Abstract
To study the Chinese human milk N-glycome over lactation, N-glycans were released and separated from serum proteins, purified by solid-phase extraction, and analyzed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS). In total, 66 different putative N-glycans were found in the colostrum (week 1) and mature milk (week 4) of seven Chinese mothers. A clear difference was observed between milk of five secretor and two nonsecretor mothers, based on the type and relative amounts of the individual N-glycans. The relative levels of the total neutral nonfucosylated and the fucosylated N-glycans in milk of five secretor mothers increased and decreased over lactation, respectively. This pattern could not be observed for the milk from the two nonsecretor mothers. Overall, this was the first study that provided detailed information on individual N-glycans in milk among mothers and over time as well as that fucosylation of N-glycans in milk was associated with the mother’s secretor status.
Keywords: glycoproteins, oligosaccharides, glycosylation, intestinal mucosal barrier
Introduction
Human milk is the best nutrition for infants during the first 6 months of life1 and stimulates the maturation of the infant’s intestinal immune system.2 Human milk contains many biofunctional components, such as proteins and human milk oligosaccharides (HMOs).3 HMOs represent complex lactose-based glycans synthesized in the mammary gland during lactation, which reach the colon intact, and are able to stimulate the development of the bifidogenic flora.4,5 Human milk proteins among others play a pivotal role in protecting the infant’s gut mucosa against pathogens.6 It has been reported that 70% of the human milk proteins in number are glycosylated.7 These protein-bound glycans among others might affect the folding and stability of proteins and modulate neonatal immunity by altering host epithelial and immune cell responses in the infant’s gut.7−10
Caseins are divided into three main types (αS1, β-, and κ-casein) and are a valuable source of amino acids, phosphate, and calcium, used for the growth of the neonate.11 The proteins αS1- and β-casein in human milk do not have any glycosylated amino acid residues, while κ-casein has multiple O-glycosylation sites at various threonine residues.12 Serum proteins such as lactoferrin, immunoglobulins, serum albumin, and α-lactalbumin form the main portion of the glycoproteins present in human milk and mainly contain N-glycans.12 These glycans are attached to the amide nitrogen of an asparagine residue of the protein.13−16 There are also serum proteins that contain only O-glycans (e.g., osteopontin) or contain both O-glycans and N-glycans (e.g., bile salt-activated lipase). Osteopontin, lactoferrin, and immunoglobulins have direct bactericidal properties.12 In addition, many other glycosylated serum proteins can be found in human milk having a reported immune activity.12
N-glycans can be assembled with four types of neutral monosaccharides: fucose (Fuc), galactose (Gal), mannose (Man), N-acetylglucosamine (GlcNAc), N-acetylgalactosamine (GalNAc). N-glycans can also contain the sialic acidic structure N-acetylneuraminic acid (NeuAc).14−19 All identified N-glycans in human milk have a pentasaccharide as the common core, consisting of three Man and two GlcNAc residues.14−16 N-glycans can be classified into three types, namely, high-mannose, complex, and hybrid N-glycans.20−23 High-mannose N-glycans typically contain between two and six Man residues attached to the pentasaccharide core, whereas complex N-glycans can be elongated at the mannose residues by GlcNAc residues, which are often further decorated by Gal and GalNAc residues.20−23 Hybrid N-glycans are composed of a pentasaccharide core, with one branch of mannose residues and another branch with one or two GlcNAc residues.20−23
Complex and hybrid N-glycans have a large variety of structures, and fucosylation might affect their conformation and functional properties.19 To date, 13 different fucosyltransferases (FUTs) have been detected in the human genome.24 The FUT8 gene encodes for the α1,6-fucosyltransferase that transfers a Fuc residue to the innermost GlcNAc unit of N-glycan chains.24,25 Fucosylation of N-glycans also might partially depend on the FUT2 and FUT3 genes,24,25 which determine the mother’s secretor (Se) and Lewis (Le) status. The FUT2 Se gene determines the presence of α1,2-linked fucosylated glycans.26 The addition of Fuc residues by an α1,3/4-linkage on the antennae of GlcNAc might be regulated by FUT3 or other α1,3-genes (FUT4, 5, 6, 7, and 9).26 The fucosylated glycotypes on serum glycoproteins in milk from 43 healthy mothers were analyzed semiquantitatively by lectin-blotting, where three specific biotinylated lectins were able to recognize and differentiate among the α1,2-, α1,3-, and α1,6-Fuc linkages.27
The type and levels of N-glycans have been previously investigated in mature milk (weeks 12–16) of three mothers.14 From the total 52 N-glycans identified, 34 (65%) N-glycans were fucosylated.14 The relative levels of the fucosylated N-glycans covered >80% of the total N-glycan content.14 However, the type and level of individual N-glycans in milk of the individual mothers have not yet been investigated.14,27 More recently, the variation of N-glycans in human milk over lactation has been studied.16 In this latter study, milk from 10 mothers was collected and combined per lactation stage (colostrum, 3 days; transition milk, 9 days; and mature milk, 40 days).16 It was reported that levels of fucosylated N-glycans dropped from ca. 61% in colostrum to 37% in transition milk and then remained constant in mature milk.16 This current study set out to investigate the individual differences in type and levels of N-glycans in milk between mothers.
The main objective was to profile and compare N-glycans in colostrum (week 1) and mature milk (week 4) of seven Chinese mothers differing in secretor status using matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF-MS).
Material and Methods
Chemicals
Ammonium bicarbonate, sodium chloride, acetonitrile (MeCN), trifluoroacetic acid (TFA), and ethanol (EtOH) were purchased from Biosolve B.V. (Valkenswaard, The Netherlands). Water was filtered using the Milli-Q water purification system of Merck Millipore (Molsheim, France). Sodium dodecyl sulfate (SDS), 2-mercaptoethanol (2-BME), 2,5-dihydroxybenzoic acid (DHB), fetuin originated from fetal bovine serum, and branched octylphenoxy poly(ethoxy)ethanol (IGEPAL CA-630) were provided by Sigma-Aldrich (St. Louis). A mixture of maltodextrins was obtained from Avebe (Veendam, The Netherlands). The complex N-glycans NA2 (No. 24) and NA2F (No. 33) and the high-mannose structures Man6 (No. 11) and Man8 (No. 27) (Table 1) were provided by Ludger (Oxfordshire, U.K.). Peptidyl N-glycosidase F (PNGase F) was bought from Asparia Glycomics (San Sebastian, Spain).
Table 1. Putative N-Glycans Identified in Colostrum (Week 1) and Mature Milk (Week 4) of Seven Chinese Mothers, Including Sugar Mass, Building Blocks, and Using Two Classification Systems.
| composition |
classification:
type |
||||||
|---|---|---|---|---|---|---|---|
| no. | mass | Hex | HexNAc | Fuc | NeuAc | Ia | IIb |
| N1 | 1056.415 | 3 | 2 | 1 | 0 | other | NF |
| N2 | 1072.315 | 4 | 2 | 0 | 0 | other | N |
| N3 | 1113.316 | 3 | 3 | 0 | 0 | other | N |
| N4 | 1202.4 | 3 | 2 | 2 | 0 | other | NF |
| N5 | 1218.415 | 4 | 2 | 1 | 0 | other | NF |
| N6 | 1234.314−16 | 5 | 2 | 0 | 0 | high mannose | N |
| N7 | 1259.316 | 3 | 3 | 1 | 0 | hybrid | NF |
| N8 | 1275.414,16 | 4 | 3 | 0 | 0 | hybrid | N |
| N9 | 1316.316 | 3 | 4 | 0 | 0 | complex | N |
| N10 | 1380.6 | 5 | 2 | 1 | 0 | other | NF |
| N11 | 1396.314−16 | 6 | 2 | 0 | 0 | high mannose | N |
| N12 | 1421.414,16 | 4 | 3 | 1 | 0 | hybrid | NF |
| N13 | 1437.415 | 5 | 3 | 0 | 0 | hybrid | N |
| N14 | 1462.514 | 3 | 4 | 1 | 0 | complex | NF |
| N15 | 1478.414,15 | 4 | 4 | 0 | 0 | complex | N |
| N16 | 1519.414,16 | 3 | 5 | 0 | 0 | complex | N |
| N17 | 1542.4 | 6 | 2 | 1 | 0 | other | NF |
| N18 | 1558.414−16 | 7 | 2 | 0 | 0 | high mannose | N |
| N19 | 1566.914,16 | 4 | 3 | 0 | 1 | hybrid | A |
| N20 | 1583.415 | 5 | 3 | 1 | 0 | hybrid | NF |
| N21 | 1599.514 | 6 | 3 | 0 | 0 | hybrid | N |
| N22 | 1607.3 | 3 | 4 | 0 | 1 | hybrid | A |
| N23 | 1624.514,15 | 4 | 4 | 1 | 0 | complex | NF |
| N24 | 1640.414,15 | 5 | 4 | 0 | 0 | complex | N |
| N25 | 1665.514,16 | 3 | 5 | 1 | 0 | complex | NF |
| N26 | 1681.514 | 4 | 5 | 0 | 0 | complex | N |
| N27 | 1720.414−16 | 8 | 2 | 0 | 0 | high mannose | N |
| N28 | 1722.5 | 3 | 6 | 0 | 0 | complex | N |
| N29 | 1728.115 | 5 | 3 | 0 | 1 | hybrid | A |
| N30 | 1729.015 | 5 | 3 | 2 | 0 | hybrid | NF |
| N31 | 1769.514 | 4 | 4 | 0 | 1 | complex | A |
| N32 | 1770.814 | 4 | 4 | 2 | 0 | complex | NF |
| N33 | 1786.514−16 | 5 | 4 | 1 | 0 | complex | NF |
| N34 | 1802.5 | 6 | 4 | 0 | 0 | complex | N |
| N35 | 1827.514,16 | 4 | 5 | 1 | 0 | complex | NF |
| N36 | 1843.5 | 5 | 5 | 0 | 0 | complex | N |
| N37 | 1874.415 | 5 | 3 | 1 | 1 | hybrid | AF |
| N38 | 1882.514−16 | 9 | 2 | 0 | 0 | high mannose | N |
| N39 | 1884.516 | 4 | 6 | 0 | 0 | complex | N |
| N40 | 1931.914−16 | 5 | 4 | 0 | 1 | complex | A |
| N41 | 1932.914,15 | 5 | 4 | 2 | 0 | complex | NF |
| N42 | 1948.514,15 | 6 | 4 | 1 | 0 | complex | NF |
| N43 | 1972.714 | 4 | 5 | 0 | 1 | complex | A |
| N44 | 1989.514,16 | 5 | 5 | 1 | 0 | complex | NF |
| N45 | 2005.6 | 6 | 5 | 0 | 0 | complex | N |
| N46 | 2014.515 | 3 | 6 | 2 | 0 | complex | NF |
| N47 | 2036.6 | 6 | 3 | 1 | 1 | complex | AF |
| N48 | 2060.9 | 4 | 4 | 0 | 2 | complex | A |
| N49 | 2077.514−16 | 5 | 4 | 1 | 1 | complex | AF |
| N50 | 2078.614,15 | 5 | 4 | 3 | 0 | complex | NF |
| N51 | 2094.615 | 6 | 4 | 2 | 0 | complex | NF |
| N52 | 2118.714,15 | 4 | 5 | 1 | 1 | complex | AF |
| N53 | 2134.714 | 5 | 5 | 0 | 1 | complex | A |
| N54 | 2135.5 | 5 | 5 | 2 | 0 | complex | NF |
| N55 | 2151.614 | 6 | 5 | 1 | 0 | complex | NF |
| N56 | 2167.6 | 7 | 5 | 0 | 0 | complex | N |
| N57 | 2176.715 | 4 | 6 | 2 | 0 | complex | NF |
| N58 | 2224.614 | 5 | 4 | 4 | 0 | complex | NF |
| N59 | 2240.715 | 6 | 4 | 3 | 0 | complex | NF |
| N60 | 2297.714,16 | 6 | 5 | 2 | 0 | complex | NF |
| N61 | 2313.6 | 7 | 5 | 1 | 0 | complex | NF |
| N62 | 2354.8 | 6 | 6 | 1 | 0 | complex | NF |
| N63 | 2370.8 | 5 | 4 | 5 | 0 | complex | NF |
| N64 | 2443.814,16 | 6 | 5 | 3 | 0 | complex | NF |
| N65 | 2459.814 | 7 | 5 | 2 | 0 | hybrid | NF |
| N66 | 2500.615 | 6 | 6 | 2 | 0 | complex | NF |
Type I classification: complex, hybrid, high-mannose, and other N-glycans.
Type II classification: N, neutral nonfucosylated N-glycans; NF, neutral fucosylated N-glycans; A, acidic nonfucosylated N-glycans; AF, acidic fucosylated N-glycans.
Human Milk Collection and the Mother’s SeLe Status
Milk samples from seven healthy mothers who delivered term (38–42 weeks) infants were assessed in week 1 (colostrum) and week 4 (mature milk) postpartum. Human milk collection was approved by the Chinese Ethics Committee of Registering Clinical Trials (ChiECRCT-20150017). Written informed consent was obtained from all mothers. The milk was categorized based on the mother’s SeLe group, using liquid chromatography-mass spectrometry (LC-MS) quantification of four secretor-status-specific HMOs, 2′fucosyllactose, lacto-N-fucopentaose I, lacto-N-difucosylhexaose I, and lacto-N-tetraose, as described previously (M1 = mother 26, M2 = 20, M3 = 22, M4 = 25, M5 = 23, M6 = 21, M7 = 24).28 Mothers 1 and 4 belong to the Se–Le+ group, whereas mothers 2, 3, 5, 6, and 7 were assigned to the Se+Le+ group. Milk samples from Se+Le– and Se–Le– mothers are not represented in this study.
Isolation of Human Milk Serum Proteins
The lipid layer was removed from the human milk samples (7 mL) after centrifugation (10 min, 1500g, 4 °C), and the obtained skim milk was transferred to ultracentrifuge tubes.11 After ultracentrifugation (90 min, 100.000g, 4 °C), the top layer represented the remaining milk fat still present, the middle layer was milk serum (consisting of serum proteins and free oligosaccharides), and the bottom layer consisted of micellar casein.11 The glycoproteins from the milk fat globule membrane, which represent 1–2% of the total protein content, were not taken into consideration in this study.6 Although these latter glycoproteins have small contributions in nutritional value, they have been reported to play an important role in various cellular processes and defense mechanisms in the newborn.6 Serum proteins were separated from the HMOs via EtOH precipitation,15 with modifications. Milk serum (3 mL) was diluted twice, and then absolute EtOH was added until a relative concentration of 67% EtOH was reached. After 67% EtOH precipitation (60 min, 4 °C) and centrifugation (15 min, 1500g, 4 °C), the supernatant containing HMOs was discarded. The pellet containing serum proteins was redissolved in 0.5 mL of water, and EtOH precipitation was repeated three times. Finally, serum proteins in the pellet were redissolved in 0.5 mL of 200 mM ammonium bicarbonate (pH 8) using alternately a vortex and an ultrasonic bath at room temperature. The final experiments (after method optimization and validation) were done in duplicate.
Release and Purification of N-Glycans from Serum Proteins
Methods were based upon previously described methods,14,15,29−32 with modifications. Briefly, 1 μL of 1 M SDS in water and 10 μL of 2-BME were combined with 100 μL of the solution containing the human milk serum proteins and kept for 10 min at 95 °C. Optimization of the denaturation steps was done with fetuin. Fetuin is folded by these disulfide bonds, and SDS was not strong enough to break down the covalent bridges.29 A sulfhydryl reducing reagent, like 2-BME, is essential to release N-glycans.
After cooling down to 37 °C, solutions with the denatured serum proteins were diluted with 50 μL of 100 mM ammonium bicarbonate and mixed with 50 μL of 4% (v/v) IGEPAL CA-630. A wide range of concentrations (range: 50–200 mM) has been used before starting incubation with PNGase F,15,29,32 and here the final concentration of the samples was 100 mM. To protect the PNGase F from denaturation by SDS, IGEPAL CA-630 as a nonionic detergent was added, although the mechanism behind this protection effect is still unknown.29 For the complete release of N-glycans from human milk serum proteins, the mixture was incubated with PNGase F (24 h, 37 °C), 6 μL of enzyme at t = 0 followed by 4 μL of enzyme after 16 h. Redigestion of the deglycosylated proteins with PNGase F after 16 h provided sufficient reaction time and guaranteed the action of the enzyme, resulting in more released N-glycans in numbers compared to other incubation times.
After incubation, the mixtures containing N-glycans and deglycosylated proteins were mixed with absolute EtOH until a relative concentration of 67% EtOH was reached and stored for 60 min at 4 °C. After centrifugation (15 min, 1500g, 4 °C), the supernatant was dried under a stream of air overnight, and the N-glycans thereafter reconstituted with 0.5 mL of water.
The N-glycans in solution were further purified by solid-phase extraction using a graphitized carbon column cartridge (bed weight: 150 mg; tube size: 4 mL; Alltech, Deerfield).14 Removing excess salts, denatured proteins, and other reagents by EtOH precipitation is necessary before definitive characterization of the N-glycans. Besides sediment by cold EtOH, solid-phase extraction was used for purification of the N-glycans. The recovery of N-glycans from graphitized cartridges was checked using a set of standards. The cartridge was prepared with 2 mL of water, followed by 2 mL of 80% MeCN containing 0.1% TFA. The cartridge was conditioned with 2 mL of water before loading 0.5 mL of the sample with N-glycans. The N-glycans on the cartridge were eluted with 0.5 mL of 10% MeCN, 0.5 mL of 20% MeCN, and 0.5 mL of 40% MeCN in water containing 0.05% TFA. The N-glycan mixtures were dried under a stream of air overnight. After reconstitution in 20 μL of water, the solution containing N-glycans was ready for MALDI-TOF-MS analysis.
Analysis of N-Glycans by MALDI-TOF-MS
Analysis of N-glycans by MALDI-TOF-MS was done, as described previously.33 MALDI-TOF mass spectra were recorded using an UltraFlextreme workstation controlled by FlexControl 3.3 software (Bruker Daltonics, Bremen, Germany) equipped with a Smartbeam II laser of 355 nm and operated in both positive and negative modes. Since the same glycans were detected in both negative and positive modes and no differences were found in the relative response between neutral and acidic glycans, spectra obtained in the positive mode were described in the result section, due to better reproducibility. Spectra were collected from 1500 laser shots with an energy level of 30%. The spectrometer was calibrated using a mixture of maltodextrins in a mass range of 500–3000 Da. The complex N-glycans NA2 (No. 24) and NA2F (No. 33) and the high-mannose structures Man6 (No. 11) and Man8 (No. 27) in solution were used as N-glycan standards. The numbers behind the four standards correspond with the numbers in Table 1. The matrix solution was prepared by mixing 25 mg of DHB in 1 mL of 50% MeCN/50% water (containing 1 mM sodium chloride) and subsequent centrifugation (5 min, 1500g, 4 °C). For each sample containing N-glycans, 1 μL was added directly on the ground steel MS target plate (Bruker Daltonics), followed by 1 μL of the matrix solution, and dried under a stream of air. DHB always crystallized as needlelike crystals along the boundary of the MS target plate, which result in heterogeneous sample distribution. Conditions of improved crystallization were checked in this study, and various matrix solutions have been tested.
Data analysis of N-glycans was performed with Flex Analysis 3.3 (Bruker Daltonics). Peak intensities of the individual N-glycans were used if the peak height of the N-glycans was 3 times higher than background noise. For data normalization, the MALDI-TOF-MS peak intensities for each N-glycan were transformed into percentages, by relating the peak intensity of each N-glycan in a sample to the total signal intensity of all of the identified N-glycans within a sample. The data of the individual N-glycans for two biological replicates were averaged. We used pure N-glycans to standardize the method, and technical replicates measured with MALDI-TOF-MS were precise, accurate, and reproducible. The structures of the N-glycans were assigned via the online database GlyTouCan using their molecular mass.34 No distinction could be made between molecular isomers. For each structure, just one possible isomer was selected for visualization. Interpretation of the N-glycan profiles in human milk was facilitated by principal component analysis (PCA) and heatmaps using R (Lucent Technologies, New York). The relative levels of the individual N-glycans in milk per mother and per lactation stage were used. Information about the mother’s SeLe status was omitted from the data set during statistical analysis. The data for the PCA analysis was mean-centered and Pareto-scaled prior to analysis. Before plotting a heatmap and dendrogram, Spearman’s rank correlations were compared, p-values were corrected with the Benjamini–Hochberg false discovery rate (FDR) method, and then represented in a heatmap with a dendrogram. For statistical analysis, an FDR adjusted p-value of <0.05 was considered significant.
Results and Discussion
Analysis of N-Glycans by MALDI-TOF-MS
To analyze all N-glycans present in milk, colostrum (week 1) and mature milk (week 4) of seven Chinese mothers were analyzed. The removal of HMOs with the right concentration of EtOH was the most crucial step to get the optimal signal-to-noise ratio by MALDI-TOF-MS and to identify more individual N-glycans. An example of a MALDI-TOF mass spectrum for colostrum (week 1) from Chinese mother 4 can be found in Figure 1, highlighting the 15 most abundant N-glycans.
Figure 1.
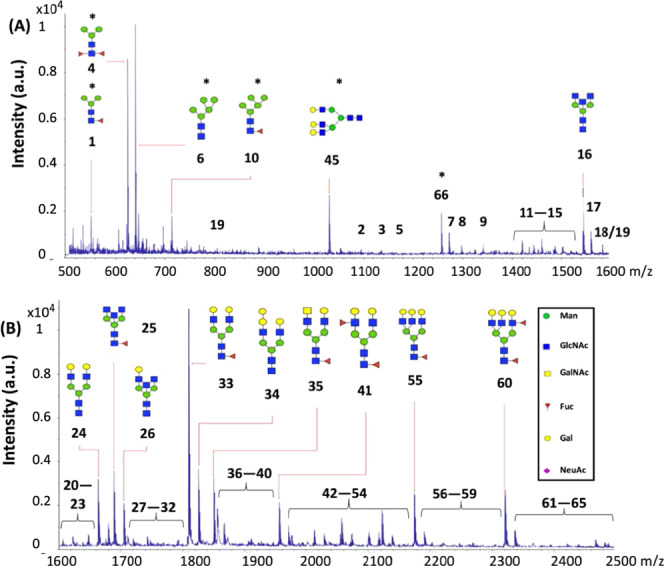
MALDI-TOF mass spectrum, highlighting the 15 most abundant N-glycans in colostrum (week 1) of Chinese mother 4. The numbers of the putative N-glycans correspond with the numbers in Table 1 and Figure 2. (A) Spectrum with m/z ranging from 500 to 1600 and (B) m/z ranging from 1600 to 2500. Just one possible isomer was selected for visualization. The N-glycans highlighted with an asterisk were doubly charged.
The structures of the different putative N-glycans numbered in Figure 1 can be found in Figure 2, which were assigned via the online database GlyTouCan.34 The top 15 N-glycans have a pentasaccharide as a common core, consisting of three Man and two GlcNAc residues (Figure 1). More than half of the top 15 N-glycans contained a Fuc residue, and none of them contained a NeuAc residue (Figure 1). No distinction could be made between the different molecular isomers by MALDI-TOF-MS. The molecular mass of the different N-glycans numbered in Figure 1 can be found in Table 1. However, not all of the 66 different putative N-glycans, as summarized in Table 1, were found in colostrum of each individual Chinese mother.
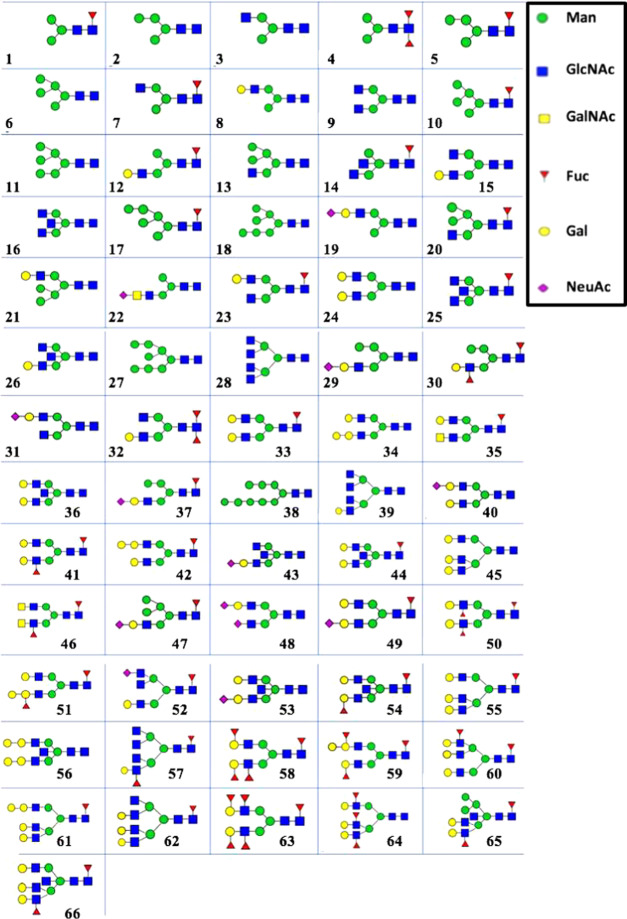
Figure 2.
Overview of 66 putative N-glycans identified in colostrum (week 1) and mature milk (week 4) of seven Chinese mothers using MALDI-TOF-MS. Numbers indicate the N-glycans displayed in Table 1. The structures of the identified N-glycans were assigned via the online GlyTouCan database based on their molecular mass.34 Just one possible isomer is shown.
Overview of Identified N-Glycans in Colostrum (Week 1) and Mature Milk (Week 4) of Seven Chinese Mothers
An overview of all of the identified putative N-glycans in human milk can be found in Figure 2 and Table 1, combining the data obtained by MALDI-TOF-MS of the seven mothers from two different lactation periods. In total, 66 different N-glycan compositions were detected in human milk over time by MALDI-TOF-MS (Table 1), a higher number than previously reported in the literature.14−16 Of these 66 N-glycans, 42 (64%) were found in all human milk samples: 48 (73%) and 43 (65%) unique structures were detected in colostrum and mature milk, respectively (Table S1). Among these 66 N-glycans, 42 (64%) structures can be classified as complex N-glycans, 5 (7%) as high mannose, and 12 (18%) as hybrid (Table 1, classification system type I15), while seven structures (11%) could not be classified in one of these three groups and are referred to in Table 1 as “other”. The other N-glycans 10 and 17 both have a Fuc residue (Figure 2), excluding them as high-mannose N-glycans. The other N-glycans 1, 2, 4, and 5 did not have 5–9 Man residues (Figure 2). High-mannose N-glycans merely consist of Man building blocks. The other N-glycan 3 lacks either a Fuc or Gal residue (Figure 2) to be classified as hybrid N-glycan and does not have two or three GlcNAc residues like the complex N-glycans (Figure 2).
Another classification system has been introduced to group the different types of N-glycans.14 Using this classification system, 11 (17%) and 55 (83%) structures can be grouped as acidic and neutral N-glycans, respectively, and 37 (56%) as fucosylated (Table 1, classification system type II14). The relative occurrence of fucosylated N-glycans in human milk has been mentioned in several studies.14−16 Two earlier studies found that the numbers of fucosylated N-glycans ranged between 65 and 75%.14,15 A more recent paper, which used a larger sample size (10 mothers and three time points), found that 16 (55%) of the 29 N-glycans found were fucosylated.16 Based on the structural features of the N-glycans, as mentioned in the literature,26,35 the fucosylated N-glycans in Figure 2 with a single Fuc residue are probably α1,6-linked by FUT8 during biosynthesis to the GlcNAc residue at the reducing end. The N-glycans containing more than one Fuc residue (Figure 2) might be formed due to the presence of other fucosyltransferases. Multiple (>50) N-glycans from different blood and tissue glycoproteins have been structurally characterized, containing α1,2-, α1,3-, and α1,6-linked Fuc linkages.35 The extra Fuc residues in the N-glycans of these blood and tissue glycoproteins were α1,2- and α1,3-linked to a Gal residue and a peripheral GlcNAc residue, respectively.35 It has also been found that fucosylation of N-glycans is modified by FUT2,24 which decorates the Fuc residues by α1,2-linkages.24 These fucose-containing N-glycans might also be important for the infant’s healthy development, as has been reported for fucosylated HMOs.36
Untargeted Statistics with the Relative Levels of Individual N-Glycans in Milk of Seven Chinese Mothers Over Lactation
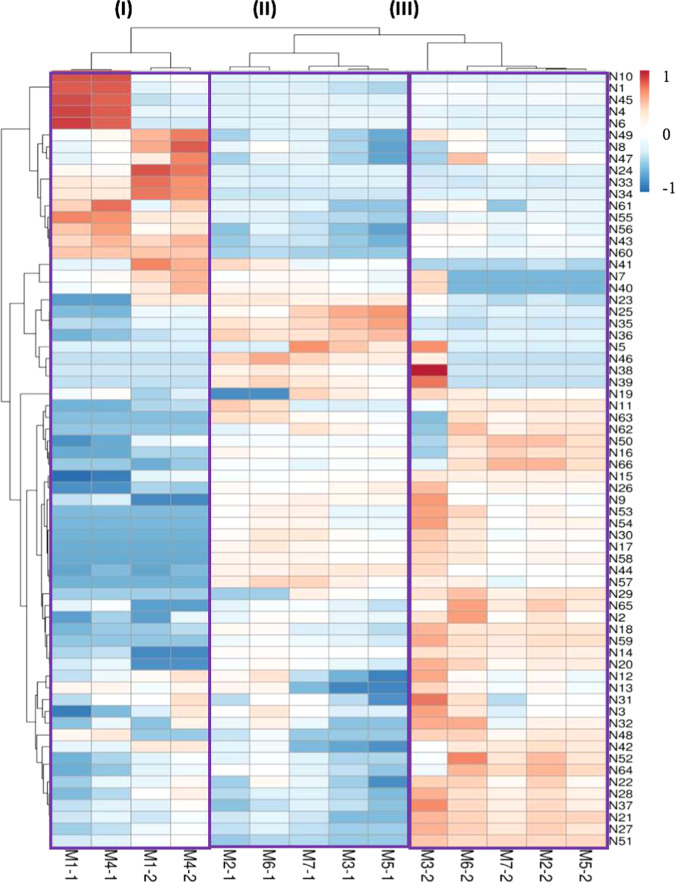
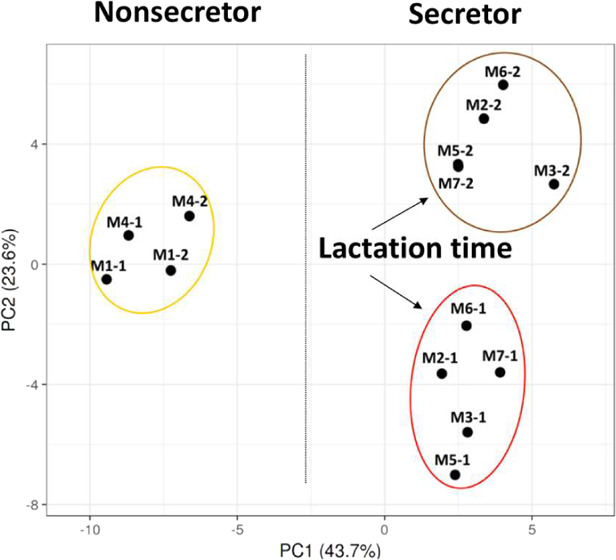
The averaged levels of the individual N-glycans per mother and per lactation stage can be found in Table S1. Separation of the different clusters coincides with the type of secretor status and lactation time as indicated in Figure 3.
Figure 3.
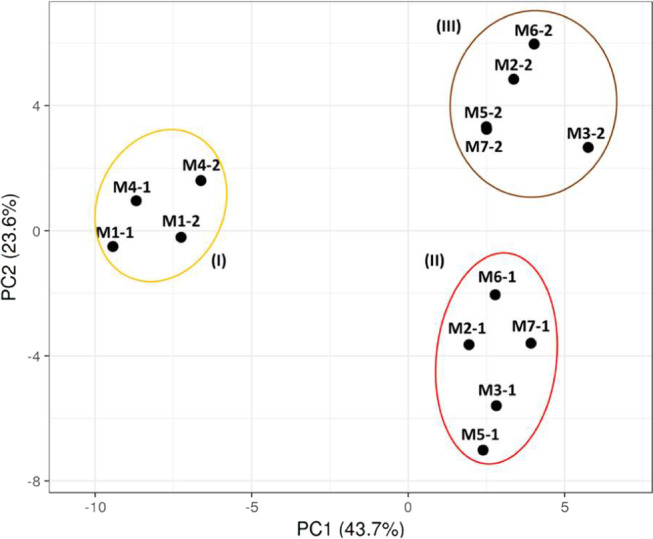
PCA plot of the Chinese human milk N-glycome over lactation, using the relative level of each single N-glycan per mother and per lactation stage. Mothers 1 and 4 were assigned to the Se–Le+ group, whereas mothers 2, 3, 5, 6, and 7 were grouped in the Se+Le+ group. The numbers after the hyphen per mother indicate colostrum (1) and mature milk (2).
Mothers 1 and 4 can be assigned to the Se–Le+ group, whereas mothers 2, 3, 5, 6, and 7 belong to the Se+Le+ group. Based on the PCA plot, three different groups could be observed (I–III). For the first time, a clear difference can be observed with respect to milk of two Se–Le+ mothers (I) and five Se+Le+ mothers (II and III), based on the levels of the individual N-glycans. Although the sample size for Se–Le+ mothers (N = 2) is too small for strong conclusions, the distinction between secretor status of the mothers can be clearly seen for both colostrum and mature milk. It can also be observed that milk of the Se+Le+ mothers was strongly grouped per lactation stage (II and III). A similar trend could be observed for the two Se–Le+ mothers; however, a larger sample size is needed for confirmation.
Individual N-Glycans in Milk of Se+Le+ and Se–Le+ Mothers Over Lactation Grouped on Classification Systems I and II
The levels of the 66 different N-glycan compositions were grouped, according to classification systems I (Figure S1) and II (Figure 4) per mother and lactation stage. Based on classification system I (high mannose, complex, hybrid, and other N-glycans), the relative levels of the total high-mannose and total other N-glycans decreased over lactation for the two Se–Le+ mothers, whereas the relative levels of the total complex N-glycans increased over time (Figure S1). The relative levels of the total hybrid N-glycans remained constant over lactation (Figure S1). The group containing the complex N-glycans covered >65% of the total N-glycan content, for all seven mothers (Figure S1). It has been reported before that complex N-glycans individually are highly abundant in human milk and the most dominant type of N-glycans present in mature milk among mothers.14
Figure 4.
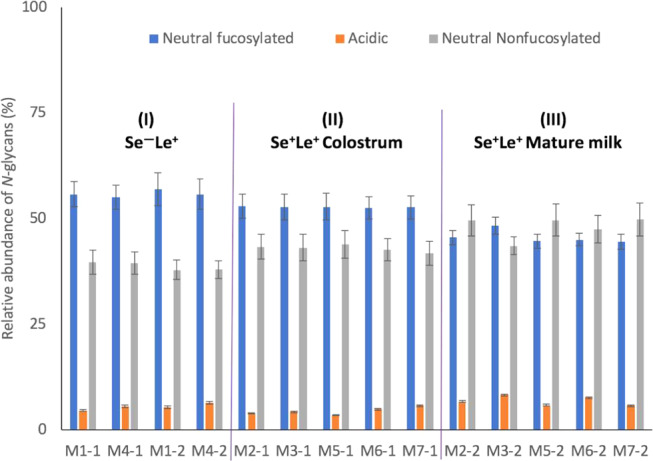
Total acidic and neutral (nonfucosylated and fucosylated) N-glycan content in milk of seven mothers from two different lactation periods. The acidic (nonfucosylated and fucosylated) N-glycans were grouped together. Mothers 1 and 4 were assigned to the Se–Le+ group, whereas mothers 2, 3, 5, 6, and 7 belong to the Se+Le+ group. The numbers after the hyphen per mother indicate colostrum (1) and mature milk (2). Roman numerals (I–III) refer to the groups in the PCA plot in Figure 3.
As mentioned above, classification system II considered all of the different structural features of the N-glycans. The levels of the total neutral fucosylated N-glycans in milk from five Se+Le+ mothers slightly decreased over lactation, while the total levels of acidic N-glycans remained constant, and the total levels of neutral nonfucosylated N-glycans increased (Figure 4). This pattern could not be observed for the milk from the two Se–Le+ mothers. The profiles of the three N-glycan groups stayed constant over lactation for the two Se–Le+ mothers (Figure 4). Despite the different patterns, the relative levels of the total neutral (sum of nonfucosylated and fucosylated) N-glycans end up being the same for both genetic groups (Figure 4). The relative levels of total neutral N-glycans covered >90% of the total N-glycan as present in the mass spectrum, for all seven mothers (Figure 4).
The patterns for the total acidic and total neutral N-glycan contents of human milk proteins over lactation (Figure 4) did not completely match with the literature.16 It has been reported by others that the levels of fucosylated N-glycans decreased from ca. 61% in colostrum (3 days) to 37% in transition milk (9 days) and then remained constant in mature milk (40 days).16 This large drop could not be observed here for neutral fucosylated N-glycans over time (Figure 4). It was also reported that the levels of the nonfucosylated N-glycans increased over time and the levels of acidic N-glycans in milk proteins over time ranged from 5 to 12%, with a little increase over lactation.16 In this current study, the relative amounts of the total acidic N-glycans ranged between 3 and 8% in milk of seven mothers over time (Figure 4). However, none of the acidic N-glycans belong here to the most abundant N-glycans (Table S2). Two other studies showed a completely different pattern for the acidic N-glycans in mature milk.14,15 By abundance, 47 and 57% of the N-glycans were sialylated.14,15
Twenty-seven N-glycans were not found in our study (Table S3), as compared to the literature,14−16 including 13 acidic N-glycans (Table S3). It seems unlikely that these acidic N-glycans in Tables 1 and S3 belong to the most abundant N-glycans. Some of the highly acidic N-glycans (e.g., 1915.7, 2881.1) were only found once in the literature (Table S3), while other structures (e.g., 40, 49) were found in low quantities by us (Table S1) and others.15,16 In addition, a recent study investigated the core fucosylation patterns of serum proteins in milk of 56 Chinese mothers. In this latter study, acidic N-glycans were also not highly abundant in human milk. The type and level of individual N-glycans in milk of the individual mothers were not investigated, as the study evaluated the role of FUT8 with respect to the formation of a healthy micriobiota.25
Individual N-Glycans in Colostrum (Week 1) and Mature Milk (Week 4) of Seven Chinese Mothers
Besides the PCA plot, also a heatmap was generated. The differences in individual N-glycans in colostrum and mature milk of the Chinese mothers can be investigated using a heatmap, showing variation in both the type and levels of specific individual N-glycans among mothers and over time (Figure 5). For example, the level of the neutral fucosylated N-glycan 25 for Chinese mother 2 was higher in colostrum than in mature milk (Figure 5). The level of the neutral fucosylated N-glycan 33 was higher in both colostrum and mature milk from Chinese mother 4 in comparison to Chinese mother 2 (Figure 5).
Figure 5.
Heatmap of N-glycans in colostrum and mature milk per mother, using the relative abundancies of each single N-glycan. The levels of individual N-glycans are represented as different colors. The colors blue and red represent the lowest and highest values of the N-glycans, respectively. Mothers 1 and 4 were assigned to the Se–Le+ group, whereas mothers 2, 3, 5, 6, and 7 were grouped in the Se+Le+ group. The numbers after the hyphen per mother indicate colostrum (1) and mature milk (2). Roman numerals (I–III) refer to the groups in the PCA plot in Figure 3.
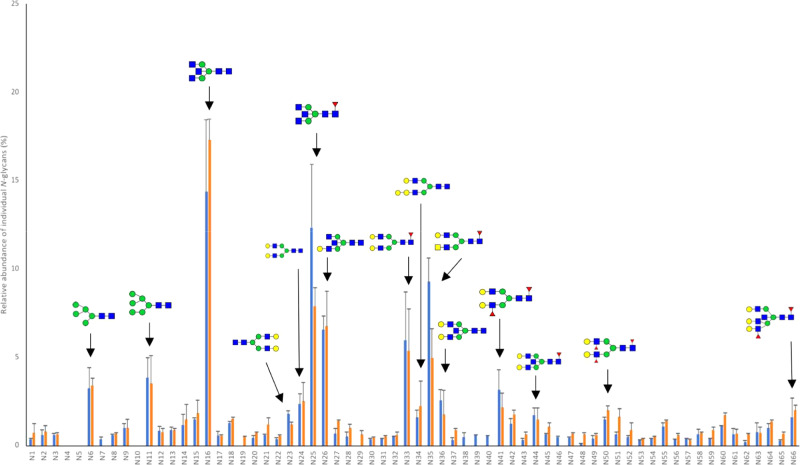
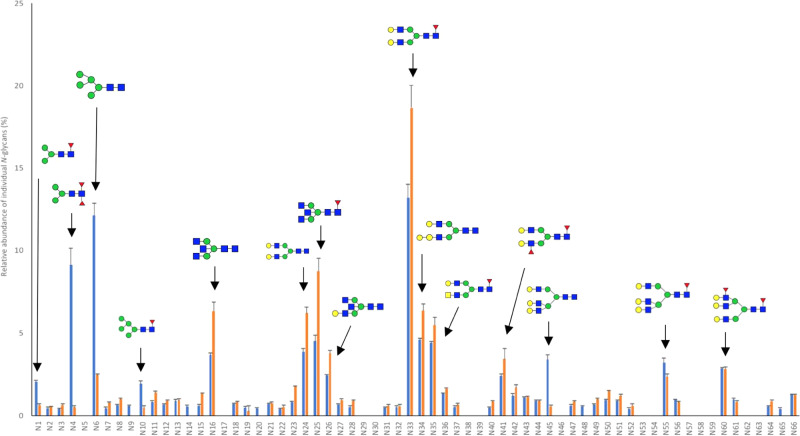
Although the heatmap (Figure 5) provided insights into the Chinese human milk N-glycome over lactation, it is quite hard to observe accurately the differences in type and levels of individual N-glycans between the individual mothers and over lactation. Therefore, the profiles of the individual N-glycans over time can be found in Figures 6 and 7 for milk of Chinese mother 2 (Se+Le+ status) and Chinese mother 4 (Se–Le+ status), respectively.
Figure 6.
Individual N-glycan profiles in colostrum (blue bars) and mature milk (orange bars) of Chinese mother 2, as measured using MALDI-TOF-MS. Mother 2 belongs to the Se+Le+ group. Numbers on the x-axis indicate the N-glycans displayed in Table 1. Biological replicates (N = 2). The structures displayed highlight the 15 most abundant N-glycans.
Figure 7.
Individual N-glycan profiles in colostrum (blue bars) and mature milk (orange bars) of Chinese mother 4, as measured using MALDI-TOF-MS. Mother 4 was grouped in the Se–Le+ group. Numbers on the x-axis indicate the N-glycans displayed in Table 1. Biological replicates (N = 2). The structures displayed highlight the 15 most abundant N-glycans.
Levels of the Individual N-Glycans in Colostrum and Mature Milk of Chinese Mother 2 with the Se+Le+ Status
It can be seen in Figure 6 that neutral nonfucosylated N-glycan 16 and fucosylated N-glycan 25 are both highly abundant in colostrum (13.0%) of Chinese mother 2. Other highly abundant neutral N-glycans in colostrum of Chinese mother 2 are structures 35, 33, 26, 11, 41, 6, 24, 36, 23, 44, 34, 15, and 50, ordered from most to least abundant (Figure 6).
The levels of the neutral nonfucosylated N-glycans 16 and 34 increased (14.4 → 17.3% and 1.6 → 2.3%) over time, respectively (Figure 6). The levels of the neutral fucosylated N-glycans 25, 35, and 41 decreased (12.3 → 7.9%, 9.3 → 5.0%, and 3.2 → 2.2%) over lactation, respectively (Figure 6). The levels of the neutral nonfucosylated N-glycan 36 also decreased (2.6 → 1.8%) from colostrum to mature milk (Figure 6). The neutral fucosylated N-glycans 4, 5, and 10 were completely absent in milk of Chinese mother 2 (Figure 6). The neutral fucosylated N-glycans 7 and 46, the nonfucosylated N-glycans 38 and 39, and acidic nonfucosylated N-glycan 40 were only present in colostrum, while the acidic nonfucosylated N-glycan 19 and neutral fucosylated N-glycan 29 were only present in mature milk (Figures 6 and 2).
Levels of the Individual N-Glycans in Colostrum and Mature Milk of Chinese Mother 4 with the Se–Le+ Status
It can be seen in Figure 7 that the neutral fucosylated N-glycan 33 is highly abundant in colostrum (13.2%) of Chinese mother 4. The other highly abundant N-glycans in colostrum of Chinese mother 4 are 6, 4, 34, 25, 35, 24, 16, 45, 55, 60, 26, 41, 1, and 10, ordered from most to least abundant (Figure 7). The majority of the top 15 structures can be categorized as neutral fucosylated N-glycans (Figure 7), despite the fact that this milk belongs to the Se–Le+ group.
The levels of the neutral fucosylated N-glycans 33, 25, and 35 increased (13.2 → 18.7%, 4.5 → 8.8%, and 4.4 → 5.5%) over lactation, respectively, while the levels of the neutral nonfucosylated N-glycans 6 and 45 decreased (12.1 → 2.5% and 3.4 → 0.6%) over time, respectively (Figure 7). The levels of the neutral fucosylated N-glycans 4, 55, 1, and 10 decreased (9.1 → 0.5%, 3.2 → 2.4%, 2.1 → 0.7%, and 1.9 → 0.5%) from colostrum to mature milk, respectively (Figure 7). The levels increased for the nonfucosylated N-glycans 34, 24, 16, 26, and 41 (4.6 → 6.4%, 3.9 → 6.2%, 3.7 → 6.3%, 2.5 → 3.8%, and 2.4 → 3.4%) over time, respectively (Figure 7).
The neutral fucosylated N-glycans 20 and 65, the neutral fucoyslated N-glycan 9, and the acidic nonfucosylated N-glycan 48 were only present in colostrum (Figure 7). The neutral fucosylated N-glycans 5, 17, 30, 46, 54, 57–59, 62, and 63, the acidic nonfucosylated N-glycans 29 and 53, and the neutral nonfucosylated N-glycans 38 and 39 were completely absent in milk of Chinese mother 4 (Figures 7 and 2).
Comparison of the Individual N-Glycans in Colostrum and Mature Milk of Chinese Mothers 2 and 4 with the Se+Le+ and Se–Le+ Status, Respectively
The neutral fucosylated N-glycan 33 was present in milk of the Se+Le+ and Se–Le+ mother, with higher levels for the Se–Le+ mother in comparison to the Se+Le+ mother (Figures 6 and 7). The levels of the neutral N-glycan 6 were also higher in colostrum for the Se–Le+ mother compared to the Se+Le+ mother. The levels of the neutral fucosylated N-glycans 25 and 35 were higher in colostrum for the Se+Le+ mother in comparison to the Se–Le+ mother. The levels of the neutral N-glycans 11 and 26 were both higher in colostrum and mature milk for the Se+Le+ mother in comparison to the Se–Le+ mother. The neutral N-glycan 34 was more dominant in colostrum and mature milk for the Se–Le+ mother in comparison to the Se+Le+ mother. From the total putative 66 N-glycans, the neutral fucosylated N-glycans 4, 5, and 10 were completely absent in the milk from the Se+Le+ mother (Figure 6), while more N-glycans 5, 17, 29–30, 38–39, 46, 53, 54, 57–59, and 62–63 were missing in the milk of the Se–Le+ mother of which 10 structures were neutral fucosylated (Figure 7). These missing structures already differentiate between the two different milk types. It might be that structures 54, 57–59, and 63, which lack an α1,2-linked Fuc residue, are not present due to the absence of the FUT2 enzyme for the Se–Le+ mother. These latter structures were only present in the milk from the Se+Le+ mothers (Table S1). Structures 15, 17, and 62 only contain α1,6-linked Fuc residues due to the enzyme FUT8. The N-glycans 30 and 46 were only present in the milk from the Se+Le+ mothers (Table S1), which suggest that these Fuc residues might be α1,2-linked to a Gal residue instead of being α1,3-linked to a peripheral GlcNAc residue (Figure 2). No distinction between molecular isomers could be made by MALDI-TOF-MS.
The levels of the neutral fucosylated N-glycans 25, 33, and 35 decreased from colostrum to mature milk for the Se+Le+ mother, whereas the neutral N-glycan 16 became more dominant over time. In contrast to the milk from the Se+Le+ mother, the levels of neutral N-glycans 6 and 45 decreased over time for the Se–Le+ mother, while the levels of the neutral fucosylated N-glycans 25, 33, and 35 increased over time. As a consequence, other neutral fucosylated N-glycans 1, 10, 33, 55, and 60 might explain why the total neutral fucosylated concentration ends up being the same for both genetic groups (Figure 4). The milk of one Se+Le+ mother and one Se–Le+ mother thus mainly differed based on neutral fucosylated N-glycans. The same top 15 in N-glycans were found in colostrum and mature milk for both Se–Le+ mothers (Table S2). A similar top 15 in N-glycans can be found for all Se+Le+ mothers over lactation. The profiles of the top 15 N-glycans for the Se+Le+ mothers 3, 5, and 7 have more in common than the Se+Le+ mothers 2 and 6 (Table S2). The patterns (increase/decrease in levels) of the individual 15 N-glycans behave differently over time for the Se+Le+ mothers 2 and 6 (Table S2) and the Se+Le+ mothers 3, 5, and 7. The 15 most abundant N-glycans covered in levels >72 and >65% of the total N-glycan content in colostrum and mature milk, respectively (Table S2). In contrast to the most abundant serum proteins11 and HMOs28 in human milk, a much larger variety in type and levels can be observed for the top 15 N-glycans among mothers and over lactation. This indicates that lesser abundant N-glycans should deserve the same attention as the relative highly abundant N-glycans. Overall, N-glycans share several building blocks with HMOs although the latter do not have Man and GalNAc residues. In addition, most N-glycan and HMO structures are fucosylated. Based on this study, it can be concluded that fucosylation of N-glycans was associated with the mother’s secretor status.
This study aimed to fill a gap in the literature by investigating the N-glycan profiles in milk of seven mothers over lactation individually. For this purpose, an accurate and reproducible method was needed. The procedure to remove HMOs was efficient, addition of 2-BME improved denaturation of serum proteins, and the incubation time and amount of the PNGase F were optimized. After method optimization and validation, a larger set of human milk samples was used. Acidic N-glycans do not belong to the 15 most abundant N-glycans, as mainly neutral fucosylated and nonfucosylated N-glycans can be found in colostrum and mature milk, for all seven mothers. The difference between secretor status was mainly based on the neutral fucosylated N-glycans.
Acknowledgments
The authors want to thank Melliana Jonathan (Food Chemistry, Wageningen University & Research) for the fruitful discussions on the isolation, extraction, and characterization of N-glycans.
Glossary
Abbreviations
- EtOH
ethanol
- Fuc
fucose
- FUT
fucosyltransferases
- Gal
galactose
- GalNAc
N-acetylgalactosamine
- GlcNAc
N-acetylglucosamine
- HMOs
human milk oligosaccharides
- IGEPAL CA-630
branched octylphenoxy poly(ethoxy)ethanol
- Le
Lewis status
- MALDI-TOF-MS
matrix-assisted laser desorption ionization time of flight mass spectrometry
- Man
mannose
- MeCN
acetonitrile
- NeuAc
sialic acid
- PCA
principal component analysis
- PNGase F
peptidyl N-glycosidase F
- Se
secretor status
- SDS
sodium dodecyl sulfate
- TFA
trifluoroacetic acid
- 2-BME
2-mercaptoethanol
- DHB
2,5-dihydroxybenzoic acid
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.0c02161.
Data of individual N-glycan levels in Chinese human milk per mother during lactation (Table S1); the 15 most abundant N-glycans (Table S2); comparison of N-glycans between different studies in the literature (Table S3); N-glycans grouped according to classification system I (Figure S1) (PDF)
Funding for this project was received from Yili Industrial Group Company.
The authors declare no competing financial interest.
Supplementary Material
References
- Kunz C.; Rodriguez-Palmero M.; Koletzko B.; Jensen R. Nutritional and biochemical properties of human milk, part I: General aspects, proteins, and carbohydrates. Clin. Perinatol. 1999, 26, 307–333. 10.1016/S0095-5108(18)30055-1. [DOI] [PubMed] [Google Scholar]
- Labbok M. H.; Clark D.; Goldman A. S. Breastfeeding: Maintaining an irreplaceable immunological resource. Nat. Rev. Immunol. 2004, 4, 565–572. 10.1038/nri1393. [DOI] [PubMed] [Google Scholar]
- Ballard O.; Morrow A. L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. North Am. 2013, 60, 49–74. 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode L. Recent advances on structure, metabolism, and function of human milk oligosaccharides. J. Nutr. 2006, 136, 2127–2130. 10.1093/jn/136.8.2127. [DOI] [PubMed] [Google Scholar]
- De Leoz M. L. A.; Wu S.; Strum J. S.; Niñonuevo M. R.; Gaerlan S. C.; Mirmiran M.; German J. B.; Mills D. A.; Lebrilla C. B.; Underwood M. A. A quantitative and comprehensive method to analyse human milk oligosaccharide structures in the urine and faeces of infants. Anal. Bioanal. Chem. 2013, 405, 4089–4105. 10.1007/s00216-013-6817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettinga K. A.; Van Valenberg H.; De Vries S.; Boeren S.; Van Hooijdonk T.; Van Arendonk J.; Vervoort J. The host defense proteome of human and bovine milk. PLoS One 2011, 6, e19433 10.1371/journal.pone.0019433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich J. W.; Dodds E. D.; Barboza M.; McJimpsey E. L.; Seipert R. R.; Francis J.; An H. J.; Freeman S.; German J. B.; Lebrilla C. B. Glycoprotein expression in human milk during lactation. J. Agric. Food Chem. 2010, 58, 6440–6448. 10.1021/jf100112x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R.; Cheah W. Y.; Grinyer J.; Packer N. Glycoconjugates in human milk: Protecting infants from disease. Glycobiology 2013, 23, 1425–1438. 10.1093/glycob/cwt072. [DOI] [PubMed] [Google Scholar]
- Karav S.; Le Parc A.; de Moura Bell J. M. L. N.; Frese S. A.; Kirmiz N.; Block D. A.; Barile D.; Mills D. E. Oligosaccharides released from milk glycoproteins are selective growth substrates for infant-associated bifidobacteria. Appl. Environ. Microbiol. 2016, 82, 3622–3630. 10.1128/AEM.00547-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmiz N.; Robinson R. C.; Shah I. M.; Barile D.; Mills D. A. Milk glycans and their interaction with the infant-gut microbiota. Annu. Rev. Food Sci. Technol. 2018, 9, 429–450. 10.1146/annurev-food-030216-030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwakiel M.; Boeren S.; Hageman J. A.; Szeto I. M.; Schols H. A.; Hettinga K. A. Variability of serum proteins in Chinese and Dutch human milk during lactation. Nutrients 2018, 11, 499 10.3390/nu11030499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnerdal B. Bioactive proteins in human milk: Health, nutrition, and implications for infant formulas. J. Pediatr. 2016, 173, S4–S9. 10.1016/j.jpeds.2016.02.070. [DOI] [PubMed] [Google Scholar]
- Picariello G.; Ferranti P.; Mamone G.; Roepstorff P.; Addeo F. Identification of N-linked glycoproteins in human milk by hydrophilic interaction liquid chromatography and mass spectrometry. Proteomics 2008, 8, 3833–3847. 10.1002/pmic.200701057. [DOI] [PubMed] [Google Scholar]
- Dallas D. C.; Martin W. F.; Strum J. S.; Zivkovic A. M.; Smilowitz J. T.; Underwood M. A.; Affolter M.; Lebrilla C. B.; German J. B. N-linked glycan profiling of mature human milk by high performance microfluidic chip liquid chromatography time of flight tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 4255–4263. 10.1021/jf104681p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwosu C. C.; Aldredge D. L.; Lee H.; Lerno L. A.; Zivkovic A. M.; German J. B.; Lebrilla C. B. Comparison of the human and bovine milk N-glycome via high-performance microfluidic chip liquid chromatography and tandem mass spectrometry. J. Proteome Res. 2012, 11, 2912–2924. 10.1021/pr300008u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.; Liu J.; Jia Y.; Yang Y.; Chen Q.; Sun L.; Song S.; Huang L.; Wang Z. Mass spectrometry analysis of changes in human milk N/O-glycopatterns at different lactation stages. J. Agric. Food Chem. 2019, 67, 10702–10712. 10.1021/acs.jafc.9b02034. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen S. S.; Schoemaker R. J. W.; Timmer C. J. A. M.; Kamerling J. P.; Dijkhuizen L. N- and O-glycosylation of a commercial bovine whey protein product. J. Agric. Food Chem. 2012, 60, 12553–12564. 10.1021/jf304000b. [DOI] [PubMed] [Google Scholar]
- Takimori S.; Shimaoka H.; Furukawa J.-I.; Yamashita T.; Amano M.; Fujitani N.; Takegawa Y.; Hammarstrom L.; Kacskovics I.; Shinohara Y.; Nishimura S.-I. Alteration of the N-glycome of bovine milk glycoproteins during early lactation. FEBS J. 2011, 278, 3769–3781. 10.1111/j.1742-4658.2011.08299.x. [DOI] [PubMed] [Google Scholar]
- Wang W.-L.; Wang L.; Du Y.-M.; Wu H.; Yu X.-B.; Ye K.-P.; Li C.-B.; Jung Y.-S.; Qian Y.-J.; Voglmeir J.; Liu L. Comparison of antipathogenic activities of the human and bovine milk N-glycome: Fucosylation is a key factor. Food Chem. 2017, 235, 167–174. 10.1016/j.foodchem.2017.05.026. [DOI] [PubMed] [Google Scholar]
- Landberg E.; Huang Y.; Stromgvist M.; Mechref Y.; Hansson L.; Lundblad A.; Novotny M. V.; Pahlsson P. Changes in glycosylation of human bile salt-activated lipase during lactation. Arch. Biochem. Biophys. 2000, 377, 246–254. 10.1006/abbi.2000.1778. [DOI] [PubMed] [Google Scholar]
- Le Parc A.; Karav S.; Rouquie C.; Maga E. A.; Bunyatratchata A.; Barile D. Characterization of recombinant human lactoferrin N-glycans expressed in the milk of transgenic cows. PLoS One 2017, 12, e0171477 10.1371/journal.pone.0171477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboza M.; Pinzon J.; Wickramasinghe S.; Froehlich J. W.; Moeller I.; Smilowitz J. T.; Ruhaak L. R.; Huang J.; Lönnerdal B.; German J. B.; Medrano J. F.; Weimer B. C.; Lebrilla C. B. Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria host interactions. Mol. Cell. Proteomics 2012, 11, M111.015248 10.1074/mcp.M111.015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle L.; Roos A.; Harvey D. J.; Wormald M. R.; Van Gijlswijk-Janssen D.; Redwan E.-R. M.; Wilson I. A.; Daha M. R.; Dwek R. A.; Rudd P. M. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J. Biol. Chem. 2003, 278, 20140–20153. 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- Bai Y.; Tao J.; Zhou J.; Fan Q.; Liu M.; Hu Y.; Xu Y.; Zhang L.; Yuan Y.; Li W.; Ze X.; Malard P.; Guo Z.; Yan J.; Li M. Fucosylated human milk oligosaccharides and N-glycans in the milk of Chinese mothers regulate gut microbiome of their breast-fed infants during different lactation stages. mSystems 2018, 3, e00206-18 10.1128/mSystems.00206-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; Bai Y.; Zhou J.; Huang W.; Yan J.; Tao J.; Fan Q.; Liu Y.; Mei D.; Yan Q.; Yuan J.; Malard P.; Wang Z.; Gu J.; Tanigchi N.; Li W. Core fucosulation of maternal milk of N-glycans evokes B cell activation by selectively promoting the L-fucose metabolism of gut Bifidobacterium spp. and Lactobacillus spp. mBio 2019, 10, e00128-19 10.1128/mBio.00128-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst S.; Wilding J. L.; Koprowska K.; Rombouts Y.; Wuhrer M. N-glycomic and transcriptomic changes associated with CDX1 mRNA expression in colorectal cancer cell lines. Cells 2019, 8, 273–294. 10.3390/cells8030273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis-Kuberka J.; Kątnik-Prastowska I.; Berghausen-Mazur M.; Orczyk-Pawilowicz M. Lectin-based analysis of fucosylated glycoproteins of human skim milk during 47 days of lactation. Glycoconjugate J. 2015, 32, 665–674. 10.1007/s10719-015-9615-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwakiel M.; Hageman J. A.; Wang W.; Szeto I. M.; van Goudoever J. B.; Hettinga K. A.; Schols H. A. Human milk oligosaccharides in colostrum and mature milk of Chinese mothers: Lewis positive secretor subgroups. J. Agric. Food Chem. 2018, 66, 7036–7043. 10.1021/acs.jafc.8b02021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelle W.; Michalski J.-C. Analysis of protein glycosylation by mass spectrometry. Nat. Protoc. 2007, 2, 1585–1602. 10.1038/nprot.2007.227. [DOI] [PubMed] [Google Scholar]
- Chu C. S.; Ninonuevo M. R.; Clowers B. H.; Perkins P. D.; An H. J.; Yin H.; Killeen K.; Miyamoto S.; Grimm R.; Lebrilla C. B. Profile of native N-linked glycan structures from human serum using high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry. Proteomics 2009, 9, 1939–1951. 10.1002/pmic.200800249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldredge D.; An H. J.; Tang N.; Waddell K.; Lebrilla C. B. Annotation of a serum N-glycan library for rapid identification of structures. J. Proteome Res. 2012, 11, 1958–1968. 10.1021/pr2011439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita Y.; Miura Y.; Furukawa J.-i.; Nakano M.; Shinohara Y.; Ohno M.; Takimoto A.; Nishimura S.-I. Quantitave glycomics of human whole serum glycoproteins based on the standardized protocol for liberating N-glycans. Mol. Cell. Proteomics 2007, 6, 1437–1445. 10.1074/mcp.t600063-mcp200. [DOI] [PubMed] [Google Scholar]
- Albrecht S.; van Muiswinkel G. C. J.; Schols H. A.; Voragen A. G. J.; Gruppen H. Introducing capillary electrophoresis with laser-induced fluorescence detection for the characterization of konjac glucomannan oligosaccharides and their in vitro fermentation behavior. J. Agric. Food Chem. 2009, 57, 3867–3876. 10.1021/jf8038956. [DOI] [PubMed] [Google Scholar]
- Tiemeyer M.; Paulson J.; York W. S.; Lisacek F.; Campbell M. P.; Fijiti A.; Shinmachi D.; Yamada I.; Ranzinger R.; Aoki-Kinoshita K. F.; et al. GlyTouCan: An accessible glycan structure repository. Glycobiology 2017, 27, 915–919. 10.1093/glycob/cwx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliegenthart J. F. G.; Dorland L.; Van Halbeek H. High resolution, 1H-nuclear magnetic resonance spectroscopy as a tool in the structural analysis of carbohydrates related to glycoproteins. Adv. Carbohydr. Chem. Biochem. 1983, 41, 209–374. 10.1016/S0065-2318(08)60059-1. [DOI] [Google Scholar]
- Plaza-Díaz J.; Fontana L.; Gil A. Human milk oligosaccharides and immune system development. Nutrients 2018, 10, 1038 10.3390/nu10081038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.