Abstract
Objective
Invasive motor cortex stimulation (iMCS) has been proposed as a treatment for intractable neuropathic pain syndromes. Although the mechanisms underlying the analgesic effect of iMCS remain largely elusive, several studies found iMCS‐related changes in regional cerebral blood flow (rCBF) in neuropathic pain patients. The aim of this study was to meta‐analyze the findings of neuroimaging studies on rCBF changes to iMCS.
Methods
PubMed, Embase, MEDLINE, Google Scholar, and the Cochrane Library were systematically searched for retrieval of relevant scientific papers. After initial assessment of relevancy by screening title and abstract by two investigators, independently, predefined inclusion and exclusion criteria were used for final inclusion of papers. Descriptive results were statistically assessed, whereas coordinates were pooled and meta‐analyzed in accordance with the activation likelihood estimation (ALE) methodology.
Results
Six studies were included in the systematic narrative analysis, suggesting rCBF increases in the cingulate gyrus, thalamus, insula, and putamen after switching the MCS device “ON” as compared to the “OFF” situation. Decreases in rCBF were found in for example the precentral gyrus and different occipital regions. Two studies did not report stereotactic coordinates and were excluded from further analysis. ALE meta‐analysis showed that, after switching the iMCS electrode “ON,” increased rCBF occurred in the (1) anterior cingulate gyrus; (2) putamen; (3) cerebral peduncle; (4) precentral gyrus; (5) superior frontal gyrus; (6) red nucleus; (7) internal part of the globus pallidus; (8) ventral lateral nucleus of the thalamus; (9) medial frontal gyrus; (10) inferior frontal gyrus; and (11) claustrum, as compared to the “OFF” situation. Reductions in rCBF were found in the posterior cingulate gyrus when the iMCS electrode was turned “OFF.”
Conclusions
These findings suggested that iMCS induces changes in principal components of the default mode‐, the salience‐, and sensorimotor network.
Keywords: Cerebral blood flow, chronic neuropathic pain, efficacy, meta‐analysis, motor cortex stimulation
INTRODUCTION
Neuropathic pain is a subjective state that arises as a direct consequence of a lesion or disease affecting the somatosensory system 1, which is clinically characterized by shooting pain and burning sensations, although established diagnostic criteria are still lacking 2. The somatosensory system that processes nociceptive information consists of an ascending system, a pain modulating descending system, and the corresponding supraspinal sites that together form the neuromatrix for pain 3, 4. Insight into the way these regions are involved in processing noxious, input is thought to be of crucial importance in understanding neuropathic pain and to improve its treatment 5. For example, Treede et al. reported that various cortical areas are involved in the processing of pain 6, 7, which provided a scientific rationale for cortical‐centered treatment methods in (pre)clinical settings to alleviate pain.
One of these treatment options concerns invasive motor cortex stimulation (iMCS). iMCS was introduced in the early 1990s as a possible treatment option for intractable central neuropathic pain 8, 9. In iMCS, an electrode is placed on a target area of the primary motor cortex which matches the somatotopic site of the corresponding pain. Stimulation of this target area below the threshold for muscle activity alleviates chronic pain in patients 10. Because the first reports, numerous studies provided evidence for the effectiveness of iMCS in experimental, double‐blinded randomized controlled trials 11, 12, 13 and in clinical settings (for a review, see ref. 14). The underlying mechanisms of action of iMCS remains elusive, but is proposed to involve a distributed network of brain regions 15. In humans, the effects of iMCS have been mainly investigated by use of neuroimaging studies measuring the modifications in rCBF. The vast majority of studies have used positron emission tomography‐computed tomography (PET‐CT) as this technique is compatible with implanted electrodes and allows for detecting changes in cerebral blood flow during stimulation. Nonetheless, a systematic overview of the neuroimaging results, using specially designed meta‐analysis methods, is lacking. One of these specially designed methods comprises activation likelihood estimation (ALE).
ALE was introduced in 2002 16 and has grown to become one of the most commonly used algorithms for coordinate‐based meta‐analyses, and it is part of the BrainMap software suite 17, 18 (http://brainmap.org/ale). The central thought in the ALE methodology is that foci reported in neuroimaging studies should be regarded as spatial probability distributions centered at given coordinates rather than dimensionless points. This approach therefore accommodates the spatial uncertainty associated with neuroimaging findings by using the reported coordinates as the most optimal point estimator, whereas it uses a (Gaussian) spatial variance model at the same time 19. ALE maps are then obtained by computing the union of activation probabilities across experiments for each voxel 20. Finally, true convergence of foci is distinguished from random clustering of foci (i.e., noise) by testing against the null‐hypothesis of random spatial association between different settings (e.g. ON vs. OFF, different experiments) 16, 21.
The present study quantitatively analyzes the neuroimaging results on rCBF alterations induced by iMCS by using the ALE method.
MATERIALS AND METHODS
Search Strategy and Assessment of Papers
Five investigators (E.G., M.v.d.H., M.K., S.L., and D.H.) each systematically searched one of the following online databases: PubMed, Embase, MEDLINE, Google Scholar, and the Cochrane Library, with assistance of an independent librarian. Searches were conducted to capture studies published between January 1986 and December 2018. Keywords included: “Motor Cortex Stimulation,” “Mechanisms,” “Neuroimaging studies,” “Physiology,” “Positron emission tomography‐computed tomography (PET‐CT),” and “Analgesic effects.” To enrich the results, Medical Subject Headings (MeSH‐) terms with major subheadings were added to the search strategy (please see the Supplementary Materials). All papers were initially screened on title and abstract to assess its relevance by two investigators, independently. When the investigators disagreed on the relevance of a paper and could not reach consensus, a third investigators (D.H.) made the final decision to include the paper for full‐text analysis or not. During the full‐text analysis, papers were assessed by use of predefined inclusion‐ and exclusion criteria. Inclusion criteria were: (1) Human studies; (2) iMCS carried out to alleviate pain (either clinical or experimental); (3) primary outcome of the paper was the PET‐CT based changes in cerebral blood flow in brain areas. Exclusion criteria comprised: (1) Computer‐model studies; (2) other forms of neuromodulation than iMCS; and (3) disorders other than neuropathic pain. Systematic reviews and articles written in other languages than English were excluded as well. Observational studies, case reports, and randomized‐controlled trials were excluded when they did not report on the mechanisms of action of neuromodulation in ameliorating pain. Preparation of data extraction was done by use of pilot testing, using a representative sample of papers. A final database was built in IBM SPSS Statistics version 25 containing (1) information on the study design, (2) number of subjects, (3) characteristics of the subjects investigated, (4) pain syndrome investigated, (5) applied methodology, and (6) PET‐CT results presented by use of Talairach‐ or Montreal Neurological Institute (MNI)‐ coordinates.
Statistical Analysis
Ginger ALE software, version 2.3.6 (http://brainmap.org/ale/) was used to assess common patterns of gray matter functional changes. ALE analysis is known to treat foci from neuroimaging studies as spatial probability distributions, centered at given coordinates rather than as single foci points. For each voxel, ALE analysis estimated the cumulative probabilities of at least one of the included papers discussed activation for that focus. ALE maps were subsequently obtained by computing the union of activation probabilities for each voxel. Differentiation between true convergence of foci and random clustering was controlled for by using a permutation procedure 19, 20, 22. By using random effects within the ALE methods, variable uncertainty based on the sample size was incorporated into the algorithm 21. Such a random effects model was considered to assume a higher than chance likelihood of consensus between different experiments, but not in relation to activation variance within each study. During an ALE analysis, each activation focus is modeled as the center of a Gaussian probability distribution and is used to generate a modeled activation map for each study. Foci coordinates that are not expressed in Talairach‐coordinates (i.e., MNI‐coordinates) were transformed into Talairach space by use of the icbm2tal function to create a homologous dataset of coordinates 23. A recommended conservative threshold of p < 0.001 was chosen with a minimum cluster size of 100 mm3 to control for publication bias with regard to reported foci (as recommended 24). All numerical statistical data was analyzed using IBM SPSS Statistics version 25 (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.).
RESULTS
In total, 232 articles were retrieved after conducting the searches. After removal of duplicates (n = 76), a total of 156 papers remained. A total of 142 papers were thereafter excluded based on title and abstract, resulting in 14 remaining articles. After in‐depth, full‐text analysis and application of the predefined inclusion and exclusion criteria, a total of six papers were included (Fig. 1). Four of these studies could be enrolled in the ALE meta‐analysis as these studies accompanied their results with Talairach‐ or MNI‐coordinates. All of the included papers used 15O‐labeled water positron emission tomography (H2 15O‐PET) to measure changes in regional cerebral blood flow (rCBF). For a detailed overview per study, see Table 1.
Figure 1.
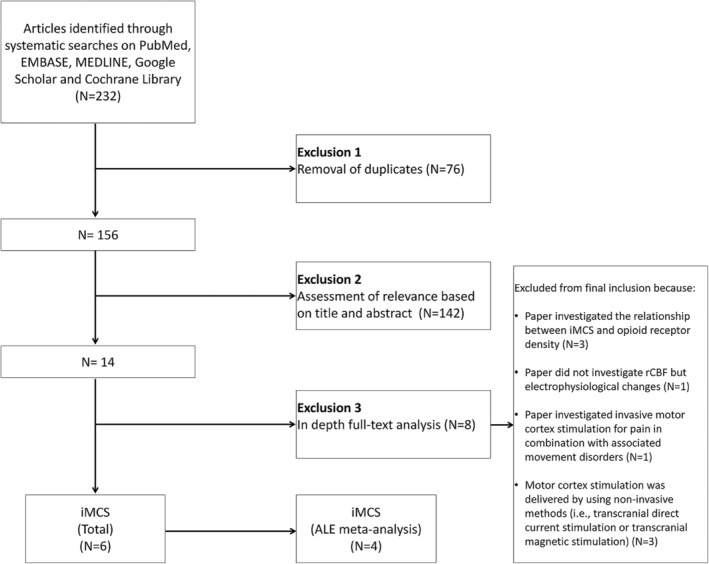
Flow‐chart describing the study selection methods. ALE meta‐analysis = activation likelihood estimation meta‐analysis; iMCS = invasive motor cortex stimulation; N = number of papers.
Table 1.
Overview of Studies That Investigate the Possible Mechanisms Involved in the Anti‐Nociceptive Effects of iMCS.
| Author (year) | N | Males (%) | Mean age (±SD) | Median duration of pain (range) | iMCS device implanted L/R | Condition | Definition of a non‐satisfactory pain relief | Findings | Conclusion | Coordinates provided |
|---|---|---|---|---|---|---|---|---|---|---|
| Peyron et al. (1995) (24) | 2 | 0 | 63.0 ± 12.7 | X |
L = 1 R = 1 |
Refractory chronic post‐stroke pain | Not specified |
(1) Increase in CBF in the ipsilateral thalamus, orbitofrontal cortex, CG, and brainstem (2) The CBF increase in the thalamus was sustained in both patients (3) The increase in CBF was rapidly reversible at the end of the stimulating period in the orbitofrontal cortex |
iMCS is associated with an increased CBF in brain regions described as being involved in pain control. The analgesic effect is likely to be mediated by a somatotopically organized mechanism leading to an inhibition of spinal nociceptive reflexes, mediated by synaptic changes | No |
| Garçia‐Larrea et al. (1997) (25) | 9 | 56 | X | X | X | Neuropathic pain | Pain relief ≤ 40% |
(1) Significant CBF increase was observed in the ipsilateral thalamic nuclei ventralis lateralis and a part of the ventralis anterior (2) Subsignificant increases in CBF were observed in the left insula, the perigenual portion of the ACC and the upper brainstem (3) Decrease in CBF was observed in the extrastriate visual regions (4) Mean blood flow in the ACC increased during iMCS only in patients with good analgesic efficacy |
Activation of thalamic nuclei directly connected with motor and premotor cortices could entail a cascade of synaptic events in other pain‐related structures, including the ACC and the PAG | No |
| Garçia‐Larrea et al. (1999) (26) | 10 | 50 | 48.4 ± 11.8 | 3.8 (2.0–16.0) |
L = 3 R = 7 |
Unilateral neuropathic pain | Pain relief ≤ 40% |
(1) iMCS increased CBF in the ventrolateral thalamus ipsilateral to stimulation, the ipsilateral medial thalamus, contralateral ACC, orbitofrontal cortex, contralateral anterior insula, and ipsilateral upper brainstem (2) Decrease in CBF was found in the occipital regions both ipsilateral and contralateral to iMCS |
Descending axons, rather than apical dendrites, are primarily activated by iMCS and the motor thalamus is the key structure in mediating functional effects of iMCS | Yes |
| Saitoh et al. (2004) (27) | 1 | 100 | 58.0 | 9.0 |
L = 0 R = 1 |
Poststroke pain | Not specified |
(1) Increases in CBF left (contralateral) rectus gyrus, left (contralateral) superior frontal gyrus, left (contralateral) anterior CG and left (contralateral) thalamus (2) Decreased CBF in the right (ipsilateral) superior temporal gyrus and in de left (contralateral) middle occipital gyrus |
iMCS induces modifications in CBF, probably mediated by synaptic changes, in the thalamus and other pain related areas | Yes |
| Peyron et al. (2007) (28) | 19 | 58 | 53.1 ± 11.3 | 4.3 (0.8–23.9) |
L = 8 R = 11 |
Neuropathic pain | Pain relief < 40% |
(1) After iMCS, CBF changes were seen in the contralateral MCC, contralateral pgACC, contralateral putamen, contralateral PAG, ipsilateral pre‐motor cortex, orbitofrontal cortex, thalami, posterior cingulate, prefrontal areas, and pons (2) There is a correlation between the pgACC and PAG, basal ganglia, and lower pons activities |
iMCS may act in part through descending inhibitory controls that involve prefrontal, orbitofrontal, and ACC as well as basal ganglia, thalamus and brainstem | Yes |
| Kishima et al. (2007) (23) | 6 | 67 | 56.5 ± 6.3 | 5.0 (3.0–27.0) |
L = 0 R = 6 |
Intractable deafferentation pain | Not specified |
(1) Increased CBF in the left (contralateral) insula, left (contralateral) posterior thalamus, right (ipsilateral) orbitofrontal cortex, and left (contralateral) ACC (2) Decreased CBF in the right (ipsilateral) precentral gyrus, and right (ipsilateral) prefrontal gyrus |
iMCS modulates the pathways from the posterior insula and orbitofrontal cortex to the posterior thalamus to upregulate the pain threshold and pathways from the posterior insula to the caudal ACC to control emotional perception | Yes |
ACC, anterior cingulate cortex; BOLD, blood oxygen‐level dependent; CG, cingulate gyrus; iMCS, invasive motor cortex stimulation; L, Left; MCC, midcingulate cortex; N, number of participants; PAG, periaqueductal gray; pgACC, pregenual anterior cingulate cortex; R, Right; SD, standard deviation; X, missing.
Clinical Effectiveness of iMCS in the Included Papers
In total, the analgesic effect of iMCS was evaluated clinically in 47 patients. Patients included in this meta‐analysis suffered from central post‐stroke pain in 32 cases (68%), brachial plexus avulsion pain in 11 cases (24%), spinal trauma in one case (2%), and intractable neuropathic radiculopathy in three cases (6%). To assess the clinical effectiveness of iMCS, the study of Kishima et al. (2007) 25 needed to be excluded as they did not provide numbers of responders (>40% pain relief in pain intensity score) and/or non‐responders (<40% pain relief in pain intensity score). In the remaining pool of patients, 51% of the cases could be labeled as responders, experiencing a fair/satisfactory pain relief. The remaining 49% of the cases were regarded as non‐responders.
Overview of the Changes of Cerebral Blood Flow Induced by iMCS
Studies suggested that rCBF increases in the cingulate gyrus and thalamus due to iMCS in chronic neuropathic pain patients 25, 26, 27, 28, 29, 30. In addition, rCBF increases were observed in the brainstem 26, 27, 28, 30, insula 25, 27, 28, the putamen 30, orbitofrontal cortex 25, 26, 28, 30, rectus gyrus 29, left superior frontal gyrus 29, and other prefrontal areas 25, 30. Decreases in rCBF were found in the precentral gyrus 25, extrastriate visual regions 27, occipital regions 28, right superior temporal gyrus, and the left middle occipital gyrus 29.
ALE Meta‐Analysis Results
The four studies totaled 36 patients (21 males, 15 females) with a median age of 53 years (range 25–72 years). Median duration of pain showed to be 6.5 years (ranging from 0.8–23.9 years). In 12 patients (33.3%), the iMCS electrode was implanted over the left primary motor cortex, whereas in 24 patients (66.6%), the iMCS electrode was implanted over the right primary motor cortex. The included patients suffered from various neuropathic pain syndromes.
ALE meta‐analysis showed that increased rCBF occurred in 11 clusters after switching “ON” the iMCS device. These clusters included the (1) anterior cingulate gyrus, (2) putamen, (3) cerebral peduncle, (4) precentral gyrus, (5) superior frontal gyrus, (6) red nucleus, (7) medial/internal part of the globus pallidus, (8) ventral lateral nucleus of the thalamus, (9) medial frontal gyrus, (10) inferior frontal gyrus, and (11) claustrum. A visual overview can be found in Fig. 2. ALE meta‐analysis suggested a decreased cerebral blood flow in the posterior cingulate gyrus after switching “ON” the iMCS device. A visual overview can be found in Fig. 3 and Table 2.
Figure 2.
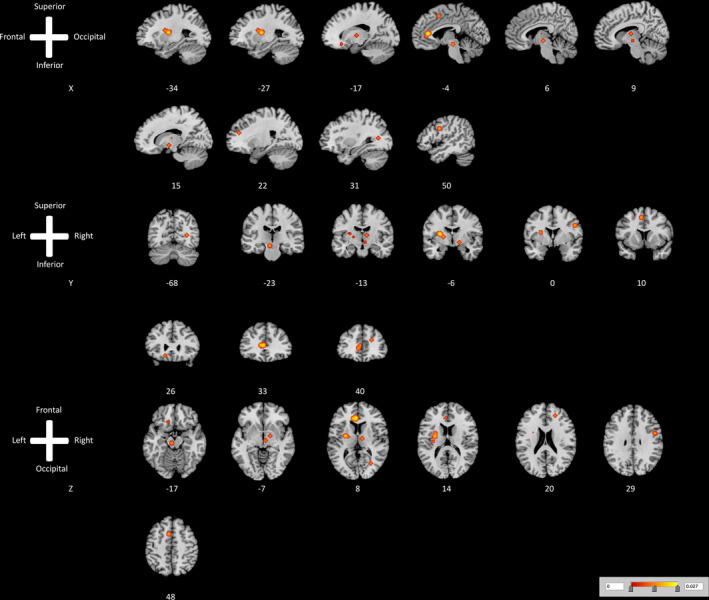
ALE map investigating increased cerebral blood flow as measured by H2 15O‐PET caused by ON vs. OFF invasive motor cortex stimulation. This image summarizes the results of all the papers involved in this meta‐analysis on changes in cerebral blood flow induced by active invasive motor cortex stimulation. Red color shows gray matter decreases (ALE maps were computed at a threshold of p < 0.001). [Color figure can be viewed at wileyonlinelibrary.com]
Figure 3.

ALE map investigating decreased cerebral blood flow as measured by H2 15O‐PET caused by ON vs. OFF invasive motor cortex stimulation. This image summarizes the results of all the papers involved in this meta‐analysis on changes in cerebral blood flow induced by active invasive motor cortex stimulation. Red color shows gray matter decreases (ALE maps were computed at a threshold of p < 0.001). [Color figure can be viewed at wileyonlinelibrary.com]
Table 2.
Regional Changes in Cerebral Blood Flow as Measured by H2 15O‐PET Induced by ON vs. OFF Invasive Motor Cortex Stimulation.
| Cluster # | Volume (mm3) | Weighted center | Extrema value | Label | L/R | Brodmann area | ||
| x | y | z | ||||||
| Regions in which increased cerebral blood flow was induced by active invasive motor cortex stimulation | ||||||||
| 1 | 2129 | −4.0 | 34.0 | 8.0 | 0.027 | Anterior cingulate gyrus | L | Brodmann area 24 |
| 2 | 2032 | −26.3 | −5.5 | 10.3 | 0.025 | Putamen | L | N/A |
| 3 | 520 | −6.1 | −22.0 | −15.7 | 0.017 | Cerebral peduncle | L | N/A |
| 4 | 456 | 50.0 | 0.0 | 30.0 | 0.017 | Precentral gyrus | R | Brodmann area 6 |
| 5 | 456 | −6.0 | 10.0 | 48.0 | 0.013 | Superior frontal gyrus | L | Brodmann area 6 |
| 6 | 264 | 6.0 | −16.0 | −8.0 | 0.013 | Red nucleus | R | N/A |
| 7 | 264 | 16.0 | −6.0 | −8.0 | 0.013 | Medial globus pallidus | R | N/A |
| 8 | 264 | 10.0 | −12.0 | 8.0 | 0.013 | Ventral lateral nucleus of the thalamus | R | N/A |
| 9 | 264 | 22.0 | 40.0 | 20.0 | 0.013 | Medial frontal gyrus | R | Brodmann area 9 |
| 10 | 208 | −14.6 | 26.2 | −15.4 | 0.011 | Inferior frontal gyrus | L | Brodmann area 47 |
| 11 | 136 | −31.9 | −18.2 | 14.9 | 0.010 | Claustrum | L | N/A |
| Regions in which decreased cerebral blood flow was induced by active invasive motor cortex stimulation | ||||||||
| 1 | 152 | 30.0 | −68.0 | 8.0 | 0.007 | Posterior cingulate gyrus | R | Brodmann area 30 |
L, Left; R, Right.
DISCUSSION
This study systematically analyzed iMCS‐related changes in rCBF in patients with neuropathic pain. Results suggested significant activation of the salience network ([mid]cingulate cortex) and sensorimotor network (thalamus, primary motor cortex, corticospinal tract/cerebral peduncle). With regard to the default mode network, mixed responses were observed. Decreased rCBF was observed in the posterior cingulate cortex, whereas the prefrontal cortex showed increased rCBF. These activation patterns can be associated with (de)activation of regions which have been revealed as key nodes in the pain matrix of the brain 31. When compared to results from studies using non‐invasive stimulation methods of the primary motor cortex, these findings are partially discordant. For example, Ohn et al. (2012) showed that the antalgic effects induced by repetitive transcranial magnetic stimulation (rTMS) of the motor cortex are mediated by decreased activity in the sensorimotor cortex, insula, anterior cingulate gyrus, and cerebellum as measured by fMRI 32. However, despite the opposite neurophysiological evidence, it has been shown that rTMS can be used to predict the effect of iMCS 33, 34. The underlying mechanisms, however, remain largely elusive.
Neuroimaging Studies on the Mechanisms of iMCS in Animal Pain Models
Animal‐based studies have extensively investigated the correlates underlying the analgesic effects of iMCS, although neuroimaging studies have been infrequently carried out. Using 2‐deoxy‐[18F]fluoro‐d‐glucose (18F‐FDG) PET‐CT in rats after iMCS, it was observed that changes occurred in glucose metabolism in various brain structures. In the striatum and thalamic area, glucose uptake was decreased by neuropathic pain. Particularly in the right‐sided striatum and thalamus, contralateral to the site of placement of the iMCS electrode, the glucose uptake was increased by iMCS. Increases in glucose uptake, as a measurement of brain activity, could be in agreement with the findings of the present ALE meta‐analysis as we suggest increased cerebral blood flow occurs in comparable regions during active iMCS. Functionally, this could possibly be explained by suppressed interhemispheric inhibition. In the cerebellum, glucose uptake was decreased by neuropathic pain and increased by iMCS 35. Another study using functional magnetic resonance imaging (fMRI) suggested significant lower blood oxygen level dependent (BOLD) responses in the primary somatosensory cortex (S1) and prefrontal cortex following noxious stimulation after iMCS 36. These areas with decreased BOLD responses are not in line with brain areas that were found significantly decreased rCBF by iMCS according to the present ALE meta‐analysis. Such disparities indicate that further research is needed.
Differences in rCBF Responses to iMCS Between Responders and Non‐Responders
Although this meta‐analysis suggests that various brain regions experience increased or decreased rCBF, the differences between responders and non‐responders could not be taken into account at a meta‐level. The present meta‐analysis found that within the included neuroimaging studies, 51% of the patients respond to iMCS, which is in agreement with larger, more epidemiological meta‐analyses on this topic 14. However, how the neuroimaging data was impacted by outcome, has only been investigated by Maarraawi et al. They showed by using PET‐CT that significant decreases of [11C]diprenorphine (a non‐selective opioid antagonist) binding capacity were found in the anterior middle cingulate cortex (MCC) and periaqueductal gray (PAG) after iMCS, which correlated with pain relief 37. Furthermore, a significant and positive correlation in levels of preoperative opioid‐binding in the insula, thalamus, PAG, anterior cingulate, and orbitofrontal cortex and pain relief due to iMCS were found 38. These opioideric regions highly correspond to the regions in which the present meta‐analysis found changes of rCBF induced by iMCS and might provide an explanation for iMCS‐induced analgesia 39. This could indicate that rCBF changes induced by active vs. non‐active iMCS might not be the most important mechanism to explain clinical outcome. Hypothetically, alterations in the activity of opioidergic brain regions might only be effective in patients with favorable preoperative opioid‐binding levels. This would indicate that the analgesic effect of iMCS is non‐dependent from rCBF, but from the preoperative opioidergic‐binding status. With regard to non‐invasive therapies, rTMS was found to reduce [11C]carfentanil (opioid) binding potential in the ventral striatum, medial orbitofrontal, prefrontal and anterior cingulate cortices, left insula, superior temporal gyrus, dorsolateral prefrontal cortex, and precentral gyrus of both hemispheres, as compared to sham stimulation 40. This is partially in agreement with the meta‐analyzed evidence presented here.
Possible Future Directions
Various studies have shown that neuroimaging can further our understanding of chronic pain and can help us to define new therapeutic options/targets (for a consensus paper, see ref. 41). Insights from neuroimaging studies could also open the field of neuromodulation for other pain disorders, for example diabetic neuropathic pain. Neuroimaging studies in diabetic neuropathic pain have shown that changes in resting state functional connectivity exists between patients and healthy controls, which includes a decreased thalamocortical resting state functional connectivity 42 and an aberrant default mode, represented by an impaired parieto‐fronto‐cingulate network 43. Furthermore, patients with painful diabetic neuropathy showed increased connectivity between left anterior cingulate cortex and posterior cingulate cortex/precuneus, and the increased connectivity between medial prefrontal cortex and left medial temporal region compared to their controls 44. Finally, it was posited that ventrolateral periaqueductal gray‐mediated descending pain modulatory system dysfunction may reflect a brain‐based pain facilitation mechanism contributing to painful diabetic polyneuropathy. Based on these findings, new studies should be conducted to investigate whether similar networks are affected by iMCS in different pain syndromes (i.e., diabetic neuropathic pain).
Strengths and Limitations
One of the strengths of this review concerns the use of the robust ALE methodology 19, 20, 21, 45 to meta‐analyze imaging results in neuromodulation treatment. Another strength of this paper comprises the homogeneity of the neuroimaging methods used in the included papers. Nevertheless, the ALE methodology also knows several limitations including the absence of null‐findings when weighing the results and the fact that the weighing of the data is mainly based on sample size. Another limitation is that other sophisticated neuroimaging methods, including various MRI techniques, were not included in this meta‐analysis. However, this limitation can be explained by the fact that the neuromodulation devices used in iMCS are not MR conditional. Another possible limitation concerns that several factors (e.g., variability in pain relief induced by iMCS, variability in included pain syndromes, variability in duration of pain, differences in acquisition protocols and timing) might have introduced heterogeneity in the included studies. However, the authors were unable to find an appropriate method to control for these factors. However, results from the included publications were rather homogeneous. Another possible limitation of this paper is that the selected studies came from only two research groups: Peyron and Garcia‐Larrea from France and Saitoh and colleagues from Japan. Nevertheless, this ALE meta‐analysis is not necessarily hindered by this limitation as this is goal dependent 46. The goal of the present ALE meta‐analysis was to provide insight into the most important theories of iMCS on a meta‐level. By investigating this theory using a method which has never been done before in iMCS imaging studies, this paper contributes to the existing literature. Furthermore, it also shows the lack neuroimaging studies which can help us to elucidate the mechanisms of action in iMCS in humans.
CONCLUSION
This ALE meta‐analysis suggests that rCBF changes were induced by active iMCS in key nodes of the default mode network (posterior cingulate cortex and prefrontal cortex), the salience network ([mid]cingulate cortex) and sensorimotor network (thalamus, primary motor cortex, corticospinal tract/cerebral peduncle). Whether these iMCS‐induced rCBF changes in principal components of the pain matrix form the neurophysiological foundation for pain relief in patients with neuropathic pain remains largely elusive and needs data from complementary methods.
Authorship Statement
Dylan Henssen undertook the actions of reviewing and analyzing the eligible publications. Henssen also wrote and edited the manuscript versions. Ronald Bartels and Erkan Kurt acted as the independent second and third reviewer of the literature. Robert van Dongen provided us with feedback regarding the grammar and the used vocabulary. Anne‐Marie van Cappellen van Walsum provided us with anatomical insights and valuable feedback on the manuscript. Ronald Bartels and Tamas Kozicz provided us with valuable feedback with regard to the applied analysis and anatomical insights. All authors approved the final version of the manuscript.
COMMENTS
The article entitled: “Invasive motor cortex stimulation influences intracerebral structures in patients with neuropathic pain; an activation likelihood estimation meta‐analysis of image data” is very interesting, even I am not sure that it adds many new things. Most results came from studies by Peyron and Maarrawi. The evidence on rTMS and its predictive value prior to invasive motor cortex stimulation is well discussed. The methodology is well described.
Jean‐Paul Nguyen, MD, PhD
Nantes, France
***
This meta analysis is carefully performed study using an innovative means of imaging analysis. It confirms findings in several studies of consistent effects of motor cortex stimulation on cerebral blood flow, especially in the anterior cingulate gyrus. The authors propose that the beneficial effects of cortical stimulation on pain levels may be a consequence of improvements in opioid binding rather than glucose uptake. Given that the pain under treatment was thought to be primarily neuropathic and thus non opioid responsive, this conclusion is contrary to common thinking. It may be an explanation for why cortical stimulation is only 50% effective, though this is still clinically significant for these difficult to treat patients.
Jeffrey A. Brown, MD
Great Neck, NY, USA
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Conflict of Interest: The authors declare that they have had no conflict of interest in the conduction of this research.
Source(s) of financial support: No funding was received for conducting this research.
REFERENCES
- 1. Treede RD, Rief W, Barke A et al. A classification of chronic pain for ICD‐11. Pain 2015;156:1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Loeser JD, Treede R‐D. The Kyoto protocol of IASP basic pain terminology. Pain 2008;137:473–477. [DOI] [PubMed] [Google Scholar]
- 3. Brooks J, Tracey I. From nociception to pain perception: imaging the spinal and supraspinal pathways. J Anat 2005;207:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron 2007;55:377–391. [DOI] [PubMed] [Google Scholar]
- 5. Melzack R. Pain and the neuromatrix in the brain. J Dent Educ 2001;65:1378–1382. [PubMed] [Google Scholar]
- 6. Treede RD, Apkarian AV, Bromm B, Greenspan JD, Lenz FA. Cortical representation of pain: functional characterization of nociceptive areas near the lateral sulcus. Pain 2000;87:113–119. [DOI] [PubMed] [Google Scholar]
- 7. Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain 1999;79:105–111. [DOI] [PubMed] [Google Scholar]
- 8. Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien) 1991;52:137–139. [DOI] [PubMed] [Google Scholar]
- 9. Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Treatment of thalamic pain by chronic motor cortex stimulation. Pacing Clin Electrophysiol 1991;14:131–134. [DOI] [PubMed] [Google Scholar]
- 10. Brown JA, Pilitsis JG. Motor cortex stimulation. Pain Med 2006;7:S140–S145. [Google Scholar]
- 11. Velasco F, Arguelles C, Carrillo‐Ruiz JD et al. Efficacy of motor cortex stimulation in the treatment of neuropathic pain: a randomized double‐blind trial. J Neurosurg 2008;108:698–706. [DOI] [PubMed] [Google Scholar]
- 12. Radic JA, Beauprie I, Chiasson P, Kiss ZH, Brownstone RM. Motor cortex stimulation for neuropathic pain: a randomized cross‐over trial. Can J Neurol Sci 2015;42:401–409. [DOI] [PubMed] [Google Scholar]
- 13. Lefaucheur JP, Drouot X, Cunin P et al. Motor cortex stimulation for the treatment of refractory peripheral neuropathic pain. Brain 2009;132:1463–1471. [DOI] [PubMed] [Google Scholar]
- 14. Henssen DJHA, Witkam RL, Dao JCML, Comes DJ, van Cappellen van Walsum AM, Kozicz T, Vandongen R, Vissers K, Bartels RHMA, de Jong G, Kurt E. Systematic review and neural network analysis to define predictive variables in implantable motor cortex stimulation to treat chronic intractable pain. J Pain 2019;20:1015–1026. [DOI] [PubMed] [Google Scholar]
- 15. Moisset X, de Andrade DC, Bouhassira D. From pulses to pain relief: an update on the mechanisms of rTMS‐induced analgesic effects. Eur J Pain 2016;20:689–700. [DOI] [PubMed] [Google Scholar]
- 16. Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta‐analysis of the functional neuroanatomy of single‐word reading: method and validation. Neuroimage 2002;16:765–780. [DOI] [PubMed] [Google Scholar]
- 17. Laird AR, Eickhoff SB, Fox PM et al. The BrainMap strategy for standardization, sharing, and meta‐analysis of neuroimaging data. BMC Res Notes 2011;4:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laird AR, Eickhoff SB, Kurth F et al. ALE meta‐analysis workflows via the Brainmap database: progress towards a probabilistic functional brain atlas. Front Neuroinform 2009;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: a random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 2009;30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within‐experiment and within‐group effects in activation likelihood estimation meta‐analyses. Hum Brain Mapp 2012;33:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta‐analysis revisited. Neuroimage 2012;59:2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laird AR, Fox PM, Price CJ et al. ALE meta‐analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 2005;25:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lancaster JL, Tordesillas‐Gutierrez D, Martinez M et al. Bias between MNI and talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 2007;28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jia ZH, Yu SY. Grey matter alterations in migraine: a systematic review and meta‐analysis. Neuroimage Clin 2017;14:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kishima H, Saitoh Y, Osaki Y et al. Motor cortex stimulation in patients with deafferentation pain: activation of the posterior insula and thalamus. J Neurosurg 2007;107:43–48. [DOI] [PubMed] [Google Scholar]
- 26. Peyron R, Garcia‐Larrea L, Deiber MP et al. Electrical stimulation of precentral cortical area in the treatment of central pain: electrophysiological and PET study. Pain 1995;62:275–286. [DOI] [PubMed] [Google Scholar]
- 27. Garcia‐Larrea L, Peyron R, Mertens P et al. Positron emission tomography during motor cortex stimulation for pain control. Stereotact Funct Neurosurg 1997;68:141–148. [DOI] [PubMed] [Google Scholar]
- 28. Garcia‐Larrea L, Peyron R, Mertens P et al. Electrical stimulation of motor cortex for pain control: A combined PET‐scan and electrophysiological study. Pain 1999;83:259–273. [DOI] [PubMed] [Google Scholar]
- 29. Saitoh Y, Osaki Y, Nishimura H et al. Increased regional cerebral blood flow in the contralateral thalamus after successful motor cortex stimulation in a patient with poststroke pain. J Neurosurg 2004;100:935–939. [DOI] [PubMed] [Google Scholar]
- 30. Peyron R, Faillenot I, Mertens P, Laurent B, Garcia‐Larrea L. Motor cortex stimulation in neuropathic pain. Correlations between analgesic effect and hemodynamic changes in the brain. A PET study. Neuroimage 2007;34:310–321. [DOI] [PubMed] [Google Scholar]
- 31. Bosma RL, Hemington KS, Davis KD. Using magnetic resonance imaging to visualize the brain in chronic pain. Pain 2017;158:1192–1193. [DOI] [PubMed] [Google Scholar]
- 32. Ohn SH, Chang WH, Park CH et al. Neural correlates of the antinociceptive effects of repetitive transcranial magnetic stimulation on central pain after stroke. Neurorehabil Neural Repair 2012;26:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andre‐Obadia N, Mertens P, Lelekov‐Boissard T, Afif A, Magnin M, Garcia‐Larrea L. Is life better after motor cortex stimulation for pain control? Results at long‐term and their prediction by preoperative rTMS. Pain Physician 2014;17:53–62. [PubMed] [Google Scholar]
- 34. Lefaucheur JP, Menard‐Lefaucheur I, Goujon C, Keravel Y, Nguyen JP. Predictive value of rTMS in the identification of responders to epidural motor cortex stimulation therapy for pain. J Pain 2011;12:1102–1111. [DOI] [PubMed] [Google Scholar]
- 35. Kim J, Ryu SB, Lee SE et al. Motor cortex stimulation and neuropathic pain: how does motor cortex stimulation affect pain‐signaling pathways? J Neurosurg 2016;124:866–876. [DOI] [PubMed] [Google Scholar]
- 36. Jiang L, Ji Y, Voulalas PJ et al. Motor cortex stimulation suppresses cortical responses to noxious hindpaw stimulation after spinal cord lesion in rats. Brain Stimul 2014;7:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maarrawi J, Peyron R, Mertens P et al. Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology 2007;69:827–834. [DOI] [PubMed] [Google Scholar]
- 38. Maarrawi J, Peyron R, Mertens P et al. Brain opioid receptor density predicts motor cortex stimulation efficacy for chronic pain. Pain 2013;154:2563–2568. [DOI] [PubMed] [Google Scholar]
- 39. Henriksen G, Willoch F. Imaging of opioid receptors in the central nervous system. Brain 2008;131:1171–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lamusuo S, Hirvonen J, Lindholm P et al. Neurotransmitters behind pain relief with transcranial magnetic stimulation ‐ positron emission tomography evidence for release of endogenous opioids. Eur J Pain 2017;21:1505–1515. [DOI] [PubMed] [Google Scholar]
- 41. Davis KD, Flor H, Greely HT et al. Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat Rev Neurol 2017;13:624–638. [DOI] [PubMed] [Google Scholar]
- 42. Cauda F, Sacco K, D'Agata F et al. Low‐frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in diabetic neuropathic pain. BMC Neurosci 2009;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cauda F, D'Agata F, Sacco K et al. Altered resting state attentional networks in diabetic neuropathic pain. J Neurol Neurosurg Psychiatry 2010;81:806–811. [DOI] [PubMed] [Google Scholar]
- 44. Sag AT, Has AC, Oztekin N, Temucin CM, Oguz KK. Tracking pain in resting state networks in patients with hereditary and diabetic neuropathy. Noro Psikiyatr Ars 2019;56:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Acar F, Seurinck R, Eickhoff SB, Moerkerke B. Assessing robustness against potential publication bias in activation likelihood estimation (ALE) meta‐analyses for fMRI. PLoS One 2018;13:e0208177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Valentine JC, Pigott TD, Rothstein HR. How many studies do you need? A primer on statistical power for meta‐analysis. J Edu Behav Stat 2010;35:215–247. [Google Scholar]


