Optimal concentrations of unbound antimicrobials are essential for a maximum microbiological effect. Although hypoalbuminemia and albumin fluid resuscitation are common in critical care, the effects of different albumin concentrations on the unbound concentrations of highly protein-bound antimicrobials are not known. The aim of this study was to compare the effects of different albumin states on total and unbound concentrations of ertapenem and ceftriaxone using an ovine model. The study design was a prospective, three-phase intervention observational study.
KEYWORDS: ceftriaxone, ertapenem, pharmacokinetics, protein bound
ABSTRACT
Optimal concentrations of unbound antimicrobials are essential for a maximum microbiological effect. Although hypoalbuminemia and albumin fluid resuscitation are common in critical care, the effects of different albumin concentrations on the unbound concentrations of highly protein-bound antimicrobials are not known. The aim of this study was to compare the effects of different albumin states on total and unbound concentrations of ertapenem and ceftriaxone using an ovine model. The study design was a prospective, three-phase intervention observational study. The subjects were healthy Merino sheep. Eight sheep were subjected to three experimental phases: normoalbuminemia, hypoalbuminemia using plasmapheresis, and albumin replacement using a 25% albumin solution. In each phase, ceftriaxone at 40 mg/kg of body weight and ertapenem at 15 mg/kg were given intravenously. Blood samples were collected at predefined intervals and analyzed using an ultrahigh-performance liquid chromatography–tandem mass spectrometry method. Pharmacokinetic parameters such as the area under the curve from 0 to 24 h (AUC0–24), plasma clearance (CL), and apparent volume of distribution in the terminal phase (V) were estimated and compared between the phases. The protein and albumin concentrations were significantly different between phases. Hypoalbuminemia resulted in a significantly lower AUC0–24 and higher CL of total and unbound concentrations of ceftriaxone than in the other phases, whereas albumin replacement led to higher AUC0–24 and lower CL than in the other phases for both drugs. The V values for total drug concentrations for both drugs were significantly lower with albumin replacement. For highly protein-bound drugs such as ceftriaxone and ertapenem, both hypoalbuminemia and albumin replacement may affect unbound drug exposure.
TEXT
Hypoalbuminemia is a common feature of most critical illnesses, and its effect on unbound antimicrobial pharmacokinetics (PK) was described previously (1), with increases in the apparent total volume of distribution (V) and plasma clearance (CL) observed. These phenomena could lead to lower unbound antimicrobial exposures and suboptimal pharmacokinetic/pharmacodynamic (PK/PD) target attainment, especially for time-dependent antimicrobials (1).
Fluid resuscitation is recommended for the treatment of septic shock, with both crystalloid and colloidal fluids commonly used for this purpose (2). A large randomized controlled trial, the Saline versus Albumin Fluid Evaluation (SAFE) study, demonstrated the safety and efficacy of albumin administration in comparison to saline (3, 4). Consequently, albumin is often used in the treatment of septic shock in critically ill patients (5).
Although albumin as a resuscitation fluid is commonly used in the management of septic shock, its effect on unbound antimicrobial concentrations is not known. It is possible that the unbound concentrations of drugs with a high protein binding capacity may be adversely affected, thus affecting infection treatment outcomes. It was suggested previously that changes in plasma protein binding have little clinical relevance (6). However, a comparison between normoalbuminemia, hypoalbuminemia, and albumin replacement in the same subject would be useful for a mechanistic characterization of the changes in the PK of highly protein-bound antimicrobial agents, hence providing an opportunity to optimize antimicrobial dosing.
The management of sepsis and septic shock also involves the use of antimicrobial agents from a number of different classes, and these agents have various protein binding properties. For drug efficacy, the unbound antimicrobial concentration at the target site (i.e., site of infection) has to be optimal to maximize the likelihood of therapeutic success. However, exposure of the unbound antimicrobial could be influenced by different factors (7). Ceftriaxone is a 3rd-generation cephalosporin with 60 to 90% (concentration-dependent) protein binding (8). This antibiotic is commonly used for the treatment of community-acquired pneumonia. Ertapenem is a carbapenem with 95% protein binding (9) and is commonly used for the treatment of sepsis resulting from resistant organisms.
The aim of this study was to compare the plasma PK of total and unbound ceftriaxone and ertapenem during normoalbuminemia, during hypoalbuminemia, and after albumin replacement using a controlled ovine model. We hypothesized that hypoalbuminemia would result in higher unbound concentrations of ceftriaxone and ertapenem and that albumin replacement would not cause significant changes in the unbound concentrations of the two beta-lactams compared to a normoalbuminemic state.
RESULTS
Eight healthy sheep (aged 3 to 4 years, weighing 36.5 to 49.2 kg) were included in this study. Compared to the normoalbuminemia phase, the mean total protein and mean albumin concentrations were significantly reduced (64 g/liter versus 55 g/liter and 29.5 g/liter versus 26.1 g/liter, respectively) in the hypoalbuminemia phase. Compared to the hypoalbuminemia phase, albumin replacement in phase 3 significantly increased the mean total protein and mean albumin concentrations (55 g/liter versus 64.3 g/liter and 26.1 g/liter versus 35.8 g/liter, respectively) (Fig. 1 and 2).
FIG 1.
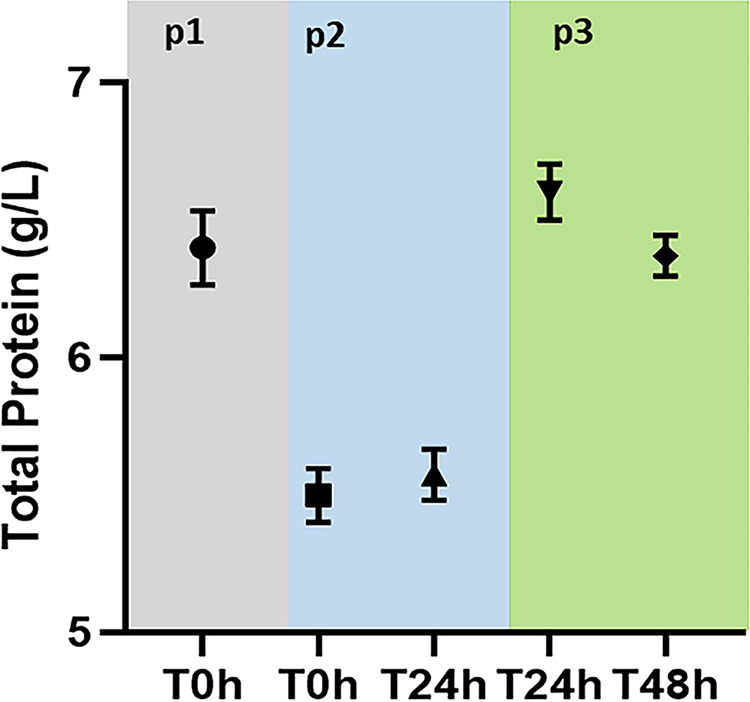
Total protein levels during the study period. p1 (phase 1), normoalbuminemia; p2, hypoalbuminemia; p3, albumin replacement.
FIG 2.
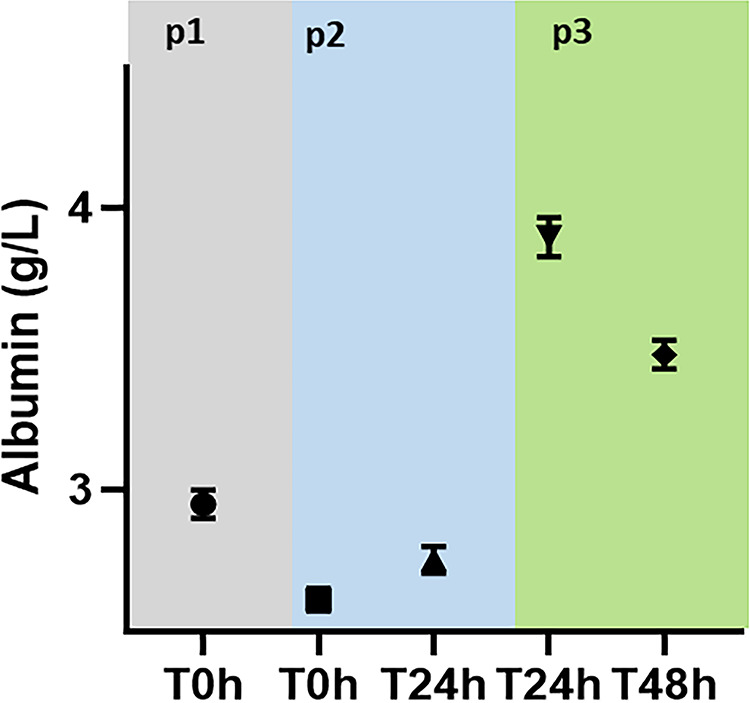
Total albumin levels during the study period. p1, normoalbuminemia; p2, hypoalbuminemia; p3, albumin replacement.
Protein binding.
The mean protein binding values for ceftriaxone and ertapenem are shown in Table 1. Significant differences were noted when comparing the normoalbuminemia- and albumin-replacement-phase groups as well as when comparing the hypoalbuminemia- and albumin-replacement-phase groups.
TABLE 1.
Protein binding percentages across the study phases
| Phasea | Mean % protein binding (SD)b
|
|
|---|---|---|
| Ceftriaxone | Ertapenem | |
| 1 | 19.9 (3.7) B | 40.6 (4.2) B |
| 2 | 19.1 (6.0) C | 42.8 (3.7) C |
| 3 | 68.0 (2.3) | 68.9 (2.3) |
Phase 1, normoalbuminemia; phase 2, hypoalbuminemia; phase 3, albumin replacement.
B, significant difference (P < 0.05) between phases 1 and 3; C, significant difference (P < 0.05) between phases 2 and 3.
Pharmacokinetic parameter estimates for ceftriaxone.
Pharmacokinetic parameter estimates for ceftriaxone are shown in Table 2.
TABLE 2.
Pharmacokinetic estimates for ceftriaxone across the study phases
| Phasea | Mean value (SD)b
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Total drug concn data |
Free drug concn data |
|||||||
| AUC0–24 (mg · h/liter) | kel (h−1) | V (liters) | CL (liters/h) | AUC0–24 (mg · h/liter) | kel (h−1) | V (liters) | CL (liters/h) | |
| 1 | 83.6 (13.4) AB | 1.2 (0.4) B | 19.9 (5.9) B | 21.9 (3.1) AB | 68.0 (11.4) AB | 1.3 (0.5) | 23.5 (7.2) | 27.0 (4.1) AB |
| 2 | 65.0 (13.5) C | 1.1 (0.2) C | 26.8 (6.2) C | 28.5 (5.1) C | 51.0 (10.9) C | 1.0 (0.1) | 36.6 (9.4) | 36.3 (6.1) C |
| 3 | 284.4 (40.1) | 0.7 (0.1) | 9.7 (1.1) | 6.4 (1.0) | 88.5 (14.0) | 1.0 (0.3) | 22.5 (4.3) | 20.7 (3.6) |
Phase 1, normoalbuminemia; phase 2, hypoalbuminemia; phase 3, albumin replacement.
AUC0–24, area under the concentration-time curve during a 24-h period; CL, clearance; kel, elimination rate constant; V, volume of distribution; A, significant difference (P < 0.05) between phases 1 and 2; B, significant difference (P < 0.05) between phases 1 and 3; C, significant difference (P < 0.05) between phases 2 and 3.
(i) Total drug concentrations. Compared to the normoalbuminemia phase, the area under the concentration-time curve from 0 to 24 h (AUC0–24) (mean, 83.6 mg · h/liter versus 65 mg · h/liter, respectively) was significantly decreased and the CL (mean, 21.9 liters/h versus 28.5 liters/h, respectively) was significantly increased in the hypoalbuminemia phase. Compared to the normoalbuminemia and hypoalbuminemia phases, the AUC0–24 (mean, 83.6 versus 65 versus 284.4 mg · h/liter, respectively) was significantly increased and the elimination rate constant (kel) (mean, 1.2 h−1 versus 1.1 h−1 versus 0.7 h−1, respectively), V (mean, 19.9 liters versus 26.8 liters versus 9.7 liters, respectively), and CL (mean 21.9 liters/h versus 28.5 liters/h versus 6.4 liters/h, respectively) were significantly decreased in the albumin replacement phase.
(ii) Unbound drug concentrations. Compared to the normoalbuminemia phase, the AUC0–24 (mean, 68 mg · h/liter versus 51 mg · h/liter, respectively) was significantly decreased and the CL (mean, 27 liters/h versus 36.3 liters/h, respectively) was significantly increased in the hypoalbuminemia phase. Compared to the normoalbuminemia and hypoalbuminemia phases, the AUC0–24 (mean, 68 mg · h/liter versus 51 mg · h/liter versus 88.5 mg · h/liter, respectively) was significantly increased and the CL (mean, 27 liters/h versus 36.3 liters/h versus 20.7 liters/h, respectively) was significantly decreased in the albumin replacement phase.
Pharmacokinetic parameter estimates for ertapenem.
Pharmacokinetic parameter estimates for ertapenem are shown in Table 3.
TABLE 3.
Pharmacokinetic estimates for ertapenem across the study phases
| Phasea | Mean value (SD)b
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Total drug concn data |
Free drug concn data |
|||||||
| AUC0–24 (mg · h/liter) | kel (h−1) | V (liters) | CL (liters/h) | AUC0–24 (mg · h/liter) | kel (h−1) | V (liters) | CL (liters/h) | |
| 1 | 26.9 (3.2) B | 0.9 (0.3) | 28.7 (7.3) B | 25.0 (3.7) B | 16.0 (2.3) B | 1.2 (0.4) | 38.4 (9.4) | 42.3 (7.5) B |
| 2 | 24.8 (7.4) C | 1.2 (0.4) | 26.8 (11.2) C | 28.8 (8.2) C | 14.5 (4.7) C | 1.2 (0.4) | 42.1 (14.2) | 49.9 (15.2) C |
| 3 | 78.0 (16.1) | 0.8 (0.1) | 10.6 (1.8) | 8.9 (2.2) | 25.5 (6.4) | 0.9 (0.2) | 30.5 (7.8) | 27.6 (7.4) |
Phase 1, normoalbuminemia; phase 2, hypoalbuminemia; phase 3, albumin replacement.
AUC0–24, area under the concentration-time curve during a 24-h period; CL, clearance; kel, elimination constant; V, volume of distribution; B, significant difference (P < 0.05) between phases 1 and 3; C, significant difference (P < 0.05) between phases 2 and 3.
(i) Total drug concentrations. There were no significant differences in the pharmacokinetic parameters between the normoalbuminemia and hypoalbuminemia phases. Compared to the normoalbuminemia and hypoalbuminemia phases, the AUC0–24 (mean, 26.9 mg · h/liter versus 24.8 mg · h/liter versus 78 mg · h/liter, respectively) was significantly increased and the V (mean, 28.7 liters versus 26.8 liters versus 10.6 liters, respectively) and CL (mean 25 liters/h versus 28.8 liters/h versus 8.9 liters/h) were significantly decreased in the albumin replacement phase.
(ii) Unbound drug concentrations. The differences in the pharmacokinetic parameters between the normoalbuminemia and hypoalbuminemia phases remained statistically insignificant. Compared to the normoalbuminemia and hypoalbuminemia phases, the AUC0–24 value (mean, 16 mg · h/liter versus 14.5 mg · h/liter versus 25.5 mg · h/liter, respectively) was significantly increased and the CL (mean, 42.3 liters/h versus 49.9 liters/h versus 27.6 liters/h, respectively) was significantly decreased in the albumin replacement phase.
The plasma drug concentrations were below the lower limit of quantification (for ertapenem, total concentration of 0.5 μg/ml and unbound concentration of 0.2 μg/ml; for ceftriaxone, total concentration of 2 μg/ml and unbound concentration of 1 μg/ml) after a mean of 3.33 h (±1.27 h) of drug administration.
DISCUSSION
In a controlled ovine model, this study showed that compared to the normoalbuminemia phase, hypoalbuminemia led to reduced exposure and increased clearance for total and unbound ceftriaxone. Albumin replacement increased the total and unbound ceftriaxone and ertapenem concentrations and reduced their clearance. Protein binding increased significantly with albumin replacement.
In the normoalbuminemia phase, the mean protein binding percentages for ceftriaxone and ertapenem were 19.9% and 40.6%, respectively. In human studies, protein binding percentages are 60 to 90% for ceftriaxone (8) and approximately 95% for ertapenem (9). Comparatively, in humans, the normal total protein (60 to 80 g/liter) and albumin (35 to 50 g/liter) concentrations (10) are different from those in sheep (total protein of 68 to 78 g/liter and albumin of 26 to 36 g/liter) (11). In our study, the lower mean serum albumin concentration (29.5 g/liter) could have had some effect on protein binding. But there could also be differences in human and sheep albumin binding affinities (12). In vitro studies investigating the interspecies differences in albumin affinity could further elucidate this phenomenon.
There was no significant difference in the protein binding percentages between the normoalbuminemia and hypoalbuminemia phases for ceftriaxone and ertapenem. The exact cause of the lack of differences is not clear. Although hypoalbuminemia is seen to reduce the protein binding percentage of highly protein-bound antimicrobials (1), the relationship of the degree of hypoalbuminemia to that of the effect on the protein binding percentage is not well defined. In addition, there is no consensus for the specific albumin levels defining hypoalbuminemia. In our study, the magnitude of the change in the albumin level (from 29.5 to 26.1 g/liter) may not be adequate to demonstrate this effect. There are also likely interspecies differences in the protein binding affinities for albumin between sheep and human.
Hypoalbuminemia was associated with an increase in the drug CL of total and unbound ceftriaxone. This led to a reduction in the plasma AUC0–24 of total and unbound ceftriaxone. These effects were similar for total and unbound ertapenem concentrations but did not achieve statistical significance, possibly due to the lower magnitude of the change in albumin levels or the small sample size. Although this finding was similar to those of previously reported studies (1), the magnitude of the difference was lower. This could be due to interspecies differences in albumin binding affinities (13).
The albumin replacement phase showed a significant increase in total plasma protein and albumin concentrations compared to the normoalbuminemia and hypoalbuminemia phases. Albumin replacement resulted in decreased CL for total and unbound ceftriaxone and ertapenem concentrations in plasma and V of total drug concentrations. This resulted in an increase in the plasma AUC0–24 of total and unbound drug concentrations. Similar findings were reported for other highly protein-bound nonantimicrobial drugs as well (14). Although an in vitro study attributed this to the presence of stabilizers in pharmaceutical-grade albumin (15), other data suggest that this is unlikely (14). The increase in the AUC0–24 of total drug concentrations is likely to be due to the increased binding of human serum albumin to the antimicrobials. However, the increase in the plasma AUC0–24 of the unbound drugs is likely to be due to increased oncotic pressure as a result of albumin replacement, thus increasing the volume of the central compartment. A study using other colloid agents would be useful to prove this hypothesis. The effect of albumin replacement on the unbound antimicrobial concentrations in tissues is not known and will need further investigation.
For antimicrobials, the unbound drug concentration at the site of infection is critical for therapeutic efficacy (7). Where the interstitial space fluid of tissues is the site of action, unbound plasma concentrations often represent a reasonable surrogate for tissue concentrations due to rapid equilibrium between plasma and tissues (16). Hence, changes in unbound plasma antimicrobial concentrations would lead to tissue concentration changes. Compared to the normoalbuminemic phase, the unbound ceftriaxone concentration decreased significantly in the hypoalbuminemia phase. The reduction in the unbound ertapenem concentrations was not statistically significant, likely due to the lower magnitude of the change in albumin levels or the small sample size. This could be due to the increased CL and V seen in hypoalbuminemia. As hypoalbuminemia is a common feature in critical illness (17), this may indicate the need for higher antimicrobial doses in this group of patients. Conversely, compared to the normoalbuminemia and hypoalbuminemia phases, albumin replacement caused significant increases in ceftriaxone and ertapenem concentrations. Decreased CL and V could lead to this phenomenon. For highly protein-bound antimicrobial drugs with a narrow therapeutic index, this may indicate the need for increased vigilance in a clinical scenario using therapeutic drug monitoring.
Previous studies have recommended therapeutic drug monitoring using direct measurement of unbound antimicrobial concentrations for highly protein-bound drugs due to the difficulty in predicting the unbound concentrations from total concentration data (18). Similarly, our study shows that although the total and unbound concentrations increased with albumin replacement, it would not be possible to predict the increment due to the nonlinear increase in the concentrations. Further studies with other highly protein-bound drugs in human subjects are necessary to confirm the findings of this study.
Strengths and limitations.
All the phases of the study were performed in the same sheep, which accounts for the interindividual variability that often affects the significance of data acquired from a small sample size. This model is also ideal for the mechanistic study of the effect of a single variable while controlling for other factors. While not definitive, these studies generate a basis for large-scale human studies in this field.
The study was conducted in sheep. Although this is a large-animal model, interspecies variation in the PK of antimicrobials could affect the results, affecting the extrapolation of the data to the human population. The antimicrobial binding affinity of highly protein-bound antimicrobials for sheep albumin is not well known and is likely to be different from that for human albumin. Finally, the study was performed in a healthy-sheep model, whereas critical illness and trauma are known to influence the antimicrobial PK; however, this approach allowed us to characterize the effect of different plasma albumin concentrations in the absence of other pathologies.
Conclusion.
Based on the study results, in hypoalbuminemia, higher doses of highly protein-bound antimicrobials may be necessary to achieve adequate unbound drug concentrations. However, with albumin replacement, dose adjustment may be necessary for drugs with a narrow therapeutic index to prevent drug toxicity. Thus, altered dosing, potentially supported by therapeutic drug monitoring using unbound concentrations, is suggested for highly protein-bound drugs to enable optimal antimicrobial therapy in critical illness.
MATERIALS AND METHODS
Ethics approval was obtained from the Institutional Animal Care and Use Committee (The University of Queensland Animal Ethics Committee; approval number SVS/395/17). The care and handling of the animals were performed in accordance with the Australian National Institutes of Health guidelines for ethical animal treatment (19).
Study design.
Merino male castrated sheep were included. All sheep were clinically normal, with normal baseline hematology and biochemistry. All sheep were maintained on ad libitum water and lucerne hay for the duration of the study.
Experimental method.
Sheep were randomly numbered 1 to 8, and bilateral jugular vein catheters were placed using a Seldinger technique at the start of the study. The right jugular venous catheter was used for drug administration, and the left one was used for blood sample acquisition. A presample of 5 ml was removed from the left catheter prior to the sample being drawn and was then returned via the catheter and flushed with 5 ml of heparinized saline.
The study was divided into three phases: normoalbuminemia (phase 1), hypoalbuminemia (phase 2), and albumin replacement (phase 3). For each phase of the study, ceftriaxone (40 mg/kg of body weight) and ertapenem (15 mg/kg) were administered as a slow bolus over 2 min via the right jugular venous catheter. Blood samples were acquired for PK evaluation 0, 0.5, 0.75, 1, 1.5, 2, 4, 6, 8, 12, and 24 h after the commencement of the antimicrobial in each of the three phases.
Hypoalbuminemia was induced by batch plasmapheresis immediately after the last PK sample in the normoalbuminemia phase was collected. Specifically, based on individual sheep weight, a calculated volume of whole blood was collected in 500-ml batches into blood collection bags (citrated) to achieve a target reduction in total protein of 20%. Centrifugation (J6-MI centrifuge; Beckman Coulter Inc., Brea, CA, USA) at 4°C was performed at 3,200 rpm for 15 min, with deceleration for an additional 5 min. After centrifugation, the protein fraction was isolated and discarded, with the remaining red cell portion returned to the sheep and with the difference in volume (equal to the removed protein volume) replaced using a balanced electrolyte solution. After hypoalbuminemia was induced (<2 h), the antimicrobials were administered, and PK sampling was repeated.
The albumin replacement phase was performed immediately after the last PK sample in the hypoalbuminemia phase was collected by using commercially available exogenous human albumin (Alburex 25; CSL Behring AG, Bern, Switzerland). Specifically, 200 ml of 25% albumin (equivalent to 1.02 to 1.37 g/kg) was administered via the right catheter over a 1-h period to return the total protein to baseline normal limits. After replacement, the antimicrobials were administered, and PK sampling was repeated. All samples were stored at −80°C for further analyses. All sheep were euthanized after the study by a lethal dose of barbiturate anesthetic.
Sample analysis.
(i) Protein analyses. Both total protein and albumin were measured on a Beckman Coulter AU480 analyzer (Beckman Coulter Diagnostic Systems Division, Melville, NY, USA) via spectrophotometry.
Total protein was measured using the Biuret method (reagent OSR6132; Olympus). Cupric ions in an alkaline solution react with proteins and polypeptides containing at least two peptide bonds to produce a violet complex. The absorbance of the complex at 540/660 nm is directly proportional to the concentration of protein in the sample.
Albumin was measured using a bromocresol green (BCG) method (reagent OSR6102; Olympus). A colored complex is formed when bromocresol green reacts with albumin. The absorbance of the albumin-BCG complex is measured bichromatically (600/800 nm) and is proportional to the albumin concentration in the sample.
(ii) Drug analyses. Ceftriaxone and ertapenem (total and unbound levels in plasma) were measured by a validated ultrahigh-performance liquid chromatography (UHPLC)–tandem mass spectrometry method on a Nexera2 UHPLC system connected to an 8030+ triple-quadrupole mass spectrometer (Shimadzu, Kyoto, Japan). Test samples were assayed in batches alongside calibrators and quality controls, and results were subject to batch acceptance criteria.
The unbound fraction was isolated by ultrafiltration at 37°C with Centrifree devices (Merck Millipore, Tullagreen, Ireland). Samples (plasma or ultrafiltered plasma, at 10 μl) were spiked with an internal standard (deuterated ceftriaxone), diluted with acetonitrile, and centrifuged. An aliquot of 0.5 μl of the supernatant was injected onto the ultrahigh-performance liquid chromatography–tandem mass spectrometer.
The stationary phase was a Luna Omega polar C18, 50- by 2.1-mm (1.6-μm) analytical column equipped with a C18 guard column (Phenomenex, Torrance, CA, USA). Mobile phase A was water with 0.2% (vol/vol) formic acid, and mobile phase B was acetonitrile with 0.2% (vol/vol) formic acid. Separations for ceftriaxone and ertapenem were performed over 2.5 min with an isocratic mobile phase (80% mobile phase A/20% mobile phase B) at a flow rate of 0.4 ml/min, producing back pressure of approximately 6,000 lb/in2. Ceftriaxone and ertapenem were monitored in the positive electrospray mode at multiple reaction monitoring (MRM) of 554.7→396.1 and 476.15→114.0, respectively. The internal standard (deuterated ceftriaxone) was monitored in the positive mode at 557.7→399.10.
Measurement of ertapenem was linear from 0.5 to 200 mg/liter (total) and from 0.2 to 200 mg/liter (unbound), with intrabatch precision within 6.1% and accuracy within 10.5%. Measurement of ceftriaxone was linear from 2 to 200 mg/liter (total) and from 1 to 200 mg/liter (unbound), with intrabatch precision within 9.5% and accuracy within 8.3%.
Data analysis.
(i) Pharmacokinetic analysis. The PK parameters were estimated using noncompartmental methods with PKsolver in MS Excel. The area under the plasma concentration-time curve from 0 h to 24 h (AUC0–24) was estimated using the linear trapezoidal rule. The CL was obtained by dividing the actual dose administered by the corresponding AUC0–24. The apparent terminal elimination rate constant (kel) was estimated by regression of the terminal log-linear concentration time points. The V was calculated as CL/kel. This method assumes linear PK.
(ii) Statistical analysis. All PK parameters were normally distributed at each study phase, as assessed by a Shapiro-Wilk test (P > 0.05). One-way repeated-measures analysis of variance (ANOVA) was used to compare PK estimates across the three study phases. Post hoc analysis with a Bonferroni adjustment was then used to determine statistically significant differences between the study phases. A P value of <0.05 was considered statistically significant in all analyses. Statistical analysis was performed using IBM SPSS Statistics v24 (IBM Corporation, Armonk, NY, USA). For statistical analysis of protein and albumin, a P value of <0.05 by one-way ANOVA using the Prism statistics program was considered significant.
ACKNOWLEDGMENTS
We acknowledge the contributions by the staff and administrators of the Medical Engineering and Research Facility (MERF), Queensland University of Technology, Chermside, Queensland, Australia.
The study was funded by an RBWH Foundation project grant. Jason A. Roberts recognizes funding from the Australian National Health and Medical Research Council for a Centre of Research Excellence grant (APP1099452) and a practitioner fellowship (APP1117065). Jayesh A. Dhanani acknowledges funding from a Metro North Clinician Researcher fellowship.
REFERENCES
- 1.Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. 2011. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet 50:99–110. doi: 10.2165/11539220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Schindler AW, Marx G. 2016. Evidence-based fluid management in the ICU. Curr Opin Anaesthesiol 29:158–165. doi: 10.1097/ACO.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 3.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R, SAFE Study Investigators . 2004. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 4.Annane D, Siami S, Jaber S, Martin C, Elatrous S, Declère AD, Preiser JC, Outin H, Troché G, Charpentier C, Trouillet JL, Kimmoun A, Forceville X, Darmon M, Lesur O, Reignier J, Abroug F, Berger P, Clec’h C, Cousson J, Thibault L, Chevret S, CRISTAL Investigators . 2013. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 310:1809–1817. doi: 10.1001/jama.2013.280502. [DOI] [PubMed] [Google Scholar]
- 5.Semler MW, Rice TW. 2016. Sepsis resuscitation: fluid choice and dose. Clin Chest Med 37:241–250. doi: 10.1016/j.ccm.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benet LZ, Hoener BA. 2002. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther 71:115–121. doi: 10.1067/mcp.2002.121829. [DOI] [PubMed] [Google Scholar]
- 7.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL, International Society of Anti-infective Pharmacology, Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases . 2014. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14:498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popick AC, Crouthamel WG, Bekersky I. 1987. Plasma protein binding of ceftriaxone. Xenobiotica 17:1139–1145. doi: 10.3109/00498258709167406. [DOI] [PubMed] [Google Scholar]
- 9.Majumdar AK, Musson DG, Birk KL, Kitchen CJ, Holland S, McCrea J, Mistry G, Hesney M, Xi L, Li SX, Haesen R, Blum RA, Lins RL, Greenberg H, Waldman S, Deutsch P, Rogers JD. 2002. Pharmacokinetics of ertapenem in healthy young volunteers. Antimicrob Agents Chemother 46:3506–3511. doi: 10.1128/AAC.46.11.3506-3511.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertholf RL. 2014. Proteins and albumin. Lab Med 45:e25–e41. doi: 10.1309/LMKRNRGW5J03APZQ. [DOI] [Google Scholar]
- 11.Roil MR, Suckling GW, Mattingley J. 1974. Serum total protein and albumin levels in grazing sheep. N Z Vet J 22:232–236. doi: 10.1080/00480169.1974.34176. [DOI] [PubMed] [Google Scholar]
- 12.Pistolozzi M, Bertucci C. 2008. Species-dependent stereoselective drug binding to albumin: a circular dichroism study. Chirality 20:552–558. doi: 10.1002/chir.20521. [DOI] [PubMed] [Google Scholar]
- 13.Beer J, Wagner CC, Zeitlinger M. 2009. Protein binding of antimicrobials: methods for quantification and for investigation of its impact on bacterial killing. AAPS J 11:1–12. doi: 10.1208/s12248-008-9072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reine PA, Kongsgaard UE, Andersen A, Thogersen AK, Olsen H. 2008. Infusion of albumin attenuates changes in serum protein binding of drugs in surgical patients compared with volume replacement with HAES. Acta Anaesthesiol Scand 52:406–412. doi: 10.1111/j.1399-6576.2007.01555.x. [DOI] [PubMed] [Google Scholar]
- 15.Olsen H, Andersen A, Nordbo A, Kongsgaard UE, Bormer OP. 2004. Pharmaceutical-grade albumin: impaired drug-binding capacity in vitro. BMC Clin Pharmacol 4:4. doi: 10.1186/1472-6904-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theuretzbacher U. 2007. Tissue penetration of antibacterial agents: how should this be incorporated into pharmacodynamic analyses? Curr Opin Pharmacol 7:498–504. doi: 10.1016/j.coph.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann FR, Safran C, Levkoff SE, Minaker KL. 1992. Serum albumin level on admission as a predictor of death, length of stay, and readmission. Arch Intern Med 152:125–130. doi: 10.1001/archinte.1992.00400130135017. [DOI] [PubMed] [Google Scholar]
- 18.Wong G, Briscoe S, Adnan S, McWhinney B, Ungerer J, Lipman J, Roberts JA. 2013. Protein binding of beta-lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob Agents Chemother 57:6165–6170. doi: 10.1128/AAC.00951-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Health and Medical Research Council 2013. Australian code for the care and use of animals for scientific purposes, 8th ed. National Health and Medical Research Council, Canberra, Australia. [Google Scholar]


