Abstract
This narrative review provides a broad and comprehensive overview of the most important discoveries on the postictal state over the past decades as well as recent developments. After a description and definition of the postictal state, we discuss postictal sypmtoms, their clinical manifestations, and related findings. Moreover, pathophysiological advances are reviewed, followed by current treatment options.
Keywords: epilepsy, postictal suppression, postictal symptoms, postictal treatment, SUDEP
Key Points.
The postictal state shows a rich phenomenology of neurological deficits and/or psychiatric symptoms, varying in severity and duration.
We define the postictal state as “a temporary brain condition following seizures (a) manifesting neurological deficits and/or psychiatric symptoms, (b) often accompanied by EEG slowing or suppression, (c) lasting minutes to days.”
Pathophysiological mechanisms are being elucidated but are mostly limited to preclinical models.
Current treatment options consist mainly of symptom suppression and are not strongly established in clinical trials.
1. INTRODUCTION
Patients with epilepsy not only have seizures, but also experience an aftermath of seizures: the postictal state. This includes a variety of sensory, cognitive, and motor deficits such as unresponsiveness, headaches, and memory impairments. 1 , 2 , 3 , 4 , 5 Furthermore, psychiatric symptoms may occur, including postictal depression and psychosis. 1 , 6 , 7 Symptoms can vary in severity and may last from minutes to hours, or even days, depending on age, type of seizures, and underlying brain disease. 4 A recent systematic review of 45 studies identified postictal clinical symptoms and characteristics in various epilepsy types. 4 Postictal unresponsiveness was most common, with a mean frequency of approximately 96%. Postictal headaches, migraines, and psychosis had a mean frequency of 33%, 16%, and 4%, respectively. The extent and intensity of the postictal state affects patients’ quality of life substantially and correlates strongly with patients’ rating of seizure severity, but has received little attention in epilepsy treatment. 1 , 8
In contrast to the ictal state, an operational definition for the postictal state is not straightforward due to the challenges of identifying exact onset and termination points. 6 Although postictal symptoms were first described in 1849 by Todd, 9 a clear definition is still lacking. In Figure 1, a timescale of the postictal state is presented. The duration of the postictal state differs in terms of clinical manifestation; T1 (red) represents the short duration of seconds to minutes; T2 (orange) includes hours, reflecting physical and cognitive symptoms; and T3 (yellow) represents days to weeks, in which psychiatric symptoms as postictal psychosis may occur. If postictal symptoms span a broader timescale, this will be indicated as T1‐T2, T2‐T3, or T1‐T3. This framework will be used throughout the review. Conceptually, the postictal state can be defined as a transient abnormal brain condition with neurologic deficits or psychiatric symptoms during the period following an epileptic seizure, which is reflected on electroencephalography (EEG) as suppression of physiological rhythms (T1‐T2; Figure 1). 6 On a fundamental level, one could define the postictal state as desynchronization of neuronal networks, for instance, resulting from disbalance in transmembrane ionic gradients (T1). Another definition of the postictal state that appears in the literature is “a manifestation of seizure‐induced reversible alterations in neuronal function, but not structure” (T1). 10 Based on these considerations, we suggest the following definition for the postictal state: “A temporary brain condition following seizures (a) manifesting neurological deficits and/or psychiatric symptoms, (b) often accompanied by EEG slowing or suppression, (c) lasting minutes to days” (T1‐T3). In this narrative review, we identified articles that mentioned the postictal state, and ordered the results in five sections: (1) clinical manifestations, (2) EEG characteristics, (3) neuroimaging and biochemistry, (4) pathophysiological mechanisms, and (5) treatment options.
FIGURE 1.
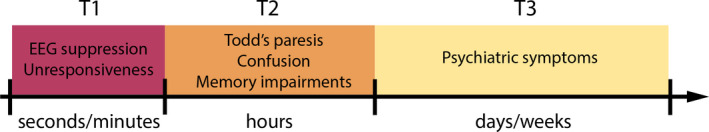
Timescales of the postictal state. The duration of the postictal state differs in terms of clinical manifestation; T1 (red) represents the short duration of seconds to minutes; T2 (orange) includes hours, reflecting physical and cognitive symptoms; T3 (yellow) represents days to weeks, in which psychiatric symptoms as postictal psychosis may occur. If postictal symptoms span a broader timescale, this will be indicated as T1‐T2, T2‐T3, or T1‐T3. This framework will be used throughout the review
2. SEARCH STRATEGY AND SELECTION CRITERIA
We searched PubMed, MEDLINE, and the Cochrane databases for English‐language articles published between January 1849 and December 2019. We searched for symptomatology and treatment strategies. The key terms used were “postictal*” in combination with “psychiatry”, “EEG”, “behaviour”, “neuroscience”, “MRI”, “epilepsy”, and “neurological deficits”. Articles were reviewed that mentioned the postictal state in the title or abstract and included human data. We excluded articles that did not focus on epilepsy, stroke‐related seizures, and study protocols.
3. CLINICAL MANIFESTATIONS
The postictal state shows a rich phenomenology of neurological deficits and/or psychiatric symptoms, summarized in Table 1.
TABLE 1.
Clinical manifestation of the postictal state
| Category | Signs and symptoms | |
|---|---|---|
| Altered consciousness |
Unresponsiveness Confusion Coma Delirium a |
|
| Cognitive dysfunction |
Declined alertness and short‐term memory Visual and spatial memory impairments Delirium a |
|
| Autonomic dysregulation |
Coughing Spitting Hypersalivation Nose rubbing Hyperthermia Cardiovascular dysfunction (arrhythmia, brady‐ and tachycardia, hypo‐ and hypertension) Myocardial infarction Neurogenic pulmonary edema Dysregulated breathing patterns |
|
| Headache |
Migraines Fatigue |
|
| Changes in mood and affect | Depressive mood and affect state disturbances | |
| Postictal paresis | Todd's paresis | |
| Visual and auditory disturbances |
Blindness/visual loss Hallucinations Palinacousis |
|
| Language dysfunction | Speech and language disturbances | |
| Dysphasia | ||
| Sleep | Sleep disturbances | |
| Psychiatric symptoms and syndromes |
Delirium a Postictal psychosis (perception and thought disturbances) Motor disturbances (catatonia) |
|
| SUDEP | Unidentifiable non‐accidental and non‐suicidal cause of death | |
| Social and economic impact |
Higher mortality and morbidity Lowered self‐esteem and increased stigma Unemployment |
|
Delirium is a syndrome in which consciousness, cognitive functions, and psychiatric symptoms (eg, hallucinations, delusions, mood disturbances) may occur.
3.1. Altered consciousness
Altered states of consciousness range from unresponsiveness to postictal coma, which is a common finding after generalized tonic‐clonic seizures (T1). 11 , 12 Recovery of consciousness may, in some cases, reveal neurological lateralized deficits, including paresis. 13 After an individual awakens from coma, memory is often temporarily impaired (T2).
3.2. Cognitive dysfunction
Cognitive functions that decline most after seizures are alertness and short‐term memory (T2). 3 Sixty‐six of 100 patients with refractory focal epilepsy showed postictal memory impairments depending on the location of seizure foci. 14 Visual memory impairments occur if seizure foci are in the nondominant hemisphere, whereas verbal memory is impaired if seizure foci are located in the dominant hemisphere. Furthermore, patients may experience clouded thinking, impaired attention and concentration, and decreased verbal skills. 1 In right temporal lobe epilepsy (TLE), a decline in visual attention and spatial orientation was reported, whereas verbal memory was impaired in left TLE. 3 The more seizures a patient experiences, the larger the likelihood of severe postictal cognitive impairment.
3.3. Autonomic dysregulation
Coughing and spitting can occur after temporal lobe seizures, presumably reflecting ictal‐induced autonomic dysfunction, or in some instances, aspiration during the seizure (T1). 15 In addition, postictal hypersalivation, nose rubbing, cardiovascular dysfunction (arrhythmia, bradycardia, and tachycardia), myocardial infarction, neurogenic pulmonary edema, and transient systemic hypotension and hypertension have been reported (T1‐T3). 3 , 16 Postictal hyperthermia resulting from ictal muscle activity was found to be related to seizure duration in several patients. 16 Neurogenic edema is an uncommon complication in the postictal state also, resulting from changes in the alveolar capillary endothelium, which typically resolves within 24 hours. 17
3.4. Headache
Postictal headache occurs in approximately 66% of patients. 4 , 18 It ranges from moderate to severe in intensity, frequently with migrainous features, and in durations from minutes to hours (T1‐T2). 19 Postictal headache is reported more often in patients with generalized tonic‐clonic focal seizures with impaired awareness, or repetitive or prolonged seizures. Gender and family history of migraines or headaches do not seem to be risk factors for postictal headache. 13 , 19
3.5. Changes in mood and affect
Postictal depressive and anxiety symptoms may occur for >24 hours and within 5 days postictally (T3). 5 In a sample of patients with focal epilepsy, 18 of 100 showed postictal depressive symptoms, characterized by anhedonia, helplessness, self‐deprecation, or suicidal thoughts. 14 Postictal anxiety may include constant worrying, agoraphobia, and unpleasant feelings due to increased self‐awareness (ie, self‐consciousness), and is often accompanied by a depressive disorder. 20 In a small (n = 5) retrospective sample, postictal mania was associated with symptoms of elevated and euphoric mood, distractibility, hyperactivity, disinhibition, pressured speech, decreased need for sleep, flight of ideas, grandiosity, and hyperreligiosity. 21 Postictal hypomania is also common (T1‐T2). 5
3.6. Postictal paresis
Todd's paresis is a specific example of a severe postictal motor impairment, which can be misdiagnosed as ischemic stroke (T2). 22 Diagnostic tools with high specificity or sensitivity to differentiate between Todd's paresis, transient ischemic attacks, or stroke (mimics) are currently lacking. 23 , 24 Careful clinical assessment including physical and neurological evaluation and brain imaging is advised. 24 Todd's paresis can develop after focal and generalized seizures involving the (contralateral) sensorimotor cortex, and can even be present bilaterally. 25 In a sample of 229 patients with focal to bilateral tonic‐clonic seizures, approximately 6% developed Todd's paresis. 26 There is a high risk that Todd's paresis may not be discovered, if not specifically sought for by clinicians, leading to an underestimation of prevalence. 13
3.7. Visual and auditory disturbances
Postictal blindness (amaurosis 27 ) is reported in two‐thirds of patients with childhood occipital epilepsy of Gastaut, 28 with seizure foci being occipital or occipitotemporal (T1‐T2). 29 , 30 Older patients may have postictal visual loss as well, if seizures started in occipital areas. 29 Postictal palinacousis is the phenomenon of preservation of an external auditory stimulus after its cessation, as, for example, a fragment of a previously heard sentence, manifesting in an auditory illusion. 31 In the few cases of palinacousis, none of the patients had electrographic seizures during the event.
3.8. Language dysfunction
Postictal speech disturbances can be indicative of seizure location and seizure spread, and they often involve postictal dysphasia (T1). 3 , 32 Approximately 38% of patients experience language impairments. 4 Most postictal speech disturbances occur in patients with TLE of the dominant hemisphere or if seizures spread to the dominant temporal lobe. 32 Postictal language delay with paraphasia was found to be longest if seizure onset is located in the nondominant temporal lobe and spreads to the contralateral dominant temporal lobe. 32
3.9. Sleep
If seizures occur during sleep, postictal phenomena may range from confusion on awakening to disturbances in sleep patterns (T1‐T3). 33 This also introduces challenges for the differential diagnoses of sleep disorders as parasomnia. For instance, sleep disturbances may affect memory consolidation and attention, but this may also be directly related to the postictal state. 33 Sleep apnea may also occur after nocturnal seizures, introducing a risk for sudden unexpected death in epilepsy (SUDEP). 34 Postictal sleep may also be a symptom related to activation of cerebral inhibitory systems to terminate seizures. 12 Postictal sleep has been reported in approximately 6% up to 45% of patients. 4 , 18
3.10. Psychiatric symptoms and syndromes
Postictal psychiatric symptoms include delirium, changes in perception (eg, hallucinations), thoughts (eg, incoherence, delusions), and motor disturbances (eg, catatonia) (T2‐T3). 18 , 35 , 36 , 37 Postictal delirium may transition to a postictal psychosis, including violent behavior. 1 , 36 , 38 In some patients, violent, bizarre, or sexual inappropriate behavior occurs. 39 , 40 Two meta‐analyses have shown that the estimated prevalence of postictal psychosis ranges between 2% and 4%, independent of type of epilepsy. 4 , 39 Diagnostic criteria include return of normal mental function within 1 week and duration of 1 day to 3 months. 1 , 41 However, no clear definition of postictal psychosis is provided in the literature. In addition, it remains unclear whether the psychosis is part of the ictal period alone or represents an underlying psychiatric illness, which makes its diagnosis and treatment challenging, 2 but diagnostic criteria designed by Logsdail and Toone may be used. 42 In a study with 100 epilepsy patients, approximately half presenting with isolated psychotic symptoms also had a history of psychiatric disorders such as depression, anxiety, and attention deficit disorders. 14 Psychic auras and grandiose and religious delusions occurred frequently in a sample of 30 patients with TLE, compared to interictal and chronic psychosis. 43 Postictal Cotard and Capgras delusion have been reported in case studies. 44 Affective symptoms may occur during postictal psychosis, 45 including the Cotard and Capgras delusion, which may lead to a discussion of whether the patient has a postictal mood disorder or psychotic disturbance. A higher incidence of violent behavior was established in postictal compared to interictal psychosis with risk for suicidal attempts and acting violently. 38 , 40 There are conflicting viewpoints on which seizure type puts patients most at risk for a postictal psychosis. Patients with generalized seizures were more likely to develop postictal psychosis than patients with focal impaired awareness seizures, 46 but others suggested the opposite relationship. 41 In which way genetic predispositions play a role in postictal psychosis is uncertain. 40 Catatonia has been reported rarely in the postictal state, as it seems to be more commonly associated with the ictal state. 47 , 48
3.11. Sudden unexpected death in epilepsy (SUDEP)
SUDEP is defined as an unidentifiable cause of death that is nonaccidental and nonsuicidal, excluding status epilepticus as possible cause, occurring during or immediately after a seizure (T1). 49 Patients with epilepsy are 20 times more likely than the general population to die unexpectedly, but the risk of SUDEP varies widely within the epilepsy population. 50 The most important risk factor for SUDEP is a high frequency of generalized tonic‐clonic seizures. 22 The MORTality in Epilepsy Monitoring Unit Study (MORTEMUS) identified postictal respiratory depression and cardiac dysfunction occurring after generalized tonic‐clonic seizures as critical factors in SUDEP. 51 Postictal generalized EEG suppression (PGES) has been observed in all monitored SUDEP cases. This could be referred to as an early postictal neurovegetative breakdown. 51 Respiratory and cardiac dysfunction has also been observed and related to SUDEP in a small sample of 42 patients. 52 Other recent work on 69 patients with focal to bilateral tonic‐clonic seizures and generalized tonic‐clonic seizures also suggests an association between the occurrence of potentially high‐risk cardiac arrhythmias and longer ictal/postictal hypoxemia. 53
Postictal hypoxemia and PGES seem to be risk factors for SUDEP (T1). 54 Recent work on periictal central apnea and SUDEP in 218 patients established that postconvulsive central apnea was associated with longer oxygen saturation recovery times to mild hypoxemia but not with total hypoxemia duration. 55 Whether prolonged ictal central apnea and postconvulsive central apnea can serve as potential biomarkers for an increased risk of SUDEP needs to be validated in larger studies. The relation between PGES and SUDEP remains controversial and poorly understood. Asadollahi and colleagues found that the risk of SUDEP increased by 1.7% for each 1 second increase in duration of PGES after generalized convulsive seizures (N = 67). 49 Contrary to this finding, SUDEP patients may have shorter durations of PGES after generalized convulsive seizures. 56 PGES and postictal hypoxemia showed a strong correlation (N = 73), which may indicate its involvement in SUDEP. 54 In a study with N = 59 patients, longer PGES (ie, >20 seconds) was no reliable predictor of SUDEP. 57 However, antiepileptic drug reduction and PGES during sleep may be associated with a higher risk of SUDEP. 57 Limited sample sizes in the aforementioned studies have to be considered.
There have been a few breakthroughs pointing to a key mechanism relating to SUDEP. 22 , 58 , 59 It is proposed that spreading depolarization propagating to brainstem cardiorespiratory centers has an active role in the postictal cardiorespiratory collapse, based on animal models of human SUDEP. 58
3.12. Social and economic impact
Patients’ health and well‐being are severely affected by postictal symptoms (T3). 60 Higher mortality and morbidity in patients with epilepsy in childhood as well as higher age are well‐known clinical characteristics. 61 , 62 , 63 , 64 Postictal aspiration remains a common threat after generalized seizures that needs to be dealt with immediately by administering oxygen, if patients are supervised in a hospital setting (T1). 13 Caregivers and loved ones are also affected by these circumstances, as patients depend on their immediate help. In the aftermath of seizures, patients can deal with injuries such as burns, fractures, and tongue biting. Patients, caregivers, and their loved ones may be confronted with an increased burden of homicidal and suicidal behavior. 38 , 41 Postictal cognitive and behavioral impairment may also lead to lowered self‐esteem, increased stigma, and employment difficulties. 65
4. ELECTROENCEPHALOGRAPHY (EEG) CHARACTERISTICS OF THE POSTICTAL STATE
The most common EEG characteristics during the postictal state are suppression and slowing of brain rhythms. 66 Postictal EEG suppression is defined as abnormal slow‐wave activity or suppression with amplitudes <10 µV within 30 seconds of seizure cessation, lasting more than 2 seconds (T1). 6 , 7 , 49 , 67 It has been found in 84% of seizures in 94% of epilepsy patients. 68 Different definitions of postictal EEG suppression have been proposed; for example, EEG attenuation of more than 2 seconds. 49 The underlying mechanism of postictal EEG suppression remains unknown. 69 It may be related to anoxia, acute hypercapnia, or cortical spreading depression. 69
As recovery of the EEG advances, initial delta slowing (<4 Hz) transitions to theta frequencies (4‐7 Hz) before returning to baseline background activity (T1). 6 , 66 Examples are shown in Figures 2 and 3. Delta slowing can be considered as the most important postictal EEG change, as it occurs after up to 81% of the seizures. This phenomenon is sometimes accompanied by intermittent epileptiform discharges (ie, postictal spikes). 66
FIGURE 2.
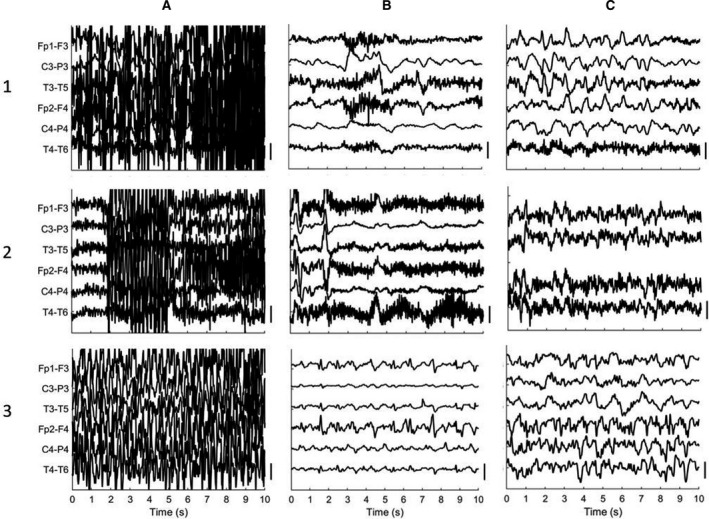
Examples of EEG recordings, showing A, seizure activity; B, postictal suppression or slowing; and C, return of background activity. Patient 1 was 88‐years‐old and had a generalized seizure that was followed by EEG suppression after approximately 3 min postictally, and postictal slowing occurring approximately 25 min postictally. Row 2 represents data from a 77‐year‐old patient with a generalized seizure. Postictal suppression occurred after approximately 1 min after seizure activity, with postictal slowing after 9 min postictally. Noisy frontal channels excluded to maintain visibility of the EEG. The last patient (3) was 65‐years‐old. After the seizure, EEG shows sporadic spikes over the frontal regions with suppressed activity (3B; t = 25 min) and subsequent appearance of rhythms in the delta and theta bands, with postictal suppression 25 min postictally and slowing approximately 1‐h postictally (3C). Filter settings 0.5‐35 Hz. Vertical scale bar: 50 µV
FIGURE 3.
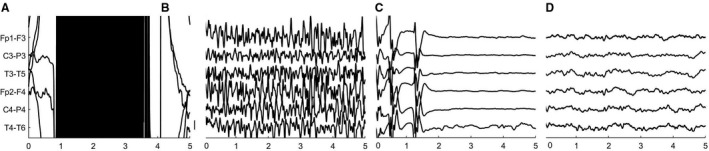
Electroencephalographic activity during and after electroconvulsive therapy (ECT). A, Artifact resulting from the ECT stimulus with (B) subsequent seizure activity; C, EEG suppression 80 s postictally; D, EEG slowing 22 min after seizure activity. Note the similarities between ECT‐evoked seizures and spontaneous epileptic seizures, shown in Figure 1. Filter settings 1‐35 Hz. Vertical scale bar: 50 µV
Postictal EEG changes can last up to 24 hours (T1‐T2). 3 Recovery to baseline EEG also depends on the use of antiepileptic drugs. For example, patients treated with levetiracetam demonstrate quicker recovery of postictal slowing to baseline compared to placebo. 3 , 70
4.1. Spatial characteristics
The spatial extent of the postictal suppression depends on the seizure type. In patients with TLE, postictal slowing may develop on the site ipsilateral to seizure onset. In generalized tonic‐clonic seizures, postictal suppression involves both hemispheres. 3
In TLE patients, increased seizure severity was associated with global postictal elevation of relative spectral delta power in several brain areas. 7 Furthermore, a regional decrease in delta power was established in ipsilateral temporal regions but increased in frontal regions. Postictal delta activity in frontal‐parietal regions has been related to seizure‐induced behavioral manifestations (eg, impairments in responsiveness and consciousness). 7 A combination of EEG attenuation and delta slowing may result in more severe postictal clinical disturbances, rather than one of these EEG changes in isolation. 66
4.2. Clinical correlates
High frequency gamma activity (>25 Hz) during postictal EEG attenuation of lower frequencies may be associated with clinical features as postictal immobility (T1). 68 These findings hint on ongoing brain activity from subcortical structures that cannot be discovered with standard scalp EEG alone. Furthermore, postictal EEG suppression has been related to a higher risk of developing postictal psychosis. 46
5. DURATION OF THE POSTICTAL STATE
5.1. Myoclonic and atonic seizures
Defining the duration of the postictal state after myoclonic seizures is challenging. Myoclonic seizures involve brief, clustered muscle jerks, often occurring while falling asleep. 6 Defining offsets for myoclonic seizures is difficult, as it is unclear whether, and if so, how these individual short‐lasting muscle jerks should be clustered. Atonic seizures present with short duration and decreased muscle tone. Reports about the postictal state in atonic seizures have been scarce. Until now, no indication has been provided about the duration of the postictal state in these seizure types. Postictal states are more likely to occur if seizures last longer, and patients with very brief absence seizures (<15 seconds) generally do not have a postictal state (T1). 6 Absence epilepsy mostly lacks a postictal state, as background EEG before and after seizures is normal and postictal hypoxia does not seem to occur, providing diagnostic value. 71 , 72
5.2. Focal with impaired awareness seizures
As early as in the year 1983, Theodore and colleagues investigated the duration of the postictal state in focal impaired awareness seizures from clinical characteristics as postictal confusion and speech disturbances. 73 Mean postictal duration was 89 seconds, with a maximum of 767 seconds, based on immediate responsiveness (T1). Baker et al showed that the revised version of the Liverpool Seizure Severity Scale could reliably identify seizure severity, focusing among others on postictal symptoms and duration of the postictal state. 18 In a sample of 97 patients with epilepsy, time to full recovery was more than 60 minutes for almost 40% of patients (T1).
5.3. Focal to bilateral tonic‐clonic seizures
In contrast, Kaibara and Blume assessed duration by focusing on EEG features, either delta or theta slowing, attenuation, or spike activation. 74 They showed that mean duration of postictal scalp EEG changes after focal seizures was 275 seconds, ranging from 7 seconds to >40 minutes (T1). 66 , 74 Others reported postictal periods of 45 minutes after generalized convulsions, 75 defining postictal duration as recovery of consciousness and motor function. The postictal state ranged from 2 minutes to 2 months based on symptom presentation, that is, postictal headache/confusion and postictal psychosis, respectively (T1‐T3). 4
There have been controversial findings for postictal duration and age. If epilepsy onset occurred after the age of 18 and if seizures started in the dominant hemisphere, patients were more likely to have longer postictal duration (T1). 29 A recent study retrospectively investigated factors associated with postictal duration after generalized seizures, with longer periods for elderly patients, longer seizure duration, and higher functional dependence (T2). 75 In contrast, Arkilo, Wang, and Thiele found that children needed on average more time to return to background EEG (120 minutes) than adults (84 minutes; T2). 76 Furthermore, postictal slowing was shorter for frontal lobe seizures than temporal lobe seizures (T2). 76
The various differences in definitions for the postictal state further illustrate the demand for adapting the definition for an accurate estimation of its duration. 4 A definition based solely on responsiveness 73 or recovery of motor function 75 questions the validity of results. We, and others, argue that the widely used criteria based on clinical observation alone 12 are too crude to provide an accurate measure of the full postictal state. 6
6. DIFFERENTIATION BETWEEN THE ICTAL, INTERICTAL, AND POSTICTAL STATES
Differentiation between the ictal, interictal, and postictal states remains challenging. 77 Depending on the type of the seizure, the transition from ictal to postictal state is more or less apparent from clinical observation. Recovery of language or motor function may reliably determine the end of the ictal state. This remains challenging, however, as during the postictal state, clinical symptoms may improve, but in some, electrographic seizures persist. 6 , 78 Marking the end of a seizure based on clinical manifestations can be therefore difficult. 78 Continuous EEG recordings may have additional value in determining the postictal state, as this helps to identify that the ictal EEG characteristics have vanished. 68 Quantitative EEG measures may aid in determining boundaries of the postictal state. In particular in the treatment of a nonconvulsive status, continuous EEG monitoring is nearly mandatory to assess transition to the postictal state and if treatment is satisfactory. 12
Pragmatically, patients should show recovery of neurological deficits within 30‐60 minutes (T1); otherwise, a nonconvulsive status must be considered. 12 Recently, various EEG criteria for nonconvulsive status epilepticus were reported as the “Salzburg consensus criteria.” 79
However, some EEG signals are associated both with seizures and the postictal state, such as rhythmic slowing in the theta and delta frequency range or periodic discharges (T1‐T2). 66 Distinguishing clinically between postictal activity and nonconvulsive status epilepticus can be difficult, because clinical signs are often subtle and nonspecific and can occur both ictally and postictally. 80 Furthermore, in some critically ill patients, the transition from the ictal to the postictal state is more gradual, and patients can even enter a state known as the “ictal‐interictal‐continuum.” 81 If the EEG shows focal or generalized periodic discharges with a frequency lower than 2.5 Hz or intermittent bursts of generalized spike‐waves, postictal activity or nonconvulsive status epilepticus is not obvious, and evaluating the clinical response to antiepileptic medication is generally advised. 77 Despite these limitations, EEG remains the most valuable tool for differentiating between the ictal and postictal states. 6
7. NEUROIMAGING AND BIOCHEMISTRY
7.1. Neuroimaging
Functional magnetic resonance imaging (fMRI) and single photon emission computed tomography (SPECT) have identified various changes in the postictal state (Table 2). The extent and duration of postictal EEG suppression (T1) in the delta frequency range was correlated with hippocampal atrophy in patients with TLE: Postictal delta power was lower on the right side if hippocampal atrophy on the ipsilateral site was also worse. 82 Deactivation of the default mode network (DMN) may persist after a seizure in epileptic patients (T1‐T2). 83 Cortical areas that were found to generate slow waves partly overlap with regions involved in the DMN.
TABLE 2.
Summary of neuroimaging studies of the postictal state (2001‐2019)
| Result | Technique | Population | Reference |
|---|---|---|---|
| Hippocampal atrophy | T1‐ and T2‐weighted MR images | Intractable focal epilepsy with impaired awareness | Olejniczak et al (2001) 82 |
| Deactivation default mode network | SPECT | Epilepsy with spontaneous secondarily generalized tonic‐clonic seizures | Blumenfeld et al (2009) 83 |
| Dysregulated cerebral blood flow | |||
| Hypoperfusion (lateral temporal lobe, hippocampus) | SPECT, ASL ‐MRI, PWI, CT | Temporal lobe epilepsy, focal epilepsy | Koepp et al (2010) 10 , Farrell et al (2016) 88 , Gaxiola‐Valdez et al (2017) 85 , Li et al (2019) 84 |
| Hyperperfusion (parahippocampal gyrus) | PWI | (Extra‐) temporal lobe epilepsy | Leonhardt et al (2005) 89 |
| Hyperperfusion (right temporal lobe, basal ganglia) | SPECT | Epilepsy after encephalitis (during postictal psychosis) | Yasumoto et al (2015) 87 |
| Hyperperfusion (left parahippocampal gyrus, bilateral fusiform gyri, left middle temporal gyrus) | ASL | Idiopathic epilepsy | Chen et al (2016) 90 |
| Dysregulated diffusion | |||
| Decreased diffusion around seizure foci | DWI | Focal status epilepticus | Koepp et al (2010) 10 |
| Increased diffusion in underlying white matter | |||
Abbreviations: ASL‐MRI, Arterial spin labelling magnetic resonance imaging; CT, computed tomography; DWI, diffusion‐weighted imaging; MR, magnetic resonance; PWI, pefusion‐weighted imaging; SPECT, single photon emission computed tomography.
7.1.1. SPECT
Neuroimaging studies have also identified significant changes in cerebral blood flow during the postictal state. 10 , 84 , 85 In one study, ictal hyperperfusion was followed by postictal hypoperfusion (T1). 10 Ictal hyperperfusion has been related to increased glucose and oxygen demand, 80 which sometimes also manifested postictally, leading to contradicting findings. 86 In a patient with epilepsy after encephalitis and postictal psychosis, hyperperfusion in the right temporal lobe and left basal ganglia manifested, indicating the possible relevance of increased cerebral blood flow in postictal psychosis (T2). 87
7.1.2. DWI
With diffusion‐weighted imaging (DWI), decreased diffusion in gray and increased diffusion in white matter was found in focal status epilepticus patients, presumably reflecting cell swelling of cortical neurons at the seizure foci (T2). 10 In a case study, decreased diffusion was located around seizure foci in the gray matter, with facilitated diffusion in the underlying white matter. 10 Another study in patients with epilepsy showed postictal decreased cerebral blood flow in regions as hippocampus, parahippocampal gyrus, and cortex. 10
7.1.3. ASL–MRI
Arterial spin labeling (ASL)–perfusion MRI provides reliable information about postictal brain perfusion and may serve as a reliable tool to identify seizure‐onset zones prior to surgery. 85 , 88 Increased ipsilateral blood flow relative to seizure onset may be found immediately after seizures terminate, which drops below baseline levels up to 1 hour after seizure termination (T1; Figure 4). 10 , 88 Another study showed that perfusion decreased postictally in the hippocampus but in turn showed a reversed (hyper‐)perfusion pattern in the parahippocampal gyrus, probably reflecting increased metabolism to restore neuronal excitability (T1‐T2). 89 In a recent study using ASL‐MRI in 21 patients with idiopathic generalized epilepsy, increased cerebral blood flow in the left parahippocampal gyrus, bilateral fusiform gyri, and left middle temporal gyrus was observed postictally, highlighting cortical hemodynamic abnormality (T3). 90 In 21 TLE patients, using ASL, postictal hypoperfusion in the lateral temporal lobe manifested prominently, while sparing the mesial temporal lobe (T2). 85 Two patients showed postictal hyperperfusion, which might be explained by the delayed scanning time and complexity of brain network distortion. 85
FIGURE 4.
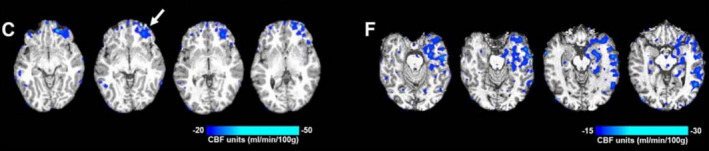
Subtraction cerebral blood flow (CBF) maps taken with arterial spin labelling magnetic resonance imaging (ASL‐MRI) in a patient with frontal lobe epilepsy (C) and temporal lobe epilepsy (F). The four CBF maps show the transition from interictal to postictal state. Both patients show left hypoperfusion relative to seizure onset (left frontal with >20 mL/100 g/min, and left temporal with >15 mL/100 g/min, respectively). Figure taken from Farrell et al 88
7.2. Biochemistry
Increased blood levels of ammonia have been observed in patients with epilepsy who did not regain complete consciousness after seizure termination (T1‐T2). 91 However, the level of consciousness was not recorded during the postictal state continuously but initially after the seizure and after 1 to 2 hours. Whether the distinction in ammonia levels can be explained by regaining consciousness or prolonged postictal impairment is unclear, as no postictal measurements were performed. It seems that hyperammonemia can occur in generalized tonic‐clonic but not focal seizures. 13
Similarly, increased levels of the hormone prolactin in serum have been observed after seizures, which may be due to disruption of hypothalamic function (T1). 92 However, baseline prolactin levels are ambiguous, as baseline levels are influenced by a multitude of factors (ie, sex, type of epilepsy, stress). 13 Seizures may also influence the release of gonadotropin‐releasing hormone that in turn regulates gonadal sex hormones. 92 Increased levels of serum creatine kinase have been found in patients with focal and tonic‐clonic seizures (T3). 93 Literature on cerebrospinal fluid lactate is scarce, but shows indication of postictal increases across seizure types. 13 Most likely, all these biochemical changes seem to be epiphenomena, and are probably not involved in termination of seizures or neuronal dysfunction in the postictal state. Studies on postictal biochemistry are scarce and mostly inconclusive.
8. PATHOPHYSIOLOGICAL MECHANISMS
The processes that are involved in the transition from the ictal to the interictal state are only partially understood. Many candidate biophysical mechanisms may terminate seizures and initiate the postictal state, summarized in Figure 5 and Table 3. 1 , 10 , 94 , 95 T1‐T3 (Figure 1) codes are used to point at the postictal time period in which mechanisms are supposed to occur.
FIGURE 5.
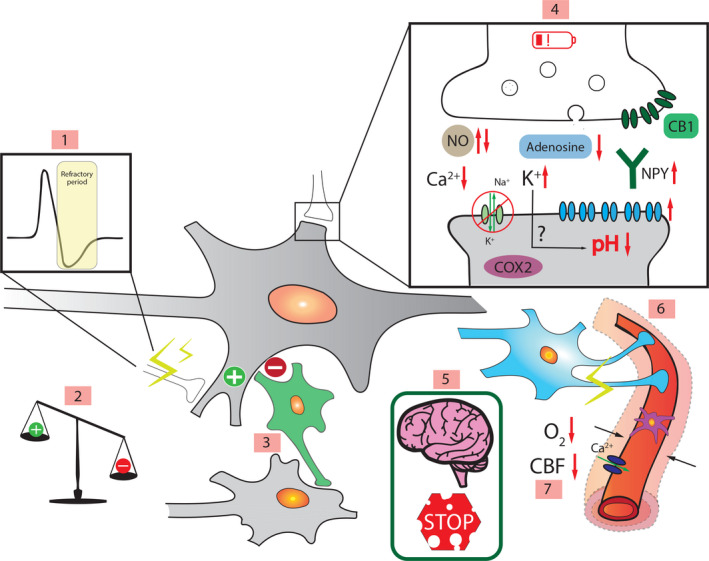
Schematic representation of pathophysiological mechanisms proposed to contribute to the postictal state. 1. Active inhibition. 2. Excitation‐inhibition imbalance. 3. Inhibitory interneurons (green). 4. Intra‐ and extracellular mechanisms: neuronal exhaustion (red battery), neurotransmitter depletion, increased extracellular potassium, decreased extracellular calcium, endocannabinoids binding to CB1 receptors 
 , increased/decreased nitric oxide (NO), cyclooxygenase 2 (COX2) activity, lower pH, lower adenosine, Na+/K+‐ATPase pump
, increased/decreased nitric oxide (NO), cyclooxygenase 2 (COX2) activity, lower pH, lower adenosine, Na+/K+‐ATPase pump  , neuropeptide Y (NPY), increased opioid receptors
, neuropeptide Y (NPY), increased opioid receptors  . 5. Blood‐brain barrier damage. 6. Neurovascular decoupling (purple pericyte, blue astrocyte). 7. Hypoperfusion/hypoxia (L‐type calcium channel
. 5. Blood‐brain barrier damage. 6. Neurovascular decoupling (purple pericyte, blue astrocyte). 7. Hypoperfusion/hypoxia (L‐type calcium channel  ).
).  represents excitation,
represents excitation,  inhibition. Black arrows indicate a direct influence or vasoconstriction, red arrows indicate an increase or decrease in activity or concentration, and green arrows show influx/efflux. Abbreviations: CBF (cerebral blood flow), CB1 and CB2 (cannabinoid receptor 1 and 2 as representation of endocannabinoids)
inhibition. Black arrows indicate a direct influence or vasoconstriction, red arrows indicate an increase or decrease in activity or concentration, and green arrows show influx/efflux. Abbreviations: CBF (cerebral blood flow), CB1 and CB2 (cannabinoid receptor 1 and 2 as representation of endocannabinoids)
TABLE 3.
Overview of proposed pathophysiological mechanisms involved in the postictal state
| Mechanism | Likelihood a | Reference |
|---|---|---|
| Neuronal exhaustion | Dismissed | Fisher & Schachter (2000) 1 |
| Excitation‐inhibition imbalance | Dismissed | Koepp et al (2010) 10 |
| Changes in opiate receptors | Unlikely |
Fisher & Schachter (2000) 1 ; Kanner et al (2010) 5 ; Theodore (2010) 29 |
| Neurotransmitter depletion | Probable | Fisher & Schachter (2000) 1 |
| Neuronal (active) inhibition | Probable |
Löscher & Köhling (2010) 100 ; Rupprecht et al (2010) 86 |
| Increased extracellular potassium | Probable | Fisher & Schachter (2000) 1 |
| Decreased extracellular calcium and intracellular acidification | Probable | Lado & Moshé (2008) 99 |
| Changes in adenosine and nitric oxide concentrations | Probable | Fisher & Schachter (2000) 1 |
| Neurovascular decoupling | Probable | Kovács et al (2018) 107 |
| Dysfunction of inhibitory interneurons | Probable | Kann et al (2014) 108 ; Englot et al (2010) 11 |
| Blood‐brain barrier dysfunction and inflammation | Probable | Gorter et al (2019) 109 |
| Hypoperfusion and hypoxia | Likely |
Farrell et al (2016) 88 ; Farrell et al (2017) 22 |
Likelihood in this table describes the probability of a mechanism being likely involved in the postictal state based on literature review. Dismissed means that a theory has been proven unlikely from experimental evidence, or lack of direct evidence. A mechanism is referred to as unlikely if there is no evidence in humans but only in animals. Probable means that a certain mechanism is likely to a certain extent, underscored by experimental evidence, but needs to be proven in future studies as there may be contradicting findings. Likely will describe a mechanism that has strong experimental evidence in support of this hypothesis.
8.1. Neuronal exhaustion (T1)
Almost two decades ago, neuronal exhaustion was dismissed as a candidate mechanism for seizure termination and the beginning of the postictal state, since neurons preserved their ability to generate action potentials after repeated intracellular stimulation at the start of the postictal state. 1
8.2. Neurotransmitter depletion (T1)
Direct evidence for neurotransmitter depletion is lacking. 1 However, vagus nerve stimulation seems to be effective in treating postictal symptoms. It may be speculated that vagus nerve stimulation increases arousal via the locus coeruleus, reducing postictal drowsiness. 96 , 97 This also suggests that neurotransmitter pathways are still intact if they are amenable to stimulation. 98 In addition, glutamate depletion might occur during seizures, which may continue into the interictal or postictal state. 99
8.3. Active inhibition and changes in ion homeostasis (T1)
Postictal symptoms may also result from active inhibition of neuronal function. 1 This hypothesis is based on the observed association between postictal refractoriness and increased seizure thresholds. 100 Postictal refractoriness may result from selective neuronal hyperpolarization that inhibits activity for several minutes or longer. 1 Shunting inhibition may be involved in this process by reducing effective neuronal coupling. 99 Decreased extracellular calcium may be involved in inhibiting synaptic transmission. 99 Network inhibition of subcortical structures may be involved in impaired consciousness after temporal lobe seizures by sending inhibitory output to neocortical neurons. 7 , 11 Another candidate mechanism is an increased concentration of extracellular potassium following seizures that may cause a depolarization block and suppression of neural activity. 1 Other mechanisms involved in the postictal state may be important for seizure termination. Intracellular acidification (lower pH), supposedly resulting from increased extracellular potassium, seems to aid in seizure termination by reducing excitability. 99 Endocannabinoids activate CB1 receptors on presynaptic receptors, resulting in a reduction of neurotransmitter release. 101 Activation of receptors on excitatory presynaptic terminals will result in a decrease of glutamate release. 99 , 102 , 103 Neuropeptide Y has endogenous anticonvulsant effects, with high postictal expression levels hours after seizures.
8.4. Opioid receptors (T2)
Another, more controversial, candidate mechanism relates to opiates and opioid receptors in the postictal state. 1 Increased levels of opioid receptors have been established after seizures, which last for several hours. 5 , 29 An opiate antagonist was shown to reverse unconsciousness after seizures in rats. In contrast, however, no change in seizure duration was discovered after administering an opiate antagonist following electroconvulsive therapy in depressed patients, questioning its role in the human postictal state. 1
8.5. Adenosine and nitric oxide (T1)
Adenosine and nitric oxide also seem to play a role in the pathophysiology of the postictal state. Increased levels of adenosine were observed in vivo during and up to 18 minutes after seizures, indicating a potential role in terminating seizures and postictal refractoriness. 104 Nitric oxide may be involved in regulating postictal cerebral blood flow. 1
8.6. Hypoperfusion and hypoxia (T2)
Recently, Farrell and colleagues showed in a study in rodents and humans that postictal behavioral symptoms result from hypoperfusion and hypoxia. 22 , 88 After both spontaneous and electrically induced seizures in rats, local blood flow and brain tissue oxygen concentration in the hippocampus decreased dramatically for more than 1 hour, mediated by local vasoconstriction. 88 Hypoperfusion and hypoxia were positively correlated with seizure duration and severity, both in animals and humans. 88 Postictal perfusion with ASL‐MRI in epilepsy patients with focal seizures also showed a decreased cerebral blood flow in the affected region. In agreement with this mechanism, caffeine, which is a well‐known vasoconstrictor, has been shown to aggravate postictal hypoxia in rodents. 105 This mechanism possibly acts via antagonistic effects on adenosine receptors.
Farrell and colleagues also propose that cyclooxygenase‐2 (COX‐2) and L‐type calcium channels are involved in the induction of postictal hypoperfusion and hypoxia. 22 They showed that by administering COX‐2 and L‐type calcium channel antagonists prior to seizure onset, postictal hypoperfusion and hypoxia were reduced in rodents. 22 , 88 These experimental findings provide new insights into the mechanisms that are related to postictal phenomena. 88
Contradicting Farrell's hypothesis, Prager et al support the view that neurovascular decoupling without hypoxia may be the main mechanism responsible for microvascular dysfunction in epilepsy. 106 They tested their hypothesis by investigating changes in capillary neurovascular coupling in hippocampal slices of rats during recurrent seizures induced by 4‐aminopyridine or low‐Mg2+ conditions. They observed, despite normoxic conditions, that neurovascular decoupling and blood‐brain barrier (BBB) dysfunction occurred, in small cortical arterioles, accompanied by perivascular cellular injury and pericyte dysfunction. Their results may exclude hypoxia as a mechanism involved in postictal hypoperfusion in epilepsy, as hypoxia may not be necessary for pericyte dysfunction. Kovács and colleagues also report that postictal phenomena may be related to hypometabolism that results from neurovascular decoupling following seizures, highlighting the role of neurovascular coupling. 107
Experimental differences between Prager et al and Farrell et al are of interest here: Prager et al used hippocampal slices to measure oxygen saturation, whereas Farrell et al implanted oxygen‐measuring probes in brains of freely moving rats. 88 , 106 Furthermore, Prager et al induced seizures with low Mg2+ or 4‐aminopyridine, whereas Farrell et al studied rats with spontaneous seizures or after kindling. These differences may have contributed to the discrepancy in findings, where the in vivo results of Farrell et al may provide stronger evidence than the in vitro results of Prager et al.
8.7. Neurovascular decoupling (T1‐T2)
Another possible mechanism is neurovascular decoupling, defined as a mismatch between neuronal energy demand (metabolism) and circulation (cerebral perfusion). 80 It remains unclear whether, and how, neurovascular decoupling takes place. During seizures, there is an increased metabolic demand, which ceases afterwards. 89 However, if seizures persist, as occurs in status epilepticus, there is a continuous demand for increased energy, which cannot be matched with the blood flow. 80 This progress leads to a decoupling of blood flow and metabolism, which in turn may result in hypoxia and glycolysis. The ensuing neuronal injury can be explained in terms of Na+/K+‐ATPase pump failure from an ATP deficiency, but may also result from glutamate release and inflammatory responses. 80
In line with the hypothesis that hypoxia may exist in the postictal state is the selective sensitivity of inhibitory interneurons to hypoxia. 108 These building blocks of cortical networks are responsible for organized gamma activity patterns (30‐100 Hz), associated with cognition and memory, and known to show extreme sensitivity to oxidative and metabolic stress. 108 Abnormal activity of interneurons may compromise information processing and may explain cognitive symptoms in a postictal hypoxic state. 108
8.8. Blood‐brain barrier (BBB) and perivascular inflammation (T1)
BBB dysfunction and subsequent perivascular inflammation after traumatic brain injury or stroke has been suggested to be involved in epileptogenesis. 106 , 109 MRI detected perivascular neuroinflammation and BBB disruption in animals as well as in patients with epilepsy. 109 BBB disruption after seizures may be involved in the postictal state on short timescales but this is presently unknown.
9. TREATMENT OPTIONS
Current treatment of the postictal state consists primarily of symptom suppression and prevention of complications (Table 4). 2
TABLE 4.
Treatment strategies targeting postictal symptoms
| Treatment strategy | Current status of development | Reference |
|---|---|---|
| Vasoconstrictive‐mediating drugs | ||
| Paracetamol | Animals | Farrell et al (2016) 88 |
| Celecoxib | ||
| Nimodipine | Human ongoing trial | NCT04028596 a , 111 |
| Oxygen treatment | Hypothesis, not yet tested | Asadollahi et al (2018) 49 |
| Psychotropic medication | ||
| Quetiapine | Humans, current treatment option | Krauss & Theodore et al (2010) 2 |
| Haloperidol | ||
| Lorazepam | ||
| Midazolam | Ohira et al (2019) 75 | |
| Antiepileptic drugs | ||
| Levetiracetam | Human trials | Schmidt (2010) 70 |
| Vagus nerve stimulation | Human trials | Vonck et al (2010) 98 |
| Drugs targeting BBBb dysfunction | Hypothesis not yet tested | Gorter et al (2019) 109 |
Abbreviations: BBB, blood‐brain barrier.
ClinicalTrials.gov ID.
9.1. Symptom suppression
Antiepileptic drugs (AEDs) may attenuate or shorten the postictal state; however, only one study found levetiracetam to be effective. 70 AEDs can alleviate postictal psychotic symptoms, but in turn may have anxiety‐ and depressive mood–promoting side effects. 2 A few case reports suggest that vagus nerve stimulation has a positive effect on the duration and symptoms of the postictal phase, independent of the effects of seizure frequency. 98
9.2. Prevention of complications
Administration of oxygen in the postictal state may counteract hypoxia. 49 Paracetamol can limit postictal headaches. 19 For most patients with mild and short‐lasting postictal delirium, no specific treatment is needed. However, if delirium is prolonged into postictal delirium or postictal psychosis, patients can be treated with antipsychotic drugs, (eg, quetiapine, haloperidol) or benzodiazepines (eg, midazolam, lorazepam), especially in cases of uncontrolled behavior and/or severe agitation. 2 , 75 These interventions are acceptable in these circumstances, even though some antipsychotic drugs carry an additional risk of seizure induction. 110
9.3. Future developments
If hypoxia caused by vasoconstriction, mediated via COX‐2, is a prominent pathophysiological mechanism, as was shown in animal studies, 88 COX‐2 inhibitors and calcium antagonists are candidate drugs to reduce the duration and intensity of the postictal state. This is currently investigated in a clinical trial with randomized cross‐over design, using electroconvulsive therapy (ECT)–induced seizures as a human model to study the postictal state (Figure 3). 111
10. CONCLUSION
In this narrative literature review, a comprehensive overview of the postictal state is presented regarding its clinical manifestations, EEG characteristics, pathophysiological mechanisms, and treatment options. Several key outstanding questions need to be answered. Which metrics can best quantify the postictal state? Which pathophysiological mechanisms contribute most to postictal symptoms? Are there multiple mechanisms interacting, or is there one mechanism responsible for a variety of symptoms? How can we best treat the postictal state?
We propose to define the postictal state as: “a temporary brain condition following seizures (a) manifesting neurological deficits and/or psychiatric symptoms, (b) often accompanied by EEG slowing or suppression, (c) lasting minutes to days.”
Clinical assessment of the postictal state is not always reliable. Arterial spin labeling and EEG can assist in objective assessment of postictal hypoperfusion or suppression of neuronal activity. Furthermore, assessment of hypoperfusion in the postictal state may serve as a reliable tool to identify the seizure‐onset zone.
Postictal symptoms and their duration are highly variable and affect multiple brain areas. Several pathophysiological mechanisms may be involved. Postictal hypoxia, resulting from vasoconstriction or spreading depression are novel candidate mechanisms, likely involved in SUDEP, too. Whether treatment of presumed postictal vasoconstriction has clinical benefit is currently studied in the SYNAPSE study. 111
CONFLICT OF INTEREST
None of the authors have any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
We thank the anonymous reviewers for their helpful suggestions and comments. Julia CM Pottkämper has received support from the Dutch National Epilepsy Fund (grant number: 309‐1611).
Pottkämper JCM, Hofmeijer J, van Waarde JA, van Putten MJAM. The postictal state—What do we know?. Epilepsia. 2020;61:1045–1061. 10.1111/epi.16519
REFERENCES
- 1. Fisher RS, Schachter SC. The postictal state: a neglected entity in the management of epilepsy. Epilepsy Behav. 2000;1:52–9. [DOI] [PubMed] [Google Scholar]
- 2. Krauss G, Theodore WH. Treatment strategies in the postictal state. Epilepsy Behav. 2010;19:188–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rémi J, Noachtar S. Clinical features of the postictal state: correlation with seizure variables. Epilepsy Behav. 2010;19:114–7. [DOI] [PubMed] [Google Scholar]
- 4. Subota A, Khan S, Josephson CB, Manji S, Lukmanji S, Roach P, et al. Signs and symptoms of the postictal period in epilepsy: a systematic review and meta‐analysis. Epilepsy Behav. 2019;94:243–51. [DOI] [PubMed] [Google Scholar]
- 5. Kanner AM, Trimble M, Schmitz B. Postictal affective episodes. Epilepsy Behav. 2010;19:156–8. [DOI] [PubMed] [Google Scholar]
- 6. Fisher RS, Engel JJ Jr. Definition of the postictal state: when does it start and end? Epilepsy Behav. 2010;19:100–4. [DOI] [PubMed] [Google Scholar]
- 7. Yang L, Worrell GA, Nelson C, Brinkmann B, He B. Spectral and spatial shifts of post‐ictal slow waves in temporal lobe seizures. Brain. 2012;135:3134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fisher RS, Nune G, Roberts SE, Cramer JA. The personal impact of epilepsy scale (PIES). Epilepsy Behav. 2015;42:140–6. [DOI] [PubMed] [Google Scholar]
- 9. Todd RB. On the pathology and treatment of convulsive diseases. Lond Med Gaz. 1849;8:661–71; 724–729; 766–772; 815–822; 837–846. [Google Scholar]
- 10. Koepp MJ, Diehl B, Woermann FG. Functional neuroimaging in the postictal state. Epilepsy Behav. 2010;19:127–30. [DOI] [PubMed] [Google Scholar]
- 11. Englot DJ, Yang LI, Hamid H, Danielson N, Bai X, Marfeo A, et al. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 2010;133:3764–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shorvon S, Trinka E. Nonconvulsive status epilepticus and the postictal state. Epilepsy Behav. 2010;19:172–5. [DOI] [PubMed] [Google Scholar]
- 13. Doudoux H, Fournier M, Vercueil L. Postictal syndrome: The forgotten continent. An overview of the clinical, biochemical and imaging features. Rev Neurol (Paris). 2020;176(1‐2):62–74. [DOI] [PubMed] [Google Scholar]
- 14. Kanner AM, Soto A, Gross‐Kanner H. Prevalence and clinical characteristics of postictal psychiatric symptoms in partial epilepsy. Neurology. 2004;62:708–13. [DOI] [PubMed] [Google Scholar]
- 15. Janszky J, Fogarasi A, Toth V, Magalova V, Gyimesi C, Kovacs N, et al. Peri‐ictal vegetative symptoms in temporal lobe epilepsy. Epilepsy Behav. 2007;11:125–9. [DOI] [PubMed] [Google Scholar]
- 16. Simon RP. Heart and lung in the postictal state. Epilepsy Behav. 2010;19:167–71. [DOI] [PubMed] [Google Scholar]
- 17. Busl KM, Bleck TP. Neurogenic pulmonary edema. Crit Care Med. 2015;43:1710–5. [DOI] [PubMed] [Google Scholar]
- 18. Baker GA, Smith DF, Jacoby A, Hayes JA, Chadwick DW. Liverpool seizure severity scale revisited. Seizure. 1998;7:201–5. [DOI] [PubMed] [Google Scholar]
- 19. Ekstein D, Schachter SC. Postictal headache. Epilepsy Behav. 2010;19:151–5. [DOI] [PubMed] [Google Scholar]
- 20. Josephson CB, Jetté N. Psychiatric comorbidities in epilepsy. Int Rev Psychiatry. 2017;29:409–24. [DOI] [PubMed] [Google Scholar]
- 21. Nishida T, Kudo T, Inoue Y, Nakamura F, Yoshimura M, Matsuda K, et al. Postictal mania versus postictal psychosis: differences in clinical features, epileptogenic zone, and brain functional changes during postictal period. Epilepsia. 2006;47:2104–14. [DOI] [PubMed] [Google Scholar]
- 22. Farrell JS, Colangeli R, Wolff MD, Wall AK, Phillips TJ, George A, et al. Postictal hypoperfusion/hypoxia provides the foundation for a unified theory of seizure‐induced brain abnormalities and behavioral dysfunction. Epilepsia. 2017;58:1493–501. [DOI] [PubMed] [Google Scholar]
- 23. Jung S, Mattle HP. Is it a vascular event and where is the lesion? warlow's stroke: practical management. Hoboken, NJ: Wiley‐Blackwell; 2019;4202368. [Google Scholar]
- 24. Brosinski CM. Implementing diagnostic reasoning to differentiate Todd's paralysis from acute ischemic stroke. Adv Emerg Nurs J. 2014;36:78–86. [DOI] [PubMed] [Google Scholar]
- 25. Werhahn KJ. Weakness and focal sensory deficits in the postictal state. Epilepsy Behav. 2010;19:138–9. [DOI] [PubMed] [Google Scholar]
- 26. Rolak LA, Rutecki P, Ashizawa T, Harati Y. Clinical features of Todd's post‐epileptic paralysis. J Neurol Neurosurg Psychiatry. 1992;55:63–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hadjikoutis S, Sawhney I. Occipital seizures presenting with bilateral visual loss. Neurol India. 2003;51:115. [PubMed] [Google Scholar]
- 28. Caraballo R, Koutroumanidis M, Panayiotopoulos CP, Fejerman N. Idiopathic childhood occipital epilepsy of Gastaut: a review and differentiation from migraine and other epilepsies. J Child Neurol. 2009;24:1536–42. [DOI] [PubMed] [Google Scholar]
- 29. Theodore WH. The postictal state: effects of age and underlying brain dysfunction. Epilepsy Behav. 2010;19:118–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rots ML, De Vos CC, Smeets‐Schouten JS, Portier R, van Putten M. Suppressors of interictal discharges in idiopathic childhood occipital epilepsy of Gastaut. Epilepsy Behav. 2012;25:189–91. [DOI] [PubMed] [Google Scholar]
- 31. Mohamed W, Ahuja N, Shah A. Palinacousis—evidence to suggest a post‐ictal phenomenon. J Neurol Sci. 2012;317:6–12. [DOI] [PubMed] [Google Scholar]
- 32. Privitera M, Kim KK. Postictal language function. Epilepsy Behav. 2010;19:140–5. [DOI] [PubMed] [Google Scholar]
- 33. Bazil CW. Effects of sleep on the postictal state. Epilepsy Behav. 2010;19:146–50. [DOI] [PubMed] [Google Scholar]
- 34. Trotti LM, Bliwise DL. Sleep apnea as a transient, post‐ictal event: report of a case. Epilepsy Res. 2009;85:325–8. [DOI] [PubMed] [Google Scholar]
- 35. Ito M. Neuropsychiatric evaluations of postictal behavioral changes. Epilepsy Behav. 2010;19:134–7. [DOI] [PubMed] [Google Scholar]
- 36. Reti IM, Krishnan A, Podlisky A, Sharp A, Melinda W, Neufeld KJ, et al. Predictors of electroconvulsive therapy postictal delirium. Psychosomatics. 2014;55:272–9. [DOI] [PubMed] [Google Scholar]
- 37. Oueslati B, Fekih‐Romdhane F, Ridha R. Postictal delirium and violent behavior in patients with post‐neurosurgical epilepsy. World Neurosurg. 2018;115:193–5. [DOI] [PubMed] [Google Scholar]
- 38. Kanemoto K, Tadokoro Y, Oshima T. Violence and postictal psychosis: a comparison of postictal psychosis, interictal psychosis, and postictal confusion. Epilepsy Behav. 2010;19:162–6. [DOI] [PubMed] [Google Scholar]
- 39. Clancy MJ, Clarke MC, Connor DJ, Cannon M, Cotter DR. The prevalence of psychosis in epilepsy; a systematic review and meta‐analysis. BMC Psychiatry. 2014;14:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hilger E, Zimprich F, Pataraia E, Aull‐Watschinger S, Jung R, Baumgartner C, et al. Psychoses in epilepsy: a comparison of postictal and interictal psychoses. Epilepsy Behav. 2016;60:58–62. [DOI] [PubMed] [Google Scholar]
- 41. Trimble M, Kanner A, Schmitz B. Postictal psychosis. Epilepsy Behav. 2010;19:159–61. [DOI] [PubMed] [Google Scholar]
- 42. Logsdail S, Toone B. Post‐ictal psychoses: a clinical and phenomenological description. Br J Psychiatry. 1988;152:246–52. [DOI] [PubMed] [Google Scholar]
- 43. Kanemoto K, Kawasaki J, Kawai I. Postictal psychosis: a comparison with acute interictal and chronic psychoses. Epilepsia. 1996;37:551–6. [DOI] [PubMed] [Google Scholar]
- 44. Gil YE, Choi JY, Kim TJ, Park SA, Huh K. Post‐ictal Cotard delusion in focal epilepsy patients. Seizure. 2019;71:80–2. [DOI] [PubMed] [Google Scholar]
- 45. Cavanna AE. Ictal (and Postictal) Psychiatric Disorders. Motion and Emotion. New York, NY: Springer; 2018. p. 141–50. [Google Scholar]
- 46. Devinsky O. Postictal psychosis: common, dangerous, and treatable. Epilepsy Curr. 2008;8:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sahaya K, Lardizabal D. Catatonia in encephalitis and nonconvulsive seizures: a case report and review of the literature. Epilepsy Behav. 2010;17:420–5. [DOI] [PubMed] [Google Scholar]
- 48. Primavera A, Fonti A, Novello P, Roccatagliata G, Cocito L. Epileptic seizures in patients with acute catatonic syndrome. J Neurol Neurosurg Psychiatry. 1994;57:1419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Asadollahi M, Noorbakhsh M, Simani L, Ramezani M, Gharagozli K. Two predictors of postictal generalized EEG suppression: Tonic phase duration and postictal immobility period. Seizure. 2018;61:135–8. [DOI] [PubMed] [Google Scholar]
- 50. Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, Langan Y, et al. Combined analysis of risk factors for SUDEP. Epilepsia. 2011;52:1150–9. [DOI] [PubMed] [Google Scholar]
- 51. Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12:966–77. [DOI] [PubMed] [Google Scholar]
- 52. Jaychandran R, Chaitanya G, Satishchandra P, Bharath RD, Thennarasu K, Sinha S. Monitoring peri‐ictal changes in heart rate variability, oxygen saturation and blood pressure in epilepsy monitoring unit. Epilepsy Res. 2016;125:10–8. [DOI] [PubMed] [Google Scholar]
- 53. Park KJ, Sharma G, Kennedy JD, Seyal M. Potentially high‐risk cardiac arrhythmias with focal to bilateral tonic–clonic seizures and generalized tonic–clonic seizures are associated with the duration of periictal hypoxemia. Epilepsia. 2017;58:2164–71. [DOI] [PubMed] [Google Scholar]
- 54. Rheims S, Alvarez BM, Alexandre V, Curot J, Maillard L, Bartolomei F, et al. Hypoxemia following generalized convulsive seizures: risk factors and effect of oxygen therapy. Neurology. 2019;92:e183–93. [DOI] [PubMed] [Google Scholar]
- 55. Vilella L, Lacuey N, Hampson JP, Rani MRS, Loparo K, Sainju RK, et al. Incidence recurrence, and risk factors for peri‐ictal central apnea and sudden unexpected death in epilepsy. Front Neurol. 2019;10:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kang JY, Rabiei AH, Myint L, Nei M. Equivocal significance of post‐ictal generalized EEG suppression as a marker of SUDEP risk. Seizure. 2017;48:28–32. [DOI] [PubMed] [Google Scholar]
- 57. Lamberts RJ, Gaitatzis A, Sander JW, Elger CE, Surges R, Thijs RD. Postictal generalized EEG suppression: an inconsistent finding in people with multiple seizures. Neurology. 2013;81:1252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci Transl Med. 2015;7:282ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cain SM, Bohnet B, LeDue J, Yung AC, Garcia E, Tyson JR, et al. In vivo imaging reveals that pregabalin inhibits cortical spreading depression and propagation to subcortical brain structures. Proc Natl Acad Sci. 2017;114:2401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schmidt D, Noachtar S. Outlook: The postictal state—future directions for research. Epilepsy Behav. 2010;19:191–2. [DOI] [PubMed] [Google Scholar]
- 61. Jennum P, Pickering L, Christensen J, Ibsen R, Kjellberg J. Morbidity and mortality of childhood‐and adolescent‐onset epilepsy: a controlled national study. Epilepsy Behav. 2017;66:80–5. [DOI] [PubMed] [Google Scholar]
- 62. Beghi E, Giussani G. Aging and the epidemiology of epilepsy. Neuroepidemiology. 2018;51:216–23. [DOI] [PubMed] [Google Scholar]
- 63. Yuen AW, Keezer MR, Sander JW. Epilepsy is a neurological and a systemic disorder. Epilepsy Behav. 2018;78:57–61. [DOI] [PubMed] [Google Scholar]
- 64. Watila MM, Balarabe SA, Ojo O, Keezer MR, Sander JW. Overall and cause‐specific premature mortality in epilepsy: a systematic review. Epilepsy Behav. 2018;87:213–25. [DOI] [PubMed] [Google Scholar]
- 65. Josephson CB, Engbers JDT, Sajobi TT, Jette N, Agha‐Khani Y, Federico P, et al. An investigation into the psychosocial effects of the postictal state. Neurology. 2016;86:723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. So NK, Blume WT. The postictal EEG. Epilepsy Behav. 2010;19:121–6. [DOI] [PubMed] [Google Scholar]
- 67. Rajakulendran S, Nashef L. Postictal generalized EEG suppression and SUDEP: a review. J Clin Neurophysiol. 2015;32:14–20. [DOI] [PubMed] [Google Scholar]
- 68. Bateman LM, Mendiratta A, Liou J‐Y, Smith EJ, Bazil CW, Choi H, et al. Postictal clinical and electroencephalographic activity following intracranially recorded bilateral tonic‐clonic seizures. Epilepsia. 2019;60:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Seyal M, Bateman LM, Li CS. Impact of periictal interventions on respiratory dysfunction, postictal EEG suppression, and postictal immobility. Epilepsia. 2013;54:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schmidt D. Effect of antiepileptic drugs on the postictal state. A critical overview. Epilepsy Behav. 2010;19:176–81. [DOI] [PubMed] [Google Scholar]
- 71. Blumenfeld H. Cellular and network mechanisms of spike‐wave seizures. Epilepsia. 2005;46:21–33. [DOI] [PubMed] [Google Scholar]
- 72. Farrell JS, Greba Q, Snutch TP, Howland JG, Teskey GC. Fast oxygen dynamics as a potential biomarker for epilepsy. Sci Rep. 2018;8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Theodore WH, Porter RJ, Penry JK. Complex partial seizures: clinical characteristics and differential diagnosis. Neurology. 1983;33:1115–21. [DOI] [PubMed] [Google Scholar]
- 74. Kaibara M, Blume WT. The postictal electroencephalogram. Electroencephalogr Clin Neurophysiol. 1988;70:99–104. [DOI] [PubMed] [Google Scholar]
- 75. Ohira J, Yoshimura H, Morimoto T, Ariyoshi K, Kohara N. Factors associated with the duration of the postictal state after a generalized convulsion. Seizure. 2019;65:101–5. [DOI] [PubMed] [Google Scholar]
- 76. Arkilo D, Wang S, Thiele EA. Time interval required for return to baseline of the background rhythm on electroencephalogram after recorded electrographic seizures. Epilepsy Res. 2013;106:288–91. [DOI] [PubMed] [Google Scholar]
- 77. Fisher RS, Scharfman HE, deCurtis M. How can we identify ictal and interictal abnormal activity? Adv Exp Med Biol. 2014;813:3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–30. [DOI] [PubMed] [Google Scholar]
- 79. Leitinger M, Beniczky S, Rohracher A, Gardella E, Kalss G, Qerama E, et al. Salzburg consensus criteria for non‐convulsive status epilepticus–approach to clinical application. Epilepsy Behav. 2015;49:158–63. [DOI] [PubMed] [Google Scholar]
- 80. Meletti S, Monti G, Mirandola L, Vaudano AE, Giovannini G. Neuroimaging of status epilepticus. Epilepsia. 2018;59:113–9. [DOI] [PubMed] [Google Scholar]
- 81. Rodríguez V, Rodden MF, LaRoche SM. Ictal–interictal continuum: a proposed treatment algorithm. Clin Neurophysiol. 2016;127:2056–64. [DOI] [PubMed] [Google Scholar]
- 82. Olejniczak PW, Mader E, Butterbaugh G, Fisch BJ, Carey M. Postictal EEG suppression and hippocampal atrophy in temporal lobe epilepsy. J Clin Neurophysiol. 2001;18:2–8. [DOI] [PubMed] [Google Scholar]
- 83. Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, et al. Cortical and subcortical networks in human secondarily generalized tonic‐clonic seizures. Brain. 2009;132:999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li E, d’Esterre CD, Lee T‐Y, Menon B, Federico P. Quantitatively detecting postictal hypoperfusion in patients with focal epilepsy using CT perfusion: Determining cross‐modality comparisons and electrode artifacts. J Neurosci Methods. 2019;314:13–20. [DOI] [PubMed] [Google Scholar]
- 85. Gaxiola‐Valdez I, Singh S, Perera T, Sandy S, Li E, Federico P. Seizure onset zone localization using postictal hypoperfusion detected by arterial spin labelling MRI. Brain. 2017;140:2895–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rupprecht S, Schwab M, Fitzek C, Witte OW, Terborg C, Hagemann G. Hemispheric hypoperfusion in postictal paresis mimics early brain ischemia. Epilepsy Res. 2010;89:355–9. [DOI] [PubMed] [Google Scholar]
- 87. Yasumoto S, Motooka H, Ito Y, Uchimura N. A change in electrographic activity and blood flow during interictal and postictal psychotic states in a patient with epilepsy. Epilepsy Behav Case Rep. 2015;4:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Farrell JS, Gaxiola‐Valdez I, Wolff MD, David LS, Dika HI, Geeraert BL, et al. Postictal behavioural impairments are due to a severe prolonged hypoperfusion/hypoxia event that is COX‐2 dependent. Elife. 2016;5:e19352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Leonhardt G, de Greiff A, Weber J, Ludwig T, Wiedemayer H, Forsting M, et al. Brain perfusion following single seizures. Epilepsia. 2005;46:1943–9. [DOI] [PubMed] [Google Scholar]
- 90. Chen G, Lei DU, Ren J, Zuo P, Suo X, Wang DJJ, et al. Patterns of postictal cerebral perfusion in idiopathic generalized epilepsy: a multi‐delay multi‐parametric arterial spin labelling perfusion MRI study. Sci Rep. 2016;6:28867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Liu K‐T, Yang S‐C, Yeh I‐J, Lin T‐J, Lee C‐W. Transient hyperammonemia associated with postictal state in generalized convulsion. Kaohsiung J Med Sci. 2011;27:453–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Luef G. Hormonal alterations following seizures. Epilepsy Behav. 2010;19:131–3. [DOI] [PubMed] [Google Scholar]
- 93. Chesson AL, Kasarskis EJ, Small VW. Postictal elevation of serum creatine kinase level. Arch Neurol. 1983;40:315–7. [DOI] [PubMed] [Google Scholar]
- 94. Kramer MA, Truccolo W, Eden UT, Lepage KQ, Hochberg LR, Eskandar EN, et al. Human seizures self‐terminate across spatial scales via a critical transition. Proc Natl Acad Sci U S A. 2012;109:21116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lopes da Silva FH, Blanes W, Kalitzin SN, Parra J, Suffczynski P, Velis DN. Dynamical diseases of brain systems: different routes to epileptic seizures. IEEE Trans Biomed Eng. 2003;50:540–8. [DOI] [PubMed] [Google Scholar]
- 96. Nasrin NMM, Alireza S. Vagal nerve stimulation for refractory epilepsy: a brief review. Neuropsychiatry. 2016;6:149–60. [Google Scholar]
- 97. Rizzo P, Beelke M, De Carli F, Canovaro P, Nobili L, Robert A, et al. Chronic vagus nerve stimulation improves alertness and reduces rapid eye movement sleep in patients affected by refractory epilepsy. Sleep. 2003;26:607–11. [DOI] [PubMed] [Google Scholar]
- 98. Vonck K, Raedt R, Boon P. Vagus nerve stimulation and the postictal state. Epilepsy Behav. 2010;19:182–5. [DOI] [PubMed] [Google Scholar]
- 99. Lado FA, Moshé SL. How do seizures stop? Epilepsia. 2008;49:1651–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Löscher W, Köhling R. Functional, metabolic, and synaptic changes after seizures as potential targets for antiepileptic therapy. Epilepsy Behav. 2010;19:105–13. [DOI] [PubMed] [Google Scholar]
- 101. Cachope R. Functional diversity on synaptic plasticity mediated by endocannabinoids. Phil. Trans. R. Soc. B. 2012;367:3242–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923. [DOI] [PubMed] [Google Scholar]
- 103. Marsicano G, Goodenough S, Monory K. CB1 cannabinoid receptors and on‐demand defense against excitotoxicity. Science. 2003;302:84–8. [DOI] [PubMed] [Google Scholar]
- 104. During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32:618–24. [DOI] [PubMed] [Google Scholar]
- 105. Phillips TJ, Gom RC, Wolff MD, Teskey GC. Caffeine Exacerbates Postictal Hypoxia. Neuroscience. 2019;422:32–43. [DOI] [PubMed] [Google Scholar]
- 106. Prager O, Kamintsky L, Hasam‐Henderson LA, Schoknecht K, Wuntke V, Papageorgiou I, et al. Seizure‐induced microvascular injury is associated with impaired neurovascular coupling and blood–brain barrier dysfunction. Epilepsia. 2019;60:322–36. [DOI] [PubMed] [Google Scholar]
- 107. Kovács R, Gerevich Z, Friedman A, Otáhal J, Prager O, Gabriel S, et al. Bioenergetic mechanisms of seizure control. Front Cell Neurosci. 2018;12:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kann O, Papageorgiou IE, Draguhn A. Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J Cereb Blood Flow Metab. 2014;34:1270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gorter JA, Aronica E, van Vliet EA. The roof is leaking and a storm is raging: repairing the blood‐brain barrier in the fight against epilepsy. Epilepsy Curr. 2019;19:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hedges D, Jeppson K, Whitehead P. Antipsychotic medication and seizures: a review. Drugs Today (Barc). 2003;39:551–7. [DOI] [PubMed] [Google Scholar]
- 111. Identifier NCT04028596, Measuring Blood Flow in the Brain After Epileptic Activity [database on the Internet]. ClinicalTrials.gov. 2019. Jul 8 [cited 2019 Oct 28]. Available from: https://clinicaltrials.gov/ct2/show/NCT04028596?term=NCT04028596&draw=2&rank=1.


