Methicillin-resistant Staphylococcus aureus (MRSA) poses significant therapeutic challenges related to its frequency in clinical infections, innate virulence properties, and propensity for multiantibiotic resistance. MRSA is among the most common causes of endovascular infections, including infective endocarditis (IE). Our objective was to employ transthoracic echocardiography (TTE) to evaluate the effect of exebacase, a novel direct lytic agent, in experimental aortic valve MRSA IE.
KEYWORDS: MRSA, phage lysin, endocarditis, echocardiography, vegetation size
ABSTRACT
Methicillin-resistant Staphylococcus aureus (MRSA) poses significant therapeutic challenges related to its frequency in clinical infections, innate virulence properties, and propensity for multiantibiotic resistance. MRSA is among the most common causes of endovascular infections, including infective endocarditis (IE). Our objective was to employ transthoracic echocardiography (TTE) to evaluate the effect of exebacase, a novel direct lytic agent, in experimental aortic valve MRSA IE. TTE was utilized to evaluate the in vivo effect of exebacase on MRSA-infected vegetation progression when combined with daptomycin (versus daptomycin alone). Primary intravegetation outcomes were maximum size, weights at sacrifice, and MRSA counts at infection baseline versus after 4 days of daptomycin treatment (alone or in addition to exebacase administered once on treatment day 1). A single dose of exebacase in addition to daptomycin cleared significantly more intravegetation MRSA than daptomycin alone. This was associated with a statistical trend toward reduced maximum vegetation size in the exebacase plus daptomycin versus the daptomycin alone therapy groups (P = 0.07). Also, mean vegetation weights in the exebacase-treated group were significantly lower than those of the daptomycin alone group (P < 0.0001). Maximum vegetation size by TTE correlated with vegetation weight (P = 0.005). In addition, intravegetation MRSA counts in the combination group were significantly lower than those of untreated controls (P < 0.0001) and the daptomycin alone group (P < 0.0001). This study suggests that exebacase has a salutary impact on MRSA-infected vegetation progression when combined with daptomycin, especially in terms of vegetation MRSA burden, size, and weight. Moreover, TTE appears to be an efficient noninvasive tool to assess therapeutic efficacies in experimental MRSA IE.
TEXT
Staphylococcus aureus is now the most common cause of endovascular infections, including infective endocarditis (IE), in the developed world and remains a major global health care concern (1, 2). Methicillin-resistant S. aureus (MRSA) strains pose a particularly significant clinical challenge and are associated with high rates of morbidity and mortality compared to those of infections caused by methicillin-susceptible isolates (MSSA) (1–3). In addition, MRSA has a particular propensity to acquire additional resistance mechanisms to a number of standard-of-care (SOC) antibiotics, including vancomycin, linezolid, fluoroquinolones, and daptomycin (1–7). Thus, the development of novel anti-MRSA therapeutic options, especially with compounds displaying unique pharmacologic properties, has become a high-priority goal.
Exebacase is a novel recombinantly produced lysin (cell wall hydrolase enzyme) that rapidly hydrolyzes cell wall peptidoglycan of the Gram-positive bacterium, S. aureus, resulting in osmotic lysis (8). Exebacase is now being developed as an adjunct to traditional SOC antistaphylococcal antibiotic treatment and displays several hallmark properties including the following: (i) ability to cleave essential bonds in the staphylococcal cell wall, (ii) rapid lysis leading to cell death, (iii) in vitro synergistic efficacy with SOC antibiotics, and (iv) ability to rapidly disrupt bacteria-induced biofilms (8–11). Moreover, exebacase interacts with two important human serum proteins (albumin and lysozyme) to amplify its antistaphylococcal effects (12). Importantly, exebacase, used together with SOC antibiotics, has shown synergistic efficacy versus antibiotics alone in experimental IE in both rats and rabbits, a classic “biofilm infection” (12). Of note, recent phase 2 clinical trials with exebacase have demonstrated ∼43% higher responder rates with a single dose of exebacase combined with SOC antibiotics versus SOC agents alone for the treatment of MRSA bacteremia including IE (13). The fact that exebacase has potent biofilm-disrupting properties may well underlie, at least in part, its efficacy in animals and patient populations with MRSA IE.
Studying preclinical antimicrobial efficacy has traditionally focused on the extent of clearance of a pathogen of interest from relevant target tissues. In the case of experimental IE, this translates to assessing reductions of bacterial burdens in cardiac vegetations as well as other key target organs (e.g., kidneys and spleen). However, many of the complications seen in IE relate to the hematogenous embolization of the infected cardiac vegetation to critical organs, especially the central nervous system, kidneys, and spleen (reviewed in reference 14). Thus, it is evident that therapies designed to forestall the progression of the intracardiac infection, both structurally and microbiologically, would be of great potential benefit.
The primary goal of this study was to examine the efficacy of exebacase in addition to an SOC anti-MRSA agent (daptomycin), both microbiologically as well as “structurally” (using real-time echocardiography), within cardiac vegetations in a rabbit IE model. For this latter structural metric, we were particularly focused on the impact of exebacase combined with this antibiotic (versus antibiotic alone) on the progression of the cardiac vegetation over time. We hypothesized that the in vitro documented antibiofilm mechanism of exebacase might reduce the capacity of these cardiac vegetations to evolve over time, especially when combined with daptomycin. Moreover, the biofilm-eradicating properties of exebacase might plausibly disrupt infected cardiac vegetations to the extent that daptomycin could penetrate these lesions more effectively, enhancing net bacterial clearance from the infected cardiac lesions.
RESULTS
Vegetation size by TTE.
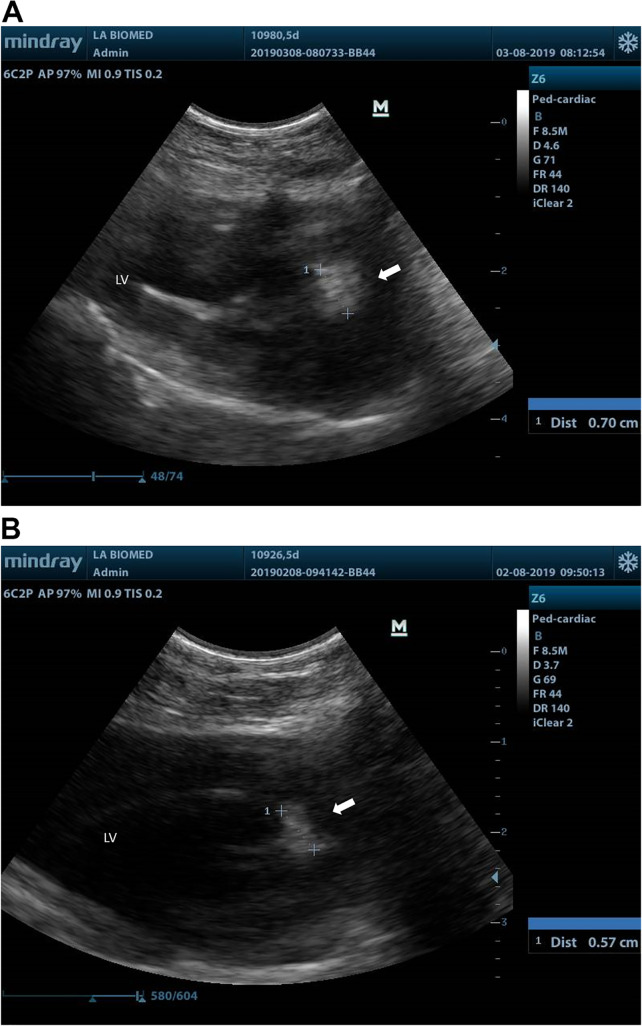
All animals (including untreated controls) underwent a baseline transthoracic echocardiography (TTE) at 24 h postinfection at the time of initiation of either daptomycin alone or daptomycin plus exebacase. Rabbits in these latter two treatment groups then had repeat TTEs at 4 h and 24 h after initiation of daptomycin (alone or in addition to exebacase), as well as 1 day after completion of daptomycin treatment (day 5). Figures 1A and B show two typical large (>0.5 cm) vegetations, each involving the juncture of the aortic valve and transvalvular catheter (see Movies S1 to S3 for a video of TTE). Table 1 shows the maximal vegetation dimensions for the largest lesions (which were serially assessed) in the untreated controls at baseline versus those in each of the animals in the two daptomycin therapy groups. In addition, the mean vegetation dimensions (±standard deviation [SD]) are shown for daptomycin-treated animals at each of the TTE interrogation time points. As noted, treatment with daptomycin alone was associated with increasing vegetation sizes in all four animals over the 4-day treatment course. In contrast, in four of the six animals receiving daptomycin in addition to exebacase, vegetation size either stabilized (within 5 to 10% of baseline) or substantially decreased over the 4 days of therapy, while vegetation size increased in the remaining two animals. These same trends toward increases in maximal vegetation size with daptomycin alone (regression coefficient = 0.03; P = 0.07) was not observed in the exebacase plus daptomycin group (regression coefficient = 0.004; P = 0.08).
FIG 1.
Sample echocardiographic images with measurement of the maximum vegetation size at 5 days following initiation of daptomycin (with or without exebacase) treatment. Parasternal long-axis view of largest vegetation measured in two animals. (A) Animal treated with daptomycin alone shows 0.7 cm vegetation (white arrow) involving the catheter and aortic valve. (B) Animal treated with daptomycin in addition to exebacase shows a vegetation of 0.57 cm (white arrow) in a similar location.
TABLE 1.
Maximum vegetation size over time measured by TTE, vegetation weights, and vegetation MRSA counts for each individual animal in each of the three groups
| Group and animal | Vegetation size (cm) |
Vegetation weight at sacrifice (g) | Vegetation MRSA counts at sacrifice (log10 CFU/g) | |||
|---|---|---|---|---|---|---|
| Baseline (24 h postinfection) | 4 h postexebacase | 24 h postexebacase | 96 h postexebacase | |||
| Untreated control | ||||||
| 1 | 0.45 | 0.49 | NDa | ND | 0.12 | 8.52 |
| 2 | 0.61 | 0.64 | ND | ND | 0.14 | 8.71 |
| 3 | 0.56 | 0.66 | ND | ND | 0.32 | 8.54 |
| 4 | 0.72 | 0.76 | ND | ND | 0.26 | 8.87 |
| 5 | 0.48 | 0.60 | ND | ND | 0.24 | 9.00 |
| Mean (±SD) | 0.56 ± 0.21 | 0.63 ± 0.08 | 0.22 ± 0.08 | 8.73 ± 0.21 | ||
| Daptomycin alone | ||||||
| 1 | 0.64 | 0.52 | 0.60 | 0.70 | 0.47 | 5.28 |
| 2 | 0.46 | 0.43 | 0.48 | 0.57 | 0.36 | 5.49 |
| 3 | 0.47 | 0.63 | 0.54 | 0.54 | 0.29 | 5.88 |
| 4 | 0.50 | 0.48 | 0.57 | 0.63 | 0.39 | 5.56 |
| Mean (±SD) | 0.52 ± 0.08 | 0.52 ± 0.09 | 0.55 ± 0.05 | 0.61 ± 0.07 | 0.38 ± 0.07 | 5.55 ± 0.25 |
| Daptomycin + exebacase | ||||||
| 1 | 0.55 | 0.52 | 0.56 | 0.59 | 0.18 | 0.74 |
| 2 | 0.32 | 0.61 | 0.52 | 0.49 | 0.16 | 0.80 |
| 3 | 0.67 | 0.61 | 0.63 | 0.48 | 0.18 | 0.74 |
| 4 | 0.49 | 0.63 | 0.59 | 0.46 | 0.19 | 0.72 |
| 5 | 0.47 | 0.47 | 0.64 | 0.57 | 0.21 | 0.68 |
| 6 | 0.60 | 0.61 | 0.57 | 0.57 | 0.24 | 0.62 |
| Mean (±SD) | 0.52 ± 0.12 | 0.58 ± 0.06 | 0.59 ± 0.05 | 0.53 ± 0.06 | 0.19 ± 0.03 | 0.72 ± 0.06 |
ND, not done.
Vegetation weights.
Table 1 shows the individual and mean (±SD) vegetation weights for animals in the untreated control as well as in the daptomycin alone and exebacase plus daptomycin treatment groups. As noted, vegetation weights in the exebacase plus daptomycin therapy group were significantly lower than those seen with daptomycin alone (mean ± SD of 0.19 g ± 0.03 g versus 0.38 g ± 0.07 g; P > 0.0001). Importantly, maximum vegetation size by TTE significantly correlated with vegetation weight (Pearson’s correlation coefficient = 0.8; P = 0.005 for pooled data).
Vegetation MRSA counts.
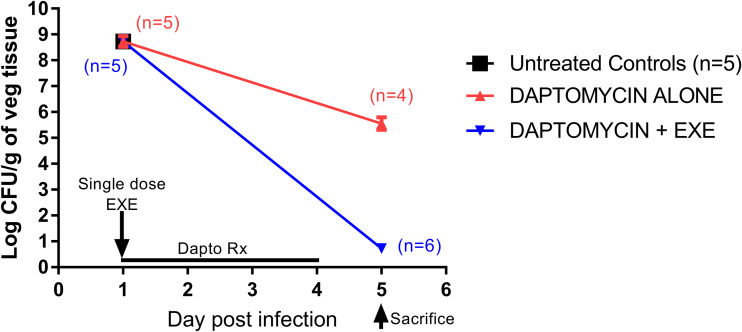
Table 1 and Fig. 2 demonstrate the individual and mean log10 CFU per gram vegetation MRSA counts (±SD) for untreated controls (at the start of daptomycin therapy alone or in addition to exebacase) versus those at the end of daptomycin therapy regimens. For daptomycin alone, there was a modest but significant reduction in MRSA counts versus untreated controls (∼3 log10 CFU/g reduction; P < 0.0001). In contrast, a single dose of exebacase plus daptomycin caused a >8 log10 CFU/g reduction in MRSA vegetation counts (P < 0.0001 versus untreated controls; P < 0.0001 versus daptomycin alone).
FIG 2.
Quantitative vegetation MRSA counts in treatment groups over time. EXE, exebacase (11 mg/kg i.v., single dose on day 1); Dapto, daptomycin treatment (4 mg/kg i.v. QD for 4 days). Symbols represent the mean (±SD) out of 5 animals in each group at each time point except on day 5, where 4 and 6 animals were included in the daptomycin alone and daptomycin plus exebacase groups, respectively.
DISCUSSION
Exebacase has several unique in vitro mechanistic properties when compared to those of traditional antibiotics, including an ability to rapidly disrupt Gram-positive bacterial cell walls and eradicate bacteria-induced biofilm (8–11). Given these in vitro properties, especially its antibiofilm mechanisms, and the known contributions of biofilm to the pathogenesis of IE (14), we hypothesized the following: (i) exebacase might well have a rapid effect on vegetation size reduction, and (ii) a secondary benefit might ensue, such that vegetation “disruption” would amplify daptomycin penetration, with concomitant reductions in intravegetation MRSA burdens. To query at least part of these metrics in real time, we employed TTE for serial assessment of vegetation size during daptomycin therapy (in the presence or absence of exebacase). Of interest, we have previously utilized this same TTE methodology in experimental S. aureus IE to demonstrate the impact of in vivo, platelet-derived host defenses on pathogenesis by comparing the following TTE-defined metrics: vegetation size, left ventricular ejection fraction, and the degree of aortic valvular regurgitation (15). These TTE metrics were then correlated with vegetation weights and intravegetation bacterial burdens (15).
Several interesting observations emerged from this current investigation. First, TTE was a facile methodology for serial assessment of vegetation size over time during specific therapeutic interventions. Second, as a microbiologic marker of IE progression, and based on our prior experimental IE studies (12), exebacase in addition to daptomycin was synergistic in vivo in terms of reducing intravegetation MRSA burdens to a significantly greater degree than daptomycin alone. Third, as anticipated from these microbiologic outcomes, serial vegetation size assessments by TTE confirmed a substantial and measurable salutary impact of exebacase in addition to daptomycin versus daptomycin alone therapies on “structural IE progression” (i.e., exebacase plus daptomycin therapy either reduced or stabilized vegetation size by TTE compared to increasing vegetation sizes over time observed by TTE in the daptomycin alone group). This outcome would speak to at least two likely events as follows: (i) biofilm disruption and (ii) reduction in MRSA burdens, a major driver toward progression and maturation of vegetations (14). Fourth, this stabilization/reduction in TTE-defined vegetation sizes correlated with reductions in vegetation weight as defined at sacrifice in the exebacase plus daptomycin versus daptomycin alone and control groups. The net vegetation weight in IE is a composite of bacterial burden, biofilm, and diverse host components, such as platelets, fibrinogen, fibronectin, and other molecules (14); these data on reduced vegetation weights in the exebacase plus daptomycin therapy group undoubtedly reflect the net impact of the lysin plus daptomycin on these factors. Lastly, the facile nature of the TTE methodology in experimental IE will allow investigators to query other important questions concerning the efficacy of exebacase in MRSA infections, such as the effects of different dose-regimens, multiple administrations, and alternate antimicrobial combination strategies. It should be pointed out that in humans with IE, many studies have implicated vegetation size with embolic risk. Also, increases in vegetation sizes by TTE during seemingly appropriate antimicrobial therapy appear to identify a subset of patients with a higher rate of complications; this outcome is independent of the presence of ongoing stigmata of IE or persistent bacteremia (14).
There are some important limitations to this study. Given the labor-intensive nature of these investigations, we studied a relatively small number of animals by TTE; this factor made it somewhat difficult to detect statistically significant differences in some group comparisons. Also, we were unable to compare the effect of exebacase (with or without daptomycin) by TTE with a concomitantly queried, untreated control group. Because of the high intrinsic virulence of this MRSA strain in experimental IE, untreated controls do not survive long enough for serial TTE evaluations. In addition, as noted above, we only employed a single-dose exebacase strategy, such as that tested in previous rabbit IE studies (12); it is conceivable that a more significant exebacase effect on vegetation microbiologic and structural metrics may be disclosed with repetitive exebacase dosing. Lastly, we only studied a single MRSA strain; future analyses will require additional strain investigations.
In conclusion, real-time TTE evaluations of animals with experimental MRSA IE are a novel way to serially assess the effect of exebacase in addition to daptomycin therapy on cardiac vegetation progression. These structural parameters as defined by TTE correlate well with impacts of such combinations on vegetation MRSA burden and vegetation weight. These data suggest that exebacase, in addition to daptomycin, can reduce the microbiologic and structural progression of experimental IE vegetations. This impact may well be related, at least in part, to the antibiofilm effect of exebacase.
MATERIALS AND METHODS
Induction of IE.
Animals were cared for in accordance with the American Associate for Accreditation of Laboratory Animal Care (AALAC). The research protocol was reviewed and approved by the Animal Research Committee (IACUC) of The Lundquist Institute (formerly The LA Biomedical Research Institute), as well as the Animal Care and Use Review Office (ACURO) at the U.S. Army Medical Research and Materiel Command (USAMRMC).
Experimental IE was induced by insertion of an indwelling vascular catheter via the right carotid artery across the aortic valve and into the left ventricle of anesthetized white New Zealand rabbits (2.2 to 2.5 kg; Irish Farms, Shafter, CA, USA) (16, 17). At 48 h after catheterization, MRSA strain MW2 was injected intravenously (i.v.) at an inoculum of 5 × 105 CFU, the previously established 95% infective dose (ID95) of this strain. MW2 has been used in a number of prior experimental IE studies in our laboratory (16, 17). The MW2 strain was grown in either tryptic soy broth (TSB) or on TSB agar plates for these experiments. The daptomycin and exebacase MIC against MW2 is 0.5 μg/ml.
Antimicrobial therapy.
At 24 h postinduction of IE, animals were randomized into the following groups: (i) untreated controls sacrificed at 24 h postinfection as a control infection baseline, (ii) daptomycin alone (4 mg/kg of body weight i.v. once a day [QD] for 4 days), or (iii) daptomycin plus a single slow bolus dose of exebacase on the first treatment day (11 mg/kg i.v.). This dose of daptomycin has been shown previously to provide a modest efficacy against this MRSA strain in the rabbit IE model (12), allowing for a “window” to define any potential in vivo synergistic impacts of daptomycin plus exebacase. The exebacase dose above was previously shown in this same model to exert a synergistic anti-MRSA effect with daptomycin (12). There was no exebacase alone group, as the lysin itself provides no microbiologic impacts on MRSA within cardiac vegetations in this model at the 11 mg/kg dose regimen (data not shown). Exebacase was provided by the ContraFect Corporation (Yonkers, NY, USA), while daptomycin was purchased from Teva Pharmaceuticals (Irvine, CA, USA).
Transthoracic echocardiography.
TTE was performed using a Mindray Z6 ultrasound system (Mahwah, NJ, USA) with a 2P2P phased-array pediatric transducer to measure the maximum vegetation size in this rabbit model of MRSA IE (15). We initially chose a group size of 6 animals per, which our statistician advised should give us an adequate sample size for analyses. Several animals died related to surgical or postsurgical complications or the general anesthesia required for maintaining animals very still for obtaining technically adequate serial echocardiograms.
TTE was carried out in the untreated control (n = 5), daptomycin alone (n = 4), and daptomycin plus exebacase (n = 6) groups. The animals were anesthetized for imaging using ketamine (50 mg/kg intramuscularly [i.m.] once) and xylazine (10 mg/kg i.m. once). Measurements were obtained serially in daptomycin-treated animals (with or without exebacase) in the parasternal long axis view at baseline (just prior to therapy) and then at 4, 24, and 96 h thereafter and again at day 5 (1 day after completion of daptomycin therapy). Although multiple vegetations were frequently visualized, the maximum dimension of the largest vegetation seen in each rabbit was recorded and then serially assessed in animals treated with daptomycin (with or without exebacase therapy). The echocardiographers (S. U. Shah and J. Iwaz) were blinded as to the treatment groups.
Animal sacrifice.
At their assigned sacrifice dates, untreated controls and daptomycin-treated animals (with or without exebacase administration) were humanely euthanized by i.v. sodium pentobarbital overdose. In daptomycin-treated animals, sacrifices were performed at least 18 to 24 h after the last antibiotic dose to minimize antibiotic carryover effects. All visible vegetations were removed from individual animals, pooled, and weighed. They were then homogenized in phosphate-buffered saline (PBS) and quantitatively cultured onto TSA plates. Vegetation MRSA counts for each rabbit were calculated as log10 CFU per gram, and the mean vegetation MRSA counts for each treatment group were calculated (±SD). For this study, we focused on intravegetation metrics and did not quantitatively culture other target organs (kidneys and spleen).
Statistical analyses.
A simple linear regression model with a corresponding regression coefficient was used to check the increasing or decreasing trends of vegetation size by TTE for each group over time. In addition, the Pearson’s correlation coefficient was used to test for a correlation between maximum vegetation size by TTE and the vegetation weight. Vegetation MRSA counts were calculated as mean log10 CFU per gram, and one-way analysis of variance (ANOVA) was used to compare vegetation counts among the control, daptomycin alone, and daptomycin plus exebacase groups with the Tukey post hoc pairwise comparisons. All outcome variables are summarized with means and standard deviations in Table 1. All statistical analyses were carried out using SAS 9.4 (SAS Institute Inc, Cary, NC, USA.).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funds from the Congressionally Directed Medical Programs (CDMRP), award number W81XWH-16-1-0245 to ContraFect Corp (R.S.). A.S.B., W.A., and Y.Q.X. were supported in part by a research subcontract from this award.
S. U. Shah, Y. Q. Xiong, J. Iwaz, W. Abdelhady, Y. Pak, and A. S. Bayer have no conflicts of interest to declare.
R. Schuch and C. Cassino are employees of ContraFect Corp.; D. Lehoux is a consultant to ContraFect Corp.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Hassoun A, Linden PK, Friedman B. 2017. Incidence, prevalence, and management of MRSA bacteremia across patient populations—a review of recent developments in MRSA management and treatment. Crit Care 21:211. doi: 10.1186/s13054-017-1801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodvold KA, McConeghy KW. 2014. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis 58(Suppl):S20–S27. doi: 10.1093/cid/cit614. [DOI] [PubMed] [Google Scholar]
- 3.Holland TL, Arnold C, Fowler VG Jr.. 2014. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 312:1330–1341. doi: 10.1001/jama.2014.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayer AS, Schneider T, Sahl HG. 2013. Mechanisms of daptomycin resistance in Staphylococcus aureus: role of the cell membrane and cell wall. Ann N Y Acad Sci 1277:139–158. doi: 10.1111/j.1749-6632.2012.06819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ernst CM, Slavetinsky CJ, Kuhn S, Hauser JN, Nega M, Mishra NN, Gekeler C, Bayer AS, Peschel A. 2018. Gain-of-function mutations in the phospholipid flippase MprF confer specific daptomycin resistance. mBio 9:e01659-18. doi: 10.1128/mBio.01659-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 7.Pfaller MA, Mendes RE, Streit JM, Hogan PA, Flamm RK. 2017. Five-year summary of in vitro activity and resistance mechanisms of linezolid against clinically important Gram-positive cocci in the United States from the LEADER surveillance program (2011 to 2015). Antimicrob Agents Chemother 61:e00609-17. doi: 10.1128/AAC.00609-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuch R, Lee HM, Schneider BC, Sauve KL, Law C, Khan BK, Rotolo JA, Horiuchi Y, Couto DE, Raz A, Fischetti VA, Huang DB, Nowinski RC, Wittekind M. 2014. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J Infect Dis 209:1469–1478. doi: 10.1093/infdis/jit637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuch R, Khan BK, Raz A, Rotolo JA, Wittekind M. 2017. Bacteriophage lysin CF-301, a potent antistaphylococcal biofilm agent. Antimicrob Agents Chemother 61:e02666-16. doi: 10.1128/AAC.02666-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson A, Oh JT, Sauve K, Bradford PA, Cassino C, Schuch R. 2019. Antimicrobial activity of exebacase (lysin CF-301) against the most common causes of infective endocarditis. Antimicrob Agents Chemother 63:e01078-19. doi: 10.1128/AAC.01078-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson A, Sauve K, Cassino C, Schuch R. 2020. Exebacase demonstrates in vitro synergy with a broad range of antibiotics against both methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 64:e01885-19. doi: 10.1128/AAC.01885-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Indiani C, Sauve K, Raz A, Abdelhady W, Xiong YQ, Cassino C, Bayer AS, Schuch R. 2019. The antistaphylococcal lysin, CF-301, activates key host factors in human blood to potentiate methicillin-resistant Staphylococcus aureus bacteriolysis. Antimicrob Agents Chemother 63:e02291-18. doi: 10.1128/AAC.02291-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowler VG, Das A, Lipka J, Schuch R, Cassino C. 2019. Exebacase (lysin CF-301) improved clinical responder rates in methicillin-resistant Staphylococcus aureus bacteremia and endocarditis compared to standard of care antibiotics alone in a first-in-patient phase 2 study. European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, Netherlands. [Google Scholar]
- 14.Holland TL, Bayer AS, Fowler VG. 2019. Endocarditis and intravascular infections, p 1068–1089. In Mandell GL, Bennett JE, Dolin R (ed), Principles and practices of infectious diseases, 9th ed Elsevier Publishing, Philadelphia, PA. [Google Scholar]
- 15.Kupferwasser LI, Yeaman MR, Shapiro SM, Nast CC, Bayer AS. 2002. In vitro susceptibility to thrombin-induced platelet microbicidal protein is associated with reduced disease progression and complication rates in experimental Staphylococcus aureus endocarditis: microbiological, histopathologic, and echocardiographic analyses. Circulation 105:746–752. doi: 10.1161/hc0602.103721. [DOI] [PubMed] [Google Scholar]
- 16.Ersoy SC, Abdelhady W, Li L, Chambers HF, Xiong YQ, Bayer AS. 2019. Bicarbonate resensitization of methicillin-resistant Staphylococcus aureus to beta-lactam antibiotics. Antimicrob Agents Chemother 63:e00496-19. doi: 10.1128/AAC.00496-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trotonda MP, Xiong YQ, Memmi G, Bayer AS, Cheung AL. 2009. Role of mgrA and sarA in methicillin-resistant Staphylococcus aureus autolysis and resistance to cell wall-active antibiotics. J Infect Dis 199:209–218. doi: 10.1086/595740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.