Cytochrome P450 (CYP) enzymes are involved in the biotransformation of chloroquine (CQ), but the role of the different profiles of metabolism of this drug in relation to Plasmodium vivax recurrences has not been properly investigated. To investigate the influence of the CYP genotypes associated with CQ metabolism on the rates of P. vivax early recurrences, a case-control study was carried out. The cases included patients presenting with an early recurrence (CQ-recurrent individuals), defined as a recurrence during the first 28 days after initial infection and plasma concentrations of CQ plus desethylchloroquine (DCQ; the major CQ metabolite) higher than 100 ng/ml.
KEYWORDS: CYP450, Plasmodium vivax, chloroquine, early recurrence, malaria
ABSTRACT
Cytochrome P450 (CYP) enzymes are involved in the biotransformation of chloroquine (CQ), but the role of the different profiles of metabolism of this drug in relation to Plasmodium vivax recurrences has not been properly investigated. To investigate the influence of the CYP genotypes associated with CQ metabolism on the rates of P. vivax early recurrences, a case-control study was carried out. The cases included patients presenting with an early recurrence (CQ-recurrent individuals), defined as a recurrence during the first 28 days after initial infection and plasma concentrations of CQ plus desethylchloroquine (DCQ; the major CQ metabolite) higher than 100 ng/ml. A control group with no parasite recurrence over the follow-up (the CQ-responsive group) was also included. CQ and DCQ plasma levels were measured on day 28. CQ-metabolizing CYP (CYP2C8, CYP3A4, and CYP3A5) genotypes were determined by real-time PCR. An ex vivo study was conducted to verify the efficacy of CQ and DCQ against P. vivax isolates. The frequency of alleles associated with normal and slow metabolism was similar between the cases and the controls for the CYP2C8 (odds ratio [OR] = 1.45, 95% confidence interval [CI] = 0.51 to 4.14, P = 0.570), CYP3A4 (OR = 2.38, 95% CI = 0.92 to 6.19, P = 0.105), and CYP3A5 (OR = 4.17, 95% CI = 0.79 to 22.04, P = 1.038) genes. DCQ levels were higher than CQ levels, regardless of the genotype. Regarding the DCQ/CQ ratio, there was no difference between groups or between those patients who had a normal genotype and those patients who had a mutant genotype. DCQ and CQ showed similar efficacy ex vivo. CYP genotypes had no influence on early recurrence rates. The similar efficacy of CQ and DCQ ex vivo could explain the absence of therapeutic failure, despite the presence of alleles associated with slow metabolism.
INTRODUCTION
Malaria remains an important public health problem worldwide. In 2018, there were 219 million cases, and 435,000 deaths were caused by malaria (1). In Brazil, the Amazon region is the main area of transmission, and Plasmodium vivax accounts for 88.4% of the reported cases in the country (2). An important obstacle to P. vivax malaria elimination in areas where P. vivax is endemic stems from the frequent recurrences caused by this parasite. These recurrences are characterized as a relapse when they are caused by activation of hypnozoites in the liver; as a reinfection if parasitemia returns due to a new infected mosquito bite; and as a recrudescence when there is an early return of asexual parasitemia despite adequate levels of chloroquine (CQ) and the metabolite desethylchloroquine (DCQ) in host plasma, which often indicates the presence of drug-resistant parasites, leading to therapeutic failures (3).
CQ undergoes hepatic biotransformation through the N-dealkylation pathway into two main metabolites: DCQ and bisdesethylchloroquine (BDCQ). DCQ is the major CQ metabolite, with the concentrations detected in plasma ranging from 20% to 50% of those of CQ (4). In contrast, BDCQ plasma or blood concentrations never reach more than 10% to 15% of CQ levels (5). The antimalarial action of DCQ, evaluated only for Plasmodium falciparum, was as active as that of CQ against a CQ-sensitive strain, but DCQ was significantly less active than the parent compound against a CQ-resistant strain (6) For P. vivax, in in vivo studies to assess resistance to CQ, only CQ and DCQ levels are generally measured (7, 8), since they are the major CQ metabolites (4).
P. vivax resistance to CQ has been reported, with a high prevalence of resistance being found in Indonesia and Papua New Guinea (9). In the Brazilian Amazon, CQ resistance rates range from 5% to 11% in vivo (8, 10, 11) and 9.8% to 10.7% ex vivo (12, 13). Although the mechanisms that lead to P. vivax resistance to CQ are not well understood, some studies have shown an association between gene expression and variations in the copy number of the pvcrt-o and pvmdr-1 genes and resistance to CQ (7, 14). CQ remains at therapeutic levels against P. vivax until 35 days after starting treatment (3, 15). After this period, with decreasing plasma levels of CQ and DCQ, the return of parasitemia is due to reinfection or relapse (9). Several studies used day 28 as the cutoff point to assess possible therapeutic failures by CQ (7, 8, 10, 11, 16), following the recommendation of the World Health Organization for monitoring the effectiveness of antimalarials (17).
Recent studies have demonstrated the importance of host genetics in antimalarial treatment outcomes (18–21), based on single-nucleotide polymorphisms (SNPs) detected in genes encoding drug-metabolizing enzymes (22). The presence of certain genotypes related to the metabolism of these enzymes may be associated with an increase in drug metabolism rates, generating adverse effects, and an increase in the elimination rate or a decrease in the rate of metabolism may lead to therapeutic failure (20, 22, 23).
The biotransformation of primaquine (PQ), mediated by cytochrome P450 (CYP) enzymes, is attributed to the CYP2D6, CYP3A4, CYP1A2, and CYP2C19 enzymes (22, 24). Therapeutic failures in the treatment of P. vivax malaria with PQ are generally attributed to the presence of CYP2D6 polymorphisms (19), a relationship that has also been reported in Brazil (21, 25). CYP2C8, CYP3A4, and CYP3A5 were reported to be the major enzymes involved in the formation of DCQ from CQ (26). An effect of the CYP2C8*2/*3/*4 gene on the gametocyte clearance rate in patients with malaria undergoing CQ and PQ treatment has been reported (27).
Pharmacogenetics has gained great importance over the last few years, since it can enable patients to received personalized drug therapy for various diseases (28). However, the frequency of CYP alleles associated with the slow metabolism of CQ in individuals from the Brazilian Amazon has not been fully studied, in particular, whether the presence of these alleles influences early recurrence. In addition, there is a paucity of studies to understand which molecule (CQ or DCQ) has the best antimalarial action on P. vivax and whether a profile of low drug metabolism contributes to increased early recurrence rates.
This study aimed to investigate the frequency of genotypes associated with slow CQ metabolism for the main metabolizing CYPs in patients from the Brazilian Amazon and verify the influence of these alleles in the early recurrence of P. vivax malaria.
RESULTS
Allele frequencies of CYPs associated with CQ metabolism.
Twenty-six cases (CQ-recurrent individuals) and 99 controls (CQ-responsive individuals) were included in this study. The clinical and laboratory characteristics of the patients included in this study are presented in Table 1. All individuals with CQ-recurrent P. vivax malaria had a positive quantitative PCR result for P. vivax, and their mean blood levels of CQ plus DCQ were greater than 100 ng/ml at day 28.
TABLE 1.
Demographic and parasitological baseline data for individuals involved in this studya
| Characteristic | Value for the following group: |
P valueb | |
|---|---|---|---|
| CQ-recurrent | CQ-responsive | ||
| No. of subjects | 26 | 99 | |
| Mean (95% CI) age (yr) | 34.69 (27.59–41.78) | 35.21 (31.94–38.47) | 0.8872 |
| No. (%) of male subjects | 19 (73.1) | 73 (73.7) | 1.0000 |
| Mean no. of parasites/μl (95% CI) on day 0 | 2,618.82 (1,359.20–3,878.43) | 2,961.6 (2,297.90–3,625.29) | 0.6368 |
| Mean no. of gametocytes/μl (95% CI) on day 0 | 45.26 (24.89–65.62) | 75.3 (39.12–111.47) | 0.4060 |
Recurrent indicates a new malaria episode by day 28, and responsive indicates no malaria episode by day 28.
P values were determined by the χ2 test, t test, or Fisher’s exact test.
The allele frequencies of CYP2C8 (P = 0.3196), CYP3A4 (P = 0.0916), and CYP3A5 (P = 0.1064) were similar in the cases and the controls. Most individuals carried alleles associated with normal enzyme activity (*1 or *1A). Alleles associated with slow enzyme activity were found in both cases and controls (Table 2).
TABLE 2.
Chloroquine-metabolizing CYP allele frequency
| Gene | Allele | CQ-recurrent group (n = 52) |
CQ-responsive group (n = 198) |
P valuea | ||
|---|---|---|---|---|---|---|
| No. of chromosomes | Frequency | No. of chromosomes | Frequency | |||
| CYP2C8 | *1 | 46 | 0.8846 | 179 | 0.9040 | 0.6137 |
| *2 | 0 | 0.0000 | 6 | 0.0303 | 0.3493 | |
| *3 | 4 | 0.0769 | 11 | 0.0556 | 0.5225 | |
| *4 | 2 | 0.0385 | 2 | 0.0101 | 0.192 | |
| CYP3A4 | *1A | 42 | 0.8077 | 178 | 0.8990 | 0.0916 |
| *1B | 10 | 0.1923 | 20 | 0.1010 | ||
| CYP3A5 | *1A | 49 | 0.9423 | 195 | 0.9848 | 0.1064 |
| *3 | 2 | 0.0385 | 2 | 0.0101 | 0.192 | |
| *6 | 1 | 0.0192 | 1 | 0.0051 | 0.3734 | |
P values were determined by the χ2 test.
The most frequent diplotypes for CYP2C8 were *1/*1 (76.2% of cases and 82.8% of controls) and *1/*3 (15.3% of cases and 10.1% of controls). For CYP3A4, the most frequent diplotypes were *1A/*1A (65.3% of cases and 81.2% of controls) and *1A/*1B (30.7% of cases and 16.1% of controls). For CYP3A5, *1/*1 (88.4% of cases and 96.9% of controls) was the most frequent diplotype, 1/*3 was found in both cases (7.6%) and controls (2.0%), and *1/*6 was found in both cases (3.8%) and controls (1.0%). The frequency of diplotypes associated with normal and slow metabolism was similar between cases and controls for the CYP2C8 (odds ratio [OR] = 1.45, 95% confidence interval [CI] = 0.51 to 4.14, P = 0.570), CYP3A4 (OR = 2.38, 95% CI = 0.92 to 6.19, P = 0.105), and CYP3A5 (OR = 4.17, 95% CI = 0.79 to 22.04, P = 1.038) genes.
Genotypes and P. vivax malaria recurrences.
In addition to following up the patients until day 28, we also investigated the occurrence of vivax malaria recurrences in patients for up to 1 year by passive case detection using the SIVEP-Malaria platform (the Brazilian official malaria epidemiological surveillance information system). When considering individuals with alleles of CYP2C8, CYP3A4, and CYP3A5 associated with normal and slow metabolism, no significant differences in the recurrence rates between the cases and the controls were found over the 1-year follow-up (Table 3).
TABLE 3.
Parasitemia clearance, drug levels, and malaria recurrence patterns between different genotype groups
| Gene, patient group, and genotype | No. of patients | No. (%) of patients with asexual parasitemia clearance ona
: |
Mean (95% CI) drug level (ng/ml) at day 28b
|
No. (%) of patients with the following no. of malaria episodes up to 1 yra,c
: |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 7 | CQ | DCQ | 0 | 1 | >1 | ||
| CYP2C8 | ||||||||||
| CQ-recurrent | ||||||||||
| *1 | 20 | 0 (0) | 11 (55) | 4 (20) | 5 (25) | 63.8 (49.33–78.26) | 108.4 (89.68–127.11) | 8 (40) | 9 (45) | 3 (15) |
| *2/*3/*4 allele carriers | 6 | 1 (16.7) | 2 (33.3) | 0 (0) | 3 (50) | 42.2 (17.37–67.02) | 130.6 (57.82–203.37) | 2 (33.3) | 3 (50) | 1 (16.7) |
| P value | 0.2308 | 0.6447 | 0.5425 | 0.3301 | 0.1293 | 0.3266 | 1.0000 | 1.0000 | 1.0000 | |
| CQ-responsive | ||||||||||
| *1 | 82 | 3 (3.7) | 36 (43.9) | 30 (36.6) | 13 (15.8) | 43.4 (37.85–48.94) | 93.3 (68.17–118.42) | 59 (71.9) | 14 (17.1) | 9 (11) |
| *2/*3/*4 allele carriers | 17 | 1 (5.9) | 9 (52.9) | 5 (29.4) | 2 (11.8) | 64.1 (23.29–104.90) | 79.9 (40.23–119.56) | 10 (58.9) | 4 (23.5) | 3 (17.6) |
| P value | 0.5354 | 0.5956 | 0.7813 | 1.0000 | 0.0528 | 0.6459 | 0.3843 | 0.5047 | 0.4277 | |
| CYP3A4 | ||||||||||
| CQ-recurrent | ||||||||||
| *1A | 17 | 1 (5.9) | 7 (41.2) | 3 (17.6) | 6 (35.3) | 62.06 (46.61–77.50) | 121.93 (94.73–149.12) | 5 (29.4) | 10 (58.8) | 2 (11.8) |
| *1B allele carriers | 9 | 0 (0) | 6 (66.7) | 1 (11.1) | 2 (22.2) | 55 (30.04–79.95) | 96.66 (75.71–117.60) | 5 (55.6) | 2 (22.2) | 2 (22.2) |
| P value | 0.4375 | 0.411 | 1.0000 | 0.6673 | 0.5842 | 0.1948 | 0.2341 | 0.11 | 0.5906 | |
| CQ-responsive | ||||||||||
| *1A | 81 | 4 (4.9) | 37 (45.7) | 27 (33.3) | 13 (16.1) | 48.68 (39.82–57.53) | 100.48 (74.65–126.30) | 56 (69.2) | 15 (18.5) | 10 (12.3) |
| *1B allele carriers | 18 | 0 (0) | 8 (44.4) | 8 (44.4) | 2 (11.1) | 39.25 (18.81–59.68) | 48.52 (29.23–67.80) | 13 (72.2) | 3 (16.7) | 2 (11.1) |
| P value | 1.0000 | 1.0000 | 0.4195 | 0.732 | 0.3706 | 0.0662 | 1.0000 | 1.0000 | 1.0000 | |
| CYP3A5 | ||||||||||
| CQ-recurrent | ||||||||||
| *1A | 23 | 1 (4.4) | 10 (43.5) | 4 (17.4) | 8 (34.7) | 61.63 (47.95–75.30) | 113 (91.80–134.19) | 7 (30.4) | 12 (52.2) | 4 (17.4) |
| *3/*6 allele carriers | 3 | 0 (0) | 3 (100) | 0 (0) | 0 (0) | 44 (6.91–81.08) | 111.66 (53.18–170.13) | 3 100 | 0 (0) | 0 (0) |
| P value | 1.0000 | 0.22 | 1.0000 | 0.5292 | 0.3570 | 0.9637 | 0.0462 | 0.2246 | 1.0000 | |
| CQ-responsive | ||||||||||
| *1A | 96 | 4 (4.2) | 42 (43.8) | 35 (36.5) | 15 (15.5) | 47.5 (39.29–55.70) | 92.46 (70.20–114.71) | 67 (69.8) | 17 (17.7) | 12 (12.5) |
| *3/*6 allele carriers | 3 | 0 (0) | 3 (100) | 0 (0) | 0 (0) | 29.92 (42.81–102.65) | 45.25 (71.03–161.53) | 2 (66.7) | 1 (33.3) | 0 (0) |
| P value | 1.0000 | 0.0905 | 0.5501 | 1.0000 | 0.4584 | 0.4615 | 1.0000 | 0.456 | 1.0000 | |
P values were determined by the χ2test.
P values were determined by the t test or Fisher’s exact test.
Determined by use of the SIVEP-Malaria platform.
Drug levels and CQ-metabolizing CYP genotypes.
When we compared the CQ and DCQ concentrations between the cases and the controls, no significant differences were found. Furthermore, no significant differences in the mean concentrations of CQ and DCQ were found between individuals carrying alleles associated with slow metabolism (Table 3). The DCQ concentration was higher than the CQ concentration irrespective of genotype, indicating that most individuals had a drug biotransformation ability, despite the mutated genotypes found (for cases with CYP2C8*1, the DCQ concentration was 108.4 nM [95% CI = 89.7 to 127.1 nM]; for cases with CYP2C8*2/*3/*4, the DCQ concentration was 130.6 nM [95% CI = 57.8 to 203.4 nM]; for controls with CYP2C8*1, the DCQ concentration was 93.3 nM [95% CI = 68.2 to 118.4 nM]; and for controls with CYP2C8*2/*3/*4, the DCQ concentration was 79.9 nM [95% CI = 40.2 to 119.6 nM]).
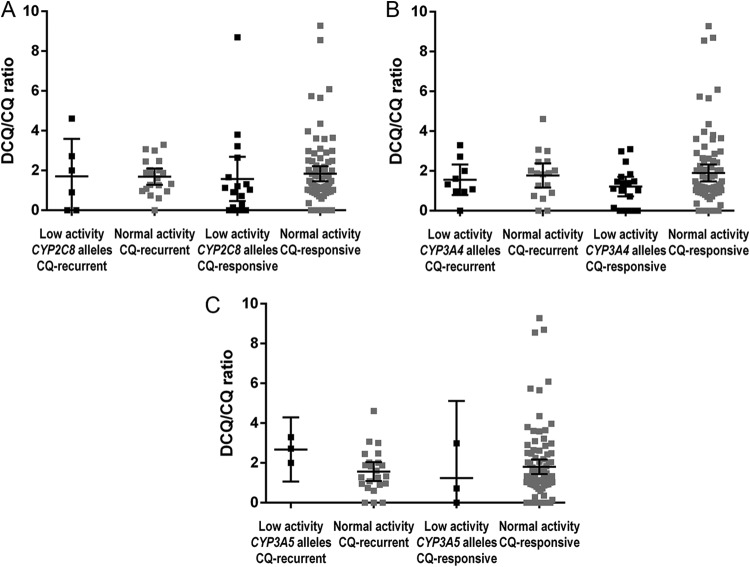
The possibility that the genotype influenced the metabolite/drug ratio was analyzed. However, there was no significant difference in the DCQ/CQ ratio between individuals carrying alleles associated with the normal metabolism and the slow metabolism of CYP2C8 and CYP3A5. In the control group, the metabolite/drug ratio was higher in individuals carrying alleles associated with normal CYP3A4 metabolism than in individuals with a mutated genotype (Fig. 1). For CYP3A4, a significant difference was observed (for cases with CYP3A4*1A the mean DCQ/CQ was 1.76 and for cases with CYP3A4*1B the mean DCQ/CQ was 1.55 [P = 0.629]; for controls with CYP3A4*1A the mean DCQ/CQ was 1.89 and for controls with CYP3A4*1B the mean DCQ/CQ was 1.21 [P = 0.0347]). No difference in CQ and DCQ levels (metabolite/drug ratio) was found between cases and controls for CYP2C8 and CYP3A5 (P > 0.05) (Fig. 1).
FIG 1.
Desethylchloroquine/chloroquine ratios of the different genotype groups. (A and C) No significant difference in the metabolite/drug ratios was found between patients with different genotypes (associated with low and normal enzyme activity) for patients with a P. vivax early recurrence (CQ-recurrent) and CQ-responsive patients with the CYP2C8 (A) and CYP3A5 (C) genotypes. (B) The ratio was higher among CQ-responsive patients with the genotype associated with normal metabolism. Values were obtained by an unpaired t test.
Clearance of P. vivax parasitemia and CQ-metabolizing CYP alleles.
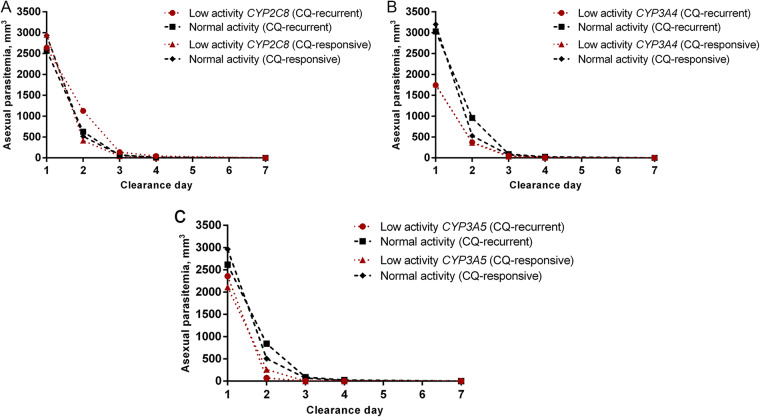
Most individuals had clearance of the asexual parasitemia on day 2 (58/125; 46.4%) or day 3 (39/125; 31.2%). Late clearance (from days 4 to 7) was recorded for 23 patients (18.4%). The slow clearance of asexual parasitemia did not predict early recurrences (OR = 2.49, 95% CI = 0.92 to 6.75, P = 0.088). Genotypes associated with normal and slow metabolism were found in these patients at a similar frequency (Table 3), which suggests that the presence of a mutated genotype is not associated with the clearance time for P. vivax. There was also no difference regarding the mean asexual parasitemia on days 1, 2, 3, and 7 between patients with alleles associated with normal activity and patients with alleles associated with low activity for all the enzymes studied (Fig. 2). Only 1 patient, belonging to the control group (1.01%, 1/99), showed gametocyte clearance on day 7. No association between late gametocyte clearance (days 3 to 7) and genotypes associated with the slow metabolism of CYP was observed in the cases (P = 1.000) or the controls (P = 0.259).
FIG 2.
Asexual parasitemia clearance between patients with genotypes with low and normal activity. The mean levels of parasitemia were evaluated at visits on days 1, 2, 3, 4, and 7. There was no significant difference in the clearance day and asexual parasitemia between patients in the CQ-recurrent and CQ-responsive groups with genotypes associated with low and normal enzyme activity for CYP2C8 (A), CYP3A4 (B), and CYP3A5 (C). Values were obtained by a paired t test.
P. vivax ex vivo assay.
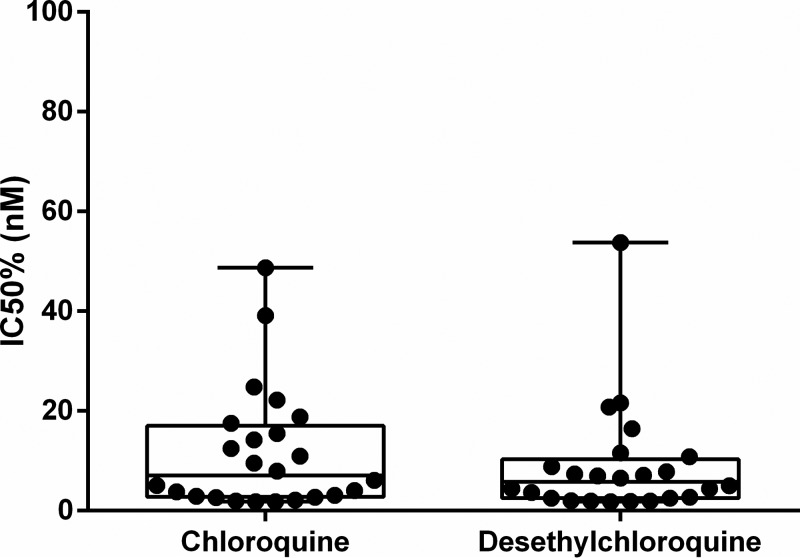
Twenty-four malaria patients not included in the cases or controls were enrolled in the ex vivo assays to evaluate the efficacy of CQ and DCQ against P. vivax. None of these isolates was resistant to CQ. The mean 50% inhibitory concentration (IC50) of CQ was 11.67 nM (95% CI = 6.520 to 16.82 nM), and the mean IC50 of DCQ was 8.95 nM (95% CI = 4.25 to 13.65 nM) (Fig. 3). None of these 24 patients presented with an early recurrence.
FIG 3.
Ex vivo efficacy of chloroquine and desethylchloroquine against P. vivax isolates. The drug and metabolite showed similar efficacy against isolates in patients from the Brazilian Amazon. Values were obtained by a paired t test.
DISCUSSION
This is the first study to investigate the role of CYP genotypes in the early recurrence of P. vivax malaria in samples from the Brazilian Amazon and the first to provide results regarding an evaluation of the sensitivity of P. vivax isolates to CQ and DCQ.
The frequency of allelic gene variants has already been used to predict the activity of drug-metabolizing enzymes (29). In Brazil, the frequency of these variants for cytochrome P450 enzymes has been described (30–33), but there is still little information regarding the influence of these genotypes on the failure of antimalarial therapy, including CQ therapy. Studies of P. falciparum have demonstrated the importance of pharmacogenetics in the elimination of this parasite (34, 35).
For the CYP2C8 gene, variant *2 is more frequent in Africans (11 to 17%) and CYP2C8*3 is more frequent in Caucasians (15%) (36). The frequency of CYP2C8*4 is higher in European populations (4 to 7%) (37). For CYP3A4, the frequency of the *1B allele is variable, ranging from 3.6% among white Americans to 76% among Africans (38). For CYP3A5, the highest frequencies of *3 are among Asians (60 to 75%) and Caucasians (85 to 95%); *6 is present in Africans (22%) and rare in Caucasians and Asians (22). The frequency of mutated alleles for CYP2C8, CYP3A4, and CYP3A5*6 found in our study is in agreement with that found in other studies of Brazilian populations (27, 30, 33). Only the frequency of the CYP3A5*3 variant was found to be lower.
The CYP2C8*2 variant is associated with a 50% reduction in metabolic activity and the CYP2C8*3 variant is associated with an 85% reduction in normal enzyme activity compared to the activity of the wild-type allele, which was found in a study with paclitaxel and arachidonic acid drugs in vitro (36, 39). In Burkina Faso and Ghana, the role of CYP2C8 variants in the response to amodiaquine and the correlation between CYP2C8*2 and low levels of enzyme metabolism have been investigated, confirming their influence on drug metabolism (40, 41). However, they did not demonstrate an association between alleles associated with low enzyme activity and the treatment outcome.
Studies conducted in Papua New Guinea and Thailand described the risk of P. vivax recurrence in individuals with late parasitemia clearance (41, 42). In our study, the clearance of asexual parasitemia at day 7 and gametocytemia after day 3 was not a predictor of an early recurrence by P. vivax and was not associated with the presence of mutated CYP genotypes. A recent study in Brazil found an association between CYP2C8*2*3/*4 and the gametocyte clearance rate in patients with malaria undergoing CQ/PQ treatment. From the baseline to the first day of treatment, individuals homozygous for wild-type CYP2C8 achieved a greater gametocyte reduction (P = 0.007) than individuals without this genotype (27). There was no difference in CQ and DCQ levels between patients with normal genotypes and patients with mutated genotypes or in the frequency of these CYP2C8 alleles between cases and controls.
The CYP3A5*3 variant is related to decreased enzyme activity, and CYP3A5*6 and CYP3A5*7 are null alleles that cause enzyme absence (29). Kim et al. (26) demonstrated a role for CYP3A5 in CQ metabolism, but another study showed a smaller role for this enzyme in the formation of DCQ in vitro (42). A recent study demonstrated that polymorphisms of CYP3A5 and CYP3A4 did not show any significant association with the blood levels of hydroxychloroquine and desethylhydroxychloroquine in patients with systemic lupus erythematosus (43). In our study, in the control group, the metabolite/drug ratio was higher in individuals carrying alleles associated with normal CYP3A4 metabolism than in individuals with mutated genotypes. However, studies conducted so far have not clarified whether the occurrence of SNPs alters the metabolism of the drug (22).
In our study, we found no association between mutated genotypes and changes in CQ and DCQ levels. Although other studies have reported a role for the alleles associated with slow metabolism (CYP2C8, CYP3A4, and CYP3A5) in the levels of drugs for clinical use (37, 44, 45), evidence of the influence of these alleles on the enzyme phenotype is still limited (46). It is known that there may be a failure in the cytochrome P450 genotype/phenotype correlation, with about 50% of errors in phenotype prediction being described. Factors such as gene splicing, transcriptional regulation, and the influence of microRNAs, in addition to other existing SNPs, may play a role in the final enzyme activity phenotype (47). These studies suggest that genotype information alone may not be sufficient to replace phenotype measurements, in this case, measurements of drug levels. In addition, the expression and activity of CYP enzymes can be affected by the inflammatory response triggered by the infection, as has already been demonstrated for P. falciparum malaria with CYP1A2 (48, 49) and CYP3A (50).
In vivo resistance to CQ signifies a persistence of P. vivax asexual stages, despite the presence of adequate levels of CQ plus DCQ (a concentration higher than 100 ng/dl) (16). For ex vivo conditions, resistance occurs when IC50 values greater than 100 nM CQ are obtained after 42 h of P. vivax culture (13, 51).
It is known that DCQ’s action is similar to that of CQ and that it is effective against avian malaria, but little is known about its action in human malaria (52). According to Fu et al. (6), DCQ is as effective as CQ against sensitive isolates of P. falciparum, but its efficacy against resistant isolates is significantly reduced. In vivo sensitivity trials with CQ and DCQ showed that DCQ was more effective and that a combination of drugs had better potential than monotherapy with just CQ. For resistant isolates, better effectiveness was reported using CQ monotherapy, and the combination of drugs was shown to be better than when CQ or DCQ was used alone (53).
A limitation of this study was that the possibility of recurrent parasitemia prior to day 28 in the presence of normally lethal CQ blood levels could not be excluded, since there may have been a relapse of the CQ-recurrent parasite after clearance of the original CQ-responsive parasitemia; however, the frequency of this event is not expected to vary between different CYP genotypes. Unfortunately, the ex vivo and in vivo studies were not performed with the same samples of P. vivax. According to our experience, the level of parasitemia on the recrudescent day (DR) is often very low and less than 50% of parasites are at the ring stage of development, making the experiment unfeasible on DR (54), but patients in the ex vivo study were followed up for 28 days, and none showed a recurrence during this period. The sample size used in the ex vivo study is not adequate to confirm CQ resistance rates.
The study had a small sample size for the CQ-recurrent group; however, the frequency of recurrence was in agreement with that reported in previous studies (8, 10, 11). No genetic marker analysis for differentiating relapse, resistance, or reinfection was performed, as some researchers have stated that there are no ideal genetic markers for these (55, 56).
Our results demonstrate that CQ and DCQ had similar efficacy against P. vivax isolates from the Amazon and can explain why alleles associated with low enzyme activity found in patients in the control group did not necessarily lead to the failure of CQ treatment and an early recurrence. The absence of drug metabolism problems, even in the occurrence of SNPs in CYP genes in the control group, indicates that these genetic host characteristics had little influence on the early recurrence caused by P. vivax.
MATERIALS AND METHODS
Ethics statement.
The study was approved by the Ethics Review Board of the Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (FMT-HVD; approval number 343/2009). Participants were instructed regarding the objectives of the study and signed an informed consent form. In the case of minors, the consent form was signed by the parents or legal guardians. Patients diagnosed with malaria were treated according to Brazilian Ministry of Health guidelines (57).
Study site.
The study was carried out between 2012 and 2014 at FMT-HVD, an infectious disease referral center located in Manaus, western Brazilian Amazon. Ex vivo studies were carried out on samples obtained from 2017 to 2018.
Selection of patients.
The study included patients of either sex who were infected with P. vivax malaria; aged 6 months to 60 years; and had a body weight of greater than 5 kg, a blood parasite density of from 250 to 100,000 parasites/ml, and an axillary temperature of 37.5°C or a history of fever in the last 48 h. Exclusion criteria were the use of antimalarials in the previous 30 days, refusal to be followed up for 28 days, and any clinical complication (58). All patients received supervised treatment with 25 mg/kg of body weight of chloroquine phosphate over a 3-day period (10 mg/kg on day 1 and 7.5 mg/kg on days 2 and 3). PQ was prescribed for a 7-day period at a dosage of 0.5 mg/kg per day, starting only at the end of the follow-up or on the day of recurrence. Patients who vomited the first dose within 30 min after drug ingestion were retreated with the same dose. Clinical and laboratory tests were performed, and interviews and sample collection were done on follow-up days 1, 2, 3, 4, 7, 14, and 28. If there were any extra days of follow-up, the same sample collection procedures were performed.
This study was conducted by using a convenience sampling from previous patient follow-ups. The enrolled patients were divided into cases (CQ-recurrent patients), when the patients presented with a P. vivax early recurrence, in which parasites returned during the first 28 days after initial infection, and plasma levels of CQ plus DCQ were higher than 100 ng/ml, and controls (CQ-responsive patients), a group that consisted of patients with no parasitemia recurrence during the 28 days of follow-up.
P. vivax malaria diagnosis.
Asexual and sexual parasitemia at day 1 and the clearance of asexual parasitemia were determined by microscopy by two experienced microscopists, using parasite counts per 500 leukocytes. Patients were actively followed up for 28 days. After day 28, recurrences were detected by passive case detection by use of the SIVEP-Malaria system, the official malaria epidemiological surveillance system used in Brazil.
Genotyping of CQ CYPs.
DNA was purified from whole-blood samples using a QIAamp DNA minikit (Qiagen, USA). We genotyped three single-nucleotide polymorphisms (SNPs) in CYP2C8 (A805T [rs11572103], C792G [rs1058930], G416A [rs11572080]), one SNP in CYP3A4 (A392G [rs2740574]), and two SNPs in CYP3A5 (G14690A [rs10264272], A6986G [rs776746]). Analyses were assessed using 7500 Fast real-time PCR software (version 2.3; Applied Biosystems). Amplification reactions and cycling parameters were determined according to the manufacturer’s protocols.
CQ and DCQ levels.
Three 100-μl aliquots of the sample collected on day 28 of follow-up were used for measuring CQ and DCQ levels. Analysis was assessed by high-performance liquid chromatography (HPLC), as previously described (59).
P. vivax ex vivo culture.
Plasmodium isolates were collected between August 2017 and June 2018 from FMT-HVD patients. Patients were recruited if they presented with a monoinfection with P. vivax, a parasitemia of ≥10,000 parasites/ml, and a majority (>50%) of parasites at the ring stage of development. Four milliliters of whole blood was collected by venipuncture. After removal of leukocytes by using CF11 cellulose (Sigma-Aldrich), infected red blood cells (IRBC) were used for ex vivo drug susceptibility testing.
The CQ and DCQ susceptibilities of the P. vivax isolates were measured using a protocol modified from the WHO microtest (60). Two hundred microliters of a 2% hematocrit blood medium mixture (BMM), which consisted of McCoy’s 5A medium plus 20% type AB human serum, was added to each well of predosed drug plates containing 11 serial concentrations (2-fold dilutions) of the antimalarials (1.95 to 1,000 ng/ml) CQ diphosphate and DCQ, and each concentration of drug was tested in quadruplicate. A candle jar was used to mature the parasites at 37.5°C for 48 h. Incubation was stopped when ≥40% of the ring-stage parasites in the drug-free control well had reached the mature schizont stage.
Thick blood smears were made from each well and then stained with 5% Giemsa solution and examined microscopically. The number of schizonts per 200 asexual-stage parasites was determined for each drug concentration and normalized to the value for the control well.
Statistical analyses.
Allele and genotype frequencies were estimated by gene counting, and haplotype frequencies and linkage disequilibrium were estimated with Haploview software. Fisher’s exact test or the χ2 test was performed to compare the CYP2C8/CYP3A4/CYP3A5 alleles and the genotype frequencies between cases and controls. The odds ratios (OR) with their respective 95% confidence intervals (95% CI) were determined to verify the risk of CQ recurrence, depending on the diplotypes found, and to relate the late clearance of parasitemia and cases. In the ex vivo essay, the percentage of mature schizonts at each drug concentration was estimated for 200 asexual-stage parasites, the results were entered in the online IC Estimator software to calculate the IC50 of each sample by nonlinear regression analysis and a t test of the average comparisons, and graph construction was performed. A P value of <0.05 was considered significant in all analyses. Analysis was performed using GraphPad Prism software.
ACKNOWLEDGMENTS
We thank all participants who collaborated with this study and Monica Costa (head of the Malaria Department at FMT-HVD).
We thank CNPq for funding our research and CAPES for awarding scholarships. W.M.M. and M.V.G.L. are CNPq fellows. We thank FAPEAM for providing financial support for publication via PAPAC Edital no. 005/2019.
Conceived and designed the experiments: G.C.M., W.M.M., M.V.G.L., and A.M.S. Sample processing: A.C.G.A., M.C.B.P., E.F.G.F., Y.E.A.R.S., and E.L.S. Performed the experiments: A.C.G.A., M.C.B.P., E.F.G.F., L.R.B., and J.L.F.V. Data entry and analyses: A.C.G.A. and M.C.B.P. Wrote the paper: A.C.G.A., M.C.B.P., G.C.M., Y.E.A.R.S., L.R.B., and M.A.M.B. All authors read and approved the final manuscript.
REFERENCES
- 1.World Health Organization. 2018. World malaria report 2018. WHO Press, Geneva, Switzerland: https://www.who.int/malaria/publications/world-malaria-report-2018/en/. [Google Scholar]
- 2.Siqueira AM, Mesones-Lapouble O, Marchesini P, Sampaio VS, Brasil P, Tauil PL, Fontes CJ, Costa FTM, Daniel-Ribeiro CT, Lacerda MVG, Damasceno CP, Santelli A. 2016. Plasmodium vivax landscape in Brazil: scenario and challenges. Am J Trop Med Hyg 95:87–96. doi: 10.4269/ajtmh.16-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird JK. 2009. Resistance to therapies for infection by Plasmodium vivax. Clin Microbiol Rev 22:508–534. doi: 10.1128/CMR.00008-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frisk-Holmberg M, Bergqvist Y, Termond E, Domeij-Nyberg B. 1984. The single dose kinetics of chloroquine and its major metabolite desethylchloroquine in healthy subjects. Eur J Clin Pharmacol 26:521–530. doi: 10.1007/bf00542151. [DOI] [PubMed] [Google Scholar]
- 5.Frisk-Holmberg M, Bergqvist Y, Domeij-Nyberg B. 1983. Steady state disposition of chloroquine in patients with rheumatoid disease. Eur J Clin Pharmacol 24:837–839. doi: 10.1007/bf00607097. [DOI] [PubMed] [Google Scholar]
- 6.Fu S, Björkman A, Wåhlin B, Ofori-Adjei D, Ericsson O, Sjöqvist F. 1986. In vitro activity of chloroquine, the two enantiomers of chloroquine, desethylchloroquine and pyronaridine against Plasmodium falciparum. Br J Clin Pharmacol 22:93–96. [PMC free article] [PubMed] [Google Scholar]
- 7.Melo GC, Monteiro WM, Siqueira AM, Silva SR, Magalhaes BM, Alencar AC, Kuehn A, del Portillo HA, Fernandez-Becerra C, Lacerda MV. 2014. Expression levels of pvcrt-o and pvmdr-1 are associated with chloroquine resistance and severe Plasmodium vivax malaria in patients of the Brazilian Amazon. PLoS One 9:e105922. doi: 10.1371/journal.pone.0105922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siqueira AM, Alencar AC, Melo GC, Magalhaes BL, Machado K, Alencar Filho AC, Kuehn A, Marques MM, Manso MC, Felger I, Vieira JL, Lameyre V, Daniel-Ribeiro CT, Lacerda MV. 2017. Fixed-dose artesunate-amodiaquine combination vs chloroquine for treatment of uncomplicated blood stage P. vivax infection in the Brazilian Amazon: an open-label randomized, controlled trial. Clin Infect Dis 64:166–174. doi: 10.1093/cid/ciw706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird KJ, Maguire JD, Price RN. 2012. Diagnosis and treatment of Plasmodium vivax malaria. Adv Parasitol 80:203–270. doi: 10.1016/B978-0-12-397900-1.00004-9. [DOI] [PubMed] [Google Scholar]
- 10.de Santana Filho FS, Arcanjo AR, Chehuan YM, Costa MR, Martinez-Espinosa FE, Vieira JL, Barbosa M, Alecrim WD, Alecrim M. 2007. Chloroquine-resistant Plasmodium vivax, Brazilian Amazon. Emerg Infect Dis 13:1125–1126. doi: 10.3201/eid1307.061386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marques MM, Costa MR, Santana Filho FS, Vieira JL, Nascimento MT, Brasil LW, Nogueira F, Silveira H, Reyes-Lecca RC, Monteiro WM, Lacerda MV, Alecrim MG. 2014. Plasmodium vivax chloroquine resistance and anemia in the western Brazilian Amazon. Antimicrob Agents Chemother 58:342–347. doi: 10.1128/AAC.02279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chehuan YF, Costa MR, Costa JS, Alecrim MG, Nogueira F, Silveira H, Brasil LW, Melo GC, Monteiro WM, Lacerda MV. 2013. In vitro chloroquine resistance for Plasmodium vivax isolates from the western Brazilian Amazon. Malar J 12:226. doi: 10.1186/1475-2875-12-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pratt-Riccio LR, Chehuan YF, Siqueira MJ, das Gracas Alecrim M, Bianco-Junior C, Druilhe P, Brasseur P, Ferreira-da-Cruz MDF, Carvalho LJ, Daniel-Ribeiro CT. 2013. Use of a colorimetric (DELI) test for the evaluation of chemoresistance of Plasmodium falciparum and Plasmodium vivax to commonly used anti-plasmodial drugs in the Brazilian Amazon. Malar J 12:281. doi: 10.1186/1475-2875-12-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva SR, Almeida ACG, da Silva GAV, Ramasawmy R, Lopes SCP, Siqueira AM, Costa GL, Sousa TN, Vieira JLF, Lacerda MVG, Monteiro WM, de Melo GC. 2018. Chloroquine resistance is associated to multi-copy pvcrt-o gene in Plasmodium vivax malaria in the Brazilian Amazon. Malar J 17:267. doi: 10.1186/s12936-018-2411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, McGready R, Fernandez C, Stepniewska K, Paw MK, Viladpai-Nguen SJ, Thwai KL, Villegas L, Singhasivanon P, Greenwood BM, White NJ, Nosten F. 2008. Chloroquine pharmacokinetics in pregnant and nonpregnant women with vivax malaria. Eur J Clin Pharmacol 64:987–992. doi: 10.1007/s00228-008-0500-z. [DOI] [PubMed] [Google Scholar]
- 16.Baird JK, Leksana B, Masbar S, Fryauff DJ, Sutanihardja MA, Suradi Wignall FS, Hoffman SL. 1997. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am J Trop Med Hyg 56:621–626. doi: 10.4269/ajtmh.1997.56.621. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. 2009. Methods for surveillance of antimalarial drug efficacy. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2009/9789241597531_eng.pdf?ua=1. [Google Scholar]
- 18.Baird JK, Louisa M, Noviyanti R, Ekawati L, Elyazar I, Subekti D, Chand K, Gayatri A, Instiaty Soebianto S, Crenna-Darusallam C, Djoko D, Hasto BD, Meriyenes D, Wesche D, Nelwan EJ, Sutanto I, Sudoyo H, Setiabudy R. 2018. Association of impaired cytochrome P450 2D6 activity genotype and phenotype with therapeutic efficacy of primaquine treatment for latent Plasmodium vivax malaria. JAMA Netw Open 1:e181449. doi: 10.1001/jamanetworkopen.2018.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, McCarthy WF, Deye G, Melendez V, Ockenhouse CF. 2013. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med 369:1381–1382. doi: 10.1056/NEJMc1301936. [DOI] [PubMed] [Google Scholar]
- 20.Elewa H, Wilby KJ. 2017. A review of pharmacogenetics of antimalarials and associated clinical implications. Eur J Drug Metab Pharmacokinet 42:745–756. doi: 10.1007/s13318-016-0399-1. [DOI] [PubMed] [Google Scholar]
- 21.Silvino AC, Costa GL, Araujo FC, Ascher DB, Pires DE, Fontes CJ, Carvalho LH, Brito CF, Sousa TN. 2016. Variation in human cytochrome P-450 drug-metabolism genes: a gateway to the understanding of Plasmodium vivax relapses. PLoS One 11:e0160172. doi: 10.1371/journal.pone.0160172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerb R, Fux R, Morike K, Kremsner PG, Gil JP, Gleiter CH, Schwab M. 2009. Pharmacogenetics of antimalarial drugs: effect on metabolism and transport. Lancet Infect Dis 9:760–774. doi: 10.1016/S1473-3099(09)70320-2. [DOI] [PubMed] [Google Scholar]
- 23.Marcsisin SR, Reichard G, Pybus BS. 2016. Primaquine pharmacology in the context of CYP 2D6 pharmacogenomics: current state of the art. Pharmacol Ther 161:1–10. doi: 10.1016/j.pharmthera.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Pybus BS, Marcsisin SR, Jin X, Deye G, Sousa JC, Li Q, Caridha D, Zeng Q, Reichard GA, Ockenhouse C, Bennett J, Walker LA, Ohrt C, Melendez V. 2013. The metabolism of primaquine to its active metabolite is dependent on CYP 2D6. Malar J 12:212. doi: 10.1186/1475-2875-12-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brasil LW, Rodrigues-Soares F, Santoro AB, Almeida ACG, Kuhn A, Ramasawmy R, Lacerda MVG, Monteiro WM, Suarez-Kurtz G. 2018. CYP2D6 activity and the risk of recurrence of Plasmodium vivax malaria in the Brazilian Amazon: a prospective cohort study. Malar J 17:57. doi: 10.1186/s12936-017-2139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KA, Park JY, Lee JS, Lim S. 2003. Cytochrome P450 2C8 and CYP3A4/5 are involved in chloroquine metabolism in human liver microsomes. Arch Pharm Res 26:631–637. doi: 10.1007/bf02976712. [DOI] [PubMed] [Google Scholar]
- 27.Sortica VA, Lindenau JD, Cunha MG, Ohnishi MD, Ventura AMR, Ribeiro-Dos-Santos ÂK, Santos SE, Guimarães LS, Hutz MH. 2016. The effect of SNPs in CYP450 in chloroquine/primaquine Plasmodium vivax malaria treatment. Pharmacogenomics 17:1903–1911. doi: 10.2217/pgs-2016-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanger UM, Schwab M. 2013. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138:103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Zanger UM, Turpeinen M, Klein K, Schwab M. 2008. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal Bioanal Chem 392:1093–1108. doi: 10.1007/s00216-008-2291-6. [DOI] [PubMed] [Google Scholar]
- 30.Bonifaz-Peña V, Contreras AV, Struchiner CJ, Roela RA, Furuya-Mazzotti TK, Chammas R, Rangel-Escareño C, Uribe-Figueroa L, Gómez-Vázquez MJ, McLeod HL, Hidalgo-Miranda A, Parra EJ, Fernández-López JC, Suarez-Kurtz G. 2014. Exploring the distribution of genetic markers of pharmacogenomics relevance in Brazilian and Mexican populations. PLoS One 9:e112640. doi: 10.1371/journal.pone.0112640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suarez-Kurtz G. 2010. Pharmacogenetics in the Brazilian population. Front Pharmacol 1:118. doi: 10.3389/fphar.2010.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suarez-Kurtz G, Genro JP, de Moraes MO, Ojopi EB, Pena SD, Perini JA, Ribeiro-dos-Santos A, Romano-Silva MA, Santana I, Struchiner CJ. 2012. Global pharmacogenomics: impact of population diversity on the distribution of polymorphisms in the CYP2C cluster among Brazilians. Pharmacogenomics J 12:267–276. doi: 10.1038/tpj.2010.89. [DOI] [PubMed] [Google Scholar]
- 33.Suarez-Kurtz G, Pena SD, Struchiner CJ, Hutz MH. 2012. Pharmacogenomic diversity among Brazilians: influence of ancestry, self-reported color, and geographical origin. Front Pharmacol 3:191. doi: 10.3389/fphar.2012.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavaco I, Martensson A, Froberg G, Msellem M, Bjorkman A, Gil JP. 2013. CYP2C8 status of patients with malaria influences selection of Plasmodium falciparum pfmdr1 alleles after amodiaquine-artesunate treatment. J Infect Dis 207:687–688. doi: 10.1093/infdis/jis736. [DOI] [PubMed] [Google Scholar]
- 35.Paganotti GM, Gallo BC, Verra F, Sirima BS, Nebie I, Diarra A, Coluzzi M, Modiano D. 2011. Human genetic variation is associated with Plasmodium falciparum drug resistance. J Infect Dis 204:1772–1778. doi: 10.1093/infdis/jir629. [DOI] [PubMed] [Google Scholar]
- 36.Daily EB, Aquilante CL. 2009. Cytochrome P450 2C8 pharmacogenetics: a review of clinical studies. Pharmacogenomics 10:1489–1510. doi: 10.2217/pgs.09.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Backman JT, Filppula AM, Niemi M, Neuvonen PJ. 2016. Role of cytochrome P450 2C8 in drug metabolism and interactions. Pharmacol Rev 68:168–241. doi: 10.1124/pr.115.011411. [DOI] [PubMed] [Google Scholar]
- 38.Cavaco I, Reis R, Gil JP, Ribeiro V. 2003. CYP3A4*1B and NAT2*14 alleles in a native African population. Clin Chem Lab Med 41:606–609. doi: 10.1515/CCLM.2003.091. [DOI] [PubMed] [Google Scholar]
- 39.Dai D, Zeldin DC, Blaisdell JA, Chanas B, Coulter SJ, Ghanayem BI, Goldstein JA. 2001. Polymorphisms in human CYP2C8 decrease metabolism of the anticancer drug paclitaxel and arachidonic acid. Pharmacogenetics 11:597–607. doi: 10.1097/00008571-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Adjei GO, Kristensen K, Goka BQ, Hoegberg LC, Alifrangis M, Rodrigues OP, Kurtzhals JA. 2008. Effect of concomitant artesunate administration and cytochrome P4502C8 polymorphisms on the pharmacokinetics of amodiaquine in Ghanaian children with uncomplicated malaria. Antimicrob Agents Chemother 52:4400–4406. doi: 10.1128/AAC.00673-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parikh S, Ouedraogo JB, Goldstein JA, Rosenthal PJ, Kroetz DL. 2007. Amodiaquine metabolism is impaired by common polymorphisms in CYP2C8: implications for malaria treatment in Africa. Clin Pharmacol Ther 82:197–203. doi: 10.1038/sj.clpt.6100122. [DOI] [PubMed] [Google Scholar]
- 42.Projean D, Baune B, Farinotti R, Flinois JP, Beaune P, Taburet AM, Ducharme J. 2003. In vitro metabolism of chloroquine: identification of CYP2C8, CYP3A4, and CYP2D6 as the main isoforms catalyzing N-desethylchloroquine formation. Drug Metab Dispos 31:748–754. doi: 10.1124/dmd.31.6.748. [DOI] [PubMed] [Google Scholar]
- 43.Lee JY, Vinayagamoorthy N, Han K, Kwok SK, Ju JH, Park KS, Jung SH, Park SW, Chung YJ, Park SH. 2016. Association of polymorphisms of cytochrome P450 2D6 with blood hydroxychloroquine levels in patients with systemic lupus erythematosus. Arthritis Rheumatol 68:184–190. doi: 10.1002/art.39402. [DOI] [PubMed] [Google Scholar]
- 44.Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, Wang D, Vinks AA, He Y, Swen JJ, Leeder JS, van Schaik R, Thummel KE, Klein TE, Caudle KE, MacPhee IA. 2015. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin Pharmacol Ther 98:19–24. doi: 10.1002/cpt.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werk AN, Cascorbi I. 2014. Functional gene variants of CYP3A4. Clin Pharmacol Ther 96:340–348. doi: 10.1038/clpt.2014.129. [DOI] [PubMed] [Google Scholar]
- 46.Tornio A, Backman JT. 2018. Cytochrome P450 in pharmacogenetics: an update. Adv Pharmacol 83:3–32. doi: 10.1016/bs.apha.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Waring RH. 2020. Cytochrome P450: genotype to phenotype. Xenobiotica 50:9–18. doi: 10.1080/00498254.2019.1648911. [DOI] [PubMed] [Google Scholar]
- 48.Akinyinka OO, Sowunmi A, Honeywell R, Renwick AG. 2000. The pharmacokinetics of caffeine in Nigerian children suffering from malaria and kwashiorkor. Eur J Clin Pharmacol 56:153–158. doi: 10.1007/s002280050734. [DOI] [PubMed] [Google Scholar]
- 49.Akinyinka OO, Sowunmi A, Honeywell R, Renwick AG. 2000. The effects of acute falciparum malaria on the disposition of caffeine and the comparison of saliva and plasma-derived pharmacokinetic parameters in adult Nigerians. Eur J Clin Pharmacol 56:159–165. doi: 10.1007/s002280050735. [DOI] [PubMed] [Google Scholar]
- 50.Pukrittayakamee S, Looareesuwan S, Keeratithakul D, Davis TM, Teja-Isavadharm P, Nagachinta B, Weber A, Smith AL, Kyle D, White NJ. 1997. A study of the factors affecting the metabolic clearance of quinine in malaria. Eur J Clin Pharmacol 52:487–493. doi: 10.1007/s002280050323. [DOI] [PubMed] [Google Scholar]
- 51.Druilhe P, Brasseur P, Blanc C, Makler M. 2007. Improved assessment of Plasmodium vivax response to antimalarial drugs by a colorimetric double-site Plasmodium lactate dehydrogenase antigen capture enzyme-linked immunosorbent assay. Antimicrob Agents Chemother 51:2112–2116. doi: 10.1128/AAC.01385-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verdier F, Le Bras J, Clavier F, Hatin I. 1984. Blood levels and in vitro activity of desethylchloroquine against Plasmodium falciparum. Lancet i:1186–1187. doi: 10.1016/S0140-6736(84)91436-3. [DOI] [PubMed] [Google Scholar]
- 53.Aderounmu AF. 1984. In vitro assessment of the antimalarial activity of chloroquine and its major metabolites. Ann Trop Med Parasitol 78:581–585. doi: 10.1080/00034983.1984.11811868. [DOI] [PubMed] [Google Scholar]
- 54.Russell B, Suwanarusk R, Malleret B, Costa FTM, Snounou G, Kevin Baird J, Nosten F, Rénia L. 2012. Human ex vivo studies on asexual Plasmodium vivax: the best way forward. Int J Parasitol 42:1063–1070. doi: 10.1016/j.ijpara.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Felger I, Snounou G, Hastings I, Moehrle JJ, Beck HP. 2020. PCR correction strategies for malaria drug trials: updates and clarifications. Lancet Infect Dis 20:e20–e25. doi: 10.1016/S1473-3099(19)30426-8. [DOI] [PubMed] [Google Scholar]
- 56.Price RN, von Seidlein L, Valecha N, Nosten F, Baird JK, White NJ. 2014. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis 14:982–991. doi: 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ministério da Saúde, Secretaria de Vigilância em Saúde. 2010. Guia prático de tratamento da malária no Brasil. Ministério da Saúde, Brasília, Brazil: http://bvsms.saude.gov.br/bvs/publicacoes/guia_pratico_malaria.pdf. [Google Scholar]
- 58.World Health Organization. 2015. Guidelines for the treatment of malaria, 3rd ed World Health Organization, Geneva, Switzerland: https://www.who.int/malaria/publications/atoz/9789241549127/en/. [Google Scholar]
- 59.Dua VK, Kar PK, Gupta NC, Sharma VP. 1999. Determination of chloroquine and desethylchloroquine in plasma and blood cells of Plasmodium vivax malaria cases using liquid chromatography. J Pharm Biomed Anal 21:199–205. doi: 10.1016/S0731-7085(99)00097-7. [DOI] [PubMed] [Google Scholar]
- 60.World Health Organization. 2001. In vitro micro-test (Mark III) for the assessment of the response of Plasmodium falciparum to chloroquine, mefloquine, quinine, amodiaquine, sulfadoxine/pyrimethamine and artemisinin. World Health Organization, Geneva, Switzerland: https://www.who.int/malaria/publications/atoz/ctd_mal_97_20_Rev_2_2001/en/. [Google Scholar]