Abstract
Objective
The present work evaluates the relationship between postoperative immune and neurovascular changes and the pathogenesis of surgery‐induced delirium superimposed on dementia.
Background and rationale
Postoperative delirium is a common complication in many older adults and in patients with dementia including Alzheimer's disease (AD). The course of delirium can be particularly debilitating, while its pathophysiology remains poorly defined.
Historical evolution
As of 2019, an estimated 5.8 million people of all ages have been diagnosed with AD, 97% of whom are >65 years of age. Each year, many of these patients require surgery. However, anesthesia and surgery can increase the risk for further cognitive decline. Surgery triggers neuroinflammation both in animal models and in humans, and a failure to resolve this inflammatory state may contribute to perioperative neurocognitive disorders as well as neurodegenerative pathology.
Updated hypothesis
We propose an immunovascular hypothesis whereby dysregulated innate immunity negatively affects the blood‐brain interface, which triggers delirium and thereby exacerbates AD neuropathology.
Early experimental data
We have developed a translational model to study delirium superimposed on dementia in APPSwDI/mNos2−/− AD mice (CVN‐AD) after orthopedic surgery. At 12 months of age, CVN‐AD showed distinct neuroimmune and vascular impairments after surgery, including acute microgliosis and amyloid‐β deposition. These changes correlated with attention deficits, a core feature of delirium‐like behavior.
Future experiments and validation studies
Future research should determine the extent to which prevention of surgery‐induced microgliosis and/or neurovascular unit dysfunction can prevent or ameliorate postoperative memory and attention deficits in animal models. Translational human studies should evaluate perioperative indices of innate immunity and neurovascular integrity and assess their potential link to perioperative neurocognitive disorders.
Major challenges for the hypothesis
Understanding the complex relationships between delirium and dementia will require mechanistic studies aimed at evaluating the role of postoperative neuroinflammation and blood‐brain barrier changes in the setting of pre‐existing neurodegenerative and/or aging‐related pathology.
Linkage to other major theories
Non‐resolving inflammation with vascular disease that leads to cognitive impairments and dementia is increasingly important in risk stratification for AD in the aging population. The interdependence of these factors with surgery‐induced neuroinflammation and cognitive dysfunction is also becoming apparent, providing a strong platform for assessing the relationship between postoperative delirium and longer term cognitive dysfunction in older adults.
Keywords: inflammation, innate immunity, neuroinflammation, neurovascular unit, surgery, vascular
1. OBJECTIVE
Here, we propose an updated immunovascular‐driven hypothesis of the mechanisms underlying delirium superimposed on dementia. We intend to: (1) use our new clinically relevant model to interrogate the pathogenesis of delirium superimposed on dementia after surgery and (2) integrate our findings with ongoing efforts, recognizing the growing awareness of neuroimmune and vascular contributions to Alzheimer's disease (AD) pathophysiology. The objective of this work is to understand the role of postoperative neuroinflammation and neurovascular dysfunction in promoting neurodegenerative disease pathology. We propose that targeted therapeutic discoveries based on selective immunoregulatory approaches to normalize postoperative blood‐brain barrier (BBB) dysfunction will be efficacious for treating and, possibly, preventing postoperative delirium and ensuing long‐term cognitive decline.
2. BACKGROUND AND RATIONALE
Every year, more than 19 million Americans over age 65 undergo anesthesia and surgery. Orthopedic surgery is routinely performed in these older adults, and as many as 50% of these patients suffer from postoperative delirium. 1 Delirium is an acute and fluctuating disturbance in awareness and attention that can also manifest through other cognitive domains. Delirium occurs not only after orthopedic surgery, but also after other types of surgery and in medical, intensive, emergency, nursing, and palliative care settings. 2 Although some studies have described higher delirium rates after cardiac surgery (reviewed in Marcantonio 3 ), other studies have found similarly increased delirium rates after other types of surgery. Importantly, preexisting medical conditions can also play a role in delirium rates. For example, postoperative neurocognitive complications occur more frequently in patients with lower baseline cognitive function. However, the extent to which surgical procedures versus individual patient risk factors contribute to delirium and cognitive dysfunction remains unclear.
Studies have suggested no difference in delirium rates based on anesthesia type (ie, general anesthesia vs regional anesthesia or nerve blocks), although these findings may be confounded in part by the significant doses of intravenous sedation given to patients who received regional anesthesia. 4 Nevertheless, because delirium has been observed following different types of major surgery and different modes of anesthesia in older adults, there may be something intrinsic to surgical trauma itself, or to perioperative care and hospitalization in general, that contributes to the risk for these perioperative neurocognitive disorders. 3 , 5
To date, the Food and Drug Administration has not approved a drug to treat delirium, which contributes an estimated $150 billion per year to the soaring health‐care costs in the United States. 6 Although some strategies have been implemented to prevent postoperative delirium, such as unit‐based targeted multifactorial intervention 7 and proactive geriatric consultation, 8 delirium consistently correlates with poor outcomes including a five‐fold increased risk for 6‐month postoperative mortality, persistent functional decline, increased nursing time per patient, increased length of hospital stay, and higher rates of nursing home placement. 8 , 9 , 10 When postoperative delirium occurs in patients with underlying dementia, the prognosis is even worse. 11 In fact, patients with dementia who suffer delirium after hip fracture surgery have a two‐fold increased risk for 1‐year mortality compared to hip fracture surgery patients without dementia or delirium. 12 Furthermore, a diagnosis of delirium superimposed on dementia can be challenging and elusive, 13 and why the synergism between dementia and postoperative delirium leads to poorer outcomes and even mortality is not clear.
3. HISTORIC EVOLUTION
Inflammation is a well‐recognized hallmark of pathology in multiple disease states. 14 Since Alois Alzheimer's early seminal discoveries of amyloid‐β (Aβ) plaques and neurofibrillary tangles in the post‐mortem AD brain, glial cells (the brain's first responders of the innate immune system) have been identified in close proximity to these characteristic pathologic lesions. Indeed, inflammation is now recognized as a significant contributor to AD pathogenesis as well as other neurodegenerative pathologies. 15 AD is currently the sixth leading cause of death in the United States, and its inexorable debilitating course has become a global pandemic without a disease‐modifying therapy. 16 The impact of surgery on the vulnerable brains of patients with either clinically diagnosed dementia or preclinical AD pathology remains largely under‐appreciated. Indeed, with more than 16 million procedures performed each year on older Americans alone, there is a growing need to understand the impact of surgery (and hospitalization in general), especially among patients in the highest risk strata.
Systemic inflammation has been described as a key driver of neuroinflammation and delirium‐like behavior. Indeed it is well known that elements of infection, such as endotoxemia, trigger behavioral changes commonly referred to as “sickness behavior.” It is also appreciated that systemic inflammation causes delirium and negatively impacts a brain prone to neurodegeneration. 17 In fact, lipopolysaccharide (LPS) induces acute behavioral changes via a cytokine storm that can accelerate neurodegenerative pathology. 17 In a disease‐prone vulnerable brain, a single systemic challenge with LPS induces microglial IL‐1β expression and contributes to subsequent neuronal death in this “immune‐primed” setting. 18 Importantly, blocking IL‐1β signaling pharmacologically and genetically reverses memory deficits after LPS, 19 as well as surgery‐induced neuroinflammation. 20
RESEARCH IN CONTEXT
Systematic review: Postoperative delirium is a common complication in many older adults and in patients with dementia, including Alzheimer's disease (AD). A growing body of evidence is highlighting the role of dysregulated innate immunity as a key driver of cognitive deficits after surgical trauma. Yet, the biologic mechanisms that contribute to delirium and neurodegeneration remain unknown and without effective therapies.
Interpretation: Our data in APPSwDI/mNos2−/− AD mice (CVN‐AD) demonstrate distinct age‐dependent neuroimmune and vascular impairments after orthopedic surgery. Importantly, surgery triggered acute Aβ deposition accompanied by microgliosis in the hippocampus of aged CVNβAD mice. These pathologic changes correlated with postoperative inattention, one of the core features of delirium.
Future directions: Future research should determine the extent to which targeted interventions aimed at protecting the neurovascular unit can curtail surgery‐induced Aβ deposition, microgliosis, and delirium‐like behavior. Translational studies are needed to define the evolution of Surgical Trauma Associated Molecular Patterns (ie, STAMPs) in postoperative delirium.
We have pioneered a clinically relevant tibial fracture surgery mouse model that causes systemic cytokine release, disrupts the BBB, allows macrophages to migrate into the brain, alters microglial morphology, and causes memory dysfunction. Notably, using Cx3cr1GFP/+ x Ccr2RFP/+ mice, we have reported that orthopedic surgery promotes the acute infiltration of monocytes into the brain parenchyma, and that this is dependent in part on TNFα/NF‐κB signaling. 21 , 22 Macrophage‐specific deletion of IKKβ, a central coordinator of TNFα activation of NF‐κB, prevents macrophage infiltration into the hippocampus following surgery. D'Mello et al. 23 have described a similar immune‐to‐central nervous system (CNS) communication pathway after hepatic inflammation. They also demonstrated that TNFα‐stimulated microglia produce monocyte chemoattractant protein (MCP)‐1/CCL2, with monocyte infiltration into the brain. One prior study in surgery patients found an association between increases in postoperative MCP‐1 levels and postoperative delirium, although this study measured MCP‐1 levels in peripheral serum rather than in cerebrospinal fluid (CSF). 24 In another prior study, preoperative CSF MCP‐1 levels did not predict postoperative delirium risk, which is consistent with our hypothesis here because the investigators did not measure postoperative CSF cytokine levels, and thus did not assess the relationship between postoperative CSF MCP‐1 levels and delirium status. 25 We and other colleagues found that postoperative CSF MCP‐1 levels were increased in one patient with delirium after orthopedic surgery. 26 Although this finding is far from definitive as it occurred only in a single patient, it does raise the question of whether MCP‐1 enters the brain from the circulation or is locally produced to chemoattract monocytes into the human brain. Although monocyte infiltration has been implicated in disease progression and neurodegeneration, 27 , 28 , 29 bone marrow–derived macrophages also have unique reparative properties, boosting tissue recovery in ways that resident microglia cannot. 30 , 31
4. UPDATED HYPOTHESIS
The objective is to examine the impact of systemic surgical trauma on the AD‐vulnerable brain by focusing on key neurovascular and neuroinflammatory markers. Our central hypothesis is that neurovascular pathology drives the development of postoperative delirium via surgical trauma associated molecular patterns (STAMPs) that impair the blood‐brain interface, which becomes more susceptible during aging with pre‐existing AD pathology. In this study, we examined immunovascular pathology leading to delirium‐like behavior in 3‐ and 12‐month‐old APPSwDI/mNos2−/− AD mice (CVN‐AD) after orthopedic surgery. We found that aged CVN‐AD mice are more susceptible to postoperative neuroinflammation due to two key age‐dependent phenomena: (1) failed resolution of inflammation, thereby perpetuating a maladaptive state during aging, and (2) increased neurovascular vulnerability due to underlying AD pathology, which renders the brain more susceptible to stressors like surgery. Indeed, the compromised BBB and neurovascular unit (NVU) are critical for allowing systemic factors to impact CNS health and homeostasis. Neurovascular pathology, such as cerebral amyloid angiopathy (CAA), is a key hallmark in human AD, 32 and is also an established feature in the brain of CVN‐AD mice. 33 Consistent with previous work that shows BBB impairment after surgery, 34 , 35 we have found a close correlation between vascular dysfunction and neurodegeneration, with a significant deposition of Aβ in the hippocampus in the acute postoperative period. Interestingly, these changes are exacerbated in aged CVN‐AD mice, which have higher systemic pro‐inflammatory cytokines with no changes in anti‐inflammatory markers such as IL‐10. This finding provides evidence for non‐resolving inflammation as a key driver of CNS pathology, especially within the context of intrinsic brain vulnerability present during aging and neurodegeneration. Early experimental data are presented in support of this theory.
5. EARLY EXPERIMENTAL DATA
5.1. Increased neuroinflammation in aged CVN‐AD mice after orthopedic surgery
Male and female CVN‐AD mice underwent an open tibial fracture of the left hind leg with intramedullary fixation under anesthesia and analgesia (see detailed methods in the supporting information). Three‐month‐old and 12‐month‐old mice were evaluated, representing a preclinical and a pathologic AD state, respectively. Both groups exhibited a robust postoperative systemic inflammatory response (Figure 1A). Plasma levels of the pro‐inflammatory cytokines G‐CSF and IL‐6 were increased on postoperative day 1, irrespective of age (P = .026 for 3‐month‐old mice and P = .0022 for 12‐month‐old mice; P = .033 for 3‐month‐old mice and P = .038 for 12‐month‐old mice, respectively). However, markers such as MCP‐1 (P = .03) and IP‐10 (P = .0033) were greatly induced in the older brain of CVN‐AD mice after surgery. TNF‐α and MIP‐1α showed similar trends, without reaching significance. Notably, the potent anti‐inflammatory cytokine IL‐10 was minimally expressed in aged mice both at baseline and postoperatively, but was detectable at baseline in 3‐month‐old mice (P = .0012).
FIGURE 1.
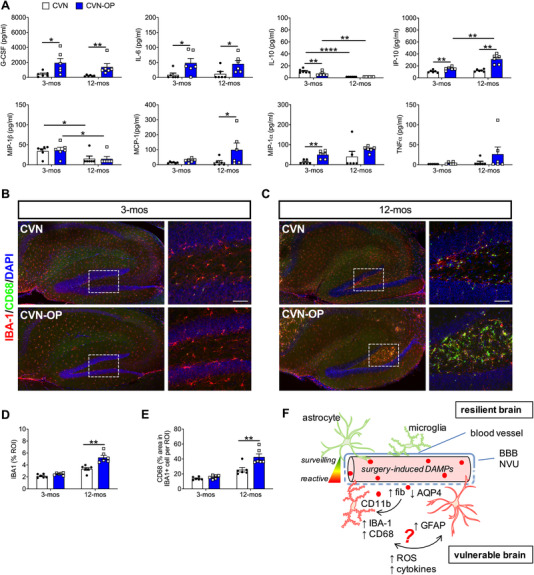
Age‐dependent inflammatory changes after orthopedic surgery in CVN‐AD mice. A, Systemic inflammatory markers in plasma from 3‐ and 12‐month‐old CVN naïve (CVN) and operated mice (CVN‐OP) 24 hours after surgery. B, Hippocampal neuroinflammation in 3‐month‐old CVN‐AD mice. The large field images were processed at 20× magnification and stitched. C, Hippocampal neuroinflammation in 12‐month‐old CVN‐AD mice. D‐E, Quantification of Iba‐1 and CD68 immunoreactivity failed to show significant changes in microglial morphology in 12‐ month‐old CVN‐AD. Inset represents the dentate gyrus (DG). F, Schematic representation of putative mechanisms of age‐depended neuroinflammation and associated blood‐brain barrier/neurovascular unit (BBB/NVU) dysfunction. In the CVN‐AD adult brain the BBB is not affected by surgical trauma associated molecular patterns (STAMPs), thus resulting in no evident morphological changes in glia cells. However, older CVN‐AD mice are “primed” and microglia respond greatly to STAMPs, including fibrinogen (see Figure 2), possibly inducing reactive astrocytes and initiating a vicious cycle that perpetuates neuroinflammation and neurotoxicity. Results are shown as mean ± standard error of the mean, n = 6/group for both 3‐ and 12‐month‐old CVN mice. **** P < .0001, **P < .001, ** P < .01, * P < .05, as measured by two‐way analysis of variance with Tukey's test for multiple comparison. Scale bar = 50 µm
Next, we focused on microglial activation in the hippocampus and cortex. Microglial activation was not observed in 3‐month‐old CVN‐AD mice after surgery (Figure 1B). However, profound microgliosis, as evidenced by changes in Iba‐1 immunoreactivity (P = .0003) and CD68 expression in Iba‐1‐positive cells (P = .0018), was observed in 12‐month‐old CVN‐AD mice after surgery (a 57% and 66% increase, respectively; Figure 1C‐E). These changes were not limited to the hippocampus, but were also observed in cortical regions (data not shown).
Together, these findings suggest that aging and underlying neurodegeneration render the BBB/NVU more susceptible to postoperative inflammatory changes. This is evidenced by limited ability of aged CVN‐AD mice to resolve inflammation, thus allowing systemic factors to enter the brain and inducing microglial activation, which are already primed in the context of established AD pathology (Figure 1F).
5.2. Neurovascular unit and blood‐brain barrier dysfunction in CVN‐AD after surgery
Neurovascular dysfunction is well characterized in the CVN‐AD mouse model, resulting in aging‐dependent astrocytic pathology (ie, GFAP activation) and reduced expression of aquaporin 4 (AQP‐4), dystrophin 1, and potassium channels. Notably, these features have also been observed in human AD tissue. 36 Here, we interrogated postoperative changes in the key neurovascular markers CD31, GFAP, and AQP‐4. Neither aging nor surgery significantly impacted expression of CD31. However, significant postoperative changes were observed in AQP‐4 and GFAP expression, which were particularly evident in 12‐month‐old CVN‐AD mice (Figure 2A‐C). Three‐month‐old mice retained normal expression of AQP‐4, with classical tubular formations (Figure 2A). This organized cytoarchitecture was abnormal in aged mice, and was further impaired 24 hours after surgery (42% decrease, P = .0082). Further, astrocytic activation was evident in the dentate gyrus area of the hippocampus in the aged group, and GFAP expression was significantly exacerbated in the hippocampus after surgery (93% increase, P = .0022; Figure 2A, C).
FIGURE 2.
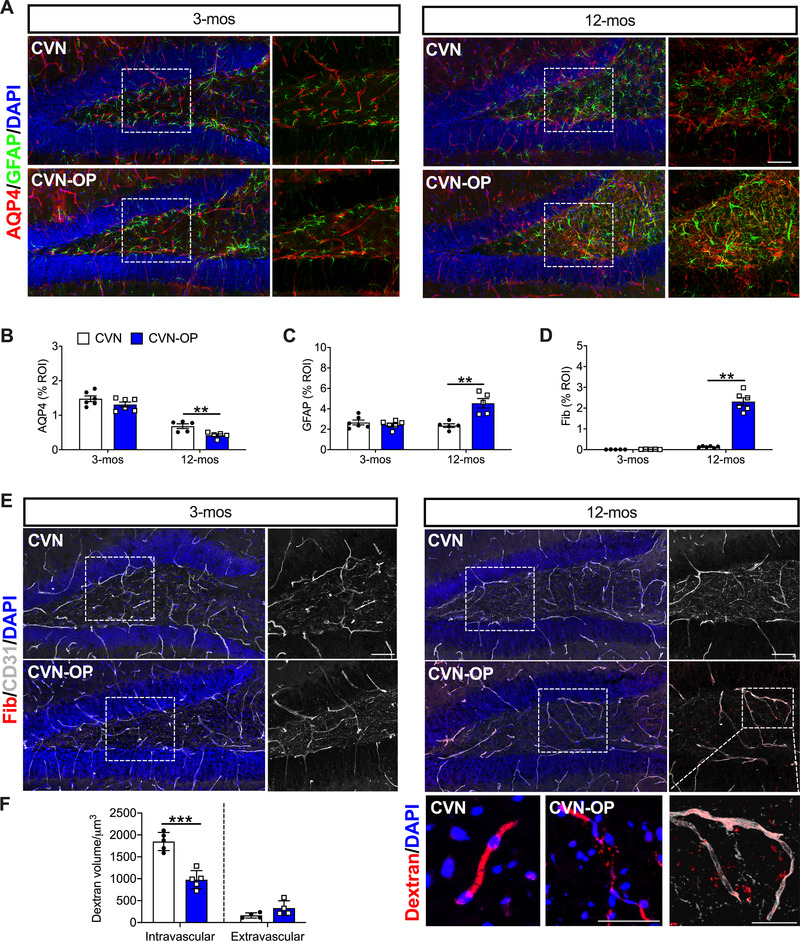
Neurovascular unit and blood‐brain barrier dysfunction in CVN‐AD after surgery. A‐B, Age and surgery significantly impaired the expression of the water channel marker AQP‐4 and increased expression of the astrocytic marker GFAP (C) in the dentate gyrus area of the hippocampus. The expression of the endothelial marker, CD31, remained constant across groups. D‐E, However, 12‐month‐old CVN‐AD mice showed increased levels of perivascular. Inset further show extravasated fibrinogen in CVN‐AD mice 24 hours after surgery. F, Dextran tracing also releveled leakiness of the blood vessels after surgery, resulting in a reduction in intravascular fluorescent dextran at 24 hours after surgery, although quantification of extravascular dextran did not reach significance. Results are shown as mean ± standard error of the mean, n = 5‐6/group. *** P < .001, ** P < .01, * P < .05, as measured by two‐tailed unpaired Student t tests. Scale bar = 50 µm
Next, we evaluated BBB function using fibrinogen and 70‐kDa dextran tracing. Fibrinogen deposition in the perivascular space was significantly increased after surgery in 12‐month‐old, but not in 3‐month‐old CVN‐AD mice (P = .0022; Figure 2D‐E). Interestingly, we also observed postoperative fibrinogen extravasation (Figure 2D inset), which we confirmed by evaluating dextran extravasation at 24 hours. Fluorescent dextran tracer also perfused systemically in 12‐month‐old CVN‐AD mice. Less intravascular dextran was observed postoperatively due to more extravasation and vascular dysfunction at 24 hours (P < .001; Figure 2F).
5.3. Acute Aβ deposition in the hippocampus after surgery
Given the greater neurodegenerative pathology in 12‐month‐old CVN‐AD mice, we evaluated the putative postoperative changes in Aβ levels in these mice. Compared to CVN‐AD controls, we observed an acute increase in both the soluble (7.07 ± 0.80 pg/mg vs 10.36 ± 0.99 pg/mg) and insoluble (21.67 ± 3.48 pg/mg vs 36.22 ± 3.40 pg/mg) fractions of Aβ42 in the hippocampus after surgery, as measured by enzyme‐linked immunosorbent assay (ELISA; Figure 3A). This indicates the presence of the more toxic and aggregation‐prone Aβ isoform. We corroborated these changes using standard Aβ immunofluorescence, and observed more plaques within 24 hours after orthopedic surgery (P = .0139; Figure 3B). To better evaluate and quantify changes in Aβ pathology and microglial dynamics, we employed tissue clarification (CLARITY) 37 (video 1 and 2 in supporting information). Three‐D reconstruction of clarified hippocampal slices after lightsheet microscopy revealed significant changes in Aβ deposition at 24 hours after orthopedic surgery (22% increase; P = .0139; Figure 3C, D). Plaques were also accompanied by reactive microglia as detected by Iba‐1 immunostaining with CLARITY, with larger cell bodies and retracted processes indicative of a pathologic state (P = .0026; Figure 3C‐D). Interestingly, we also found 80% greater Aβ engulfment by microglia after surgery (P = .0362; Figure 3C, E). Here, we propose an acute interaction between STAMPs and Aβ processing in the context of postoperative delirium superimposed on dementia. Immunity can alter AD‐related transcription factors such as amyloid precursor protein (APP), which is critical in the production of Aβ. 38 The robust immune response that occurs after orthopedic surgery can rapidly elevate Aβ levels in the brain, thus contributing to a neurotoxic environment. Primed microglia react to these changes; however, the function of these cells remains largely undefined, and the greater phagocytic activity described here may serve as a protective mechanism in response to the surgical stress (Figure 3F).
FIGURE 3.
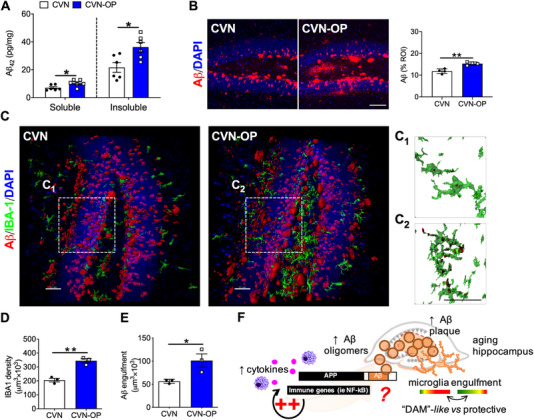
Acute amyloid‐β deposition in the hippocampus after surgery in 12‐month‐old CVNs. A, Levels of soluble and insoluble Aβ1‐42 in hippocampal lysate were assessed using enzyme‐linked immunosorbent assay. Surgery increases both factions of Aβ1‐42 at 24 hours in older CVN‐AD mice. B, Immunofluorescence and quantification of Aβ in the dentage gyrus area showed a rapid increase in hippocampal plaque load 24 hours after surgery compared to control mice. C, Tissue clarification of hippocampal slices was also performed 24 hours after orthopedic surgery using CLARITY. Three‐D reconstructions of lightsheet images showed similar changes in the pan‐microglial marker (Iba‐1) together with amyloid β (Aβ) deposits after surgery. Increased Aβ engulfment (inset, renderings C1 and C2) was observed in CVN‐OP mice compared to controls. D‐E, Quantification of Iba‐1 immunoreactivity and Aβ engulfment by microglia in tissue clarified slides. Amyloid was detected using a rabbit monoclonal anti‐Aβ was used to visualize amyloid (D54D2, Cell Signaling Technologies). F, Putative model for acute postoperative Aβ elevation driven by immune genes like NF‐κB, thus elevating oligomers in the context of an AD‐vulnerable brain. The function of microglial cells in response to surgical trauma associated molecular patterns (STAMPs)‐induced Aβ is not yet defined and requires in‐depth evaluation. Results are shown as mean ± standard error of the mean, **P < .01, * P < .05, as measured by two‐tailed unpaired Student t test. Scale bar = 50 µm
5.4. Delirium‐like behavior in CVN‐AD mice
Impaired attention is key feature for delirium. We used the 5‐choice serial‐reaction time task (5‐CSRTT), a validated procedure for the assessment of attention in mice 39 to characterize attention in age‐matched 12‐month‐old C57BL/J (C57) and CVN‐AD mice. During a trial, a nose poke funnel is illuminated for 1.5 seconds followed by a 5‐second response phase with extinguished nose funnels. The training and testing protocols are described in detail in the supporting information. All mice sustained at least an 80% response rate out of 40 daily trails throughout testing (Figure 4A). Within the responded trials, the correct responses were more than 90% in both C57 and CVN‐AD mice across the baseline 3 days prior to tibial fracture (Figure 4B). However, on post‐surgery day 1 and 2, CVN‐AD mice showed significant increases for incorrect responses compared to baseline (average of 3 days prior to surgery, P = .004 and 0.045 respectively, Figure 4B). Although the incidence of incorrect responses also increased in C57 mice after surgery, no statistic difference was observed compared to baseline. The percentage of incorrect responses on post‐surgery day 1 was also significantly higher in CVN‐AD mice (40% ± 8%) compared to C57 (23% ± 6%, P = .043; Figure 4B). In addition, the increase of incorrect responses from average baseline to post‐surgery day 1 (Δ incorrect responses) was significantly higher for CVN‐AD mice compared to C57 (15% ± 6% vs 35% ± 8%, P = .0497; Figure 4C). Because the overall behavioral response ratio in 40 trials, whether correct or incorrect, remained stable in both groups after surgery compared to baseline (Figure 4A), the reduction in accuracy of behavior responses is most likely caused by incorrect choices. Therefore, our data suggest that compared to C57 mice, the CVN‐AD mice experience more profound attention impairment following surgery.
FIGURE 4.
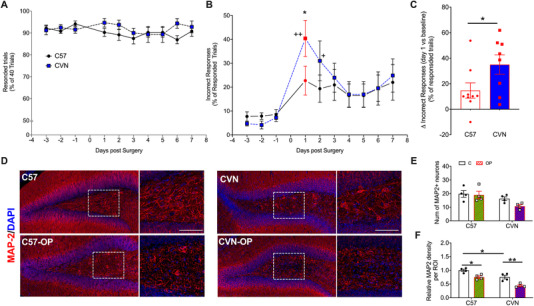
Orthopedic surgery triggers deficits in attention processes in CVN‐AD mice and impairs neuronal markers. Attention was assessed using the 5‐choice serial reaction time task (5‐CSRTT) in 12‐month‐old C57 and CVN‐AD mice. A, All the mice had over 80% responses (including correct and incorrect responses) in 40 daily trials throughout the testing days. B, On postoperative day 1 and 2, CVD‐AD mice showed significantly higher incidence of incorrect responses compared to baseline. Furthermore, the incorrect response ratio on postoperative day 1 was significantly higher in CVN‐AD mice compared to C57. C, The increase of incorrect response from baseline (average of results of 3 days prior to surgery) to postoperative day 1 was higher in CVN‐AD mice compared to C57. D‐F, Neuronal cytoarchitecture in the dentate gyrus (DG) area of the hippocampus. The expression of MAP2 was reduced in the DG of operated 12‐month‐old C57 as well as CVN‐AD mice, although the number of neurons were not significantly affected. Results are shown as mean ± standard error of the mean, n = 8‐9/group (behavior) and n = 4/group (histology). **P < .01, * P < .05, as determined by repeated measures analysis of variance (ANOVA) with Bonferroni corrections for 5‐CSRTT and as measured by two‐way ANOVA with Tukey's test for multiple comparison for histology. Scale bar = 50 µm
In a separate cohort, we assessed neuronal markers at 24 hours after surgery, the peak of the attention deficit. Immunostaining showed that the number of MAP2+ neurons in 12‐month‐old CVN‐AD mice was decreased although it did not reach significance from age‐matched C57BL6/J mice (Figure 4E). After surgery, neurites were significantly impaired in both C57 and CVN‐AD mice, although more significantly in the latter (25% reduction, P = .0147; and 42% reduction, P = .0032, respectively; Figure 4D and F).
6. FUTURE EXPERIMENTS AND VALIDATION STUDIES
Future experiments should focus on defining the causal link between STAMPs and specific behavioral deficits. Based on our early experimental data, we propose to focus future work on the following two key areas.
6.1. BBB and neurodegenerative hallmarks
We used the CVN‐AD mouse model to specifically address the increased vulnerability of the NVU, part of the CAA pathology that these mice develop by 12 months of age. 40 Indeed, many limitations are intrinsic to the use of rodent models to address complex human diseases, especially to represent the more common, late‐onset, form of AD. 40 The generation of reliable sporadic AD models will likely have a larger impact on delirium and aging‐associated neurological disorders. In addition, models of other risk factors common to dementia and delirium, for example, metabolic syndrome and diabetes, should also be tested. Because AD pathology develops over decades before becoming clinically manifest, 41 it remains crucial to determine how surgery may impact cognitive trajectories in animal models that attempt to recapitulate preclinical and mild cognitive impairment states. Additional experiments should evaluate relationships between trauma‐induced factors and BBB/NVU dysfunction, and between BBB permeability and AD pathologic hallmarks such as Aβ, tau, and other proteinopathies.
Studies are under way to evaluate BBB dysfunction as a risk factor for postoperative delirium. In cardiac surgery the BBB can become compromised and this may have implications for postoperative delirium. 42 Moreover, biomarkers of oxidative stress and neuronal injury are elevated in this surgical population, and are amplified in patients with BBB dysfunction, as detected by plasma levels of S100 calcium‐binding protein B, which is usually released by astrocytes. 43 Endothelial dysfunction involving the BBB is also a feature of delirium in critically ill patients, 44 and studies in several animal models suggest changes in BBB permeability in perioperative neurocognitive disorders. 35 , 45
Future studies must also evaluate the kinetics of BBB opening after surgery, and determine whether specific biomarkers may help to identify patients at risk for developing delirium. One such biomarker is fibrinogen. For example, we found fibrinogen extravasated after orthopedic surgery in adult wild‐type 34 , 46 and CVN‐AD mice (Figure 2). Fibrinogen can bind to the CD11b receptor expressed on microglia, which is associated with dendritic and spine loss in AD‐like mice, 47 , 48 and can additionally interact with Aβ 49 (Figure 5). In addition, Aβ can interact with other inflammatory genes, such as NF‐κB, which is upregulated via pro‐inflammatory cytokines during surgery; thus, NF‐κB can interact directly with APPs to transcribe oligomeric Aβ1‐42. 38 As an acute‐phase response to surgical trauma, this “immunocentric” function of Aβ must be further assessed.
FIGURE 5.
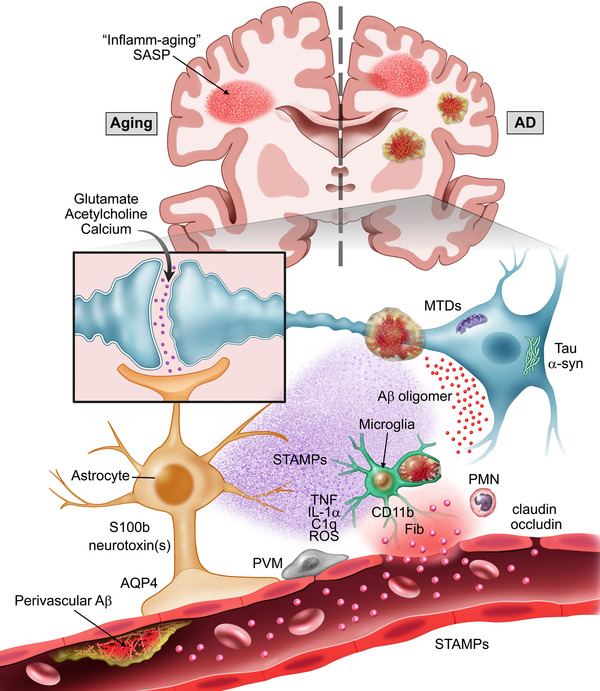
Visual abstract of putative mechanisms involved in the pathogenesis of postoperative delirium. Aging and dementia are the most significant risk factors for delirium. Both states are characterized by chronic inflammation (including inflamm‐aging) as well as upregulation of senescent cells (SASP). The Alzheimer's disease (AD) brain has additional hallmarks of vulnerability, herein we focus on pre‐existing amyloid β (Aβ) pathology. Together with the classical plaques and tangles, vascular pathology (including perivascular Aβ/cerebral amyloid angiopathy) are commonly found in the AD brain. This ongoing pathology compromises the blood‐brain barrier/neurovascular unit (BBB/NVU), which becomes more susceptible to additional stressors like surgery. Surgical trauma associated molecular patterns (STAMPs) can disrupt the BBB and preexisting BBB pathology amplifies delirium risk in patients. Detailed characterization of STAMPs (including cytokines, damage‐associated molecular patterns [DAMPs], resolvins, etc) is needed. A putative factor, fibrinogen, enters the brain parenchyma and activates microglia via CD11b signaling. The time course of postoperative glial activation needs significant investigation. “A1” mediators like TNF, IL‐1α, and C1q can activate astrocytes contributing to neuronal loss. A similar process may explain postoperative delirium; however, astrocytes may also be an earlier responder in settings in which the BBB is already compromised. Markers like S100b are detectable at baseline in patients with perioperative neurocognitive disorders, thus STAMPs affecting astrocytic‐end feet, water channels (like AQP‐4) and tight junctions (claudin, occludin, etc) may in turn trigger microglial activation. Whether astrocytes directly release specific neurotoxins after surgery that compromise surrounding neurons is unknown. Thus, STAMPs may directly disrupt synaptic plasticity and/or by upregulating other factors like neurotoxic Aβ. These can impair calcium signaling, neurotransmitter release, and induce oxidative stress. Finally, peripheral immune cells may further contribute to this pathology and selective therapies to prevent infiltration may reduce delirium incidence in the vulnerable brain. Additional abbreviations: MTDs, mitochondrial damage associated molecular patterns; PMN, polymorphonuclear leukocytes; ROS, reactive oxygen species; PVM, perivascular macrophage
Inflammation is likely an influential event in the pathophysiology of AD. 50 Delirium can acutely accelerate existing neurodegeneration; however, such pathologic changes in the vulnerable brain may be unique to delirium superimposed on dementia. In fact, delirium in non‐demented patients does not induce long‐term changes in Aβ load, according to positron emission tomography (PET) imaging, 51 , 52 and similar findings are described when measuring CSF Aβ biomarkers. 53 , 54
However, more detailed evaluation of vascular Aβ pathology is required to determine if this is a protective response to acute trauma or a pathogenic hallmark of delirium superimposed on dementia, possibly predictable in the context of elective surgery. This is especially relevant in the context of Aβ‐directed therapies as many have failed in recent clinical trials for mild to moderate AD, with a remaining hope for efficacy in early “preclinical AD.” 55 Thus, targeting Aβ within the context of a predictable injury, that is, surgery, will require careful validation in patients at risk for postoperative delirium.
6.2. Neuroinflammation and delirium‐like behavior
Dysregulated innate immunity is regarded as a key hallmark of many neurologic diseases including AD. 56 Further work is needed to define the signaling mechanisms involved in the transduction of systemic inflammation to the CNS, including defining if specific STAMPs are causally related to delirium onset. In this regard, using models of isochronic and heterochronic parabiosis, pioneering studies from Wyss‐Coray et al. have demonstrated the important contribution of hematogenous factors to aging‐related cognitive decline. 57 Because peripheral inflammatory stimuli are sufficient to disrupt synaptic communication and functional brain connectivity, it will be important to determine the extent to which perioperative increases in neuroinflammation, for example, PET scan ligands, or cytokines in CSF, are associated with changes in brain activity/connectivity as measured by electroencephalogram (EEG [or functional magnetic resonance imaging (fMRI)]).
Multiple surgical models and clinical trials have been used in rodents and humans, respectively, to study perioperative neurocognitive disorders. These range from minimally invasive abdominal procedures to more invasive procedures such as cardiac, orthopedic, and vascular surgery (for a recent review of the preclinical field, see Eckenhoff et al. 58 ). Although the contribution of different immune factors and damage‐associated molecular patterns (DAMPs) may vary depending on the procedure, neuroinflammation has been reported as a common overarching phenomenon that may presage delirium. Surgery activates glia, including microglia and astrocytes, which contribute to neuronal impairment and behavioral deficits. In our own work, we have found virtually no evidence that brief isoflurane anesthesia causes neuroinflammation or behavioral deficits, suggesting that the immune response to trauma is the main driver of cognitive deficits. 20 , 59 Further, others have described inattention with acute changes in α‐synuclein 24 hours after abdominal surgery with isoflurane anesthesia. 60 Also, anesthesia with surgery, but not anesthesia alone, caused long‐term cognitive deficits with signs of microglial activation, amyloidopathy, and tauopathy in 3xTgAD mice. 61 Thus, anesthesia combined with surgery leads to a cascade of immune events, although anesthesia per se may influence inflammatory and neurodegenerative pathologic hallmarks, for example. 62 , 63
More detailed definitions of neuroinflammation that specifically focus on the function of microglial and astrocyte modulation by anesthesia and surgery are urgently needed in the perioperative field. Comprehensive transcriptomic studies are helping to define molecular signatures of highly heterogenous immune cells in health and disease. 64 Approaches that use single‐cell RNA sequencing are identifying novel clusters with functional implications, such as disease‐associated microglia (DAM), 65 that strongly associate with pathologic immunity in AD and other neurodegenerative disease. Future work should define and compare markers of perioperative immunity, including immune‐metabolic signatures, with established datasets of rodent and human biofluids. Such cross‐species comparisons are critical to validating the rodent models and to understanding similarities and differences in pathologic processes that accompany delirium and neurodegenerative disease. Current human data can be accessed via large programs such as Accelerating Alzheimer's Research and Drug Development (AMP‐AD), which is supported by the National Institutes of Health (NIH) with multiple stakeholders in industry, academia, and non‐profit agencies. 66
Finally, efforts should be devoted to safely targeting perioperative immunity with new therapeutic approaches. Therapies aimed at dampening the immune response, for example, with nonsteroidal anti‐inflammatory drugs or glucocorticoids, have shown limited or no efficacy in treating dementia (INTREPAD trial 67 ) or perioperative neurocognitive disorders (DECS trial 68 ). These drugs may also interfere with wound healing. Thus, more specific approaches may be required, especially in the context of a predictable stressor like an elective surgical procedure. Such approaches should focus on selective strategies that safely and effectively regulate perioperative immune responses (and/or specific STAMPs) without inappropriately activating or prolonging their effects. To ensure safety and selectivity, promising strategies may involve administration of specialized pro‐resolving mediators, for example, resolvins, lipoxins, and maresins, or stimulation of neuronal pathways such as the vagus nerve, to regulate innate immunity. 69 , 70 Finally, one of the most promising interventions to reset homeostasis between a compromised BBB, pathologic microgliosis and synaptic dysfunction is a “broad‐spectrum” brain‐penetrant small molecule inhibitor to mixed lineage kinases, URMC‐099. 71 We have recently demonstrated its efficacy in preventing delirium and safety with respect to bone healing in our orthopedic model of hip fracture. 72 The utility of this agent is further strengthened by its ability to reverse neuroinflammatory and synaptic damage in the APP/PS‐1 model of AD. 73
7. MAJOR CHALLENGES FOR THE HYPOTHESIS
Delirium is an emerging challenge in neuropsychiatry, gerontology, and perioperative medicine. Aging and dementia are the strongest risk factors for postoperative delirium. According to the network hypothesis of aging, “inflamm‐aging” has been proposed as a critical driver of senescence via continuous low‐level exposure to antigenic load and stress 74 (Figure 5). Surgery, especially in older frail adults, is associated with a potent antigenic load capable of inducing sterile inflammation through the release of DAMPs and cytokines. In vulnerable systems, DAMPs and cytokines can trigger postoperative complications such as delirium. In fact, older adults who develop delirium have significantly elevated levels of pro‐inflammatory cytokines in the CSF and plasma. 26 IL‐6, a classic biomarker of inflammation, can induce pleiotropic effects both in health and disease by modulating the NF‐κB transcription factor. 75 Indeed, plasma IL‐6 and C‐reactive protein are the most powerful predictors of morbidity and mortality as well as postoperative delirium in older adults. 76 , 77 In our study, we found similar changes in pro‐inflammatory systemic markers, including IL‐6 and the monocytic chemoattractant proteins MCP‐1, MIP, and G‐CSF. A key question remains: Does the increased incidence of postoperative delirium in older adults reflect a greater CNS inflammatory response with aging, or are older adults more cognitively sensitive to a similar magnitude of postoperative neuroinflammatory response? Although identifying more specific biomarkers may help to predict risk for developing delirium, deep immunophenotyping of elderly surgical candidate has the potential to provide further insights in this area.
Delirium and dementia are complex multifactorial disorders with multiple risk factors that can influence each other. Indeed, dementia is a strong predictor of delirium; however, many of the genes that are known to increase risk for AD are not clearly linked to delirium. 78 The apolipoprotein E (APOE) genotype is perhaps the most studied of these genes, given its predominant role in late onset AD. In the Successful AGing after Elective Surgery (SAGES) cohort of 560 patients aged ≥70 years who did not have recognized dementia or a history of delirium, APOE ε4 was not associated with higher delirium incidence, nor was ε2 carrier status, which is associated with decreased AD risk and with lower delirium incidence. 79 Similarly, in older adults undergoing elective primary hip or knee arthroplasty, APOE genotype and postoperative delirium were not associated. 80 However, Ely et al. found that the APOE ε4 genotype is the strongest predictor of delirium duration in critically ill patients. 81 This finding is consistent with a large retrospective analysis of ε4 carriers undergoing general anesthesia and surgery, which showed that these patients experienced worse long‐term cognitive outcome and an accelerated rate of decline. 82
APOE ε4 negatively affects tau pathology and tau‐meditated neurodegeneration independently of Aβ, 83 thereby providing a possible bridge between neurodegeneration and inflammation, especially in the context of glial activation and Aβ plaque phagocytosis. 84 , 85 Thus, baseline immune status and/or immune cell‐specific susceptibilities may underlie the different outcomes reported in the clinical studies above. Although host inflammatory responses are well conserved and ubiquitous following critical illness, trauma, or surgery, immune signatures may be unique to each patient. For example, a specific peripheral CD14+ monocyte subset was shown to predice postoperative clinical outcomes in hip surgery patients. 86 This finding suggests that more in‐depth immunophenotyping of individual patients pre‐/post‐surgery is necessary to assign immune risk scores that may predict cognitive changes over time.
Interactions between delirium and dementia are becoming apparent; yet, the factors that contribute to progression are not known. Some studies have identified hospitalization as a strong risk factor for subsequent dementia. 87 , 88 Based on our immunocentric model of delirium, it is plausible that early stressors impact brain function and accelerate individual predisposition to neurodegeneration. Indeed, several aspects of the innate immune response are evolutionarily conserved, and thus our surgical model has important translational implications. In fact, we previously demonstrated that BBB opening allows macrophage migration into the brain parenchyma, and promotes changes in microglial activity, which is associated with deficits in declarative memory and hippocampal neuroplasticity. 20 , 22 , 59 , 89 , 90 The interplay between glial cells and a potentially protective role of microglia and astrocytes is largely unexplored in perioperative medicine. Reactive “A1” astrocytes are present in several neurologic conditions including aging and AD, and have been observed after peripheral surgery. 89 , 91 , 92 Liddelow et al. 91 demonstrated that microglia activate A1 astrocytes via cytokines and complement factors (Figure 5). Surgery triggers similar immunologic changes that are often transient and self‐resolving; however, these changes may become maladaptive in co‐morbid states such as advanced age, metabolic syndrome, and neurodegeneration.
According to this theory, an early stressor can predispose the individual to subsequent changes in neuroimmune functions, thus eliciting such phenomena as microglial priming. 93 Indeed, the blood‐derived protein fibrinogen, which has been detected in the brain after postoperative decline (Terrando et al. 21 and this study), promotes microglial activation, peripheral macrophage recruitment, and synapse loss. 48 , 94 The mechanism by which systemic inflammation drives postoperative neuroinflammation remains unclear. Does it simply involve passive flux across the permeable BBB, 42 or are cytokines produced in situ within the brain itself, perhaps due to a neural relay from the peripheral trauma site into the brain? 95 The latter model is supported by at least one study that reports higher levels of inflammatory cytokines in the CSF than in the peripheral serum after non‐neurologic surgery. 96 In the CVN‐AD mouse model, we detected high levels of systemic cytokines and chemokines after surgery, which may indicate an important role for the peripheral milieu in driving subsequent neuroinflammatory changes in the brain. In either case, the extent to which acute postoperative neuroinflammation contributes to long‐term cognitive decline or even dementia after postoperative delirium remains unanswered. Work by Davis et al. demonstrated that baseline neurodegeneration increases the risk, severity, and duration of delirium in mice and humans. 97 However, the impact of one, or multiple, episodes of delirium on a nervous system without evidence of neurodegeneration will require further studies. For example, the differential impact of delirium on dementia subtypes or other neurodegenerative conditions such as tauopathies and Parkinson's and Lewy body diseases, is poorly described in the current literature.
Importantly, a recent analysis of autopsy and Medicare records that included cognitive outcomes data on >500 older adults showed higher rates of cognitive decline after hospitalization in subjects with tau tangles and neocortical Lewy bodies. 98
Finally, we do not yet know definitively how surgery affects select areas of the brain. CVN‐AD mice exhibit postoperative age‐dependent pathologic changes in the NVU, which are associated with changes in astrocytes and pericytes.37 Surgery selectively impairs the hippocampal vasculature, which in turn can impact Aβ distribution and CAA pathology. 99 However, hippocampal capillary damage is also observed in patients with early cognitive decline, suggesting that this damage may occur before dementia onset. 100 , 101 Indeed, these pathologic changes correlate with memory and cognitive impairments. Inattention, one feature of delirium, is largely attributed to impaired frontoparietal and frontostriatal networks in humans but is somewhat difficult to define in rodents. Thus, there is a critical need to study the effect of postoperative neuroinflammation on networks in the human brain that are related to dementia and delirium. Using fMRI and other brain imaging modalities will allow us to map postoperative changes in these networks and to identify other brain regions of interest.
8. TRANSLATIONAL POTENTIAL
Postoperative delirium provides a unique clinical setting because we know exactly when inflammatory processes begin as the elective surgical procedures are typically scheduled weeks to months in advance. Therefore, therapeutic strategies (including those that have failed in AD) may find repurposing for conditions like delirium or for delirium superimposed on dementia.
Ongoing clinical studies are examining whether postoperative neuroinflammation leads to postoperative delirium with an attempt at identifying putative biomarkers for prevention. For example, advanced proteomic analyses comparing pre‐ and postoperative markers have identified plasma C‐reactive protein as a perioperative marker of delirium. 102 The application of blood‐ based biomarkers is attractive compared to CSF, which is more invasive and carries some risks. With the advances in blood‐based biomarkers that accurately reflect AD pathology, 103 there are newer opportunities to detect autoantibodies, exosomes, and microRNAs that may readily identify high‐risk patients for delirium. 104 Intraoperative markers of oxidative stress, including F2‐isoprostanes and isofurans, were recently correlated with postoperative ubiquitin carboxyl‐terminal hydrolase isozyme L1 in patients with delirium after cardiac surgery. This change was exacerbated in subjects with elevated S100 calcium‐binding protein B, suggesting a vulnerable BBB hastens delirium. 43 PET imaging and dynamic contrast‐enhanced magnetic resonance imaging are providing more selective approaches for interrogating changes in glial activity and NVU/BBB opening in older adults following surgery 105 and for those with neurodegeneration. 100 Ongoing studies like RISE (Role of Inflammation after Surgery for Elders) 106 and ASCRIBED (the impact of Acute SystematiC inflammation upon CSF and blood BiomarkErs of brain inflammation and injury in dementia) 107 will provide large cohort data on immune biomarkers in orthopedic patients during aging and ongoing neurodegeneration. These combined approaches are likely to contribute to a better understanding of delirium and AD pathology to refine therapeutic targets.
9. LINKS TO OTHER MAJOR THEORIES
Evidence of impaired neurovascular function and neuroinflammation are increasingly recognized in the AD field. 108 The cerebral vasculature plays a pivotal role in regulating the blood supply to the brain and the BBB interface. Changes in the cerebral vasculature can lead to neuronal dysfunction, and have been implicated in aging‐related cognitive impairment including AD. 109 , 110 Epidemiologic studies have shown that midlife vascular risk factors such as metabolic disorders and hypertension are associated with increased risk for dementia 111 and cerebral amyloid deposition later in life. 112 A separate population‐based study revealed that demented patients are two times more likely to develop cerebral vascular pathology, for example, infarcts, atherosclerosis, and small vascular diseases, than are non‐demented patients. In addition, patients who have vascular pathology plus AD‐type lesions, that is, neurofibrillary tangles and Aβ plaques, have nearly twice the incremental risk for dementia compared to patients with only AD‐type lesions. 113 Thus, vascular dysfunction plays a critical role in the pathogenesis and development of dementias, and is one of the research priorities presented at the 2019 Alzheimer's Disease‐Related Dementia Summit held at the NIH.
Dysregulated calcium signaling has been implicated in processes of neurodegeneration and associated cognitive loss, including AD. 114 Indeed, Aβ plaques can alter calcium levels and overload synaptodendritic signaling; 115 this in turns activates calcineurin that initiates long‐term depression and decreases synaptic efficacy. 116 Impaired calcium signaling also contributes to neurotoxic effects, including oxidative stress, mitochondrial damage, and neuroapoptosis by promoting opening of the ryanodine and inositol 1,4,5‐trisphosphate receptors. 117 Importantly, these receptors can be activated by anesthetics such as isoflurane, thus contributing to further toxicity in the vulnerable brain 118 and synergize with the immune system 119 (Figure 5).
Finally, aging itself is characterized by a low‐grade, chronic, inflammatory state that can be exacerbated by multiple factors including co‐morbidities; infection; trauma; and in some cases, surgery. Failed resolution of inflammation has been implicated in several pathologies and inhibits the return to homeostasis necessary for premorbid health. 120 , 121 , 122 , 123 As our aging population increases in number, surgical procedures increase as well to maintain quality of life. Thus, strategies to prevent pathologic activation of innate immune responses in the aging brain become even more important to insure brain health.
Supporting information
Material and Methods. Detailed description of the methods related to the Early experimental data.
Video 1. Representative video depicting Iba‐1 immunoreactivity and Aβ deposition in a CVN‐AD control (12‐month‐old) clarified hippocampus.
Video 2. Representative video depicting Iba‐1 immunoreactivity and Aβ deposition in a CVN‐AD operated (12‐month‐old) clarified hippocampus.
ACKNOWLEDGMENTS
Supported by the National Institutes of Health (NIH) grants R01AG057525 (NT), R21 AG055877‐01A1 (NT), R03 AG064260‐01 (NT), K76‐AG057022 (MB); additional support from the Duke Claude D. Pepper Older American Independence Center (P30AG028716); the Duke Anesthesiology Department (NT and MB); the Alzheimer's Association (NT). The equipment for the attention test was purchased with a grant from the North Carolina Biotechnology Center (to WCW). The Zeiss light sheet was purchased with a NIH S10 grant 1S10OD020010‐01A1 (Dr. Lisa Cameron). Biomarker profiling was performed under the management of Dr. Andrew N. Macintyre and direction of Dr. Gregory D. Sempowski in the Immunology Unit of the Duke Regional Biocontainment Laboratory, which received partial support for construction from the NIH, National Institute of Allergy and Infectious Diseases (UC6‐AI058607). We thank Stuart Sundseth (Colton Lab, Duke University Medical Center) for assistance with the MSD assays; Benjamin Carlson, PhD (Light Microscopy Core Facility, Duke University) for guidance on imaging analyses; Kathy Gage, BS (Duke University Medical Center) for editorial assistance; and Katerina Akassoglou, PhD (Gladstone Institutes, UCSF) for discussions.
Wang P, Velagapudi R, Kong C, et al. Neurovascular and immune mechanisms that regulate postoperative delirium superimposed on dementia. Alzheimer's Dement. 2020;16:734–749. 10.1002/alz.12064
REFERENCES
- 1. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ags/Nia Delirium Conference Writing Group, P.C. and Faculty , The American Geriatrics Society/National Institute on Aging Bedside‐to‐Bench Conference: research agenda on delirium in older adults. J Am Geriatr Soc. 2015;63(5):843‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456‐1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mason SE, Noel‐Storr A, Ritchie CW. The impact of general and regional anesthesia on the incidence of post‐operative cognitive dysfunction and post‐operative delirium: a systematic review with meta‐analysis. J Alzheimers Dis. 2010;22(Suppl 3):67‐79. [DOI] [PubMed] [Google Scholar]
- 5. Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112(5):1179‐1185. [DOI] [PubMed] [Google Scholar]
- 6. Leslie DL, Marcantonio ER, Zhang Y, Leo‐Summers L, Inouye SK. One‐year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1): 27‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669‐676. [DOI] [PubMed] [Google Scholar]
- 8. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516‐522. [DOI] [PubMed] [Google Scholar]
- 9. Han JH, Shintani A, Eden S, et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010;56(3):244‐252 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Terrando N, Eriksson LI, Eckenhoff RG. Perioperative neurotoxicity in the elderly: summary of the 4th International Workshop. Anesth Analg. 2015;120(3):649‐652. [DOI] [PubMed] [Google Scholar]
- 11. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50(10):1723‐1732. [DOI] [PubMed] [Google Scholar]
- 12. Lee HB, Oldham MA, Sieber FE, Oh ES. Impact of delirium after hip fracture surgery on one‐year mortality in patients with or without dementia: a case of effect modification. Am J Geriatr Psychiatry. 2017;25(3):308‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morandi A, Davis D, Bellelli G, et al. The diagnosis of delirium superimposed on dementia: an emerging challenge. J Am Med Dir Assoc. 2017;18(1):12‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nathan C, Ding A, Nonresolving inflammation. Cell. 2010;140(6):871‐882. [DOI] [PubMed] [Google Scholar]
- 15. Van Eldik LJ, Carrillo MC, Cole PE, et al. The roles of inflammation and immune mechanisms in Alzheimer's disease. Alzheimers Dement. 2016;2(2):99‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. 2019 Alzheimer's disease facts and figures. Alzheimer's & Dementia. 2019;15(3):321‐387. [Google Scholar]
- 17. Cunningham C, Campion S, Lunnon K, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65(4):304‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH, Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25(40):9275‐9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skelly DT, Griffin ÉW, Murray CL, et al. Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL‐1‐dependent mechanisms. Mol Psychiatry. 2019;24(10):1533‐1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cibelli M, Fidalgo AR, Terrando N, et al. Role of interleukin‐1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68(3):360‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Terrando N, Eriksson LI, Ryu JK, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70(6):986‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Degos V, Vacas S, Han Z, et al. Depletion of bone marrow‐derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology. 2013;118(3):527‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. D'Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29(7):2089‐2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skrede K, Wyller TB, Watne LO, Seljeflot I, Juliebø V. Is there a role for monocyte chemoattractant protein‐1 in delirium? Novel observations in elderly hip fracture patients. BMC Res Notes. 2015;8:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Westhoff D, Witlox J, Koenderman L, et al. Preoperative cerebrospinal fluid cytokine levels and the risk of postoperative delirium in elderly hip fracture patients. J Neuroinflammation. 2013;10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hirsch J, Vacas S, Terrando N, et al. Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. J Neuroinflammation. 2016;13(1): 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morganti JM, Jopson TD, Liu S, et al., CCR2 antagonism alters brain macrophage polarization and ameliorates cognitive dysfunction induced by traumatic brain injury. J Neurosci, 2015;35(2):748‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamasaki R, Lu H, Butovsky O, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med. 2014;211(8):1533‐1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Varvel NH, Neher JJ, Bosch A et al. Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. Proc Natl Acad Sci U S A. 2016;113(38):E5665‐e5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shechter R, London A, Varol C, et al. Infiltrating blood‐derived macrophages are vital cells playing an anti‐ inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6(7):e1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wattananit S, Tornero D, Graubardt N, et al., Monocyte‐derived macrophages contribute to spontaneous long‐term functional recovery after stroke in mice. J Neurosci. 2016;36(15):4182‐4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iadecola C, Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5(5):347‐360. [DOI] [PubMed] [Google Scholar]
- 33. Wilcock DM, Lewis MR, Van Nostrand WE, et al. Progression of amyloid pathology to Alzheimer's disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J Neurosci. 2008;28(7):1537‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Terrando N, Eriksson LI, Ryu JK, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70(6):986‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang T, Xu G, Newton PF, et al. Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders. Br J Anaesth. 2019;122(3):350‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilcock DM, Vitek MP, Colton CA. Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer's disease. Neuroscience. 2009;159(3):1055‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chung K, Deisseroth K.. CLARITY for mapping the nervous system. Nat Methods. 2013;10(6):508‐513. [DOI] [PubMed] [Google Scholar]
- 38. Grilli M, Ribola M, Alberici A, Valerio A, Memo M, Spano P. Identification and characterization of a kappa B/Rel binding site in the regulatory region of the amyloid precursor protein gene. J Biol Chem. 1995;270(45):26774‐26777. [DOI] [PubMed] [Google Scholar]
- 39. Patel S, Stolerman IP, Asherson P, Sluyter F. Attentional performance of C57BL/6 and DBA/2 mice in the 5‐choice serial reaction time task. Behav Brain Res. 2006;170(2):197‐203. [DOI] [PubMed] [Google Scholar]
- 40. King A, The search for better animal models of Alzheimer's disease. Nature. 2018;559(7715):S13‐S15. [DOI] [PubMed] [Google Scholar]
- 41. Jack CR, Jr , Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9(1):119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Merino JG, Latour LL, Tso A, et al., Blood‐brain barrier disruption after cardiac surgery. AJNR Am J Neuroradiol. 2013;34(3):518‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lopez MG, Hughes CG, DeMatteo A, et al. Intraoperative oxidative damage and delirium after cardiac surgery. Anesthesiology. 2020;132(3)551‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hughes CG, Pandharipande PP, Thompson JL, et al. Endothelial activation and blood‐brain barrier injury as risk factors for delirium in critically Ill patients. Crit Care Med. 2016;44(9):e809‐e817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang S, Gu C, Mandeville ET, et al. Anesthesia and surgery impair blood‐brain barrier and cognitive function in mice. Front Immunol. 2017;8:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Velagapudi R, Subramaniyan S, Xiong C, et al. Orthopedic surgery triggers attention deficits in a delirium‐like mouse model. Front Immunol. 2019;10:2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Adams RA, Bauer J, Flick MJ, et al. The fibrin‐derived gamma377‐395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med. 2007;204(3):571‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Merlini M, Rafalski VA, Rios Coronado PE, et al. Fibrinogen induces microglia‐mediated spine elimination and cognitive impairment in an alzheimer's disease model. Neuron. 2019;101(6):1099‐1108 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cortes‐Canteli M, Paul J, Norris EH, et al. Fibrinogen and beta‐amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer's disease. Neuron. 2010;66(5):695‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Newcombe EA, Camats‐Perna J, Silva ML, Valmas N, Huat TJ, Medeiros R. Inflammation: the link between comorbidities, genetics, and Alzheimer's disease . J Neuroinflammation. 2018;15(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rolandi E, Cavedo E, Pievani M, et al. Association of postoperative delirium with markers of neurodegeneration and brain amyloidosis: a pilot study. Neurobiol Aging. 2018;61:93‐101. [DOI] [PubMed] [Google Scholar]
- 52. Jackson JC, Warrington HJ, Kessler R, Kiehl AL, Ely WE. Florbetapir‐PET beta‐amyloid imaging and associated neuropsychological trajectories in survivors of critical illness: A case series. J Crit Care. 2018;44:331‐336. [DOI] [PubMed] [Google Scholar]
- 53. Witlox J, Kalisvaart KJ, de Jonghe JF, et al. Cerebrospinal fluid beta‐amyloid and tau are not associated with risk of delirium: a prospective cohort study in older adults with hip fracture. J Am Geriatr Soc. 2011;59(7):1260‐1267. [DOI] [PubMed] [Google Scholar]
- 54. Xie Z, Swain CA, Ward SAP, et al. Preoperative cerebrospinal fluid beta‐Amyloid/Tau ratio and postoperative delirium. Ann Clin Transl Neurol. 2014;1(5):319‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid‐beta‐targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019;15(2):73‐88. [DOI] [PubMed] [Google Scholar]
- 56. Morgan AR, Touchard S, Leckey C et al. Inflammatory biomarkers in Alzheimer's disease plasma. Alzheimers Dement. 2019;15(6):776‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eckenhoff RG, Maze M, Xie Z, et al. Perioperative neurocognitive disorder: state of the preclinical science. Anesthesiology, 2020;132(1):55‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang MD, Barde S, Yang T, et al. Orthopedic surgery modulates neuropeptides and BDNF expression at the spinal and hippocampal levels. Proc Natl Acad Sci U S A. 2016;113(43):E6686‐E6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ren Q, Peng M, Dong Y, et al. Surgery plus anesthesia induces loss of attention in mice. Front Cell Neurosci. 2015;9:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tang JX, Mardini F, Janik LS, et al. Modulation of murine Alzheimer pathogenesis and behavior by surgery. Ann Surg. 2013;257(3):439‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Eckenhoff RG, Johansson JS, Wei H, et al. Inhaled anesthetic enhancement of amyloid‐beta oligomerization and cytotoxicity. Anesthesiology. 2004;101(3):703‐709. [DOI] [PubMed] [Google Scholar]
- 63. Okuno T, Koutsogiannaki S, Hou L, et al. Volatile anesthetics isoflurane and sevoflurane directly target and attenuate Toll‐ like receptor 4 system. FASEB J. 2019;33(12):fj201901570R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hammond TR, Dufort C, Dissing‐Olesen L, et al. Single‐cell rna sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell‐state changes. Immunity. 2019;50(1):253‐271 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Keren‐Shaul H, Spinrad A, Weiner A, et al. A unique microglia type associated with restricting development of alzheimer's disease. Cell. 2017;169(7):1276‐1290 e17. [DOI] [PubMed] [Google Scholar]
- 66. Accelerating Medicines Partnership ‐ Alzheimer's Disease (AMP‐AD). August 15, 2019]; Available from: https://www.nia.nih.gov/research/amp-ad.
- 67. Meyer PF, Tremblay‐Mercier J, Leoutsakos J, et al. INTREPAD: A randomized trial of naproxen to slow progress of presymptomatic Alzheimer disease. Neurology. 2019;92(18):e2070‐e2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ottens TH, Dieleman JM, Sauër AM, et al. Effects of dexamethasone on cognitive decline after cardiac surgery: a randomized clinical trial. Anesthesiology. 2014;121(3):492‐500. [DOI] [PubMed] [Google Scholar]
- 69. Pavlov VA, Chavan SS, Tracey KJ. Bioelectronic medicine: from preclinical studies on the inflammatory reflex to new approaches in disease diagnosis and treatment. Cold Spring Harb Perspect Med. 2020;10(3):a034140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro‐resolving superfamily of mediators. J Clin Invest. 2018;128(7):2657‐2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Goodfellow VS, Loweth CJ, Ravula SB, et al. Discovery, synthesis, and characterization of an orally bioavailable, brain penetrant inhibitor of mixed lineage kinase 3. J Med Chem. 2013;56(20):8032‐8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Miller‐Rhodes P, Kong C, Baht GS, et al. The broad spectrum mixed‐lineage kinase 3 inhibitor URMC‐099 prevents acute microgliosis and cognitive decline in a mouse model of perioperative neurocognitive disorders. J Neuroinflammation. 201916(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kiyota T, Machhi J, Lu Y, et al. URMC‐099 facilitates amyloid‐beta clearance in a murine model of Alzheimer's disease. J Neuroinflammation. 2018;15(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Franceschi C, Bonafè M, Valensin S, et al. Inflamm‐aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244‐254. [DOI] [PubMed] [Google Scholar]
- 75. Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin‐6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61(6):575‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fong TG, Chan NY, Dillon ST, et al. Identification of plasma proteome signatures associated with surgery using SOMAscan. Ann Surg, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin‐6 and C‐reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506‐512. [DOI] [PubMed] [Google Scholar]
- 78. Adamis D, Meagher D, Williams J, Mulligan O, McCarthy G. A systematic review and meta‐analysis of the association between the apolipoprotein E genotype and delirium. Psychiatr Genet. 2016;26(2):53‐59. [DOI] [PubMed] [Google Scholar]
- 79. Vasunilashorn S, Ngo L, Kosar CM, et al., Does apolipoprotein e genotype increase risk of postoperative delirium? Am J Geriatr Psychiatry. 2015;23(10):1029‐1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cunningham EL, Mawhinney T, Beverland D, et al. Observational cohort study examining apolipoprotein E status and preoperative neuropsychological performance as predictors of post‐operative delirium in an older elective arthroplasty population. Age Ageing. 2017;46(5):779‐786. [DOI] [PubMed] [Google Scholar]
- 81. Ely EW, Girard TD, Shintani AK, et al. Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Crit Care Med. 2007;35(1):112‐117. [DOI] [PubMed] [Google Scholar]
- 82. Schenning KJ, Murchison CF, Mattek NC, Silbert LC, Kaye JA, Quinn JF. Surgery is associated with ventricular enlargement as well as cognitive and functional decline. Alzheimers Dement. 2016;12(5):590‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shi Y, Yamada K, Liddelow SA, et al., ApoE4 markedly exacerbates tau‐mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549(7673):523‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tzioras M, Davies C, Newman A, Jackson R, Spires‐Jones T. Invited Review: APOE at the interface of inflammation, neurodegeneration and pathological protein spread in Alzheimer's disease. Neuropathol Appl Neurobiol. 2019;45(4):327‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vitek MP, Brown CM, Colton CA. APOE genotype‐specific differences in the innate immune response. Neurobiol Aging. 2009;30(9):1350‐1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gaudilliere B, Fragiadakis GK, Bruggner RV, et al., Clinical recovery from surgery correlates with single‐cell immune signatures. Sci Transl Med., 2014;6(255):255ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Eriksson LI, Lundholm C, Narasimhalu K, et al. Hospitalization, surgery, and incident dementia. Alzheimers Dement. 2019;15(4):534‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8):763‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Terrando N, Gómez‐Galán M, Yang T, et al. Aspirin‐triggered resolvin D1 prevents surgery‐induced cognitive decline. FASEB J. 2013;27(9):3564‐3571. [DOI] [PubMed] [Google Scholar]
- 90. Terrando N, Monaco C, Ma D, et al. Tumor necrosis factor‐alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107(47):20518‐20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Clarke LE, Liddelow SA, Chakraborty C, et al. Normal aging induces A1‐like astrocyte reactivity. Proc Natl Acad Sci U S A. 2018;115(8):E1896‐E1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10(4):217‐224. [DOI] [PubMed] [Google Scholar]
- 94. Petersen MA, Ryu JK, Akassoglou K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci. 2018;19(5):283‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pavlov VA, Tracey KJ. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci. 2017;20(2):156‐166. [DOI] [PubMed] [Google Scholar]
- 96. Bromander S, Anckarsäter R, Kristiansson M, et al. Changes in serum and cerebrospinal fluid cytokines in response to non‐ neurological surgery: an observational study. J Neuroinflammation. 2012;9:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Davis DHJ, Skelly DT, Murray C, et al. Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium. Am J Geriatr Psychiatry. 2015;23(4):403‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. James BD, Wilson RS, Capuano AW, et al., Hospitalization, Alzheimer's disease and related neuropathologies, and cognitive decline. Ann Neurol. 2019;86(6):844‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sudduth TL, Weekman EM, Brothers HM, Braun K, Wilcock DM. Beta‐amyloid deposition is shifted to the vasculature and memory impairment is exacerbated when hyperhomocysteinemia is induced in APP/PS1 transgenic mice. Alzheimers Res Ther. 2014;6(3):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nation DA, Sweeney MD, Montagne A, et al. Blood‐brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Montagne A, Barnes SR, Sweeney MD, et al. Blood‐brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dillon ST, Vasunilashorn SM, Ngo L, et al. Higher c‐reactive protein levels predict postoperative delirium in older patients undergoing major elective surgery: a longitudinal nested case‐control study. Biol Psychiatry. 2017;81(2):145‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. O'Bryant SE, Mielke MM, Rissman RA, et al. Blood‐based biomarkers in Alzheimer disease: Current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimers Dement. 2017;13(1):45‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. DeMarshall C, Oh E, Kheirkhah R, et al. Detection of early‐stage Alzheimer's pathology using blood‐based autoantibody biomarkers in elderly hip fracture repair patients. PLoS One. 2019;14(11):e0225178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Forsberg A, Cervenka S, Jonsson Fagerlund M,et al. The immune response of the human brain to abdominal surgery. Ann Neurol. 2017;81(4):572‐582. [DOI] [PubMed] [Google Scholar]
- 106. Hshieh TT, Vasunilashorn SM, D'Aquila ML, et al. The Role of Inflammation after Surgery for Elders (RISE) study: Study design, procedures, and cohort profile. Alzheimers Dement. 2019;11:752‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Leavey N, Hammond SP, Shepstone L, et al. Study protocol: ASCRIBED: the impact of Acute SystematiC inflammation upon cerebRospinal fluId and blood BiomarkErs of brain inflammation and injury in dementia: a study in acute hip fracture patients. BMC Neurol. 2019;19(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction‐The disregarded partner of Alzheimer's disease. Alzheimers Dement. 2019;15(1):158‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Iadecola C, Duering M, Hachinski V, et al. Vascular cognitive impairment and dementia: jacc scientific expert panel. J Am Coll Cardiol. 2019;73(25):3326‐3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25‐year incident dementia in the atherosclerosis risk in communities (ARIC) cohort. JAMA Neurol. 2017;74(10):1246‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gottesman RF, Schneider AL, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition.JAMA. 2017;317(14):1443‐1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Azarpazhooh MR, Avan A, Cipriano LE, Munoz DG, Sposato LA, Hachinski V. Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimers Dement. 2018;14(2):148‐156. [DOI] [PubMed] [Google Scholar]
- 114. Wang Y, Shi Y, Wei H. Calcium Dysregulation in Alzheimer's Disease: A Target for New Drug Development. J Alzheimers Dis Parkinsonism. 2017;7(5):374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59(2):214‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor‐1 phosphatase cascade in hippocampal long‐term depression. Nature. 1994;369(6480):486‐488. [DOI] [PubMed] [Google Scholar]
- 117. Ferreiro E, Oliveira CR, Pereira C. Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1,4,5‐triphosphate receptors in the neurotoxic effects induced by the amyloid‐beta peptide. J Neurosci Res. 2004;76(6):872‐880. [DOI] [PubMed] [Google Scholar]
- 118. Zhao Y, Liang G, Chen Q, et al. Anesthetic‐induced neurodegeneration mediated via inositol 1,4,5‐trisphosphate receptors. J Pharmacol Exp Ther. 2010;333(1):14‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Vig M, Kinet JP. Calcium signaling in immune cells. Nat Immunol. 2009;10(1):21‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang X, Zhu M, Hjorth E, et al. Resolution of inflammation is altered in Alzheimer's disease. Alzheimers Dement. 2015;11(1):40‐50 e1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lukiw WJ, Cui JG, Marcheselli VL, et al. A role for docosahexaenoic acid‐derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115(10):2774‐2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Fiala M, Kooij G, Wagner K, et al. Modulation of innate immunity of patients with Alzheimer's disease by omega‐3 fatty acids. FASEB J. 2017;31(8):3229‐3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti‐inflammatory and pro‐resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Material and Methods. Detailed description of the methods related to the Early experimental data.
Video 1. Representative video depicting Iba‐1 immunoreactivity and Aβ deposition in a CVN‐AD control (12‐month‐old) clarified hippocampus.
Video 2. Representative video depicting Iba‐1 immunoreactivity and Aβ deposition in a CVN‐AD operated (12‐month‐old) clarified hippocampus.


