Abstract
BACKGROUND
Dural arteriovenous fistulas (DAVFs) can be categorized based on location.
OBJECTIVE
To compare stereotactic radiosurgery (SRS) outcomes between cavernous sinus (CS) and non-CS DAVFs and to identify respective outcome predictors.
METHODS
This is a retrospective study of DAVFs treated with SRS between 1988 and 2016 at 10 institutions. Patients’ variables, DAVF characters, and SRS parameters were included for analyses. Favorable clinical outcome was defined as angiography-confirmed obliteration without radiological radiation-induced changes (RIC) or post-SRS hemorrhage. Other outcomes were DAVFs obliteration and adverse events (including RIC, symptomatic RIC, and post-SRS hemorrhage).
RESULTS
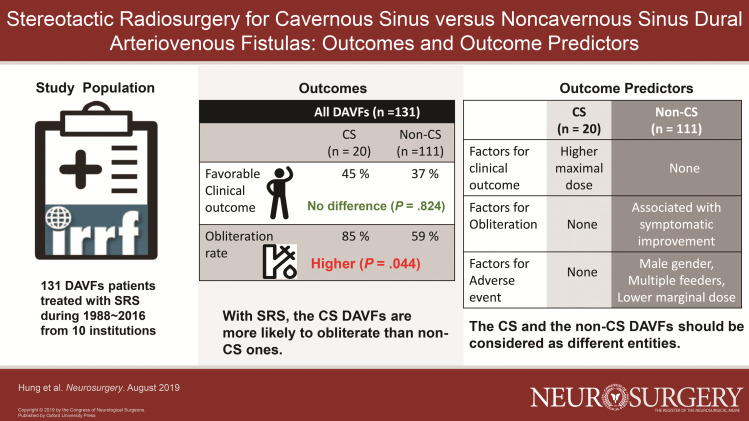
The overall study cohort comprised 131 patients, including 20 patients with CS DAVFs (15%) and 111 patients with non-CS DAVFs (85%). Rates of favorable clinical outcome were comparable between the 2 groups (45% vs 37%, P = .824). Obliteration rate after SRS was higher in the CS DAVFs group, even adjusted for baseline difference (OR = 4.189, P = .044). Predictors of favorable clinical outcome included higher maximum dose (P = .014) for CS DAVFs. Symptomatic improvement was associated with obliteration in non-CS DAVFs (P = .005), but symptoms improved regardless of whether obliteration was confirmed in CS DAVFs. Non-CS DAVFs patients with adverse events after SRS were more likely to be male (P = .020), multiple arterial feeding fistulas (P = .018), and lower maximum dose (P = .041).
CONCLUSION
After SRS, CS DAVFs are more likely to obliterate than non-CS ones. Because these 2 groups have different total predictors for clinical and radiologic outcomes after SRS, they should be considered as different entities.
Keywords: Cavernous sinus, Dural arteriovenous fistulas, Gamma Knife, Intracranial, Predictor, Radiosurgery
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- DAVFs
dural arteriovenous fistulas
- SRS
stereotactic radiosurgery
- CS
cavernous sinus
- RIC
radiation-induced changes
- CVD
cortical venous drainage
- CVR
cortical venous reflux
- MRI
magnetic resonance imaging
- DSA
digital subtraction angiography
- CTA
computed tomography-angiography
- MRA
magnetic resonance angiography
- aOR
adjusted odd ratio
- AVMs
arteriovenous malformations
Intracranial dural arteriovenous fistula (DAVFs) are thought to be connections between meningeal arteries and dural venous sinuses or cortical veins. These lesions comprise 10% to 15% of all intracranial vascular malformations.1-7 DAVFs represent a heterogeneous group of vascular lesions that vary by location, pattern of arterial supply and venous drainage, and symptomology. Similarly, interventions for DAVFs are heterogeneous, and options include endovascular embolization, surgical ligation, stereotactic radiosurgery (SRS), or multimodality therapy. Because of the immediate DAVFs obliteration offered by endovascular embolization and surgical ligation, SRS is typically reserved for lesions that cannot be obliterated with endovascular or surgical approaches, or for patients are unable to undergo embolization or surgery because of medical comorbidities.6,8-13
In addition to classification by the presence of cortical venous drainage (CVD) or cortical venous reflux (CVR), as exemplified by the Borden and Cognard grading systems, DAVFs can be categorized based on location.4,14 Cavernous sinus (CS) DAVFs are often considered to be distinct entities from non-CS DAVFs. CS DAVFs are located extradurally. Their natural history is often benign, and symptoms often include blurry vision, bruit, diplopia, exophthalmos, chemosis, and glaucoma.15 In contrast, the natural history of non-CS DAVFs is variable and dependent upon pattern of venous drainage and presence of CVD or CVR.12,16-18 Consequently, variations in pattern of arterial supply and venous drainage, and symptomology between CS and non-CS DAVFs may contribute to differences in treatment outcomes. Hence, the goals of this study are to compare outcomes between patients with CS and non-CS DAVFs and to identify outcome predictors in these patients treated with SRS in a multicenter cohort.
METHODS
Study Design and Participants
Retrospectively, patients who underwent SRS for DAVFs between 1988 and 2016 at 10 institutions participating (6 in the United States, 2 in Canada, 1 in Czech Republic, and 1 in Puerto Rico) in the International Radiosurgery Research Foundation (IRRF) were identified and reviewed under the approval of the institutional review boards at individual institutions. The consent form was not required. The criteria for inclusion were patients with intracranial DAVFs treated with SRS with sufficient baseline data to assess demographic information, fistula angioarchitecture, radiosurgery parameters, ≥6 mo of neuroimaging, and clinical follow-up, and SRS performed in a single session. Patients who had procedure- or DAVFs-related complications within 6 mo of follow-up were also included. The data from each individual institution were de-identified and pooled by an independent third party. Discrepancies and ambiguities in data were addressed by the respective contributing institutions. The pooled data were subsequently sent to the institution of the first and senior authors for statistical analysis and drafting of the initial manuscript.
Baseline Data and Variables
The baseline data comprised patient demographic, DAVFs angioarchitecture, and SRS variables. The patient demographic variables included were age, sex, presenting symptoms, hemorrhagic presentation, and prior DAVFs treatment (microsurgery or endovascular embolization). DAVFs angioarchitecture variables comprised DAVFs location (CS or non-CS), presence of multiple arterial feeding fistula, Borden grade, presence of CVD or CVR, presence of ante-grade or retrograde venous sinus drainage, presence of venous ectasia, and presence of spinal venous drainage. SRS variables included iso-dose line and margin and maximum dose.
SRS Technique
SRS was performed using the Gamma Knife unit (Elekta AB, Stockholm, Sweden), and the specific model used (including models U, B, C, 4C, Perfexion) differed by year and availability at each participating institution. Briefly, under local or monitored anesthesia, a Leksell Model G stereotactic frame (Elekta AB, Stockholm, Sweden) was affixed to the calvarium. When magnetic resonance imaging (MRI) was contraindicated, digital subtraction angiography (DSA) and thin-slice MRI with intravenous contrast or computed tomography-angiography (CTA) were performed for fistula definition and radiosurgical planning. All DAVFs were treated with the intention for complete obliteration, and no DAVF was treated for palliation. Dose planning and SRS delivery were performed by multidisciplinary teams comprising radiation oncologists, medical physicists, and neurosurgeons.
Clinical and Neuroimaging Follow-Up
Clinical and neuroimaging assessments were generally performed at intervals of 6 mo for the first 2 yr after radiosurgery, and then yearly afterwards using MRI and magnetic resonance angiography (MRA). When MRI/MRA was contraindicated, CTA was performed instead. Additional neuroimaging may be performed in patients with neurological changes during the follow-up period. Once MRI/MRA was suggestive of DAVFs obliteration, the patient was recommended to undergo DSA to confirm obliteration. All images were reviewed by the treating clinical team. Patients were recommended to undergo additional neuroimaging follow-ups at intervals of 1 to 5 yr after DAVFs obliteration long-term complication surveillance. Whenever possible, clinical follow-up was obtained concurrently with routine neuroimaging follow-up. When in-person follow-up was not feasible, clinical and neuroimaging data from other institutions or physicians were transferred to the treating institution for review. All clinical and neuroimaging data were compared with data obtained at the time of SRS.
Outcomes
Favorable clinical outcome of the study was defined as DAVFs obliteration confirmed on DSA without radiological radiation-induced changes (RIC) or post-SRS hemorrhage and radiological follow-up ≥12 mo. DAVFs obliteration on MRI and MRA was defined as absence of abnormal flow voids and regression of abnormal contrast enhancement. Definitive DAVFs obliteration was confirmed on DSA by the lack of abnormal early arteriovenous shunting. Radiologically evident RIC was defined as peri-DAVFs hyperintensity on T2 or fluid-attenuated inversion recovery MRI sequences. Permanent symptomatic RIC was radiologically evident RIC associated with a persist deterioration of neurological status. Functional outcomes were favorable clinical outcome and symptomatic improvement. Radiological outcomes were DAVFs obliteration (on MRI/MRA or DSA) and DAVFs obliteration confirmed on DSA. Adverse events recorded were RIC, permanent symptomatic RIC, post-SRS hemorrhage, and all adverse events.
Statistical Analysis
All statistical analyses were performed using SPSS (version 24.0, IBM Corporation, Armonk, New York). Calculations of normality were assessed graphically and carried out by ladder of powers. Patients were dichotomized based on DAVFs location into CS and non-CS DAVFs groups. Baseline variables between these 2 groups were compared using Pearson's chi-square or Fisher's exact tests and independent t or Wilcoxon rank-sum tests for categorical and continuous variables, respectively, as appropriate. Functional outcome, radiological outcomes, and adverse events between CS and non-CS DAVFs groups were compared using univariate binary logistic regression analysis or Fisher's exact test, as appropriate.
To identify predictors of favorable clinical outcome, DAVFs obliteration, and adverse events in CS and non-CS DAVFs groups, patients in each group were dichotomized based on achievement of favorable clinical outcome, DAVFs obliteration, and adverse events. Univariate comparisons of baseline variables were performed using binary logistic regression analysis or Fisher's exact test, as appropriate. Baseline variables with P < .15 were entered into multivariate binary logistic regression models to identify independent predictors of favorable clinical outcome, DAVFs obliteration, and adverse events in CS and non-CS DAVFs groups. Statistical significance was defined as a P value of <.05, and all tests were 2 sided.
RESULTS
Study Cohort and Participants
After exclusion of 16 patients without neuroimaging follow-up of ≥6 mo from the original database of 147 patients, the remaining study cohort for analysis comprised 131 patients, including 20 patients with CS DAVFs (15%) and 111 patients with non-CS DAVFs (85%). Table 1 compares the baseline patient demographics, DAVFs angioarchitecture, and SRS parameters between the 2 groups. The CS DAVFs group had more females (70% vs 39%, P = .009). Rates of previous embolization (20% vs 49%, P = .015), and hemorrhagic presentation (5% vs 23%, P = .048) were lower in the CS DAVFs group. CVD was more frequently encountered in non-CS DAVFs (36% vs 10%, P = .016). Table 2 outlines the specific presenting symptoms of patients with CS and non-CS DAVFs.
TABLE 1.
Comparison of Baseline Patient Demographic, DAVFs Angioarchitecture, and SRS Variables between CS and Non-CS DAVFs
| Variables | CS DAVFs (n = 20) | Non-CS DAVFs (n = 111) | P value |
|---|---|---|---|
| Demographic variables | |||
| Female sex, n (%) | 14/20 (70%) | 43/111 (39%) | .009 |
| Age, median/mean yr (SD) | 63/60 (11.9) | 56/54 (13.5) | .058a |
| Previous embolization, n (%) | 4/20 (20%) | 54/111 (49%) | .015 |
| Previous surgery, n (%) | 0/20 (0%) | 5/111 (5%) | .431 |
| Hemorrhagic presentation, n (%) | 1/20 (5%) | 26/111 (23%) | .048 |
| DAVF angioarchitecture, n (%) | |||
| Multi-hole fistula | 5/20 (25%) | 41/111 (37%) | .303 |
| Borden grade I | 11/20 (55%) | 34/104 (33%) | .057 |
| Cortical venous reflex | 7/20 (35%) | 61/111 (55%) | .100 |
| Cortical venous drainage | 2/20 (10%) | 40/111 (36%) | .016 |
| Antegrade flow within sinus | 9/20 (45%) | 53/111 (48%) | .821 |
| Retrograde flow within sinus | 4/20 (20%) | 25/111 (23%) | .802 |
| Venous ectasia | 4/20 (20%) | 27/111 (24%) | .461 |
| Spinal drainage | 3/20 (15%) | 20/111 (18%) | .459 |
| SRS parameters | |||
| Margin dose, median/mean Gy (SD) | 22/22 (4.3) | 21/21(3.0) | .559a |
| Maximum dose > 45 Gy, n (%) | 9/20 (45%) | 29/111 (26%) | .087 |
| Isodose line, median/mean % (SD) | 50/51 (8.6) | 50/54 (11.0) | .166a |
| Radiological follow-up, median/mean mo (SD) | 34/30 (17.5) | 26/36 (34.1) | .523a |
| Clinical follow-up, median/mean mo (SD) | 34/31 (17.5) | 31/44 (40.4) | .015 a |
CS = cavernous sinus; DAVFs = dural arteriovenous fistulas; SRS = stereotactic radiosurgery; n = number; SD = standard deviation; mo = months.
aIndependent t-test.
Boldface type indicates statistical significance.
TABLE 2.
Clinical Presentations of Patients with CS and Non-CS DAVFs
| Clinical presentation, n (%) | CS DAVFs (n = 20) | Non-CS DAVFs (n = 111) |
|---|---|---|
| Asymptomatic | 1/20 (5%) | 12/111 (11%) |
| Headache | 9/20 (45%) | 59/111 (53%) |
| Tinnitus | 7/20 (35%) | 36/111 (32%) |
| Visual deficit | 13/20 (65%) | 22/111 (20%) |
| Seizure | 0/20 (0%) | 10/111 (9%) |
| Focal neurological deficit | 9/20 (45%) | 34/111 (31%) |
| Bruit | 9/20 (45%) | 2/111 (2%) |
| Ptosis | 5/20 (25%) | 5/111 (5%) |
CS = cavernous sinus; DAVFs = dural arteriovenous fistulas.
Outcome Comparison between CS and Non-CS DAVFs Groups
Table 3 compares the outcomes between the 2 groups. Rates of favorable clinical outcome were comparable between the 2 groups after SRS. The rates remained comparable even after adjustments for baseline differences. Obliteration rate after SRS was higher in the CS DAVFs group (85%) compared to the non-CS DAVFs group (59%) (odd ratio [OR] = 4.010, P = .034). Obliterate rate for the CS DAVFs group remained higher after adjustments for baseline differences (adjusted odd ratio [aOR] = 4.189, P = .044). Rates of DSA-confirmed obliteration and symptomatic improvement were comparable between the groups. Although rate of all RIC showed significance after adjustments for baseline differences, most of the RICs were radiological or transient. Rate of permanent symptomatic RIC was higher in the CS DAVFs group (OR = 18.562, P = .014). However, this difference was no longer significant after the adjustment for baseline differences (OR = 12.319, P = .091).
TABLE 3.
Comparison of Outcomes between Patients with CS and Non-CS DAVFs
| CS DAVFs (n = 20) | Non-CS DAVFs (n = 111) | Unadjusted OR (95% CI) | Unadjusted P value | Adjusted ORb (95% CI) | AdjustedbP value | |
|---|---|---|---|---|---|---|
| Functional outcome | ||||||
| Favorable clinical outcome, n (%) | 9/20 (45%) | 41/111 (37%) | 1.397 (0.534-3.654) | .496 | 1.133 (0.377-3.410) | .824 |
| Symptomatic improvement, n (%) | 16/20 (80%) | 67/87 (77%) | 1.194 (0.358-3.981) | .773 | 2.617 (0.562-12.187) | .220 |
| Radiological outcomes | ||||||
| Obliteration, n (%) | 17/20 (85%) | 65/111 (59%) | 4.010 (1.110-14.484) | .034 | 4.189 (1.041-16.850) | .044 |
| Obliteration on DSA, n (%) | 13/16 (81%) | 50/71 (70%) | 1.820 (0.470- 7.055) | .386 | 2.468 (0.814-7.485) | .111 |
| Adverse events | ||||||
| All RICa, n (%) | 5/19 (26%) | 14/105 (13%) | 2.321 (0.723-7.449) | .157 | 9.871 (1.793-54.338) | .009 |
| Permanent Sym. RIC, n (%) | 3/19 (16%) | 1/100 (1%) | 18.562 (1.817-189.637) | .014 | 12.319 (0.673-225.611) | .091 |
| Post-SRS hemorrhage, n (%) | 0/20 (0%) | 4/108 (4%) | – | .502c | – | – |
| All adverse events, n (%) | 5/20 (25%) | 18/109 (17%) | 1.722 (0.556-5.337) | .346 | 4.128 (0.992-17.182) | .051 |
CS = cavernous sinus; DAVFs = dural arteriovenous fistulas; OR = odds ratio; n = number; CI = confidence interval; DSA = digital subtraction angiography; RIC = radiation-induced changes; Sym. RIC = symptomatic RIC; SRS = stereotactic radiosurgery.
aAll RIC: all radiation induced change, including radiological, transient and permanent RIC.
bAdjusted for covariates of: female sex, age, previous embolization, hemorrhagic presentation, Borden grade I, cortical venous drainage, cortical venous reflex, and maximum dose >45 Gy.
cFisher's exact test.
Boldface type indicates statistical significance.
Predictors of Favorable Clinical Outcome
Table 4 describes the predictors of favorable clinical outcome in patients with CS and non-CS DAVFs after SRS. In the CS DAVFs group, maximum dose >45 Gy was associated with favorable clinical outcome (78% vs 18%, P = .014), and a lower rate of symptomatic improvement was associated with favorable clinical outcome (56% vs 100%, P = .026). Counterintuitive, in the non-CS DAVFs group, higher rate of symptomatic improvement was associated with favorable clinical outcome in the univariate model (78% vs 49%, P = .034) but failed statistical significance in the multivariable model (aOR = 3.428, P = .050). Longer clinical follow-up was associated with the favorable clinical outcome in the non-CS DAVFs group (mean 39 vs 36 mo, P = .010). However, in the multivariate model, this approached significance (aOR = 1.02, P = .050).
TABLE 4.
Predictors of Favorable Clinical Outcome for CS and Non-CS DAVFs
| CS DAVFs (n = 20) | Non-CS DAVFs (n = 111) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Favorable clinical outcomeb | Univariable | Multivariable | Favorable clinical outcomeb | Univariable | Multivariable | |||||||
| Variables | Yes (n = 9) | No (n = 11) | OR (95% CI) | P value | OR (95% CI) | P value | Yes (n = 41) | No (n = 70) | OR (95% CI) | P value | OR (95% CI) | P value |
| Maximum dose >45 Gy, n (%) | 7 (78%) | 2 (18%) | 15.750 (1.754-141.404) | .014 | – | – | 11 (27%) | 18 (25%) | 1.059 (0.422-2.539) | .897 | – | – |
| Margin dose, mean Gy (SD) | 23(5.5) | 21(3.1) | 1.092 (0.865-1.378) | .460 | – | – | 21 (2.6) | 21 (3.2) | 0.985 (0.863-1.124) | .821 | – | – |
| Symptomatic improvement, n (%) | 5 (56%) | 11(100%) | - | .026 a | – | – | 32(78%) | 35(49%) | 3.657 (1.106-12.093) | .034 | 3.428 (0.998-11.783) | .050 |
| Radiological follow-up mean mo (SD) | 29 (10.3) | 31 (22.2) | 0.994 (0.943-1.047) | .812 | – | – | 36 (31.0) | 32 (35.4) | 1.010 (0.998-1.022) | .098 | 0.985 (0.962-1.008) | .196 |
| Clinical follow-up mean mo (SD) | 29 (10.3) | 31 (22.2) | 0.994 (0.943-1.047) | .812 | – | – | 39 (39.9) | 36 (38.9) | 1.014 (1.003-1.024) | .010 | 1.021 (1.000-1.043) | .050 |
CS = cavernous sinus; DAVFs = dural arteriovenous fistulas; OR = odds ratio; CI = confidence interval; n = number; SD = standard deviation; mo = months.
aFisher's exact test.
bFavorable clinical outcome: defined as DAVFs obliteration confirmed on digital subtraction angiography (DSA) without radiological radiation-induced changes (RIC) or post- stereotactic radiosurgery hemorrhage and follow-up ≥12 mo.
Only factors with P < .15 in the univariable analysis were listed in the multivariable analysis.
Boldface type indicates statistical significance.
Predictors of DAVFs Obliteration Confirmed on DSA
Table 5 describes the predictors of DAVFs obliteration for CS and non-CS DAVFs after SRS. In the CS DAVFs group, no specific predictor of obliteration was identified. In the non-CS DAVFs group, higher rates of symptomatic improvement were associated with obliteration in both the univariate (71% vs 46%, P = .003) and multivariate (aOR = 5.193, P = .005) models.
TABLE 5.
Predictors of DAVF Obliteration for CS and Non-CS DAVFs
| CS DAVFs (n = 20) | Non-CS DAVFs (n = 111) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAVFs obliteration | Univariable | Multivariable | DAVFs obliteration | Univariable | Multivariable | |||||||
| Variables | Yes (n = 17) | No (n = 3) | OR (95% CI) | P value | OR (95% CI) | P value | Yes (n = 65) | No (n = 46) | OR (95% CI) | P value | OR (95% CI) | P value |
| Venous ectasia, n (%) | 3 (18%) | 1 (33%) | 0.429 (0.029-6.409) | .539 | – | – | 12 (18%) | 15 (33%) | 0.468 (0.194-1.127) | .090 | 0.296 (0.083-1.053) | .060 |
| Maximum dose >45 Gy, n (%) | 9 (53%) | 0 (0%) | – | .491a | – | – | 14 (22%) | 15 (33%) | 0.433 (0.149-1.253) | .122 | 0.782 (0.271- 2.254) | .649 |
| Margin dose, mean Gy (SD) | 22 (4.5) | 19 (1.2) | 1.155 (0.883-1.510) | .293 | – | – | 21 (2.6) | 21 (3.5) | 1.002 (0.881-1.139) | .976 | – | – |
| Symptomatic improvement, n (%) | 13 (76%) | 3 (100%) | – | .491a | – | – | 46 (71%) | 21 (46%) | 5.111 (1.724-13.153) | .003 | 5.193 (1.657-16.277) | .005 |
| Clinical follow-up mean mo (SD) | 31 (17.8) | 29 (19.5) | 1.006 (0.932-1.086) | .873 | – | – | 49 (38.8) | 37 (42.1) | 1.008 (0.997-1.018) | .140 | 1.007 (0.995-1.019) | .253 |
CS = cavernous sinus; DAVFs = dural arteriovenous fistulas; OR = odds ratio; CI = confidence interval; n = number; SD = standard deviation; mo = months.
aFisher's exact test.
Only factors with P < .15 in the univariable analysis were listed in the multivariable analysis.
Boldface type indicates statistical significance.
Predictors of Adverse Events
Table 6 describes the predictors of adverse events for CS and non-CS DAVFs after SRS. In the current study, 19 patients with RIC (included 13 patients with radiologic RIC, 2 patients with temporary symptomatic RIC, and 4 patients with permanent symptomatic RIC) and 4 patients with post-SRS hemorrhage were recorded. No patient demographic, DAVF angioarchitectural, or radiosurgical predictors of adverse event for CS DAVFs were identified. In the non-CS DAVF group, patients without adverse events after SRS were more likely to be female in both the univariate (45% vs 11%, P = .016) and multivariate models (aOR = 0.111, P = .020). Multiple arterial feeding (multihole) fistulas were associated with adverse events in both the univariate (61% vs 31%, P = .018) and multivariate (aOR = 5.416, P = .018) models. Higher maximum dose (>45 Gy) were associated with less adverse events in multivariate (aOR = 0.135, P = .041) models. Patients without adverse events had longer clinical follow-up compared to those with adverse events in the multivariate model (aOR = 0.975, P = .015). CVR (P = .035) and CVD (P = .049) were the only univariate predictors of adverse events.
TABLE 6.
Predictors of Adverse Events for CS and Non-CS DAVFs
| CS DAVF (n = 20) | Non-CS DAVF (n = 111) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adverse event | Univariable | Multivariable | Adverse event | Univariable | Multivariable | |||||||
| Variables | Yes (n = 5) | No (n = 15) | OR (95% CI) | P value | OR (95% CI) | P value | Yes (n = 18) | No (n = 91) | OR (95% CI) | P value | OR (95% CI) | P value |
| Female sex, n (%) | 3 (60%) | 11 (73%) | 0.545 (0.065-4.562) | .576 | – | – | 2 (11%) | 41 (45%) | 0.152 (0.033-0.702) | .016 | 0.111 (0.018-0.703) | .020 |
| Age, mean yr (SD) | 64 (12.0) | 59 (12.1) | 1.042 (0.941-1.153) | .432 | – | – | 49 (12.8) | 56 (13.2) | 0.967 (0.932-1.003) | .071 | 0.964 (0.918-1.011) | .132 |
| Hemorrhagic presentation, n (%) | 0 (0%) | 1 (7%) | – | .750b | – | – | 7 (39%) | 18 (20%) | 2.581 (0.877-7.591) | .085 | 3.370 (0.808-14.055) | .095 |
| Multi-hole fistulaa, n (%) | 2 (40%) | 3 (20%) | 2.667 (0.298-23.858) | .380 | – | – | 11 (61%) | 28 (31%) | 3.536 (1.241-10.073) | .018 | 5.416 (1.333-22.005) | .018 |
| Borden grade I, n (%) | 3 (60%) | 8 (53%) | 1.313 (0.168-10.264) | .796 | – | – | 3 (17%) | 31 (34%) | 0.342 (0.092-1.275) | .110 | 1.100 (0.054-22.210) | .951 |
| Cortical venous reflux, n (%) | 2 (40%) | 5 (33%) | 1.333 (0.165-10.743) | .787 | – | – | 14 (78%) | 45 (49%) | 3.578 (1.094-11.699) | .035 | 2.024 (0.123-33.408) | .622 |
| Cortical venous drainage, n (%) | 1 (20%) | 1 (7%) | 3.500 (0.177-69.339) | .411 | – | – | 10 (56%) | 28 (31%) | 2.813 (1.003-7.884) | .049 | 1.127 (0.233-5.461) | .882 |
| Maximum dose >45 Gy, n (%) | 2 (40%) | 7 (47%) | 0.762 (0.097-5.958) | .796 | – | – | 2 (11%) | 29 (29%) | 0.313 (0.067-1.456) | .138 | 0.135 (0.020- 0.921) | .041 |
| Margin dose, mean Gy (SD) | 24 (3.3) | 21 (4.5) | 1.179 (0.851-1.634) | .322 | – | – | 22 (2.6) | 21 (3.1) | 1.105 (0.932-1.310) | .251 | – | – |
| Clinical follow-up mean mo (SD) | 18 (5.5) | 35 (18.2) | 0.893 (0.795-1.004) | .058 | – | – | 31 (32.9) | 47.4 (41.6) | 0.986 (0.968-1.004) | .123 | 0.975 (0.959-0.995) | .015 |
CS = cavernous sinus; DAVFs = dural arteriovenous fistulas; OR = odds ratio; CI = confidence interval; n = number; SD = standard deviation; Multi-hole fistulaa = multiple arterial feeding; mo = months.
aFavorable clinical outcome: defined as DAVFs obliteration confirmed on digital subtraction angiography (DSA) without radiological radiation-induced changes (RIC) or post-stereotactic radiosurgery hemorrhage and follow-up ≥ 12 mo.
bFisher's exact test
Only factors with P < .15 in the univariable analysis were listed in the multivariable analysis.
Boldface type indicates statistical significance.
Treatments after SRS
In CS DAVFs group, 2 patients received further endovascular therapy. In the non-CS DAVFs group, 11 patients received endovascular therapy, one patient underwent surgery, and 4 patients had repeat SRS.
DISCUSSION
SRS for DAVFs
Despite the 70% to 90% obliteration rates that can be achieved with embolization for DAVFs in the literature, obliteration remains challenging in a subset of patients harboring complex DAVFs with multiple arterial feeders and CVD.19-27 SRS has become an important adjuvant/salvage therapy for DAVFs since the initial reports published by Barcia-Salorio et al28,29 in 1982 and 1994. The current study is the largest series to date and proposed that SRS provided 85% and 59% obliteration rates for CS and non-CS DAVFs. Obliteration rate after SRS was higher in CS DAVFs, even adjusted for baseline difference (OR = 4.189, P = .044).
In the literature, the obliteration rate of CS and non-CS DAVF were 70% to 94% and 59% to 64%, respectively.18,27,30 The possible reasons could be that CS DAVFs might have been detected earlier because of visual and facial symptoms and tend to be smaller in size, more tolerant of higher radiation dose because of their extradural location. However, the conclusion could not be made because of the limitation of literature and case numbers. In a meta-analysis of 323 CS DAVFs and 377 non-CS DAVFs, despite the higher of obliteration for CS DAVFs compared to non-CS DAVFs (73% vs 58%), Chen et al9 found no difference in obliteration rates between the 2 groups (OR = 1.72, P = .27). The current study supports the prognostic role of CS location in SRS for DAVFs.
Prognostic Factors in CS and Non-CS DAVFs
In the current study, outcome predictors in CS and non-CS DAVFs were analyzed separately with multivariate model. Higher maximum dose was associated with favorable clinical outcome in the CS DAVFs group, whereas symptomatic improvement was associated with obliteration in the non-CS DAVFs group. The discrepancies between symptomatic improvement and DAVFs obliteration were reported in several studies, especially in non-CS DAVFs.12,27,30 In a meta-analysis of 349 DAVFs without CVR or CVD treated with SRS, Tonetti et al31 observed resolution of pulsatile tinnitus in 77% of the patients, with 79% DAVF (mainly located in transverse-sigmoid sinus) obliteration rate. In the same study, for CS DAVF patients, the ocular symptoms improved rates were 95%, 90%, and 96% for chemosis, ophthalmoparesis, and proptosis. However, the obliteration was achieved in only 76.2%, the same as the finding of the current study.
Optimal Therapeutic Dose
The optimal radiation dose for DAVFs is not concluded. Maximum central radiation doses between 18 and 38 Gy have been prescribed in some study,27,32 and up to 50 Gy in others.12,18,30,33 Because of the limited literature, we extrapolated on the pathophysiology of radiation effects on brain arteriovenous malformations (AVMs). The possible mechanisms of SRS to vascular lesions include smooth muscle cell proliferation and extracellular collagen production, with resulting vascular narrowing and eventual obliteration.34-36 The role of maximum dose remains somewhat controversial. Dose heterogeneity with “hot spots” in the target volume and the use of isodose lines around 50% have been associated with certain radiobiological advantages. Given the extraparenchymal SRS targets for CS DAVFs, higher radiation doses may be considered. However, radiation dose to the nearby cranial nerves should be limited. In the treatment plan of AVM, treatment volume is an important factor. Nevertheless, in the current study, treatment volume was not considered because of several reasons. First, these cases were accrued over more than 3 decades. Some of the early planning was done with angiogram alone or angiogram and CT. Later, MRI-based planning was routine. The measurement variations of DAVFs on these different imaging modalities would lead to appreciable bias. Also, early GK planning did not use contouring of the target and many plans cannot be reconstructed to permit this calculation retrospectively. Finally, for SRS outcomes, DAVFs, which are on the surface of the brain, may not have the same importance with regard to nidus volume that AVMs have.
All adverse effects were observed in 25% and 17% of CS and non-CS DAVFs in the current study. Unlike SRS for AVMs, increasing margin dose within the range utilized did not correlate with radiation toxicity in our DAVFs study (Figures, Supplemental Digital Content 1 and 2). Despite higher rate of adverse events in the CS DAVFs group, maximum dose >45 Gy in this group was not associated with adverse events. In non-CS DAVFs group, high maximum dose even associated with less adverse events in multivariate analysis. Although no independent predictors of adverse events were identified in the CS DAVFs group, factors that influence adverse event rates in the non-CS DAVFs group included female sex, multiple arterial feeding, maximal dose, and clinical follow-up.
SRS for DAVFs and AVMs
Although SRS has the therapeutic effect to close the nidi of AVMs and the shunting within DAVFs, they have important distinctions. SRS is considered a first-line treatment for small to moderately sized AVMs. Embolization would only be considered for flow-related aneurysm, hemorrhage, or combined treatment for large AVMs or as a precursor to resection.37 On the other hand, SRS is often reserved for DAVFs that cannot be obliterated with endovascular or surgical approaches.6,8-13. In the treatment of AVMs, a margin dose below 18 Gy appears to decrease the obliteration rate.27,37 In the current study of DAVF, the dose-related decrease of obliteration rate at doses below 18 Gy was not observed. The obliteration rates of AVMs were 56%, 80%, and 82% at 3, 5, and 10 yr in a series that included 996 patients.38 The obliteration rates of DAVFs were 41%, 61%, and 82% at 3, 5, and 10 yr, respectively, proposed by Starke et al,39 with the same database of the current study. The overall annual hemorrhage rates after SRS were 1.3% and 0.9% in AVM and DAVF studies, respectively.38,39
Study Limitations
As with any retrospective study, the current study is subject to the inherent biases at each contributing institution including selection and referral biases. Our results are contingent upon the accuracy and reliability data provided by each contributing institution. Therefore, this study may be subject to reporting bias. Because of the rarity of DAVFs, the sample size of this multicenter study remains relatively small in comparison to the AVM counterparts.40,41 Hence, statistical power may be limited. The third, the obliteration of DAVFs was evaluated with MRI/MRA and DSA, not DSA alone. Although this result was slightly less reliable, MRI/MRA also had a high correlation with obliteration rate in vascular lesions.42 The fourth, the treated volume, as well as volume of tissue receiving 12 Gy, was not considered as SRS parameters in the current study. In addition, findings of the functional/radiological outcomes, and subsequent analyses must be interpreted with caution, because the multiple tests performed in the current study may be associated with an elevated false discovery rate. The definition of favorable clinical outcome, which considers asymptomatic imaging changes to be an unfavorable event, could also lower the perceived effectiveness of the intervention. Lastly, the results of the current study may not be generalizable to all DAVFs, as SRS often serve as adjuvant or salvage therapy for DAVFs, and these patients may represent a highly selected group.
CONCLUSION
Rates of favorable clinical outcome were comparable between CS and non-CS DAVFs after SRS. Obliteration rate after SRS was higher in the CS DAVFs group, even adjusted for baseline difference. Because these 2 groups have different total predictors for clinical and radiologic outcomes after SRS, they should be considered as different entities.
Disclosures
Dr Grills reports having stock ownership and serving on the Board of Directors in a company called Greater Michigan Gamma Knife and receiving funding for nonstudy-related research from Elekta through her institution. Dr Lunsford reports owning stock in Elekta AB. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Supplemental Digital Content 1. Figure. Kjellberg Curves of maximum dose to DAVF obliteration rates and adverse event rates.
Supplemental Digital Content 2. Figure. Kjellberg Curve of margin dose to DAVF obliteration rates and adverse event rates.
REFERENCES
- 1. Chaudhary MY, Sachdev VP, Cho SH, Weitzner I Jr, Puljic S, Huang YP. Dural arteriovenous malformation of the major venous sinuses: an acquired lesion. AJNR Am J Neuroradiol. 1982;3(1):13-19. [PMC free article] [PubMed] [Google Scholar]
- 2. Houser OW, Campbell JK, Campbell RJ, Sundt TM Jr. Arteriovenous malformation affecting the transverse dural venous sinus–an acquired lesion. Mayo Clin Proc. 1979;54(10):651-661. [PubMed] [Google Scholar]
- 3. Nishijima M, Takaku A, Endo S et al.. Etiological evaluation of dural arteriovenous malformations of the lateral and sigmoid sinuses based on histopathological examinations. J Neurosurg. 1992;76(4):600-606. [DOI] [PubMed] [Google Scholar]
- 4. Cognard C, Gobin YP, Pierot L et al.. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194(3):671-680. [DOI] [PubMed] [Google Scholar]
- 5. Gupta A, Periakaruppan A. Intracranial dural arteriovenous fistulas: a review. Indian J Radiol Imaging. 2009;19(1):43-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soderman M, Edner G, Ericson K et al.. Gamma knife surgery for dural arteriovenous shunts: 25 years of experience. J Neurosurg. 2006;104(6):867-875. [DOI] [PubMed] [Google Scholar]
- 7. Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology. 1969;93(5):1071-1078. [DOI] [PubMed] [Google Scholar]
- 8. Pan DH, Wu HM, Kuo YH, Chung WY, Lee CC, Guo WY. Intracranial dural arteriovenous fistulas: natural history and rationale for treatment with stereotactic radiosurgery. Prog Neurol Surg. 2013;27:176-194. [DOI] [PubMed] [Google Scholar]
- 9. Chen CJ, Lee CC, Ding D et al.. Stereotactic radiosurgery for intracranial dural arteriovenous fistulas: a systematic review. J Neurosurg. 2015;122(2):353-362. [DOI] [PubMed] [Google Scholar]
- 10. Piippo A, Niemela M, van Popta J et al.. Characteristics and long-term outcome of 251 patients with dural arteriovenous fistulas in a defined population. J Neurosurg. 2013;118(5):923-934. [DOI] [PubMed] [Google Scholar]
- 11. Oh JT, Chung SY, Lanzino G et al.. Intracranial dural arteriovenous fistulas: clinical characteristics and management based on location and hemodynamics. J Cerebrovasc Endovasc Neurosurg. 2012;14(3):192-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanakita S, Koga T, Shin M, Shojima M, Igaki H, Saito N. Role of Gamma Knife surgery in the treatment of intracranial dural arteriovenous fistulas. J Neurosurg. 2012;117(Special_Suppl):158-163. [DOI] [PubMed] [Google Scholar]
- 13. Yang H, Kano H, Kondziolka D et al.. Stereotactic radiosurgery with or without embolization for intracranial dural arteriovenous fistulas. Prog Neurol Surg. 2013;27:195-204. [DOI] [PubMed] [Google Scholar]
- 14. Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82(2):166-179. [DOI] [PubMed] [Google Scholar]
- 15. Awad IA, Little JR, Akarawi WP, Ahl J. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg. 1990;72(6):839-850. [DOI] [PubMed] [Google Scholar]
- 16. van Dijk JM, terBrugge KG, Willinsky RA, Wallace MC. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke. 2002;33(5):1233-1236. [DOI] [PubMed] [Google Scholar]
- 17. Hamada Y, Goto K, Inoue T et al.. Histopathological aspects of dural arteriovenous fistulas in the transverse-sigmoid sinus region in nine patients. Neurosurgery. 1997;40(3):452-456; discussion 456–458. [DOI] [PubMed] [Google Scholar]
- 18. Cifarelli CP, Kaptain G, Yen CP, Schlesinger D, Sheehan JP. Gamma knife radiosurgery for dural arteriovenous fistulas. Neurosurgery. 2010;67(5):1230-1235; discussion 1235. [DOI] [PubMed] [Google Scholar]
- 19. Lv X, Li Y, Jiang C, Wu Z. Endovascular treatment of brain arteriovenous fistulas. AJNR Am J Neuroradiol. 2009;30(4):851-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu YC, Newman CB, Dashti SR, Albuquerque FC, McDougall CG. Cranial dural arteriovenous fistula: transarterial Onyx embolization experience and technical nuances. J Neurointerv Surg. 2011;3(1):5-13. [DOI] [PubMed] [Google Scholar]
- 21. Macdonald JH, Millar JS, Barker CS. Endovascular treatment of cranial dural arteriovenous fistulae: a single-centre, 14-year experience and the impact of Onyx on local practise. Neuroradiology. 2010;52(5):387-395. [DOI] [PubMed] [Google Scholar]
- 22. Abud TG, Nguyen A, Saint-Maurice JP et al.. The use of Onyx in different types of intracranial dural arteriovenous fistula. AJNR Am J Neuroradiol. 2011;32(11):2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Natarajan SK, Ghodke B, Kim LJ, Hallam DK, Britz GW, Sekhar LN. Multimodality treatment of intracranial dural arteriovenous fistulas in the Onyx era: a single center experience. World Neurosurg. 2010;73(4):365-379. [DOI] [PubMed] [Google Scholar]
- 24. van Rooij WJ, Sluzewski M. Curative embolization with Onyx of dural arteriovenous fistulas with cortical venous drainage. AJNR Am J Neuroradiol. 2010;31(8):1516-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gonzalez LF, Chalouhi N, Jabbour P, Teufack S, Albuquerque FC, Spetzler RF. Rapid and progressive venous thrombosis after occlusion of high-flow arteriovenous fistula. World Neurosurg. 2013;80(6):e359-e365. [DOI] [PubMed] [Google Scholar]
- 26. Bink A, Goller K, Luchtenberg M et al.. Long-term outcome after coil embolization of cavernous sinus arteriovenous fistulas. AJNR Am J Neuroradiol. 2010;31(7):1216-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan DH, Lee CC, Wu HM, Chung WY, Yang HC, Lin CJ. Gamma Knife radiosurgery for the management of intracranial dural arteriovenous fistulas. Acta Neurochir Suppl. 2013;116:113-119. [DOI] [PubMed] [Google Scholar]
- 28. Barcia-Salorio JL, Soler F, Barcia JA, Hernandez G. Stereotactic radiosurgery for the treatment of low-flow carotid-cavernous fistulae: results in a series of 25 cases. Stereotact Funct Neurosurg. 1994;63(1-4):266-270. [DOI] [PubMed] [Google Scholar]
- 29. Barcia-Salorio JL, Herandez G, Broseta J, Gonzalez-Darder J, Ciudad J. Radiosurgical treatment of carotid-cavernous fistula. Appl Neurophysiol. 1982;45(4-5):520-522. [DOI] [PubMed] [Google Scholar]
- 30. Yang HC, Kano H, Kondziolka D et al.. Stereotactic radiosurgery with or without embolization for intracranial dural arteriovenous fistulas. Neurosurgery. 2010;67(5):1276-1285; discussion 1284-1275. [DOI] [PubMed] [Google Scholar]
- 31. Tonetti DA, Gross BA, Jankowitz BT et al.. Stereotactic radiosurgery for dural arteriovenous fistulas without cortical venous reflux. World Neurosurg. 2017;107:371-375. [DOI] [PubMed] [Google Scholar]
- 32. Park SH, Park KS, Kang DH, Hwang JH, Hwang SK. Stereotactic radiosurgery for dural carotid cavernous sinus fistulas. World Neurosurg. 2017;106:836-843. [DOI] [PubMed] [Google Scholar]
- 33. Wu HM, Pan DH, Chung WY et al.. Gamma Knife surgery for the management of intracranial dural arteriovenous fistulas. J Neurosurg. 2006;105(Suppl):43-51. [DOI] [PubMed] [Google Scholar]
- 34. Schneider BF, Eberhard DA, Steiner LE. Histopathology of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 1997;87(3):352-357. [DOI] [PubMed] [Google Scholar]
- 35. Hopewell JW, Millar WT, Paddick I, Lindquist C. Impact of decaying dose-rate in gamma knife radiosurgery. J Radiosurg. SBRT. 2013;2(3):251-253. [PMC free article] [PubMed] [Google Scholar]
- 36. Coderre JA, Morris GM, Micca PL et al.. Late effects of radiation on the central nervous system: Role of vascular endothelial damage and glial stem cell survival. Radiat Res. 2006;166(3):495-503. [DOI] [PubMed] [Google Scholar]
- 37. Cuccurullo V. L. Dade Lunsford and Jason P. Sheehan (eds): Intracranial stereotactic radiosurgery. Eur J Nucl Med Mol Imaging. 2010;37(3):652-652. [Google Scholar]
- 38. Kano H, Kondziolka D, Flickinger JC et al.. Aneurysms increase the risk of rebleeding after stereotactic radiosurgery for hemorrhagic arteriovenous malformations. Stroke. 2012;43(10):2586-2591. [DOI] [PubMed] [Google Scholar]
- 39. Starke RM, McCarthy DJ, Chen CJ et al.. Evaluation of stereotactic radiosurgery for cerebral dural arteriovenous fistulas in a multicenter international consortium. J Neurosurg. 2019:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen CJ, Ding D, Kano H et al.. Stereotactic radiosurgery for pediatric versus adult brain arteriovenous malformations: a multicenter study. Stroke. 2018:49(8):1939-1945. [DOI] [PubMed] [Google Scholar]
- 41. Patibandla MR, Ding D, Kano H et al.. Effect of treatment period on outcomes after stereotactic radiosurgery for brain arteriovenous malformations: an international multicenter study. J Neurosurg. 2018: 123(1):136-144. [DOI] [PubMed] [Google Scholar]
- 42. Lee CC, Reardon MA, Ball BZ et al.. The predictive value of magnetic resonance imaging in evaluating intracranial arteriovenous malformation obliteration after stereotactic radiosurgery. J Neurosurg. 2015;123(1):136-144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.