Comparative time-kill experiments with Staphylococcus aureus bacteriophage (phage) Sb-1 alone and phage-antibiotic combinations (PACs) against two methicillin-resistant S. aureus (MRSA) strains have shown synergy with both daptomycin-phage and vancomycin-phage combinations. PACs prevented development of phage resistance and demonstrated bactericidal activity for all triple combinations. In addition, the extracellular membrane vesicle (MV) formation and the potential impact of phage on MV suppression were examined.
KEYWORDS: Staphylococcus aureus, bacteriophage therapy, combination therapy
ABSTRACT
Comparative time-kill experiments with Staphylococcus aureus bacteriophage (phage) Sb-1 alone and phage-antibiotic combinations (PACs) against two methicillin-resistant S. aureus (MRSA) strains have shown synergy with both daptomycin-phage and vancomycin-phage combinations. PACs prevented development of phage resistance and demonstrated bactericidal activity for all triple combinations. In addition, the extracellular membrane vesicle (MV) formation and the potential impact of phage on MV suppression were examined. Our results demonstrate the potential of PAC for combating MRSA infections.
INTRODUCTION
Staphylococcus aureus is the leading cause of bacteremia and infective endocarditis that contribute significantly to morbidity and mortality (1–6). For decades, the antimicrobial treatment of choice for invasive methicillin-resistant S. aureus (MRSA) infections has been vancomycin (VAN); however, treatment failures and development of heterogeneous VAN intermediate-resistant S. aureus (hVISA), and VAN intermediate-resistant S. aureus (VISA) strains have led to the use of daptomycin (DAP) and ceftaroline (CPT), with bactericidal activity against MRSA (7–10). Despite the use of these alternatives, persistent bacteremia, development of DAP-nonsusceptible (DNS) strains, and mortality are still common in severe infections caused by S. aureus (11, 12).
Bacteriophages (phages), as natural predators of bacteria (13, 14), can evade the resistance developed to antibiotics through their different mechanisms of action (15–19). The hypothesis behind testing phage-antibiotic combinations (PACs) is that two distinctive selective pressures on bacterial growth can be more impactful than either alone (14, 20). Interestingly, Gram-positive bacteria, including S. aureus, naturally produce extracellular membrane vesicles (MVs) into their extracellular environment (21). These MVs may play a critical role in host immune evasion and transfer of virulence factors through the agr regulatory system and phenol-soluble modulins (22–29). The objective of this work was to investigate PAC therapy particularly in the context of existing antimicrobial resistance to individual agents (30–37) and the impact of PAC on MV formation with a bacteriophage used in Georgian hospitals (38).
S. aureus strain selection and in vitro susceptibility.
Eight well-characterized MRSA strains were screened for Sb-1 and antibiotic susceptibility (Table 1). Purified Sb-1 bacteriophage (a myophage categorized as Herelleviridae; GenBank accession no. HQ163896) was purchased commercially (38–40). Phage-mediated lysis and release of phage progeny particles were confirmed by formation of individual plaques on selected S. aureus strains (41). Sb-1 efficiency of plating (EOP) for strains D712 (DNS VISA, agr2) and MW2 (MRSA, agr2,3) (42) was equal to 1, 10−3, respectively. The two strains were used for 24-h time-kill experiments and MV studies. Bactericidal activity was defined as a >3-log10-CFU/ml reduction from baseline. Synergy between two agents was defined as a >2-log10-CFU/ml reduction compared with the most potent agent. Statistical analysis was performed using one-way analysis of variance with Tukey’s multiple-comparison test (with P < 0.05 considered significant).
TABLE 1.
MIC values and Sb-1 sensitivity screening tests
| Isolate | Phenotype | Strain type | Sb-1 sensitivitya | MIC (mg/liter) ofb
: |
|||
|---|---|---|---|---|---|---|---|
| VAN | DAP | CPT | CFZ | ||||
| COL | MRSA | USA100/ST250 | C | 2 | 0.25 | 0.25 | 32 |
| D712 | DNS VISA | USA100/ST5 | C | 4 | 4 | 0.5 | >64 |
| JH1 | MRSA | USA100/ST5 | C | 1 | 0.25 | 0.25 | 16 |
| 494 | MRSA | USA100/ST5 | C | 1 | 0.5 | 0.5 | 16 |
| N315 | MRSA | USA100/ST5 | T | 0.5 | <0.13 | 0.25 | 64 |
| MW2 | MRSA | USA400/ST1 | T | 0.5 | 0.25 | 0.25 | >64 |
| D592 | hVISA | USA100/ST5 | T | 2 | 0.5 | 2 | >64 |
| JO3 | DNS | USA300/ST8 | R | 1 | 4 | 0.25 | 8 |
Sb-1 produced clear or turbid spots or was resistant to phage infection. C, clear or high sensitivity to Sb-1 bacteriophage with distinct plaque formation (EOP of 0.1 to 1 compared with reference strain); T, turbid or medium sensitivity to Sb-1 with distinct plaque formation (EOP of 0.0001 to 0.01 compared with reference strain); R, resistant or no disturbance of bacterial lawn and no plaque formation.
VAN, vancomycin; DAP, daptomycin; CPT, ceftaroline; CFZ, cefazolin.
Time-kill analyses for assessing the PAC efficacy.
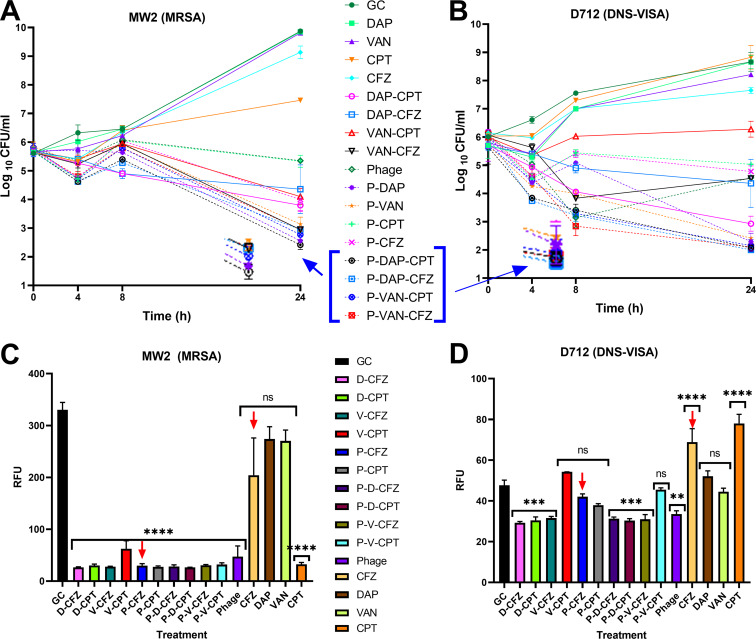
All triple combinations against D712 reached below the detection limit in 24 h (Fig. 1). Even though D712 is a DNS VISA strain, combinations of phage-DAP or phage-VAN were synergistic with significant reduction in log10 CFU/ml versus the single antibiotics DAP and VAN, respectively (P < 0.0001). Our hypothesis regarding this observation is that the complementary action of PACs reduces colony counts while inhibiting the developed mechanisms for phage resistance, because PAC is not impacted by the antibiotic resistance status of the bacterial cell (14, 43, 44). Phage monotherapy revealed static activity with a 1.45-log10-CFU/ml reduction from the initial inoculum, which was greater than those of any of the other individual agents. Other dual therapies, i.e., phage-cefazolin (CFZ) and phage-CPT, were as effective as phage alone (P > 0.05).
FIG 1.
(A, B) Time-kill experiments versus MRSA strain MW2 and DNS VISA strain D712. Triple combinations are highlighted as they demonstrated bactericidal activity compared with single antibiotics at the end of 24 h exposure. (C, D) Relative fluorescence units as identifiers of MV formation versus various treatments. Comparisons are versus growth control for each strain. VAN, vancomycin; DAP, D, daptomycin; CPT, ceftaroline; CFZ, cefazolin; Phage, P, bacteriophage Sb-1; ns, not significant.
Regarding the MW2 strain, phage monotherapy caused a 0.4-log10-CFU/ml reduction from the initial inoculum. This observation correlates with our EOP assays categorizing MW2 phage susceptibility as intermediate. The phage-DAP and phage-VAN regimens were synergistic, causing significant reduction in log10 CFU/ml versus the single antibiotics DAP and VAN, respectively (P < 0.0001). Phage-CPT was as effective as phage alone (P > 0.05), whereas the phage-CFZ regimen caused a 1.4-log10-CFU/ml reduction compared with single-phage therapy (P = 0.005).
Resistance tests.
Resistance screening was performed using the double-drop method (41, 45) (Table 2). No evidence of bacterial resistance to Sb-1 was observed at the end of 24 h in time-kill samples with PAC, whereas phage-alone regimens developed resistance. Antibiotic resistance tests were performed as described previously against DAP, VAN, CPT, and CFZ (46, 47), and no resistance/elevated MICs were observed in any of the regimens against strain D712 at the end of 24 h.
TABLE 2.
Resistance check for bacteriophage and antibiotics at the end of 24 h exposure
| Regimena | D712 |
MW2 |
||
|---|---|---|---|---|
| Phage resistanceb | Antibiotic resistance/elevated MIC (μg/ml) | Phage resistanceb | Antibiotic resistance/elevated MIC (μg/ml) | |
| P | R | –c | R | –c |
| P-VAN | S | S | ||
| VAN | NA | NA | ||
| P-VAN-CFZ | S | S | ||
| P-VAN-CPT | S | S | ||
| P-DAP | S | S | ||
| DAP | NA | NA | ||
| P-DAP-CFZ | S | S | ||
| P-DAP-CPT | S | S | ||
| P-CFZ | S | S | ||
| P-CPT | S | S | ||
| GC | S | –c | S | –c |
VAN, vancomycin; DAP, daptomycin; CPT, ceftaroline; CFZ, cefazolin; P, bacteriophage Sb-1; GC, growth control.
Bacterial isolates were susceptible at the end of 24-h time-kill experiment. S, clear spot in double-drop method; R, resistant (phage activity was not observed within bacterial spot). Regimens not including bacteriophage were not tested for bacteriophage resistance. NA, not applicable.
–, antibiotic resistance/elevated MIC values were not detected. The resistance/elevated MIC test was performed against DAP, VAN, and CPT. D712 already has MIC values of >64 against CFZ.
Membrane vesicle formation measurements.
Our hypothesis regarding MV experiments was that phages may stimulate vesicle shedding through AgrA-mediated quorum sensing (24) (Fig. 1). To test this hypothesis, we measured membrane vesicle formation in all regimens/treatments, including growth control, using modified Bolte method (48). Although MVs activate the immune response, which is critical in cases like bacteremia, they can also worsen the disease state in circumstances such as infective endocarditis due to high production of specific proinflammatory cytokines (49, 50). Moreover, reduction in MV formation might improve overall antimicrobial efficacy if MVs act as “sinks” or decoys capable of binding to phages or antibacterials and effectively reducing the local concentration of active drug (51).
Strain D712 demonstrated significantly higher MV formation with β-lactam antibiotics than with either β-lactam-phage combination (P < 0.0001). A similar trend was observed with strain MW2, in that CFZ, VAN, or DAP regimens formed significantly greater amounts of MVs than corresponding PAC regimens (P < 0.0001), whereas CPT did not follow this trend in strain MW2. Interestingly, the MW2 strain exhibited 6-fold-higher MVs for growth control than the D712 strain. Therefore, suppression of MV formation was more pronounced with MW2 than with D712, and suppression of MVs in PACs versus D712 was not significant (P > 0.5).
Phage quantification and the impact of antibiotics on phage replication.
Phage quantification was assessed using the modified small-drop agar overlay method (41). Phage populations at the end of the 24-h time-kill analysis ranged between 104 and 106 PFU/ml for all treatments and bacterial strains. While the phage population (attached plus unattached) at the start of the experiment was 6 × 105 PFU/ml (bacteriophage-to-bacterial ratio, 0.6), the number of unattached bacteriophages increased up to 106 during the experiment. In general, mean phage counts for MW2 were less than D712 because of lower sensitivity. However, no specific pattern of phage increase/decrease was observed with addition of antibiotic(s).
The limitations of this study include the limited number of tested organisms, inclusion of only one bacteriophage, and lack of dose escalation/de-escalation experiments. The findings of this study introduce PAC as a promising alternative for combating multidrug-resistant infections. In addition, PAC may allow for a reduction in required antibiotic exposures, minimizing antibiotic adverse events and strengthening antibiotic stewardship efforts.
ACKNOWLEDGMENTS
We thank Allergan Pharmaceuticals for providing ceftaroline powder. Furthermore, we acknowledge our collaborator Andrew Berti regarding MV experiment consultations.
REFERENCES
- 1.World Health Organization. 2020. Drug resistance. World Health Organization, Geneva, Switzerland: https://www.who.int/drugresistance/AMR_Importance/en. [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2020. Biggest threats and data. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/drugresistance/threat-report-2013. [Google Scholar]
- 3.Mendy A, Vieira ER, Albatineh AN, Gasana J. 2016. Staphylococcus aureus colonization and long-term risk for death, United States. Emerg Infect Dis 22:1966–1969. doi: 10.3201/eid2211.160220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakr A, Brégeon F, Mège J-L, Rolain J-M, Blin O. 2018. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections. Front Microbiol 9:2419. doi: 10.3389/fmicb.2018.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casapao AM, Leonard SN, Davis SL, Lodise TP, Patel N, Goff DA, LaPlante KL, Potoski BA, Rybak MJ. 2013. Clinical outcomes in patients with heterogeneous vancomycin-intermediate Staphylococcus aureus bloodstream infection. Antimicrob Agents Chemother 57:4252–4259. doi: 10.1128/AAC.00380-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, Stellrecht K. 2008. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother 52:3315–3320. doi: 10.1128/AAC.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Hal SJ, Fowler VG. 2013. Is it time to replace vancomycin in the treatment of methicillin-resistant Staphylococcus aureus infections? Clin Infect Dis 56:1779–1788. doi: 10.1093/cid/cit178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kollef MH. 2007. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin Infect Dis 45 (Suppl 3):S191–S195. doi: 10.1086/519470. [DOI] [PubMed] [Google Scholar]
- 9.Choo EJ, Chambers HF. 2016. Treatment of methicillin-resistant Staphylococcus aureus bacteremia. Infect Chemother 48:267–273. doi: 10.3947/ic.2016.48.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JR, Arya A, Yim J, Barber KE, Hallesy J, Singh NB, Rybak MJ. 2016. Daptomycin in combination with ceftolozane-tazobactam or cefazolin against daptomycin-susceptible and -nonsusceptible Staphylococcus aureus in an in vitro, hollow-fiber model. Antimicrob Agents Chemother 60:3970–3975. doi: 10.1128/AAC.01666-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakoulas G, Brown J, Lamp KC, Friedrich LV, Lindfield KC. 2009. Clinical outcomes of patients receiving daptomycin for the treatment of Staphylococcus aureus infections and assessment of clinical factors for daptomycin failure: a retrospective cohort study utilizing the Cubicin Outcomes Registry and Experience. Clin Ther 31:1936–1945. doi: 10.1016/j.clinthera.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Marty FM, Yeh WW, Wennersten CB, Venkataraman L, Albano E, Alyea EP, Gold HS, Baden LR, Pillai SK. 2006. Emergence of a clinical daptomycin-resistant Staphylococcus aureus isolate during treatment of methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. J Clin Microbiol 44:595–597. doi: 10.1128/JCM.44.2.595-597.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samson JE, Magadán AH, Sabri M, Moineau S. 2013. Revenge of the phages: defeating bacterial defences. 10. Nat Rev Microbiol 11:675–687. doi: 10.1038/nrmicro3096. [DOI] [PubMed] [Google Scholar]
- 14.Torres-Barceló C, Hochberg ME. 2016. Evolutionary rationale for phages as complements of antibiotics. Trends Microbiol 24:249–256. doi: 10.1016/j.tim.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Gordillo Altamirano FL, Barr JJ. 2019. Phage therapy in the postantibiotic era. Clin Microbiol Rev 32:e00066-18. doi: 10.1128/CMR.00066-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinbauer MG, Rassoulzadegan F. 2004. Are viruses driving microbial diversification and diversity? Environ Microbiol 6:1–11. doi: 10.1046/j.1462-2920.2003.00539.x. [DOI] [PubMed] [Google Scholar]
- 17.Delbrück M. 1940. The growth of bacteriophage and lysis of the host. J Gen Physiol 23:643–660. doi: 10.1085/jgp.23.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larbi D, Decaris B, Simonet JM. 1992. Different bacteriophage resistance mechanisms in Streptococcus salivarius subsp. thermophilus. J Dairy Res 59:349–357. doi: 10.1017/s0022029900030624. [DOI] [PubMed] [Google Scholar]
- 19.Filippov AA, Sergueev KV, He Y, Huang X-Z, Gnade BT, Mueller AJ, Fernandez-Prada CM, Nikolich MP. 2011. Bacteriophage-resistant mutants in Yersinia pestis: identification of phage receptors and attenuation for mice. PLoS One 6:e25486. doi: 10.1371/journal.pone.0025486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan EM, Alkawareek MY, Donnelly RF, Gilmore BF. 2012. Synergistic phage-antibiotic combinations for the control of Escherichia coli biofilms in vitro. FEMS Immunol Med Microbiol 65:395–398. doi: 10.1111/j.1574-695X.2012.00977.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, Kim SH, Desiderio DM, Kim YK, Kim KP, Gho YS. 2009. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 9:5425–5436. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- 22.Resch U, Tsatsaronis JA, Rhun AL, Stübiger G, Rohde M, Kasvandik S, Holzmeister S, Tinnefeld P, Wai SN, Charpentier E. 2016. A two-component regulatory system impacts extracellular membrane-derived vesicle production in group a Streptococcus. mBio 7:e00207-16. doi: 10.1128/mBio.00207-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Athman JJ, Wang Y, McDonald DJ, Boom WH, Harding CV, Wearsch PA. 2015. Bacterial membrane vesicles mediate the release of Mycobacterium tuberculosis lipoglycans and lipoproteins from infected macrophages. J Immunol 195:1044–1053. doi: 10.4049/jimmunol.1402894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Im H, Lee S, Soper SA, Mitchell RJ. 2017. Staphylococcus aureus extracellular vesicles (EVs): surface-binding antagonists of biofilm formation. Mol Biosyst 13:2704–2714. doi: 10.1039/c7mb00365j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan L, Li SR, Jiang B, Hu XM, Li S. 2018. Therapeutic targeting of the Staphylococcus aureus accessory gene regulator (agr) system. Front Microbiol 9:55. doi: 10.3389/fmicb.2018.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laabei M, Jamieson WD, Massey RC, Jenkins A. 2014. Staphylococcus aureus interaction with phospholipid vesicles – a new method to accurately determine accessory gene regulator (agr) activity. PLoS One 9:e87270. doi: 10.1371/journal.pone.0087270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Askarian F, Lapek JD, Dongre M, Tsai C-M, Kumaraswamy M, Kousha A, Valderrama JA, Ludviksen JA, Cavanagh JP, Uchiyama S, Mollnes TE, Gonzalez DJ, Wai SN, Nizet V, Johannessen M. 2018. Staphylococcus aureus membrane-derived vesicles promote bacterial virulence and confer protective immunity in murine infection models. Front Microbiol 9:262. doi: 10.3389/fmicb.2018.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown L, Wolf JM, Prados-Rosales R, Casadevall A. 2015. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol 13:620–630. doi: 10.1038/nrmicro3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreoni F, Toyofuku M, Menzi C, Kalawong R, Mairpady Shambat S, François P, Zinkernagel AS, Eberl L. 2019. Antibiotics stimulate formation of vesicles in Staphylococcus aureus in both phage-dependent and -independent fashions and via different routes. Antimicrob Agents Chemother 63:e01439-18. doi: 10.1128/AAC.01439-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comeau AM, Tétart F, Trojet SN, Prère M-F, Krisch HM. 2007. Phage-antibiotic synergy (PAS): β-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS One 2:e799. doi: 10.1371/journal.pone.0000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhry WN, Concepción-Acevedo J, Park T, Andleeb S, Bull JJ, Levin BR. 2017. Synergy and order effects of antibiotics and phages in killing Pseudomonas aeruginosa biofilms. PLoS One 12:e0168615. doi: 10.1371/journal.pone.0168615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan BK, Sistrom M, Wertz JE, Kortright KE, Narayan D, Turner PE. 2016. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci Rep 6:26717–26718. doi: 10.1038/srep26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirby AE. 2012. Synergistic action of gentamicin and bacteriophage in a continuous culture population of Staphylococcus aureus. PLoS One 7:e51017. doi: 10.1371/journal.pone.0051017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickey J, Perrot V. 2019. Adjunct phage treatment enhances the effectiveness of low antibiotic concentration against Staphylococcus aureus biofilms in vitro. PLoS One 14:e0209390. doi: 10.1371/journal.pone.0209390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahman M, Kim S, Kim SM, Seol SY, Kim J. 2011. Characterization of induced Staphylococcus aureus bacteriophage SAP-26 and its anti-biofilm activity with rifampicin. Biofouling 27:1087–1093. doi: 10.1080/08927014.2011.631169. [DOI] [PubMed] [Google Scholar]
- 36.Peng C, Hanawa T, Azam AH, LeBlanc C, Ung P, Matsuda T, Onishi H, Miyanaga K, Tanji Y. 2019. Silviavirus phage ΦMR003 displays a broad host range against methicillin-resistant Staphylococcus aureus of human origin. Appl Microbiol Biotechnol 103:7751–7765. doi: 10.1007/s00253-019-10039-2. [DOI] [PubMed] [Google Scholar]
- 37.Kumaran D, Taha M, Yi Q, Ramirez-Arcos S, Diallo J-S, Carli A, Abdelbary H. 2018. Does treatment order matter? Investigating the ability of bacteriophage to augment antibiotic activity against Staphylococcus aureus biofilms. Front Microbiol 9:127. doi: 10.3389/fmicb.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kvachadze L, Balarjishvili N, Meskhi T, Tevdoradze E, Skhirtladze N, Pataridze T, Adamia R, Topuria T, Kutter E, Rohde C, Kutateladze M. 2011. Evaluation of lytic activity of staphylococcal bacteriophage Sb-1 against freshly isolated clinical pathogens. Microb Biotechnol 4:643–650. doi: 10.1111/j.1751-7915.2011.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tkhilaishvili T, Lombardi L, Klatt A-B, Trampuz A, Di Luca M. 2018. Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. Int J Antimicrob Agents 52:842–853. doi: 10.1016/j.ijantimicag.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Barylski J, Enault F, Dutilh BE, Schuller MB, Edwards RA, Gillis A, Klumpp J, Knezevic P, Krupovic M, Kuhn JH, Lavigne R, Oksanen HM, Sullivan MB, Jang HB, Simmonds P, Aiewsakun P, Wittmann J, Tolstoy I, Brister JR, Kropinski AM, Adriaenssens EM. 2020. Analysis of spounaviruses as a case study for the overdue reclassification of tailed phages. Syst Biol 69:110–123. doi: 10.1093/sysbio/syz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazzocco A, Waddell TE, Lingohr E, Johnson RP. 2009. Enumeration of bacteriophages using the small drop plaque assay system. Methods Mol Biol 501:81–85. doi: 10.1007/978-1-60327-164-6_9. [DOI] [PubMed] [Google Scholar]
- 42.Queck SY, Jameson-Lee M, Villaruz AE, Bach T-H, Khan BA, Sturdevant DE, Ricklefs SM, Li M, Otto M. 2008. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell 32:150–158. doi: 10.1016/j.molcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamal F, Dennis JJ. 2015. Burkholderia cepacia complex phage-antibiotic synergy (PAS): antibiotics stimulate lytic phage activity. Appl Environ Microbiol 81:1132–1138. doi: 10.1128/AEM.02850-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levin BR, Bull JJ. 2004. Population and evolutionary dynamics of phage therapy. Nat Rev Microbiol 2:166–173. doi: 10.1038/nrmicro822. [DOI] [PubMed] [Google Scholar]
- 45.Lehman SM, Mearns G, Rankin D, Cole RA, Smrekar F, Branston SD, Morales S. 2019. Design and preclinical development of a phage product for the treatment of antibiotic-resistant Staphylococcus aureus infections. Viruses 11:88. doi: 10.3390/v11010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kebriaei R, Rice SA, Singh KV, Stamper KC, Dinh AQ, Rios R, Diaz L, Murray BE, Munita JM, Tran TT, Arias CA, Rybak MJ. 2018. Influence of inoculum effect on the efficacy of daptomycin monotherapy and in combination with β-lactams against daptomycin-susceptible Enterococcus faecium harboring LiaSR substitutions. Antimicrob Agents Chemother 62:e00315-18. doi: 10.1128/AAC.00315-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kebriaei R, Rice SA, Stamper KC, Rybak MJ. 2019. Dalbavancin alone and in combination with ceftaroline against four different phenotypes of Staphylococcus aureus in a simulated pharmacodynamic/pharmacokinetic model. Antimicrob Agents Chemother 63:e01743-18. doi: 10.1128/AAC.01743-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B. 2004. FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J Microsc 214:159–173. doi: 10.1111/j.0022-2720.2004.01348.x. [DOI] [PubMed] [Google Scholar]
- 49.Dey S, Gudipati S, Giuliano C, Zervos MJ, Monk JM, Szubin R, Jorgensen SCJ, Sakoulas G, Berti AD. 2019. Reduced production of bacterial membrane vesicles predicts mortality in ST45/USA600 methicillin-resistant Staphylococcus aureus bacteremia. Antibiotics (Basel) 9:2. doi: 10.3390/antibiotics9010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toyofuku M, Cárcamo-Oyarce G, Yamamoto T, Eisenstein F, Hsiao C-C, Kurosawa M, Gademann K, Pilhofer M, Nomura N, Eberl L. 2017. Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat Commun 8:481. doi: 10.1038/s41467-017-00492-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manning AJ, Kuehn MJ. 2011. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol 11:258. doi: 10.1186/1471-2180-11-258. [DOI] [PMC free article] [PubMed] [Google Scholar]