐job effectiveness) were observed and may equate to lost wages and economic insecurity.
A few studies have examined depression in patients with HS. Matusiak et al.7 and Onderijk et al.12 both found that 21% of patients with HS were suffering from depression. Kurek et al.23 demonstrated that the incidence of depression was significantly greater in patients with HS (39%) compared with age‐, gender‐ and BMI‐matched controls with a significant correlation between disease severity and the HADS depression score. Crowley et al.24 examined baseline comorbidities of patients enrolled in a randomized controlled trial of adalimumab in HS and showed that almost half experienced depression, with more severe HS disease correlating with a greater risk of depression. Results from these published studies are consistent with our analysis where 30.3% of adult patients had some symptoms or probable presence of depression as assessed by the HADS.
A comparative analysis of published clinical trial results showed that HS has a greater impact on HRQoL than moderate to severe psoriasis, with higher DLQI, overall work productivity impairment and pain scores observed for patients with HS.25 Mean DLQI and overall work productivity impairment scores in the Hamzavi et al. study25 are similar to those observed in patients enrolled in the UNITE registry. It should also be noted that the mean baseline DLQI scores in the UNITE registry and the Nordic HS registry are similar (12.6 and 13.9, respectively).26 In addition, mean baseline DLQI scores in the UNITE registry are greater than those reported in patients with plaque psoriasis (9.8–11.8)27 and atopic dermatitis (8.8).28 Thus, both clinical trials and registry data indicate that HS has a substantial negative impact on HRQoL that is greater than other chronic dermatologic diseases.
Approximately 30% of adults regarded HS‐related tenderness, redness, swollenness and itchiness as particularly burdensome symptoms. Adults also rated the emotional impact of HS higher than the mobility impact, with more than 45% indicating that being self‐conscious, worried, embarrassed, sad or bothered by their HS had a substantial impact on their intimate relationships. These findings indicate that HS affects multiple aspects of patients’ lives, and the HSSA/HSIA may be useful to clinicians for assessing the effectiveness of therapeutic interventions from their patient's perspective.
A major strength of the UNITE registry is that the data collected reflect the clinical care received for the average patient with HS and represent outcomes in a real‐world, clinical setting. The number of patients available for assessment is relatively large for a rare disease, such as HS. Future UNITE registry analyses can assess psychosocial and HRQoL outcomes and treatment patterns over time.
Several limitations need to be considered when interpreting the results of this analysis. This is a descriptive analysis presenting unmatched and unadjusted data; therefore, there may be inconsistencies or selective recall in reporting the data. There may also be selection bias, as patients who choose to enrol in the registry may be different from those that do not enrol. In the absence of HSSA/HSIA score interpretations, a score of ≥7 was regarded as being substantially burdensome to the patient. This threshold equated to a score that was ≥0.2 standard deviations above the mean for all items and ≥0.5 standard deviations above the mean for many of the items of the HSSA/HSIA. Formal score interpretations are underway, and the resulting thresholds may differ. The impact of HS on patients’ lives may be underestimated as many of the patients who entered the registry were on treatment. As in any observational study, there is the possibility of unidentified confounding factors.
Conclusion
The results of this study show that HS has a dramatic negative effect on the physical, social and emotional aspects of life, suggesting that patients may strongly benefit from a multidisciplinary approach that includes pharmacologic treatment, psychological therapy and social support. These interventions will need to address not only the HS‐related physical symptoms, such as chronic pain, tenderness, redness, swollenness and itchiness, but also psychosocial aspects and daily life activities, such as depression, anxiety, sexual health, school, work and financial well‐being.
Supporting information
Figure S1. Percentage of adults reporting how their HS symptoms affected various aspects of their quality of life based on DLQI.
Figure S2. Percentage of adolescents reporting how their HS symptoms affected various aspects of their quality of life based on CDLQI.
Acknowledgements
Medical writing support was provided by Joann Hettasch, PhD of JK Associates, Inc., a member of the Fishawack Group of Companies, Conshohocken, PA, and was funded by AbbVie.
Conflicts of interest
A Kimball receives honoraria as a consultant and grants as an investigator from Janssen, AbbVie, Novartis, Lilly and UCB; and receives fellowship funding from Janssen and AbbVie. J Crowley receives honoraria for consultant and speaker services from AbbVie, Amgen, Lilly, Novartis, Sun, UCB, Sanofi and Regeneron; and grants for investigator service from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Celgene, Janssen, Lilly, Maruho, Merck, Novartis, Pfizer, Regeneron, MC2 Therapeutics, Verrica and Sandoz. K Papp receives honoraria for advisory board participation and panel services from AbbVie, Allergan, Amgen, Apotex, Baxter, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Galderma, Genentech, Janssen, LEO Pharma, Merck, Merck‐Serono, Novartis, Pfizer, Regeneron, Schering Plough, UCB and Wyeth; for consultant services from AbbVie, Allergan, Amgen, Astellas, Baxter, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Centocor, Eli Lilly, Galderma, Genentech, Janssen, Kyowa Kirin, LEO Pharma, Merck, Merck‐Serono, Novartis, Pfizer, Regeneron, Schering Plough, Takeda, UCB and Wyeth; for speaker services from AbbVie, Allergan, Amgen, Astellas, Celgene, Centocor, Eli Lilly, Galderma, Genentech, Janssen, Janssen‐Cilag, LEO Pharma, Merck, Merck‐Serono, Novartis, Pfizer, Schering Plough and Wyeth; and has received grants for investigator services from Allergan, Amgen, Astellas, Baxter, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Centocor, Eli Lilly, Galderma, Genentech, GlaxoSmithKline Beecham, Janssen, Kyowa Kirin, LEO Pharma, Merck, Merck‐Serono, Novartis, Pfizer, Regeneron, Schering Plough, Takeda, UCB and Wyeth. B Calimlim and Y Duan receive a salary as AbbVie employees, and may have also received stocks and/or stock options. A Fleischer received a salary as an AbbVie employee at the time this research was conducted and may have also received stocks and/or stock options during the period this research was conducted. He is a consultant for Dermavant, Incyte, Qurient and SCM Lifescience and an investigator for Galderma, Menlo and Trevi. J Sobell receives honoraria and research grants as a consultant, speaker and/or investigator from AbbVie, Amgen, Eli Lilly, Celgene, Merck, Novartis, UCB, Sun, Bristol Myers‐Squibb and Regeneron.
Funding source
This work was supported by AbbVie. The study sponsor participated in the interpretation of data, review and approval of the article.
References
- 1. van der Zee HH, Jemec GB. New insights into the diagnosis of hidradenitis suppurativa: clinical presentations and phenotypes. J Am Acad Dermatol 2015; 73: S23–S26. [DOI] [PubMed] [Google Scholar]
- 2. Jemec GB. Clinical practice. Hidradenitis suppurativa. N Engl J Med 2012; 366: 158–164. [DOI] [PubMed] [Google Scholar]
- 3. Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol 2009; 60: 539–561; quiz 62–3. [DOI] [PubMed] [Google Scholar]
- 4. Buimer MG, Wobbes T, Klinkenbijl JH. Hidradenitis suppurativa. Br J Surg 2009; 96: 350–360. [DOI] [PubMed] [Google Scholar]
- 5. Shah N. Hidradenitis suppurativa: a treatment challenge. Am Fam Physician 2005; 72: 1547–1552. [PubMed] [Google Scholar]
- 6. Esmann S, Jemec GB. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol 2011; 91: 328–332. [DOI] [PubMed] [Google Scholar]
- 7. Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol 2010; 90: 264–268. [DOI] [PubMed] [Google Scholar]
- 8. Porter ML, Golbari NM, Lockwood SJ et al Overview and update on biologic therapy for moderate‐to‐severe hidradenitis suppurativa. Semin Cutan Med Surg 2018; 37: 182–189. [DOI] [PubMed] [Google Scholar]
- 9. Shanmugam VK, Zaman NM, McNish S et al Review of current immunologic therapies for hidradenitis suppurativa. Int J Rheumatol 2017; 2017: 8018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee RA, Eisen DB. Treatment of hidradenitis suppurativa with biologic medications. J Am Acad Dermatol 2015; 73: S82–S88. [DOI] [PubMed] [Google Scholar]
- 11. Maarouf M, Clark AK, Lee DE et al Targeted treatments for hidradenitis suppurativa: a review of the current literature and ongoing clinical trials. J Dermatolog Treat 2018; 29: 441–449. [DOI] [PubMed] [Google Scholar]
- 12. Onderdijk AJ, van der Zee HH, Esmann S et al Depression in patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2013; 27: 473–478. [DOI] [PubMed] [Google Scholar]
- 13. Matusiak L, Bieniek A, Szepietowski JC. Hidradenitis suppurativa markedly decreases quality of life and professional activity. J Am Acad Dermatol 2010; 62: 706–708 8.e1. [DOI] [PubMed] [Google Scholar]
- 14. Wolkenstein P, Loundou A, Barrau K et al Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol 2007; 56: 621–623. [DOI] [PubMed] [Google Scholar]
- 15. Alavi A, Anooshirvani N, Kim WB et al Quality‐of‐life impairment in patients with hidradenitis suppurativa: a Canadian study. Am J Clin Dermatol 2015; 16: 61–65. [DOI] [PubMed] [Google Scholar]
- 16. Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes 2003; 1: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kimball AB, Sundaram M, Banderas B et al Development and initial psychometric evaluation of patient‐reported outcome questionnaires to evaluate the symptoms and impact of hidradenitis suppurativa. J Dermatolog Treat 2018; 29: 152–164. [DOI] [PubMed] [Google Scholar]
- 18. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993; 4: 353–365. [DOI] [PubMed] [Google Scholar]
- 19. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 20. Lewis‐Jones MS, Finlay AY. The children's dermatology life quality index (CDLQI): initial validation and practical use. Br J Dermatol 1995; 132: 942–949. [DOI] [PubMed] [Google Scholar]
- 21. Reich A, Heisig M, Phan NQ et al Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol 2012; 92: 497–501. [DOI] [PubMed] [Google Scholar]
- 22. Vakharia PP, Chopra R, Sacotte R et al Severity strata for five patient‐reported outcomes in adults with atopic dermatitis. Br J Dermatol 2018; 178: 925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kurek A, Johanne Peters EM, Sabat R et al Depression is a frequent co‐morbidity in patients with acne inversa. J Dtsch Dermatol Ges 2013; 11: 743–749, ‐50. [DOI] [PubMed] [Google Scholar]
- 24. Crowley JJ, Mekkes JR, Zouboulis CC et al Association of hidradenitis suppurativa disease severity with increased risk for systemic comorbidities. Br J Dermatol 2014; 171: 1561–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamzavi IH, Sundaram M, Nicholson C et al Uncovering burden disparity: a comparative analysis of the impact of moderate‐to‐severe psoriasis and hidradenitis suppurativa. J Am Acad Dermatol 2017; 77: 1038–1046. [DOI] [PubMed] [Google Scholar]
- 26. Grimstad O, Tzellos T, Dufour DN et al Evaluation of medical and surgical treatments for hidradenitis suppurativa using real‐life data from the Scandinavian registry (HISREG). J Eur Acad Dermatol Venereol 2018; 33: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 27. Revicki D, Willian MK, Saurat JH et al Impact of adalimumab treatment on health‐related quality of life and other patient‐reported outcomes: results from a 16‐week randomized controlled trial in patients with moderate to severe plaque psoriasis. Br J Dermatol 2008; 158: 549–557. [DOI] [PubMed] [Google Scholar]
- 28. Misery L, Finlay AY, Martin N et al Atopic dermatitis: impact on the quality of life of patients and their partners. Dermatology 2007; 215: 123–129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Percentage of adults reporting how their HS symptoms affected various aspects of their quality of life based on DLQI.
Figure S2. Percentage of adolescents reporting how their HS symptoms affected various aspects of their quality of life based on CDLQI.


