The aim of this study was to assess the safety of early oral switch (EOS) prior to 14 days for low-risk Staphylococcus aureus bacteremia (LR-SAB), which is the primary treatment strategy used at our institution. The usual recommended therapy is 14 days of intravenous (i.v.) antibiotics. All patients with SAB at our hospital were identified between 1 January 2014 and 31 December 2018. Those meeting low-risk criteria (health care-associated, no evidence of deep infection or demonstrated involvement of prosthetic material, and no further positive blood cultures after 72 h) were included in the study.
KEYWORDS: Staphylococcus aureus bacteremia, low risk, early oral switch, beta-lactam
ABSTRACT
The aim of this study was to assess the safety of early oral switch (EOS) prior to 14 days for low-risk Staphylococcus aureus bacteremia (LR-SAB), which is the primary treatment strategy used at our institution. The usual recommended therapy is 14 days of intravenous (i.v.) antibiotics. All patients with SAB at our hospital were identified between 1 January 2014 and 31 December 2018. Those meeting low-risk criteria (health care-associated, no evidence of deep infection or demonstrated involvement of prosthetic material, and no further positive blood cultures after 72 h) were included in the study. The primary outcome was occurrence of a SAB-related complication within 90 days. There were 469 SAB episodes during the study period, 100 (21%) of whom met inclusion criteria. EOS was performed in 84 patients. In this group, line infection was the source in 79%, methicillin-susceptible S. aureus caused 95% of SABs and 74% of patients received i.v. flucloxacillin. The median durations of i.v. and oral antibiotics in the EOS group were 5 days (interquartile range [IQR], 4 to 6) and 10 days (IQR, 9 to 14), respectively. A total of 71% of patients received flucloxacillin as their EOS agent. Overall, 86% of oral step-down therapy was with beta-lactams. One patient (1%) undergoing EOS had SAB relapse within 90 days. No deaths attributable to SAB occurred within 90 days. In this low-MRSA-prevalence LR-SAB cohort, EOS was associated with a low incidence of SAB-related complications. This was achieved with oral beta-lactam therapy in most patients. Larger prospective studies are needed to confirm these findings.
INTRODUCTION
Staphylococcus aureus bacteremia (SAB) is both common and serious; however, there remains a lack of high-quality evidence to guide several aspects of management (1, 2). There is consensus that low-risk SAB (LR-SAB) may be safely treated with 2 weeks of therapy, but the proportion of intravenous (i.v.) or oral therapy within that 2-week window is unclear (2–5). Surveys have identified wide variation and diverse opinion in clinical practice (6–10). A number of recent studies have shown that oral is safer than i.v. therapy with respect to treatment complications and, for many conditions, has equivalent microbiological outcomes (11, 12). S. aureus has been well represented in these studies (23.4 and 36.2% in the oral treatment arms of the POET and OVIVA trials, respectively [11, 12]); however, early oral treatment of SAB remains unsupported by relevant guidelines (2, 13). We have taken the approach that with careful patient selection and monitoring, a switch to oral antibiotics is clinically safe. The aim of this retrospective cohort study was to objectively describe the outcomes of patients with LR-SAB treated at our institution, with particular emphasis on early i.v. to oral switch (EOS) of antibiotic treatment.
RESULTS
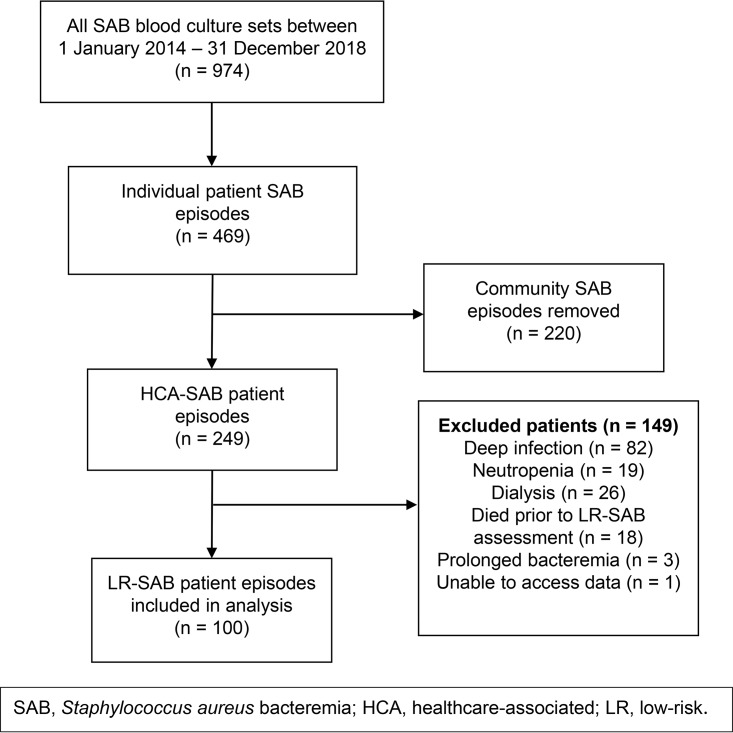
There were 469 patient episodes of SAB over the 5-year period, of which 100 (21%) patients were included (Fig. 1 and 2). Follow-up data were missing for three patients, as outlined below. Eighty-four patients were managed with EOS. Baseline characteristics of the EOS and i.v. groups are given in Table 1. The two groups were broadly similar, but differed statistically in terms of gender and a higher proportion of patients with implanted prosthetic material in the i.v. group (43.8% versus 17.9% in the EOS group, P = 0.04). Line infection was the source of SAB in 79 and 88% of the EOS and i.v. groups, respectively. Only 5% of SABs overall were due to methicillin-resistant S. aureus (MRSA).
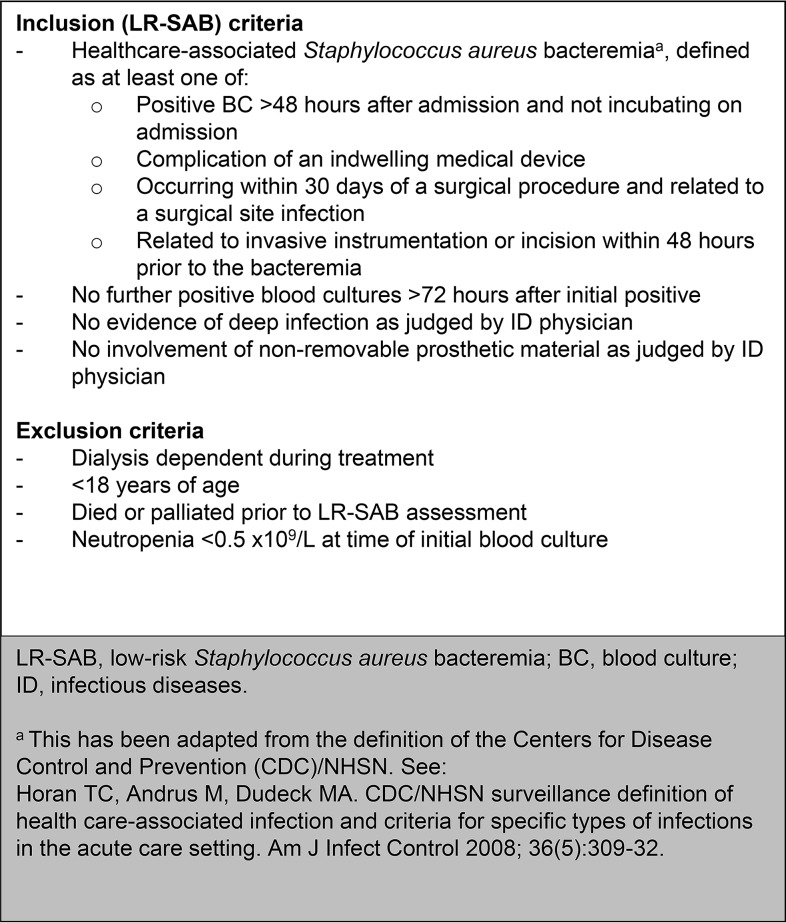
FIG 1.
Inclusion and exclusion criteria.
FIG 2.
Cohort selection process.
TABLE 1.
Baseline demographic and clinical characteristics of cohorta
| Characteristic | No. (%) |
P | |
|---|---|---|---|
| EOS group (n = 84) | i.v. group (n = 16) | ||
| Median age, yrs (range) | 63 (18–93) | 67 (38–91) | 0.14 |
| Male | 58 (69) | 15 (94) | 0.04 |
| Ethnicity | |||
| New Zealand European | 54 (64) | 10 (63) | 0.89 |
| Maori | 10 (12) | 4 (25) | 0.17 |
| Pacific | 9 (11) | 0 (0) | 0.17 |
| Other | 11 (13) | 2 (13) | 0.95 |
| Median wt, kg (range) | 78 (48–196) | 85 (60–127) | 0.27 |
| Admission specialty | |||
| Cardiology | 18 (21) | 4 (2) | 0.75 |
| General medicine | 18 (21) | 2 (13) | 0.41 |
| Hematology-oncology | 14 (17) | 1 (6) | 0.28 |
| General surgery | 13 (16) | 1 (6) | 0.33 |
| Cardiothoracic surgery | 5 (6) | 3 (19) | 0.08 |
| Other surgical specialties | 8 (10) | 3 (19) | 0.28 |
| Other medical specialties | 8 (10) | 2 (13) | 0.72 |
| Site of infection | |||
| Line | 66 (79) | 14 (88) | 0.41 |
| Peripheral cannula | 45 (54) | 8 (50) | 0.79 |
| PICC | 10 (12) | 2 (13) | 0.94 |
| Central venous line | 8 (10) | 3 (19) | 0.28 |
| Tunneled line or port | 3 (4) | 1 (6) | 0.62 |
| Skin and soft tissue | 8 (10) | 1 (6) | 0.67 |
| Unknown source | 5 (6) | 1 (6) | 0.96 |
| Urinary tract | 3 (4) | 0 (0) | 0.44 |
| Respiratory tract | 2 (2) | 0 (0) | 0.54 |
| Comorbidity | |||
| Cardiovascular disease | 53 (63) | 14 (88) | 0.06 |
| Cancer | 20 (24) | 5 (31) | 0.53 |
| Neurological disease | 18 (21) | 4 (25) | 0.75 |
| Diabetes | 19 (23) | 2 (13) | 0.36 |
| Immunosuppressionb | 16 (19) | 2 (13) | 0.54 |
| Renal disease | 17 (20) | 2 (13) | 0.47 |
| Valvular/congenital heart disease | 8 (10) | 1 (6) | 0.67 |
| Presence of prosthetic material | 15 (18) | 7 (44) | 0.04 |
| Joint or other orthopedic metalware | 7 (8) | 6 (38) | 0.01 |
| Vascular graft | 5 (6) | 1 (6) | 0.96 |
| Cardiac device or valve | 5 (6) | 0 (0) | 0.32 |
| Susceptibility pattern of organism | |||
| PSSA | 26 (31) | 4 (25) | 0.63 |
| MSSA | 54 (64) | 11 (69) | 0.73 |
| MRSA | 4 (5) | 1 (6) | 0.80 |
| Defervescence within 72 h | 80 (95) | 14 (88) | 0.23 |
| No. of positive blood cultures, mean (SD) | 1.6 (0.9) | 2.0 (1.1) | 0.11 |
EOS, early oral switch; PICC, peripherally inserted central catheter; PSSA, penicillin-susceptible S. aureus; MSSA, methicillin-susceptible S. aureus; MRSA, methicillin-resistant S. aureus. Data indicate the “numbers (%) of patients” unless noted otherwise in column 1.
Corticosteroid equivalent to >20 mg of prednisone for >14 days or other immunosuppressive drugs.
Clinical management information is given in Table 2. The all-i.v. group was more likely to have had an echocardiogram performed and less likely to have appropriate surveillance BCs, otherwise there were no significant differences noted on univariate comparisons. Flucloxacillin was the commonest i.v. antibiotic used (74 and 81% in the EOS and i.v. groups, respectively; standard dose of 2 g every 6 h), followed by cefazolin (7 and 13%, respectively; standard dose of 2 g every 8 h). Flucloxacillin was also the most common oral antibiotic used (71 and 13%, respectively; standard dose of 1 g three times daily), followed by cefalexin (8 and 6%, respectively; standard dose of 1 g three times daily). Overall, 86% of oral therapy in the EOS group was with beta-lactams. In the EOS group, the median durations for i.v. and oral antibiotics were 5 days (interquartile range [IQR], 4 to 6) and 10 days (IQR, 9 to 14), respectively, and 76% were switched before 7 days of i.v. therapy. Other standard antibiotic doses used are provided in Table S1 in the supplemental material.
TABLE 2.
Clinical management characteristics of cohorta
| Characteristic | No. (%) |
P | |
|---|---|---|---|
| EOS group (n = 84) | i.v. group (n = 16) | ||
| ID consultation | 76 (91) | 13 (81) | 0.28 |
| Negative surveillance BC performedb | 73 (87) | 12 (75) | 0.22 |
| Within 72 h of initial positive BC | 59 (70) | 7 (44) | 0.05 |
| Echocardiogram performed | 41 (49) | 13 (81) | 0.03 |
| TTE alone | 32 (38) | 9 (56) | 0.18 |
| TEE alone | 6 (7) | 2 (13) | 0.47 |
| Both TTE and TEE | 3 (4) | 2 (13) | <0.01 |
| No echocardiography | 43 (51) | 3 (19) | 0.03 |
| i.v. antibiotic therapy | |||
| Flucloxacillin | 62 (74) | 13 (81) | 0.53 |
| Cefazolin | 6 (7) | 2 (13) | 0.47 |
| Vancomycin | 5 (6) | 1 (6) | 0.96 |
| Benzylpenicillin | 5 (6) | 0 (0) | 0.32 |
| Other beta-lactam | 4 (5) | 0 (0) | 0.37 |
| No i.v. antibiotic | 2 (2) | 0 (0) | 0.54 |
| Oral antibiotic therapy | |||
| Flucloxacillin | 60 (71) | 2 (13) | |
| Cefalexin | 7 (8) | 1 (6) | |
| Other beta-lactam | 5 (6) | 0 (0) | |
| Co-trimoxazole | 5 (6) | 0 (0) | |
| Clindamycin | 3 (4) | 0 (0) | |
| Adjunctive probenecid | 5 (6) | 0 (0) | |
| No oral antibiotic | 4 (5)c | 13 (81) | |
| Median antibiotic therapy duration, days (IQR) | |||
| i.v. therapy | 5 (4–6) | 14 (14–15) | |
| Oral therapy | 10 (9–14) | 6 (2–12) | |
| Total | 16 (14–18) | 14 (14–17) | |
| EOS | |||
| EOS before 7 days of i.v. therapy | 64 (76) | ||
| EOS before 10 days of i.v. therapy | 82 (98) | ||
| Reasons for no oral switch prior to 14 days | |||
| Clinical decision by ID team not to switch | 5 (31) | ||
| EOS advice from ID team not followed | 8 (50) | ||
| Compromised oral intake/absorption | 3 (19) | ||
Data indicate the numbers (%) of patients unless noted otherwise in column 1. EOS, early oral switch; ID, infectious diseases; BC, blood culture; TTE, transthoracic echocardiogram; TEE, transesophageal echocardiogram.
The inclusion criterion for the study was the absence of a further positive BC after 72 h, rather than a documented negative, which is why not all patients had clearance BCs.
EOS is defined as a switch from i.v. treatment prior to 14 days.
Of the 16 patients who did not undergo EOS, there was an intention to treat 11 of them with EOS, but either infectious disease (ID) advice was not followed or oral administration was not feasible (Table 2). Five patients meeting LR-SAB criteria did not undergo EOS on the advice of the ID service (Table S2).
Ninety-day follow-up data were available for all except three patients who returned to their home countries after discharge. SAB recurrence within 90 days occurred in three patients (4%) and one patient (6%, P = 0.64) in the EOS and i.v. groups, respectively (Table 3). Two of these recurrences were new infections, confirmed by major differences in susceptibility pattern and/or spa typing (Table S3). One relapse occurred 19 days after the initial positive blood culture (BC) in a patient in the EOS group who was treated with oral clindamycin alone for 14 days due to penicillin allergy (the organism was confirmed as susceptible to clindamycin). The patient subsequently received 14 days of i.v. cefazolin and was cured. One relapse occurred in the i.v. group 23 days after the first positive BC in a patient who was initially treated with 14 days of i.v. flucloxacillin, and on relapse it was determined that this patient’s endovascular aneurysm repair (EVAR) graft was likely involved during the initial episode. The patient subsequently received 5 weeks of i.v. flucloxacillin, followed by lifelong oral doxycycline. Thirty (37%) and six (38%, P = 0.97) patients in each group were readmitted within 90 days; of these patients, four admissions (4%) were related to the initial SAB (Table S4).
TABLE 3.
Outcomes at 90 days after initial blood culturea
| Outcome measure | No. (%) |
P | |
|---|---|---|---|
| EOS group (n = 81) | i.v. group (n = 16) | ||
| Recurrence of SAB | 3 (4) | 1 (6) | 0.64 |
| Relapse | 1 (1) | 1 (6) | 0.20 |
| New infection | 2 (2) | 0 (0) | 0.52 |
| Recurrence of deep infection | 0 (0) | 1 (6) | 0.02 |
| Readmission to hospital | 30 (37) | 6 (38) | 0.97 |
| Related to SAB | 3 (4) | 1 (6) | 0.64 |
| Mortality | 2 (2) | 1 (6) | 0.42 |
| Attributable to SAB | 0 (0) | 0 (0) | |
EOS, early oral switch; SAB, S. aureus bacteremia.
Two patients (2%) and one patient (6%, P = 0.42) from the EOS and i.v. groups, respectively, died within 90 days. No deaths were attributable to the SAB. The causes of death were due to preexisting progressive diseases: progressive cardiac failure, progressive graft-versus-host disease, and metastatic lung cancer.
DISCUSSION
This small single-center study demonstrates that EOS for LR-SAB provides patient outcomes that are consistent with published outcomes using standard i.v. therapy (14–18). Many patients received very short i.v. courses, and the majority of patients undergoing EOS were treated with oral beta-lactams, among which there were no relapses.
There are limited studies evaluating the efficacy of oral antibiotics for SAB. A randomized study assessing oral therapy of fleroxacin plus rifampin versus standard parenteral therapy showed a comparable cure rate (19). However, the study population was small and heterogeneous, including deep infections, as well as catheter-associated coagulase-negative staphylococcal infections. A more recent prospective cohort study compared EOS with linezolid to standard parenteral therapy in LR-SAB patients, showing no difference in 90-day relapse (2.2% versus 4.4%) (18).
We believe our study adds to this limited literature in two main ways. First, it adds weight to the evidence supporting EOS for LR-SAB. It has demonstrated a low incidence of adverse outcomes in a clearly defined population. Furthermore, there was a very consistent approach to EOS, with only five patients in the entire cohort not undergoing EOS based on SAB-related clinical judgement from the ID service. This uniformity of approach means treatment allocation bias will have been minimized and suggests clinicians felt comfortable performing EOS in patients meeting our LR-SAB criteria. Second, the outcomes in the EOS group were achieved predominantly with oral beta-lactam therapy. Oral beta-lactams have not typically been recommended for bacteremia due to limited data and bioavailability concerns, with only highly bioavailable agents chosen for assessment in clinical trials (20). The SABATO trial, currently in progress, and a recent observational study both use favored alternative agents, such as linezolid, co-trimoxazole, and clindamycin for this reason, leaving the oral beta-lactam question unanswered (18, 21). Beta-lactams have several advantages over agents such as linezolid, including widespread availability, excellent tolerability, and low cost. We believe this study supports a clinical equipoise argument for beta-lactam oral therapy, and their inclusion in clinical trials of EOS for SAB. In addition, our results highlight the well-established importance of ID consultation in the management of SAB (22). Our LR-SAB criteria required exclusion of deep infection, as judged by an ID physician, thus emphasizing the integral role of ID consultation in this treatment strategy.
One patient relapsed in the EOS group. This may have been due to being treated with oral clindamycin alone, which carries a high risk of treatment failure (23). The other patient who had SAB relapse was in the i.v. group and had a subsequent diagnosis of probable EVAR graft infection, which was not clinically apparent at the initial LR-SAB assessment. There was a higher proportion of patients in the i.v. group with implanted prosthetic material, and it may be that clinicians favored i.v. therapy in these patients. Our LR-SAB criteria included patients with prosthetic material, provided there was no evidence of involvement (Fig. 1), whereas other definitions for LR-SAB exclude such patients (24, 25). We would therefore advise caution when generalizing these results to such patients. The clinical rationale behind our approach is that short course therapy is in effect a treatment trial in such patients, because no amount of antibiotic therapy would cure prosthetic infection without surgical intervention. Close clinical follow-up ensures satisfactory treatment response and early identification of failure.
Some LR-SAB criteria require negative echocardiography (26). Only 49% of the EOS group had any form of echocardiography performed versus 81% in the i.v. group (P = 0.03). This is consistent with recent evidence suggesting a limited benefit from echocardiography in LR-SAB (27–29). Much of the echocardiography done in both groups was not under the recommendation of the ID service, since we do not typically request it for patients with LR-SAB. It appears that the higher proportion of patients receiving echocardiography in the i.v. group was at least in part due to ID advice not being followed. This is also reflected in the lower numbers having clearance BCs within 72 h (70% in the EOS group versus 44% in the i.v. group; P = 0.05) and the eight patients in which EOS advice was not followed (Table 2).
Twenty-one percent of all SABs seen at our hospital met LR-SAB criteria, meaning a significant proportion of SAB patients avoided prolonged i.v. access. Compared to a standard 14-day i.v. course, we estimated a saving of 930 line-days during the course of the study. This will have avoided potential line-associated complications, such as thrombosis, infection, and insertion-related complications. Infectious complications alone occur at an incidence of 1.65 to 6.8 events per 1,000 central line-days (30, 31). This will also have had numerous other benefits, including reduced pressure on outpatient i.v. resources, increased patient independence by avoiding elastomeric pumps and attached tubing, and financial savings for our institution.
Important limitations of this study include its observational nature and single center design. The high degree of adherence to EOS reduced the size of the i.v. comparison group, limiting meaningful statistical comparisons. However, the purpose of this study was not to compare EOS with standard i.v. therapy but to report the outcomes of a cohort treated predominantly with EOS in a real-world setting. A further limitation was the small sample size. However, in the context of a solely LR-SAB population treated with a consistent EOS approach, our cohort was in fact larger than much of what is reported in the literature to date, most of which has been drawn from cohorts with a high degree of heterogeneity in their clinical management (3). This cohort was drawn from a general tertiary hospital, and our findings should be generalizable to other similar patient groups with methicillin-susceptible S. aureus (MSSA). Our cohort included few patients with methicillin-resistant S. aureus (MRSA), a risk-factor for poor outcome (32), precluding generalization of these results to such patients. The 90-day mortality rates in our cohort (2 and 6% in the EOS and i.v. groups, respectively) were low for a hospitalized population. This is in fact comparable to other studies of LR-SAB and likely reflects the fact that patients meeting low-risk criteria tend to be less comorbid (18, 33).
This study demonstrates that it is possible to use EOS to oral beta-lactam therapy in selected patients with LR-SAB. It is too small to provide high-level evidence to support EOS, but we hope that it will lead to larger, properly powered prospective studies to establish whether simpler, less expensive treatment is safe. We are reassured that our local practice is associated with patient outcomes comparable to all-i.v. therapy for LR-SAB.
MATERIALS AND METHODS
Wellington Regional Hospital is a teaching hospital in Wellington, New Zealand, providing secondary and tertiary services to 400,000 people. Data on all patients with bacteremia are routinely recorded by clinical microbiology. Data include basic demographics, source of bacteremia, medical device involvement, and place of acquisition. All patients with SAB are referred to an infectious diseases (ID) physician, and there are weekly review meetings between clinical microbiology and ID to ensure accurate data recording and consistent patient management decisions.
Patients aged ≥18 years with health care-associated SAB between 1 January 2014 and 31 December 2018 were screened for inclusion in the study. Demographic and clinical data of those with LR-SAB without relevant exclusion criteria (Fig. 1) were collected from the electronic and paper records. Antibiotic duration was recorded from the initial positive blood culture (BC) or when started in response to the BC, and EOS was defined as a switch from i.v. therapy prior to 14 days. Primary outcome measures were occurrence of a SAB-related complication (recurrence of SAB, deep-seated infection, readmission, or attributable mortality) within 90 days of the initial positive BC. Recurrence of SAB within 90 days was defined as relapse if the repeat isolate was indistinguishable from the initial isolate by antibiogram or spa typing, and as a new infection if it was clearly distinguishable via these measures. Attributable mortality was judged via chart review by two authors (O.B.-I. and M.B.). The electronic records of all local hospitals that could potentially readmit patients were searched for readmission events. Laboratory results for these hospitals in our region are stored in a combined repository; this was searched for SAB recurrences. Hospital coding data were also used to identify any readmissions or deaths within 90 days. For patients transferred back to other regions, we manually searched their local hospital electronic records.
The Fisher exact test, the chi-squared test, and the Mann-Whitney U test were used to compare proportions and medians between groups, where appropriate. The statistical analysis was limited to univariate comparisons due to small numbers in the i.v. treatment group. Hospital and minimal-risk ethics approval were obtained. The study was a retrospective analysis of an existing clinical practice at our institution. The decision to treat patients with EOS was independent from, and unrelated to, this study. The study has been reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines (34).
Supplementary Material
ACKNOWLEDGMENT
We thank Roxana Vasiu for help in obtaining patient paper records.
Footnotes
Supplemental material is available online only.
For a commentary on this article, see https://doi.org/10.1128/AAC.00317-20.
REFERENCES
- 1.Holland TL, Chambers HF, Boucher HW, Corey GR, Coleman R, Castaneda-Ruiz B, Fowler VG. 2019. Considerations for clinical trials of Staphylococcus aureus bloodstream infection in adults. Clin Infect Dis 68:865–872. doi: 10.1093/cid/ciy774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu C, Infectious Diseases Society of America, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 3.Thwaites GE, UK Clinical Infection Research Group, Edgeworth JD, Gkrania-Klotsas E, Kirby A, Tilley R, Török ME, Walker S, Wertheim HF, Wilson P, Llewelyn MJ. 2011. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis 11:208–222. doi: 10.1016/S1473-3099(10)70285-1. [DOI] [PubMed] [Google Scholar]
- 4.Gould FK, Brindle R, Chadwick PR, Fraise AP, Hill S, Nathwani D, Ridgway GL, Spry MJ, Warren RE, MRSA Working Party of the British Society for Antimicrobial Chemotherapy . 2009. Guidelines (2008) for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the United Kingdom. J Antimicrob Chemother 63:849–861. doi: 10.1093/jac/dkp065. [DOI] [PubMed] [Google Scholar]
- 5.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJA, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, Strnad L, Beekmann SE, Polgreen PM, Chambers HF. 2019. Clinical practice variation among adult infectious disease physicians in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 69:530–533. doi: 10.1093/cid/ciy1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thwaites GE, United Kingdom Clinical Infection Research Group . 2010. The management of Staphylococcus aureus bacteremia in the United Kingdom and Vietnam: a multi-centre evaluation. PLoS One 5:e14170. doi: 10.1371/journal.pone.0014170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong SYC, Campbell A, Bowen AC, Davis JS. 2019. A survey of infectious diseases and microbiology clinicians in Australia and New Zealand about the management of Staphylococcus aureus bacteremia. Clin Infect Dis 69:1835–1836. doi: 10.1093/cid/ciz275. [DOI] [PubMed] [Google Scholar]
- 9.Ammerlaan H, Seifert H, Harbarth S, Brun-Buisson C, Torres A, Antonelli M, Kluytmans J, Bonten M, European Practices of Infections with Staphylococcus aureus (SEPIA) Study Group . 2009. Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteremia in 9 Western European countries. Clin Infect Dis 49:997–1005. doi: 10.1086/605555. [DOI] [PubMed] [Google Scholar]
- 10.Dryden M, Andrasevic AT, Bassetti M, Bouza E, Chastre J, Cornaglia G, Esposito S, French G, Giamarellou H, Gyssens IC, Nathwani D, Unal S, Voss A. 2010. A European survey of antibiotic management of methicillin-resistant Staphylococcus aureus infection: current clinical opinion and practice. Clin Microbiol Infect 16 Suppl 1:3–30. doi: 10.1111/j.1469-0691.2010.03135.x. [DOI] [PubMed] [Google Scholar]
- 11.Li H-K, Rombach I, Zambellas R, Walker AS, McNally MA, Atkins BL, Lipsky BA, Hughes HC, Bose D, Kümin M, Scarborough C, Matthews PC, Brent AJ, Lomas J, Gundle R, Rogers M, Taylor A, Angus B, Byren I, Berendt AR, Warren S, Fitzgerald FE, Mack DJF, Hopkins S, Folb J, Reynolds HE, Moore E, Marshall J, Jenkins N, Moran CE, Woodhouse AF, Stafford S, Seaton RA, Vallance C, Hemsley CJ, Bisnauthsing K, Sandoe JAT, Aggarwal I, Ellis SC, Bunn DJ, Sutherland RK, Barlow G, Cooper C, Geue C, McMeekin N, Briggs AH, Sendi P, Khatamzas E, Wangrangsimakul T, Wong THN, Barrett LK, Alvand A, Old CF, Bostock J, Paul J, Cooke G, Thwaites GE, Bejon P, Scarborough M. 2019. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 380:425–436. doi: 10.1056/NEJMoa1710926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iversen K, Ihlemann N, Gill SU, Madsen T, Elming H, Jensen KT, Bruun NE, Høfsten DE, Fursted K, Christensen JJ, Schultz M, Klein CF, Fosbøll EL, Rosenvinge F, Schønheyder HC, Køber L, Torp-Pedersen C, Helweg-Larsen J, Tønder N, Moser C, Bundgaard H. 2019. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med 380:415–424. doi: 10.1056/NEJMoa1808312. [DOI] [PubMed] [Google Scholar]
- 13.Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. 2015. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong YP, Moon SM, Bang K-M, Park HJ, Park S-Y, Kim M-N, Park K-H, Kim S-H, Lee S-O, Choi S-H, Jeong J-Y, Woo JH, Kim YS. 2013. Treatment duration for uncomplicated Staphylococcus aureus bacteremia to prevent relapse: analysis of a prospective observational cohort study. Antimicrob Agents Chemother 57:1150–1156. doi: 10.1128/AAC.01021-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung E, McKenzie MG, Colon LB, Kaye KS, Petty L, Martin ET, Marini BL, Perissinotti AJ, Eschenauer G, Alaniz C, Wallace KL, Patel TS. 2018. 1071. Impact of standard versus prolonged courses of antibiotics for the treatment of uncomplicated Staphylococcus aureus bacteremia (SAB) in patients with hematologic malignancies. Open Forum Infect Dis 5:S320–S321. doi: 10.1093/ofid/ofy210.908. [DOI] [Google Scholar]
- 16.Kaasch AJ, Fowler VG, Rieg S, Peyerl-Hoffmann G, Birkholz H, Hellmich M, Kern WV, Seifert H. 2011. Use of a simple criteria set for guiding echocardiography in nosocomial Staphylococcus aureus bacteremia. Clin Infect Dis 53:1–9. doi: 10.1093/cid/cir320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jernigan JA, Farr BM. 1993. Short-course therapy of catheter-related Staphylococcus aureus bacteremia: a meta-analysis. Ann Intern Med 119:304–311. doi: 10.7326/0003-4819-119-4-199308150-00010. [DOI] [PubMed] [Google Scholar]
- 18.Willekens R, Puig-Asensio M, Ruiz-Camps I, Larrosa MN, González-López JJ, Rodríguez-Pardo D, Fernández-Hidalgo N, Pigrau C, Almirante B. 2019. Early oral switch to linezolid for low-risk patients with Staphylococcus aureus bloodstream infections: a propensity-matched cohort study. Clin Infect Dis 69:381–387. doi: 10.1093/cid/ciy916. [DOI] [PubMed] [Google Scholar]
- 19.Schrenzel J, Harbarth S, Schockmel G, Genné D, Bregenzer T, Flueckiger U, Petignat C, Jacobs F, Francioli P, Zimmerli W, Lew DP, Swiss Staphylococcal Study Group . 2004. A randomized clinical trial to compare fleroxacin-rifampicin with flucloxacillin or vancomycin for the treatment of staphylococcal infection. Clin Infect Dis 39:1285–1292. doi: 10.1086/424506. [DOI] [PubMed] [Google Scholar]
- 20.Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. 2018. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med 13:328–335. doi: 10.12788/jhm.2949. [DOI] [PubMed] [Google Scholar]
- 21.Kaasch AJ, Fätkenheuer G, Prinz-Langenohl R, Paulus U, Hellmich M, Weiss V, Jung N, Rieg S, Kern WV, Seifert H, for the SABATO Trial Group . 2015. Early oral switch therapy in low-risk Staphylococcus aureus bloodstream infection (SABATO): study protocol for a randomized controlled trial. Trials 16:450. doi: 10.1186/s13063-015-0973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogel M, Schmitz RPH, Hagel S, Pletz MW, Gagelmann N, Scherag A, Schlattmann P, Brunkhorst FM. 2016. Infectious disease consultation for Staphylococcus aureus bacteremia: a systematic review and meta-analysis. J Infect 72:19–28. doi: 10.1016/j.jinf.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Watanakunakorn C. 1976. Clindamycin therapy of Staphylococcus aureus endocarditis. Clinical relapse and development of resistance to clindamycin, lincomycin and erythromycin. Am J Med 60:419–425. doi: 10.1016/0002-9343(76)90758-0. [DOI] [PubMed] [Google Scholar]
- 24.Fowler VG, Sanders LL, Sexton DJ, Kong L, Marr KA, Gopal AK, Gottlieb G, McClelland RS, Corey GR. 1998. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 27:478–486. doi: 10.1086/514686. [DOI] [PubMed] [Google Scholar]
- 25.Naber CK. 2009. Staphylococcus aureus bacteremia: epidemiology, pathophysiology, and management strategies. Clin Infect Dis 48Suppl 4:S231–S237. doi: 10.1086/598189. [DOI] [PubMed] [Google Scholar]
- 26.Heriot GS, Cronin K, Tong SYC, Cheng AC, Liew D. 2017. Criteria for identifying patients with Staphylococcus aureus bacteremia who are at low risk of endocarditis: a systematic review. Open Forum Infect Dis 4:ofx261. doi: 10.1093/ofid/ofx261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heriot G, Yeoh J, Street A, Ratnam I. 2015. Echocardiography has minimal yield and may not be warranted in Staphylococcus aureus bacteremia without clinical risk factors for endocarditis. Eur J Clin Microbiol Infect Dis 34:1231–1236. doi: 10.1007/s10096-015-2352-7. [DOI] [PubMed] [Google Scholar]
- 28.Khatib R, Sharma M. 2013. Echocardiography is dispensable in uncomplicated Staphylococcus aureus bacteremia. Medicine 92:182–188. doi: 10.1097/MD.0b013e318294a710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heriot GS, Tong SYC, Cheng AC, Liew D. 2018. Benefit of echocardiography in patients with Staphylococcus aureus bacteremia at low risk of endocarditis. Open Forum Infect Dis 5:ofy303. doi: 10.1093/ofid/ofy303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenthal VD, Bijie H, Maki DG, Mehta Y, Apisarnthanarak A, Medeiros EA, Leblebicioglu H, Fisher D, Álvarez-Moreno C, Khader IA, Del Rocío González Martínez M, Cuellar LE, Navoa-Ng JA, Abouqal R, Guanche-Garcell H, Mitrev Z, Pirez García MC, Hamdi A, Dueñas L, Cancel E, Gurskis V, Rasslan O, Ahmed A, Kanj SS, Ugalde OC, Mapp T, Raka L, Yuet Meng C, Thu LTA, Ghazal S, Gikas A, Narváez LP, Mejía N, Hadjieva N, Gamar-Elanbya MO, Guzmán-Siritt ME, Jayatilleke K, INICC members . 2012. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004–2009. Am J Infect Control 40:396–407. doi: 10.1016/j.ajic.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. 2011. Vital signs: central line–associated blood stream infections—United States, 2001, 2008, and 2009. Ann Emerg Med 58:447–450. doi: 10.1016/j.annemergmed.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 32.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 33.Thomas MG, Morris AJ. 2005. Cannula-associated Staphylococcus aureus bacteraemia: outcome in relation to treatment. Intern Med J 35:319–330. doi: 10.1111/j.1445-5994.2005.00823.x. [DOI] [PubMed] [Google Scholar]
- 34.von EE, STROBE Initiative, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. 2007. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.