Abstract
Patients with chronic kidney disease often develop secondary hyperparathyroidism (SHPT), marked by high levels of circulating parathyroid hormone (PTH) and increased risk of morbidity and mortality. Patients with SHPT are treated with a therapeutic combination that commonly includes calcimimetics, which have recently become popular in clinical settings, and other agents such as vitamin D preparations. Calcimimetics are a drug class that reduces PTH levels by targeting the calcium‐sensing receptor. Cinacalcet, a representative calcimimetic, is widely used; however, a high incidence of upper gastrointestinal (GI) tract‐related adverse events (AEs) can result in insufficient dosage and poor long‐term compliance. The newly approved evocalcet has equivalent efficacy to cinacalcet at a lower clinical dose, with improved bioavailability, fewer upper GI tract‐related AEs, and fewer safety concerns. This review gives an overview of calcimimetic agents, with a special focus on evocalcet, and describes the clinical advantages of evocalcet in the treatment of dialysis patients with SHPT.
Keywords: Calcimimetic agents, Calcium‐sensing receptor, Dialysis, Parathyroid hormone, Secondary hyperparathyroidism
Patients with chronic kidney disease (CKD) who have dysfunctional mineral metabolism have an increased risk of fracture, vascular calcification, and cardiac dysfunction, leading to cardiovascular events and reduced survival; this is termed CKD‐mineral and bone disorder 1. CKD has a mean prevalence of approximately 13% globally 2. In healthy individuals, parathyroid hormone (PTH) controls mineral metabolism to maintain homeostasis. Extracellular ionized calcium (Ca2+) concentration is the main factor regulating the release of PTH by parathyroid gland cells 3. Declining kidney function in patients with CKD leads to physiological changes that can result in dysfunctional mineral metabolism 3, 4. These changes include decreased phosphorus excretion into the urine and increased levels of phosphorus in the blood. The resulting phosphorus accumulation enhances the production and secretion of fibroblast growth factor 23 (FGF‐23) and decreases the production of active vitamin D. Consequently, there is decreased Ca2+ absorption in the intestinal tract and decreased blood Ca2+ concentrations, leading to a disruption of Ca2+ and phosphorus homeostasis. These changes—namely increased phosphorus, and decreased vitamin D and Ca2+ levels—promote the synthesis and hypersecretion of PTH as well as parathyroid cell proliferation, resulting in secondary hyperparathyroidism (SHPT) 3, 4, 5.
Patients with SHPT have enlarged parathyroid glands, which secrete excessive amounts of PTH 5. Persistent elevated PTH promotes bone turnover and accelerates the release of Ca2+ and phosphorus from bone, which may lead to fibrous osteitis, bone and joint pain, and fractures 4, 5. Excessive Ca2+ and phosphorus release from bone may increase morbidity by causing ectopic calcification in blood vessels and cardiac valves. Excessive PTH also leads to immune dysfunction, hypertension, anemia, pruritus, sexual dysfunction, and other health concerns 5 and may contribute to the development of heart and vascular disease and increased mortality in these patients 1, 6. Patients with severe SHPT may require parathyroidectomy, which has been shown to reduce the risk of fracture, cardiovascular events, and mortality 5, 7.
PTH secretion from parathyroid gland cells is regulated by the calcium‐sensing receptor (CaR), a cell surface G protein‐coupled receptor 8. The tight regulation of PTH secretion by the CaR makes it an attractive therapeutic target for SHPT. Current treatment guidelines for patients with SHPT are outlined globally in the Kidney Disease Outcomes Quality Initiative (KDOQI) 9 and Kidney Disease: Improving Global Outcomes (KDIGO) 10 as well as in Japan in the Japanese Society for Dialysis Therapy (JSDT) guidelines 11. Treatment includes dietary restriction of phosphorus and calcium, adequate dialysis dose, vitamin D receptor activators, phosphate binders, and calcimimetics 12. A comprehensive review of SHPT disease development and current treatment strategies has been published 3. The focus of this review was to give an overview of the discovery, safety, and efficacy of the new oral calcimimetic agent, evocalcet, and to describe the clinical advantages that evocalcet has over previous calcimimetics in the treatment of patients with SHPT.
CONVENTIONAL CALCIMIMETICS
Calcimimetics are compounds that mimic or potentiate the effects of extracellular Ca2+ on the CaR, resulting in decreased secretion of PTH and inhibition of parathyroid gland cell proliferation 3, 13, 14. Early efforts to target the CaR for treatment of SHPT led to the discovery of NPS R‐568 (tecalcet), a calcimimetic agent that increased the concentration of cytoplasmic Ca2+ and inhibited the secretion of PTH in the presence of extracellular Ca2+ in vitro 14. Though NPS R‐568 was developed as the first calcimimetic agent, its clinical development was suspended because of undesirable pharmacokinetic properties such as extremely low bioavailability and being mainly metabolized by the highly polymorphic cytochrome P450 (CYP) enzyme, CYP2D6 13. Next‐generation calcimimetic agents have been subsequently developed with the goal of overcoming these issues.
Cinacalcet
Cinacalcet hydrochloride (cinacalcet), an oral calcimimetic and analogue of NPS R‐568 13, binds to the transmembrane domain of the CaR and decreases PTH, calcium, and phosphorus levels 15. It was shown to be primarily metabolized by CYP3A4, CYP2D6, and CYP1A2, eliminating the likelihood of serious adverse events (AEs) posed by NPS R‐568. Although the bioavailability of cinacalcet was improved compared with NPS R‐568, it was still low 13.
Several clinical studies reported improved outcomes in SHPT patients treated with cinacalcet compared with placebo or no cinacalcet treatment 16, 17, 18, 19, 20, 21. Cinacalcet gained worldwide approval as the first calcimimetic agent on the market and is used for treatment of dialysis patients with SHPT 13. After cinacalcet was approved in Japan in 2008, previously uncontrollable nodular hyperplasia was better managed in these patients as evidenced by the drastic reduction in the rate of parathyroidectomy that has remained low; there were 1059 cases in 2008 compared with 276 cases in 2013 for patients with SHPT 15, 22.
Regarding cardiovascular outcomes, the primary composite end point with lag‐censoring analysis in the EVOLVE study suggested a reduced risk of cardiovascular events in hemodialysis (HD) patients using cinacalcet compared with placebo 17, and the ADVANCE study showed that cinacalcet plus low‐dose vitamin D sterols attenuated vascular calcification when compared with flexible dosing of vitamin D sterols alone 18.
The large, prospective, observational Mineral and Bone Disorders Outcomes Study for Japanese CKD Stage 5D Patients (MBD‐5D) conducted in Japan showed improved achievement rates of guideline‐specified therapeutic goals associated with cinacalcet and vitamin D dose reduction 19. The initiation of cinacalcet treatment in patients with an intact parathyroid hormone (iPTH) of ≥500 pg/mL was associated with a decrease (~50%) in all‐cause mortality 6, 19.
Despite these documented improvements for SHPT patients, several undesirable effects have been noted with cinacalcet use. Important limiting side effects are gastrointestinal (GI) tract‐related AEs, particularly nausea and vomiting. The EVOLVE study reported that the incidences of nausea and vomiting were significantly higher in patients receiving cinacalcet (29.1% and 25.6%, respectively) compared with those in the placebo group (15.5% and 13.7%, respectively) (P < 0.001 for both) 17. In the ADVANCE study, GI tract‐related AEs occurred in 21% of patients treated with cinacalcet 17, 18. Furthermore, in a Japanese study, approximately 30% of patients treated with cinacalcet experienced drug‐related upper GI tract‐related AEs (nausea, vomiting, stomach discomfort, and appetite loss) 23. The relatively high incidence of GI tract‐related disorders attributed to cinacalcet use may contribute to insufficient dosage and poor patient compliance with long‐term treatment 21, 24.
The mechanism by which cinacalcet stimulates the upper GI tract is largely unknown; however, CaR expression has been reported in gastrin‐secreting cells (G cells) 25 and in the submucosal plexus of the GI tract 26, suggesting that CaR stimulation by cinacalcet may induce upper GI tract disorders. Furthermore, cinacalcet has been reported to affect the afferent action potential of the abdominal vagus nerve when administered either orally or intravenously to miniature pigs. This suggests that cinacalcet directly stimulates the upper GI tract and may cause nausea and vomiting through the afferent vagus nerve 27.
In addition to the AEs associated with cinacalcet use, there are potential drawbacks to using cinacalcet concomitantly with drugs that are metabolized by CYP2D6, such as tramadol or codeine, because cinacalcet is a potent CYP2D6 inhibitor 28.
Etelcalcetide
Because of the aforementioned potential drawbacks of cinacalcet, development of new calcimimetics is warranted 21. Etelcalcetide is a recently developed injectable calcimimetic that binds to the extracellular domain of the CaR and acts as a direct agonist 29. Several clinical studies have showed the safety and efficacy of etelcalcetide in HD patients with SHPT 30, 31, 32, 33. Etelcalcetide was approved in 2017 as the first intravenous calcimimetic agent for the treatment of SHPT patients undergoing HD 29.
A Phase 3 study showed that etelcalcetide lowered serum iPTH levels and was associated with reductions in serum albumin‐corrected calcium, phosphorus, and FGF‐23 30 compared with placebo, all of which are expected to improve patient outcomes 4, 7, 12. A comparative study conducted in the United States, Canada, Europe, Russia, and New Zealand verified the noninferiority of etelcalcetide to cinacalcet. In that study, 57.7% of patients randomized to cinacalcet and 68.2% of patients randomized to etelcalcetide achieved a ≥30% reduction in serum iPTH over 26 weeks 32. Etelcalcetide was shown to be at least as effective as cinacalcet and has become a new treatment option for patients with low adherence to cinacalcet treatment 29. In the Phase 3 study conducted in Japan comparing etelcalcetide with placebo (no cinacalcet group), treatment‐related AEs of nausea and vomiting were reported in 1.3% and 3.8% of patients receiving etelcalcetide, respectively 30. However, in the comparative study conducted overseas, the incidence of upper GI disorders with etelcalcetide use was almost the same as that of cinacalcet, which was unexpected, leaving the issue of adverse reactions to calcimimetics unresolved 32.
EVOCALCET
Mechanism of action and pharmacological characteristics
Evocalcet was developed and subsequently approved in Japan in 2018 for the treatment of HD and peritoneal dialysis (PD) patients with SHPT 34. It is the newest calcimimetic in the world and was developed in Japan with the aim of resolving the issues of the upper GI effects, drug–drug interactions, and low bioavailability observed with cinacalcet while retaining its efficacy on PTH reduction and improvement of SHPT (Fig. 1) 35, 36. Improvement of the safety profile is of particular importance to PD patients as the GI effects of cinacalcet are difficult to tolerate. In PD patients, oral administration is preferred 37.
Figure 1.
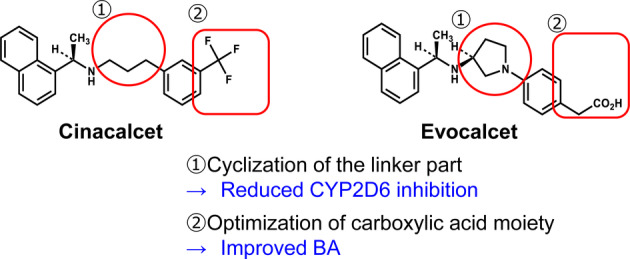
Structural characteristics of evocalcet. BA, bioavailability; CYP2D6, cytochrome P450 2D6.
To develop evocalcet, cinacalcet analogs were generated to reduce the off‐target effects of cinacalcet, which may be responsible for the upper GI disorders. The addition of a five‐membered ring (pyrrolidine ring) to the structure of cinacalcet was found to decrease its affinity for and inhibitory effect on CYP2D6 by hindering the basic amine surroundings 36. Modifications to improve bioavailability included adjustments to the configuration of the pyrrolidine ring and to the size of the ring structure, as well as optimization of the carboxylic acid position 36. The resulting compound, evocalcet, has a naphthylethylamine structure 36. Its basic nitrogen is predicted to interact with Glu837 of the CaR and, like cinacalcet, is thought to bind to the transmembrane domain of the CaR (Fig. 2 and Table 1) 35, 36, 38.
Figure 2.
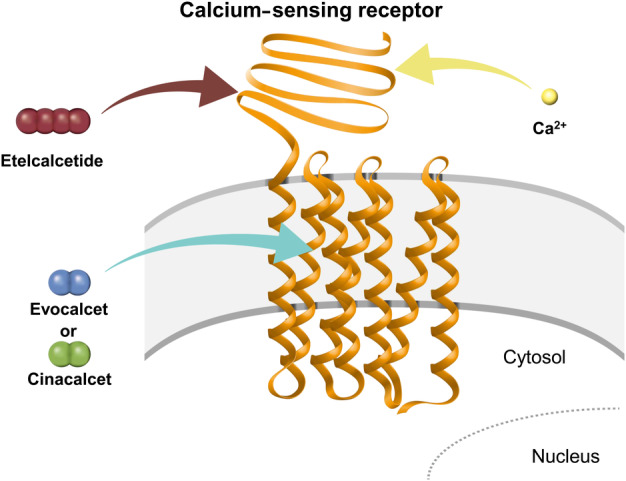
Binding of etelcalcetide and cinacalcet/evocalcet to the calcium‐sensing receptor. Figure adapted from Miyazaki et al. 36. Ca2+, ionized calcium.
Table 1.
Comparison of calcimimetics
| Evocalcet | Cinacalcet 38 | Etelcalcetide 38 | |
|---|---|---|---|
| Molecular formula | C24H26N2O2 | C22H23F3N | C38H73N21O10S2 |
| Molecular weight (Da) | 374 | 394 | 1048 |
| Mode of action at CaR | Allosteric modulator | Allosteric modulator | Allosteric modulator and direct agonist |
| Location of interaction with CaR | Transmembrane domain | Transmembrane domain | Extracellular domain |
| Mode of administration | Daily, oral | Daily, oral | Three times weekly, intravenously at the end of hemodialysis session |
CaR, calcium‐sensing receptor.
The aforementioned modifications resulted in a phenylacetic acid compound that exhibited greatly improved bioavailability, demonstrating 84% bioavailability in rats compared with 1–2% for cinacalcet 35, 36. In humans, a mass balance study showed high bioavailability (62.7%), which was a significant improvement compared with cinacalcet (5–30%) (unpublished data, Kyowa Kirin Co., Ltd.).
Pharmacokinetic studies revealed that evocalcet showed linear pharmacokinetics across a dose range of 1–12 mg in both healthy volunteers and SHPT patients 39, 40. Evocalcet reached maximum plasma concentration approximately 4 h after oral administration with a terminal elimination half‐life of approximately 20 h in SHPT patients 40. The plasma drug concentrations of evocalcet following multiple doses over 7–14 days remained constant from Day 3 to the end of treatment, with no evidence of drug accumulation 41. Of note, the clearance of evocalcet does not appear to be influenced by dialysis in patients undergoing either HD 41 or PD 37; the plasma concentration of evocalcet and other pharmacokinetic parameters in PD patients were similar to those observed in HD patients 37.
Regarding pharmacodynamics, a Phase 1b/2a study of HD patients with SHPT who were treated with a single dose of evocalcet (1, 4, or 12 mg) reported that evocalcet dose‐dependently reduced iPTH levels at 4 h after administration; iPTH levels returned to baseline at around 72 h 40. Serum‐corrected calcium levels were also reduced in a dose‐dependent manner, phosphorus levels tended to decrease, and FGF‐23 levels decreased and persisted for a longer duration with increased evocalcet dosing 40. PD patients taking evocalcet showed similar pharmacodynamics, resulting in a reduction in both iPTH and serum calcium 37. These studies and others show that evocalcet has pharmacological effects which are similar to cinacalcet 42 in both HD 40, 41 and PD 37 patients.
Preclinical assays showed no notable inhibition of the specific activities of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4/5, with IC50 values of evocalcet exceeding 50 μmol, a concentration higher than that found in healthy adults given a therapeutic dose 36. No clear interactions between evocalcet and several CYP substrates were observed in a clinical drug–drug interaction study 43. Thus, the concerns of CYP2D6 inhibition associated with cinacalcet have been eliminated with evocalcet.
Efficacy and safety
HD patients
Evocalcet has showed both efficacy and safety in short‐ and long‐term clinical trials. The optimal starting dose of evocalcet was determined in a Phase 2b study of Japanese HD patients with SHPT. This trial confirmed the safety and efficacy of ≥1 mg of evocalcet and found that a dose of 2 mg evocalcet elicited an iPTH‐lowering effect similar to that of 25 mg cinacalcet, demonstrating that improved bioavailability allowed for a lower dose 44. From this study, it was determined that a starting dose of 1 mg was appropriate for HD patients, and if warranted, a starting dose of 2 mg could be used in patients with high iPTH or calcium levels 44.
To compare evocalcet to cinacalcet, the two agents were tested in a head‐to‐head, Phase 3, noninferiority study of Japanese HD patients with SHPT 45. Patients receiving evocalcet were initially treated with either 1 mg (iPHT < 500 pg/mL) or 2 mg (iPTH ≥ 500 pg/mL) and those receiving cinacalcet were initially treated with 25 mg. Dose adjustments of 1 mg or 25 mg increase and 1 mg or 12.5–25 mg decrease within the dose range of 1–8 mg or 12.5–100 mg (evocalcet or cinacalcet, respectively) were allowed. To obtain a parallel comparison, patients who received cinacalcet prior to the study were subjected to a wash‐out period (≥2 weeks). In addition, the use of vitamin D was restricted during the study. The efficacy of evocalcet was noninferior to cinacalcet in terms of the proportion of patients achieving target range of iPTH as recommended in the JSDT guidelines (60–240 pg/mL) 11 (Fig. 3A,B) over a 28–30‐week evaluation period 45. Serum calcium, phosphorous, and FGF‐23 levels also decreased over time in both the evocalcet and cinacalcet groups (Fig. 3C–E), and both groups showed a similar trend in decreased mean levels of bone turnover markers (bone‐specific alkaline phosphatase [BAP], tartrate‐resistant acid phosphatase 5b [TRACT‐5b], and total N‐terminal propeptide of type 1 procollagen [P1NP]) 45. Importantly, the efficacy parameters examined in this study were impacted similarly by evocalcet (1–8 mg) and cinacalcet (12.5–100 mg) treatment.
Figure 3.
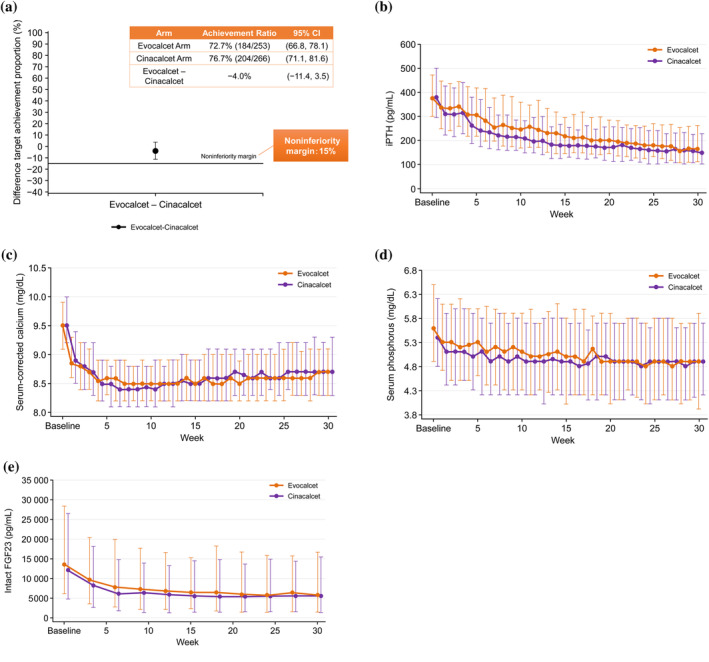
Comparison of evocalcet and cinacalcet treatment. (A) Comparison of outcomes for evocalcet, cinacalcet, and combination evocalcet/cinacalcet therapy. Time‐course of changes in (B) intact parathyroid hormone, (C) serum‐corrected calcium, (D) serum phosphorus, and (E) intact fibroblast growth factor 23 in patients treated with evocalcet or cinacalcet. Figure adapted from Table 2 and Figure 2a,d,e,f in Fukagawa et al. 45. CI, confidence interval; FGF23, fibroblast growth factor 23; iPTH, intact parathyroid hormone.
To determine the impact of transitioning patients from cinacalcet to evocalcet, the safety and efficacy of switching from cinacalcet to evocalcet in a real‐world setting, as well as the long‐term safety and efficacy of evocalcet, were examined in the evocalcet dose range of 1–12 mg in a 52‐week study of HD patients with SHPT 46. In this Phase 3 trial, patients who had been administered cinacalcet prior to the study switched from cinacalcet to evocalcet without a washout. Most patients (82.5% [113/137]) switched from cinacalcet to evocalcet. Patients who were pretreated with cinacalcet had a lower median baseline iPTH level than patients who were cinacalcet naïve, which indicated that cinacalcet pretreatment had achieved some level of efficacy in these patients. At baseline, 49.6% (56/113) of cinacalcet pretreated patients had achieved a target iPTH level. Of clinical interest, switched patients experienced an initial transient increase in iPTH levels in a cinacalcet dose‐dependent manner. It is assumed that this temporary increase was likely owing to the substantially lower initial dose of evocalcet (1–2 mg) compared with the previous dose of cinacalcet (12.5–100 mg). The dose of cinacalcet received prior to switching impacted the duration of the temporary increase; in all switched patients, iPTH decreased gradually with evocalcet dose adjustment and returned to the original value thereafter (Fig. 4).
Figure 4.
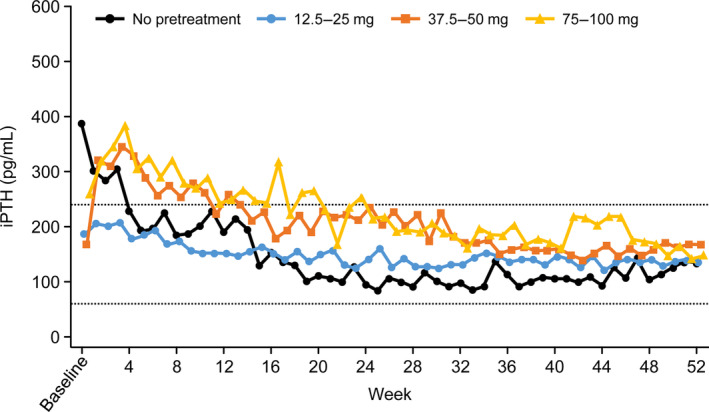
Time‐course of changes in intact parathyroid hormone in patients treated with evocalcet, stratified by pretreatment cinacalcet dose. Figure adapted from Figure 3a in Yokoyama et al. 46. iPTH, intact parathyroid hormone.
The percentage of switched patients who achieved target iPTH (60–240 pg/mL) at the end of the study was 70.8% (80/113), indicating further improvement in patients after switching to evocalcet. When patients who discontinued the study were removed from analysis, target iPTH was achieved in 86.0% (80/93) of patients 46. A reduction in iPTH of ≥30% was also achieved in 38.1% (43/113) of patients who switched, although the baseline iPTH of these patients was lower (median: 210 pg/mL). Of note, some patients achieved the JSDT guidelines target iPTH for the first time with 12 mg evocalcet. In cinacalcet naïve patients, the achievement rate of the JSTD guidelines iPTH target rate increased from 0% to 79.2% (19/24). A reduction in iPTH of ≥30% was achieved in 79.2% (19/24) of these patients and the mean percent change in iPTH concentration from baseline was −64.9%. The results in this patient population were similar to those previously reported in a study of patients treated with evocalcet after a wash‐out period 45.
To reflect real‐world clinical circumstances, the use of vitamin D and calcium preparations was not restricted during this trial; coadministration of vitamin D and calcium preparations generally increased throughout the trial to recover the decrease in serum calcium levels that resulted from evocalcet dose escalation. This combination treatment may have worked well. Additional efficacy parameters of mean corrected serum calcium, ionized calcium, phosphorus, FGF‐23, and corrected serum calcium‐phosphorus product levels were relatively unchanged; BAP, TRACT‐5b, and total P1NP decreased throughout the study.
Regarding the safety of evocalcet in HD patients, the Phase 3 comparative study reported a significantly lower incidence of upper GI tract‐related AEs with evocalcet vs. cinacalcet (18.6% vs. 32.8%; P < 0.001) (Fig. 5A,B); and unlike cinacalcet, the incidence of upper GI tract‐related AEs was not evocalcet dose‐dependent 45. The mechanism by which evocalcet reduces GI tract‐related AEs is yet to be elucidated. However, the improved bioavailability of evocalcet compared with cinacalcet, which results in reductions of the dose of drug required for efficacy and in the direct stimulation of the GI tract 35, and the improved CaR selectivity 47 are thought to contribute to the reduction of GI tract‐related AEs observed in patients treated with evocalcet compared with those treated with cinacalcet. No differences were reported in the safety profiles of patient groups with or without previous cinacalcet treatment in the 52‐week study of evocalcet. Importantly, there were no significant safety events as a result of dose increases and it was confirmed that the incidence of upper GI‐related AEs was not dose‐dependent 46. These and other trials have shown that evocalcet is safe, reporting lower incidences of drug‐related AEs and GI‐related AEs for evocalcet 45, 46, 49 compared with cinacalcet 48; the problem of upper GI tract‐related AEs as a result of calcimimetic agent use has been eliminated with evocalcet.
Figure 5.
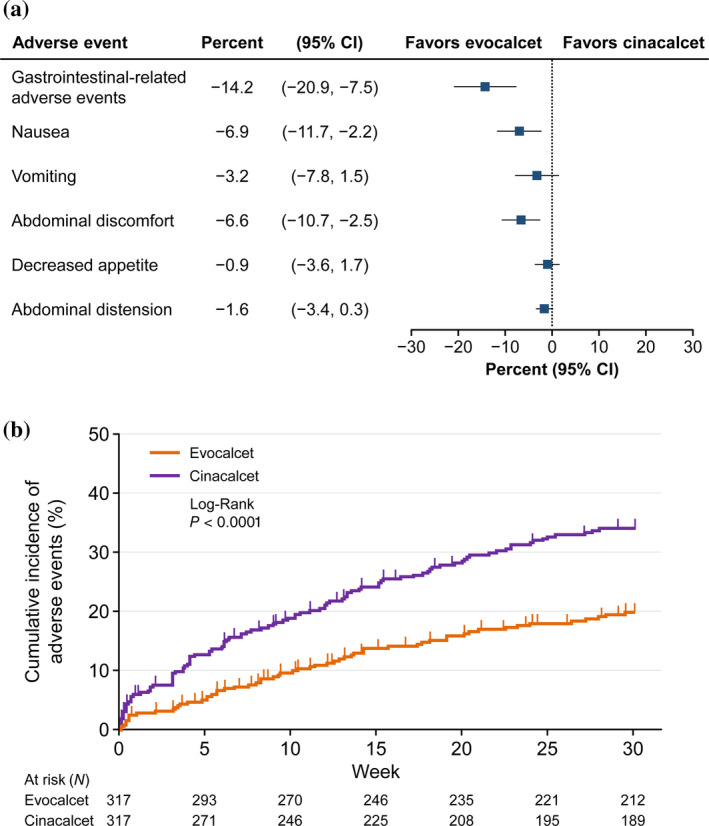
Gastrointestinal‐related adverse events for cinacalcet and evocalcet. (A) Forest plot for gastrointestinal‐related adverse events and (B) Kaplan–Meier plots of the cumulative incidence of gastrointestinal‐related adverse events at the first occurrence. Figures adapted from Figure 3 and Figure S2 in Fukagawa et al. 45. CI, confidence interval.
Other AEs of interest such as the incidences of calcium decrease‐related AEs 44, 45 and QT prolongation in HD patients 41, 45, 49 were comparable between evocalcet and cinacalcet. Regarding the incidence of heart failure with evocalcet treatment, one incident each of congestive heart failure and cardiomyopathy were reported in HD patients, both of which either improved or were resolved 46. Altogether, these studies show that evocalcet is safe in HD patients with SHPT over the long‐term and that switching from cinacalcet to evocalcet in a real‐world setting does not create any new safety concerns.
PD patients
Long‐term efficacy was also examined in a single‐arm, open‐label, dose‐adjustment study of evocalcet in Japanese PD patients with SHPT 37. Because the pharmacokinetics of evocalcet in PD patients were similar to those in HD patients 37, the starting dose and dose range in this study were set the same as in the studies of evocalcet in HD patients 45, 46. The starting dose was 1 mg in patients with an iPTH <500 pg/mL or 2 mg in patients with an iPTH ≥500 pg/mL. Patients received evocalcet (1–8 mg) for 32 weeks (30‐week dose adjustment period and 2‐week evaluation period) followed by a 20‐week extension period (1–12 mg). The target mean iPTH level (60–240 pg/mL) was achieved by 71.8% (28/39) and 83.3% (20/24) of patients during the evaluation period (Weeks 30–32) and at the end of the extension period (Week 52), respectively. A consistent reduction in mean iPTH over time was demonstrated (evaluation period [Weeks 30–32]: −64.4 ± 26.0%, Week 52: −63.5 ± 22.3%) and a ≥30% decrease in iPTH levels from baseline was achieved in a respective 74.4% (29/39) and 87.5% (21/24) of patients. No significant differences were observed in efficacy when patients were stratified by type of dialysis, residual renal function, prior cinacalcet treatment, and evocalcet starting dose 37. After an initial decrease (Week 2) in corrected serum calcium, levels remained stable throughout the study; serum ionized calcium and phosphorous were relatively constant over the course of the study; and median intact FGF‐23 levels were significantly decreased at the end of the study (Week 52). Regarding bone turnover markers, BAP, TRACP‐5b, and total P1NP decreased from baseline to Week 32, after which BAP and TRACP‐5b remained stable and P1NP continued to decrease through Week 52. No significant differences in efficacy were reported after subgroup analysis in which patients were stratified by residual renal function, demonstrating that even in patients with no residual renal function, evocalcet was able to reduce markers associated with efficacy. This study showed the efficacy of evocalcet in PD patients and that it was similar to that observed in HD patients with SHPT.
Evocalcet safety was also examined in this study. GI tract‐related AEs that were considered drug‐related occurred only in one patient (2.6%) over the course of the 52‐week study 37. Together with the studies of the safety of evocalcet in HD patients, substantial evidence has been collected to show that evocalcet has an improved safety profile regarding upper GI tract‐related AEs compared with cinacalcet. In addition, the incidence of cardiac‐related AEs was reported to be low in PD patients after evocalcet treatment, with no incidences of heart failure and only one incident of drug‐related myocardial ischemia (2.6%) 37.
CONCLUSIONS
Evocalcet, a newly approved calcimimetic in Japan, exhibits improved bioavailability compared with cinacalcet, equivalent efficacy at a lower clinical dose, and lower rates of GI tract‐related AEs based on findings of head‐to‐head clinical studies. Calcium decrease and QT prolongation are equivalent between evocalcet and cinacalcet treatment. Evocalcet has fewer concerns of CYP‐mediated drug interactions and reduced off‐target effects, namely, non‐CaR‐mediated effects associated with calcimimetic use. Overall, the results indicate that evocalcet has fewer safety concerns than cinacalcet.
The bioavailability of evocalcet is inferior to injectable etelcalcetide (100%); however, while there has not been a direct comparison between etelcalcetide and evocalcet, reductions in the GI tract‐related AEs observed with evocalcet suggest that it may be preferable to etelcalcetide. Additional clinical studies directly comparing evocalcet and etelcalcetide are necessary to show such differences. From these clinical trials in Japan, evocalcet is expected to gain global usage in clinical practice as the preferred and best available calcimimetic because of its improved pharmacologic characteristics, more flexible dosing, and better safety profile compared with other currently available calcimimetics.
Disclosures
Tadao Akizawa has received consulting fees from Kyowa Kirin Co., Ltd., Astellas Pharma Inc., Bayer Yakuhin, Ltd., Japan Tobacco Inc., Ono Pharmaceutical Co., Ltd., Sanwa Chemical Co., Ltd. and Torii Pharmaceutical Co., Ltd.; lecture fees from Kyowa Kirin Co., Ltd., Chugai Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., Kissei Pharmaceutical Co., Ltd., Torii Pharmaceutical Co., Ltd. and Ono Pharmaceutical Co., Ltd.; research funding from Kyowa Kirin Co., Ltd.; and manuscript fees from Astellas Pharma Inc. Kazuaki Ikejiri, Yuichiro Kondo and Yuichi Endo are employees of Kyowa Kirin Co., Ltd. Masafumi Fukagawa has received consulting fees from Kyowa Kirin Co., Ltd., Bayer Yakuhin, Ltd., Torii Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd. and Chugai Pharmaceutical Co., Ltd.; lecture fees from Kyowa Kirin Co., Ltd.; grants from Kyowa Kirin Co., Ltd., Ono Pharmaceutical Co., Ltd. and Chugai Pharmaceutical Co., Ltd.; and research funding from Kyowa Kirin Co., Ltd.
Acknowledgments
Research and development of this review article was funded by Kyowa Kirin Co., Ltd. We thank Sarah Bubeck, PhD, of Edanz Medical Writing for providing medical writing support, which was funded by Kyowa Kirin Co., Ltd.
[Correction added on 6 November 2019, after first online publication: the affiliations of Yuichiro Kondo and Yuichi Endo have been corrected.]
REFERENCES
- 1. Moe S, Drüeke T, Cunningham J et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006;69:1945–53. [DOI] [PubMed] [Google Scholar]
- 2. Hill NR, Fatoba ST, Oke JL et al. Global prevalence of chronic kidney disease – a systematic review and meta‐analysis. PLoS One 2016;11:e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mizobuchi M, Ogata H, Koiwa F. Secondary hyperparathyroidism: pathogenesis and latest treatment. Ther Apher Dial 2018;23:309–18. [DOI] [PubMed] [Google Scholar]
- 4. Komaba H, Kakuta T, Fukagawa M. Diseases of the parathyroid gland in chronic kidney disease. Clin Exp Nephrol 2011;15:797–809. [DOI] [PubMed] [Google Scholar]
- 5. Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 2011;6:913–21. [DOI] [PubMed] [Google Scholar]
- 6. Fukagawa M, Kido R, Komaba H et al. Abnormal mineral metabolism and mortality in hemodialysis patients with secondary hyperparathyroidism: evidence from marginal structural models used to adjust for time‐dependent confounding. Am J Kidney Dis 2014;63:979–87. [DOI] [PubMed] [Google Scholar]
- 7. Komaba H, Nakamura M, Fukagawa M. Resurgence of parathyroidectomy: evidence and outcomes. Curr Opin Nephrol Hypertens 2017;26:243–9. [DOI] [PubMed] [Google Scholar]
- 8. D'Souza‐Li L. The calcium‐sensing receptor and related diseases. Arg Bras Endocrinol Metabol 2006;50:628–39. [DOI] [PubMed] [Google Scholar]
- 9. National Kidney Foundation . K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42 (4 Suppl 3):S1–S201. [PubMed] [Google Scholar]
- 10. Kidney Disease: Improving Global Outcomes (KDIGO) CKD‐MBD Work Group . KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease‐Mineral and Bone Disorder (CKD‐MBD). Kidney Int Suppl 2009;76 (Suppl 113):S1–130. [DOI] [PubMed] [Google Scholar]
- 11. Fukagawa M, Yokoyama K, Koiwa F et al. Clinical practice guideline for the management of chronic kidney disease‐mineral and bone disorder. Ther Apher Dial 2013;17:247–88. [DOI] [PubMed] [Google Scholar]
- 12. Taniguchi M, Fukagawa M, Fujii N et al. Serum phosphate and calcium should be primarily and consistently controlled in prevalent hemodialysis patients. Ther Apher Dial 2013;17:221–8. [DOI] [PubMed] [Google Scholar]
- 13. Nagano N. Pharmacological and clinical properties of calcimimetics: calcium receptor activators that afford an innovative approach to controlling hyperparathyroidism. Pharmacol Ther 2006;109:339–65. [DOI] [PubMed] [Google Scholar]
- 14. Nemeth EF, Steffey ME, Hammerland LG et al. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci USA 1998;95:4040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tominaga Y, Kakuta T, Yasunaga C, Nakamura M, Kadokura Y, Tahara H. Evaluation of parathyroidectomy for secondary and tertiary hyperparathyroidism by the parathyroid Surgeons' Society of Japan. Ther Apher Dial 2016;20:6–11. [DOI] [PubMed] [Google Scholar]
- 16. Block GA, Zaun D, Smits G et al. Cinacalcet hydrochloride treatment significantly improves all‐cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int 2010;78:578–89. [DOI] [PubMed] [Google Scholar]
- 17. EVOLVE Trial Investigators , Chertow GM, Block GA et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 2012;367:2482–94. [DOI] [PubMed] [Google Scholar]
- 18. Raggi P, Chertow GM, Torres PU et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low‐dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 2011;26:1327–39. [DOI] [PubMed] [Google Scholar]
- 19. Akizawa T, Kurita N, Mizobuchi M et al. PTH‐dependence of the effectiveness of cinacalcet in hemodialysis patients with secondary hyperparathyroidism. Sci Rep 2016;6:19612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health‐related quality of life in secondary hyperparathyroidism. Kidney Int 2005;68:1793–800. [DOI] [PubMed] [Google Scholar]
- 21. Komaba H, Fukagawa M. Cinacalcet and clinical outcomes in dialysis. Semin Dial 2015;28:594–603. [DOI] [PubMed] [Google Scholar]
- 22. Tentori F, Wang M, Bieber BA et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol 2015;10:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fukagawa M, Yumita S, Akizawa T et al. Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrol Dial Transplant 2008;23:328–35. [DOI] [PubMed] [Google Scholar]
- 24. Gincherman Y, Moloney K, McKee C, Coyne DW. Assessment of adherence to cinacalcet by prescription refill rates in hemodialysis patients. Hemodial Int 2010;14:68–72. [DOI] [PubMed] [Google Scholar]
- 25. Ray JM, Squires PE, Curtis SB, Meloche MR, Buchan AM. Expression of the calcium‐sensing receptor on human antral gastrin cells in culture. J Clin Invest 1997;99:2328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chattopadhyay N, Cheng I, Rogers K et al. Identification and localization of extracellular Ca2+‐sensing receptor in rat intestine. Am J Physiol 1998;274:G122–30. [DOI] [PubMed] [Google Scholar]
- 27. Kondo Y, Katou M, Hosomi A, Nakamura H, Kawata T. The effects of cinacalcet on vagus nerve in miniature pigs. Kidney dial 2017;82:455–60 (In Japanese). [Google Scholar]
- 28. Nakashima D, Takama H, Ogasawara Y et al. Effect of cinacalcet hydrochloride, a new calcimimetic agent, on the pharmacokinetics of dextromethorphan: in vitro and clinical studies. J Clin Pharmacol 2007;47:1311–9. [DOI] [PubMed] [Google Scholar]
- 29. Patel J, Bridgeman MB. Etelcalcetide (parsabiv) for secondary hyperparathyroidism in adults with chronic kidney disease on hemodialysis. P T 2018;43:396–9. [PMC free article] [PubMed] [Google Scholar]
- 30. Fukagawa M, Yokoyama K, Shigematsu T et al. A phase 3, muticentre, randomized, double‐blind, placebo‐controlled, parallel‐group study to evaluate the efficacy and safety of etelcalcetide (ONO‐5163/AMG 416), a novel intravenous calcimimetic, for secondary hyperparathyroidism in Japanese haemodialysis patients. Nephrol Dial Transplant 2017;32:1723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yokoyama K, Fukagawa M, Shigematsu T et al. A single‐ and multiple‐dose, multicenter study of etelcalcetide in Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int Rep 2017;2:634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Block GA, Bushinsky DA, Cheng S et al. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA 2017;317:156–64. [DOI] [PubMed] [Google Scholar]
- 33. Block GA, Bushinsky DA, Cunningham J et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA 2017;317:146–55. [DOI] [PubMed] [Google Scholar]
- 34.Kyowa Hakko Kirin receives approval for ORKEDIA® Tablets (Evocalcet) for the treatment of secondary hyperparathyroidism in patients on maintenance dialysis in Japan. [Accessed 23 Jan 2019.] Available from URL: https://www.kyowa-kirin.com/news_releases/2018/pdf/e20180323_01.pdf
- 35. Kawata T, Tokunaga S, Murai M et al. A novel calcimimetic agent, evocalcet (MT‐4580/KHK7580), suppresses the parathyroid cell function with little effect on the gastrointestinal tract or CYP isozymes in vivo and in vitro . PLoS One 2018;13:e0195316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miyazaki H, Ikeda Y, Sakurai O et al. Discovery of evocalcet, a next‐generation calcium‐sensing receptor agonist for the treatment of hyperparathyroidism. Bioorg Med Chem Lett 2018;28:2055–60. [DOI] [PubMed] [Google Scholar]
- 37. Tsuruya K, Shimazaki R, Fukagawa M, Akizawa T, Evocalcet Study Group . Efficacy and safety of evocalcet in Japanese peritoneal dialysis patients. Clin Exp Nephrol 2019;23:739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Friedl C, Zitt E. Role of etelcalcetide in the management of secondary hyperparathyroidism in hemodialysis patients: a review on current data and place in therapy. Drug Des Devel Ther 2018;12:1589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Akizawa T, Shimazaki R, Shiramoto M, Fukagawa M, Evocalcet Study Group . Pharmacokinetics, pharmacodynamics, and safety of the novel calcimimetic agent evocalcet in healthy Japanese subjects: first‐in‐human phase I study. Clin Drug Investig 2018;38:945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shigematsu T, Shimazaki R, Fukagawa M, Akizawa T. Pharmacokinetics of evocalcet in secondary hyperparathyroidism patients receiving hemodialysis: first‐in‐patient clinical trial in Japan. Clin Pharm 2018;10:101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shigematsu T, Shimazaki R, Fukagawa M, Akizawa T, Evocalcet Study Group . Pharmacodynamics of evocalcet for secondary hyperparathyroidism in Japanese hemodialysis patients. Clin Exp Nephrol 2019;23:258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barman Balfour JA, Scott LJ. Cinacalcet hydrochloride. Drugs 2005;65:271–81. [DOI] [PubMed] [Google Scholar]
- 43. Narushima K, Maeda H, Shiramoto M et al. Assessment of CYP‐mediated drug interactions for evocalcet, a new calcimimetic agent, based on in vitro investigations and a cocktail study in humans. Clin Transl Sci 2019;12:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Akizawa T, Shimazaki R, Fukagawa M, Evocalcet Study Group . Phase 2b study of evocalcet (KHK7580), a novel calcimimetic, in Japanese patients with secondary hyperparathyroidism undergoing hemodialysis: a randomized, double‐blind, placebo‐controlled, dose‐finding study. PLoS One 2018;13:e0204896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fukagawa M, Shimazaki R, Akizawa T, Evocalcet Study Group . Head‐to‐head comparison of the new calcimimetic agent evocalcet with cinacalcet in Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int 2018;94:818–25. [DOI] [PubMed] [Google Scholar]
- 46. Yokoyama K, Shimazaki R, Fukagawa M, Akizawa T, Evocalcet Study Group . Long‐term efficacy and safety of evocalcet in Japanese patients with secondary hyperparathyroidism receiving hemodialysis. Sci Rep 2019;9:6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tokunaga S, Endo Y, Kawata T. Pharmacological and clinical profiles of a novel calcimimetic, evocalcet (ORKEDIA). Nippon Yakurigaku Zasshi 2019;154:35–43 (In Japanese). [DOI] [PubMed] [Google Scholar]
- 48. Shigematsu T, Akizawa T, Uchida E et al. Long‐term cinacalcet HCl treatment improved metabolism in Japanese hemodialysis patients with secondary hyperthyroidism. Am J Nephrol 2009;29:230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Akizawa T, Shimazaki R, Shiramoto M, Fukagawa M, Evocalcet Study Group . Response to comments on pharmacokinetics, pharmacodynamics, and safety of the novel calcimimetic agent evocalcet in healthy Japanese subjects: first‐in‐human phase I study. Clin Drug Investig 2019;39:109–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


