The rise of extensively drug-resistant and multidrug-resistant strains of Neisseria gonorrhoeae has occurred in parallel with the increasing demand for new drugs. However, the current methods of drug discovery are burdened with rigorous assessments and require more time than can be spared until gonococcal infections become difficult to control. To address this urgency, we utilized a drug-repurposing strategy and identified three clinically approved anthranilic acid drugs (tolfenamic acid, flufenamic acid, and meclofenamic acid) with potent antigonococcal activity, inhibiting 50% of the strains (MIC50) from 4 to 16 μg/ml.
KEYWORDS: drug repurposing, multidrug resistant, IL-8, endocervical cells, Neisseria, drug resistant, fenamic acids
ABSTRACT
The rise of extensively drug-resistant and multidrug-resistant strains of Neisseria gonorrhoeae has occurred in parallel with the increasing demand for new drugs. However, the current methods of drug discovery are burdened with rigorous assessments and require more time than can be spared until gonococcal infections become difficult to control. To address this urgency, we utilized a drug-repurposing strategy and identified three clinically approved anthranilic acid drugs (tolfenamic acid, flufenamic acid, and meclofenamic acid) with potent antigonococcal activity, inhibiting 50% of the strains (MIC50) from 4 to 16 μg/ml. Furthermore, tolfenamic acid showed indifferent activity with antibiotics of choice for gonococcal infections, azithromycin and ceftriaxone, in checkerboard assays with a fractional inhibitory concentration index ranging from 0.75 to 1.5. Fenamic acids reduced a high inoculum of N. gonorrhoeae below the limit of detection within 12 h and exhibited a low frequency of resistance. Interestingly, the fenamic acids did not inhibit the growth of commensal Lactobacillus spp. that comprise the healthy female genital microbiota. Fenamic acids were also superior to ceftriaxone in reducing the burden of intracellular N. gonorrhoeae within infected endocervical cells by 99%. Furthermore, all three fenamic acids significantly reduced the expression of proinflammatory cytokines by infected endocervical cells. Finally, fenamic acids and other structurally related anthranilic acid derivatives were evaluated to ascertain a more in-depth structure-activity relationship (SAR) that revealed N-phenylanthranilic acid as a novel antigonorrheal scaffold. This SAR study will pave the road to repositioning more potent fenamic acids analogues against N. gonorrhoeae.
INTRODUCTION
Neisseria gonorrhoeae is linked to 78 million gonococcal infections globally, ranking second on the list of all sexually transmitted bacterial infections (1). More importantly, N. gonorrhoeae has acquired resistance to antimicrobial agents, including most currently available antibiotics, and strains of extensively multidrug-resistant N. gonorrhoeae are beginning to emerge. Clearly, the extraordinary ability of N. gonorrhoeae to gain resistance to antibiotics necessitates an immediate effort to identify and develop new drugs.
Traditional de novo drug discovery is both resource-intensive and time-consuming. Compounding this problem further, over 90% of all new molecules entering clinical trials fail to reach the clinic (2–4). Drug repurposing, evaluating previously approved drugs for an alternative disease indication, represents a novel strategy to overcome the dilemma of de novo antibiotic drug development (5–12). Repurposing can expedite the approval process and alleviate the financial burden associated with de novo drug discovery but also is generally more likely to succeed, as the drug’s toxicity and pharmacological properties are better characterized than those of newly synthesized compounds (12, 13). Recently, this strategy has gained momentum, as it has resulted in successes in a number of disease areas and has accounted for approximately 30% of newly FDA-approved drugs and vaccines (12, 14, 15).
Utilizing a drug-repurposing strategy, we discovered fenamic acids as novel anti-Neisseria antibacterial agents. In this study, we assessed the antibacterial activities of three fenamic acids (tolfenamic, flufenamic, and meclofenamic acids) and derivatives against 45 different clinical isolates of multidrug-resistant N. gonorrhoeae. Additionally, we evaluated these agents against important members of healthy vaginal microbiota that inhibit N. gonorrhoeae colonization (16, 17). Furthermore, the possibility of using fenamic acids in combination with antibiotics currently used to treat gonorrhea was explored.
The ability of fenamic acids to reduce the burden of intracellular N. gonorrhoeae as well as their immunomodulatory effect were examined in an endocervical cell line infected with N. gonorrhoeae. The killing kinetics, postantibiotic effect (PAE) of fenamic acids, as well as their frequency of spontaneous resistance mutations were also determined. Finally, the structure-activity relationship (SAR) of the fenamic acids was investigated by analyzing the antibacterial activities of other structurally related anthranilic acid derivatives against N. gonorrhoeae.
RESULTS
Susceptibility analysis of fenamic acids against clinical isolates of N. gonorrhoeae.
Three fenamic acids (tolfenamic acid, flufenamic acid, and meclofenamic acid) were evaluated against a wide panel of N. gonorrhoeae clinical isolates. As presented in Table 1, the fenamic acids inhibited the growth of all tested N. gonorrhoeae isolates at concentrations ranging from 2 to 32 μg/ml. Tolfenamic acid and flufenamic acid were the most potent agents, as they inhibited the growth of 50% of strains (MIC50) at 4 and 8 μg/ml, respectively. Both drugs inhibited the growth of 90% of the strains (MIC90) at 8 μg/ml. Meclofenamic acid was moderately effective (MIC50 = 16 μg/ml and MIC90 = 32 μg/ml). In comparison, azithromycin exhibited an MIC50 of 0.5 μg/ml and an MIC90 of 16 μg/ml against the same isolates tested. Overall, the MIC values for each drug were consistent throughout the panel tested, with no more than a 2-fold difference against most isolates. To confirm the MIC values obtained by the broth dilution assay, the agar dilution method was utilized against five strains (strains 167, 175, 179, 181, and 194). We observed only a 1-fold increase in the MIC values for all three fenamic acids using the agar dilution method in comparison to the broth dilution method (Table 2).
TABLE 1.
MICs of fenamic acids (tolfenamic acid, flufenamic acid, and meclofenamic acid) and control antibiotics (azithromycin and ceftriaxone) against 45 clinical isolates of N. gonorrhoeae
| Strain | MIC (μg/ml) |
||||
|---|---|---|---|---|---|
| Tolfenamic acid | Flufenamic acid | Meclofenamic acid | Azithromycin | Ceftriaxone | |
| 166 | 4 | 8 | 16 | 0.25 | 0.125 |
| 167 | 2 | 2 | 8 | 16 | 0.015 |
| 168 | 4 | 8 | 16 | 0.25 | 0.06 |
| 169 | 4 | 4 | 8 | 0.25 | 0.06 |
| 171 | 4 | 4 | 8 | 1 | 0.06 |
| 172 | 4 | 4 | 16 | 0.5 | 0.06 |
| 173 | 8 | 8 | 16 | 0.25 | 0.06 |
| 175 | 4 | 4 | 8 | 16 | 0.008 |
| 176 | 4 | 4 | 16 | 0.25 | 0.06 |
| 178 | 4 | 8 | 16 | 1 | 0.06 |
| 179 | 2 | 4 | 4 | 16 | 0.008 |
| 180 | 4 | 4 | 16 | 0.25 | 0.06 |
| 181 | 2 | 2 | 8 | 512 | 0.03 |
| 182 | 4 | 4 | 16 | 1 | 0.06 |
| 184 | 2 | 4 | 8 | 0.5 | 0.06 |
| 185 | 8 | 8 | 16 | 0.25 | 0.06 |
| 186 | 4 | 8 | 16 | 0.25 | 0.06 |
| 187 | 4 | 8 | 8 | 2 | 0.06 |
| 188 | 4 | 4 | 16 | 0.5 | 0.06 |
| 190 | 8 | 8 | 16 | 0.5 | 0.125 |
| 191 | 8 | 8 | 16 | 0.5 | 0.06 |
| 192 | 4 | 8 | 16 | 2 | 0.06 |
| 193 | 8 | 8 | 16 | 1 | 0.06 |
| 194 | 4 | 4 | 8 | 0.25 | 0.5 |
| 197 | 4 | 4 | 4 | 2 | 0.03 |
| 198 | 4 | 8 | 16 | 0.5 | 0.06 |
| 199 | 8 | 8 | 16 | 1 | 0.03 |
| 200 | 8 | 8 | 32 | 0.5 | 0.125 |
| 201 | 8 | 8 | 32 | 0.5 | 0.125 |
| 202 | 8 | 8 | 16 | 8 | 0.015 |
| 203 | 8 | 8 | 32 | 1 | 0.125 |
| 204 | 4 | 8 | 16 | 0.5 | 0.06 |
| 205 | 8 | 8 | 16 | 1 | 0.06 |
| 206 | 8 | 8 | 16 | 1 | 0.06 |
| 207 | 8 | 8 | 32 | 1 | 0.06 |
| 208 | 4 | 4 | 16 | 1 | 0.06 |
| 209 | 4 | 8 | 16 | 1 | 0.06 |
| 211 | 8 | 8 | 16 | 1 | 0.06 |
| 213 | 4 | 4 | 16 | 0.5 | 0.06 |
| 214 | 4 | 4 | 16 | 0.5 | 0.125 |
| WHO F | 8 | 4 | 8 | 0.25 | <0.008 |
| WHO G | 4 | 8 | 8 | 0.5 | <0.008 |
| WHO M | 8 | 4 | 16 | 0.5 | 0.015 |
| WHO N | 4 | 8 | 16 | 0.5 | 0.015 |
| WHO O | 4 | 8 | 16 | 0.5 | 0.015 |
| MIC50 | 4 | 8 | 16 | 0.5 | 0.06 |
| MIC90 | 8 | 8 | 32 | 16 | 0.03 |
TABLE 2.
MICs of fenamic acids (tolfenamic acid, flufenamic acid, and meclofenamic acid) and azithromycin against four strains of N. gonorrhoeae by the agar dilution method
| Strain | MIC (μg/ml) |
|||
|---|---|---|---|---|
| Tolfenamic acid | Flufenamic acid | Meclofenamic acid | Azithromycin | |
| N. gonorrhoeae 167 | 4 | 4 | 16 | 16 |
| N. gonorrhoeae 175 | 8 | 8 | 16 | 0.5 |
| N. gonorrhoeae 179 | 4 | 4 | 8 | 4 |
| N. gonorrhoeae 181 | 2 | 4 | 16 | 265 |
Antibacterial activity of fenamic acids against species of Lactobacillus present in the vaginal microbiome.
The fenamic acids and N-phenylanthranilic acid derivatives were evaluated for antibacterial activity against commensal bacteria present in the lower female reproductive tract of healthy individuals, such as Lactobacillus gasseri, L. jensenii, L. rhamnosus, and L. crispatus (Table 3). The fenamic acids and N-phenylanthranilic acid derivatives exhibited a good selectivity profile, as they were generally inactive against all species of Lactobacillus tested, with MIC values that exceeded 128 μg/ml. Flufenamic acid exhibited weak activity (MIC ranging from 64 to 128 μg/ml) against four strains of L. jensenii and L. crispatus. In contrast, azithromycin inhibited the growth of all strains of Lactobacillus tested at a concentration of <1 μg/ml.
TABLE 3.
MICs of fenamic acids (tolfenamic acid, flufenamic acid, and meclofenamic acid), their analogues [dichlorophenyl-ABA, N-(2-amino-4-chlorophenyl) anthranilic acid, N-phenylanthranilic acid, 3-amino-2-(3,5-dimethyl-phenylamino) benzoic acid, 3-amino-2-p-tolylamino benzoic acid, and 3-amino-2-phenylamino benzoic acid], and a control antibiotic (azithromycin) against various species of Lactobacillus
| Strain, description | Tolfenamic acid | Flufenamic acid | Meclofenamic acid | Dichlorophenyl-ABA | N-(2-Amino-4-chlorophenyl) anthranilic acid | N-Phenylanthranilic acid | 3-Amino-2-(3,5-dimethyl-phenylamino) benzoic acid | 3-Amino-2-p-tolylamino benzoic acid | 3-Amino-2-phenylamino benzoic acid | Azithromycin |
|---|---|---|---|---|---|---|---|---|---|---|
| L. gasseri MV-22, isolated in 2007 from the vaginal mucosa of a healthy U.S. woman of childbearing age | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | <1 |
| L. johnsonii 135-1-CHN, isolated in 2007 from the vaginal mucosa of a Chinese woman | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | <1 |
| L. gasseri SV-16A-US, isolated in 2007 from the vagina of a healthy U.S. woman | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | <1 |
| L. jensenii 115-3-CHN, isolated in 2007 from the vaginal mucosa of a healthy Chinese woman | >128 | 64 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | <1 |
| L. jensenii SJ-7A-US, isolated in 2007 from the vaginal mucosa of a healthy U.S. woman | >128 | 128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | <1 |
| L. crispatus 125-2-CHN, isolated in 2007 from the vagina of a healthy Chinese woman | >128 | 128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | <1 |
| L. rhamnosus LMS2-1, isolated from the human gastrointestinal tract | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | <1 |
| L. jensenii JV-V16, human isolate from Texas | >128 | 128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | <1 |
Time-kill assay of fenamic acids against N. gonorrhoeae.
After confirming the antibacterial activity of tolfenamic acid, flufenamic acid, and meclofenamic acid against N. gonorrhoeae (and finding that they exhibited weak or no activity against Lactobacillus species), we examined whether these agents exhibit bacteriostatic or bactericidal activity. Tolfenamic acid, flufenamic acid, meclofenamic acid, azithromycin, and tetracycline were evaluated against N. gonorrhoeae strain 178 via a standard time-kill assay. Both flufenamic acid and meclofenamic acid reduced the bacterial inoculum by 3 log10 units after 8 h, similar to azithromycin, indicating that these agents (at 5× MIC) are bactericidal in vitro (Fig. 1). Tolfenamic acid achieved the same effect after 10 h. The starting inoculum of ∼1 × 106 CFU/ml N. gonorrhoeae was reduced below the limit of detection within 12 h of treatment with each fenamic acid tested. Azithromycin, another bactericidal drug used in dual therapy for gonococcal infections, was used as a control. Azithromycin effectively reduced the bacterial inoculum below the limit of detection after 10 h. Tetracycline, in contrast, exhibited a bacteriostatic effect against N. gonorrhoeae.
FIG 1.
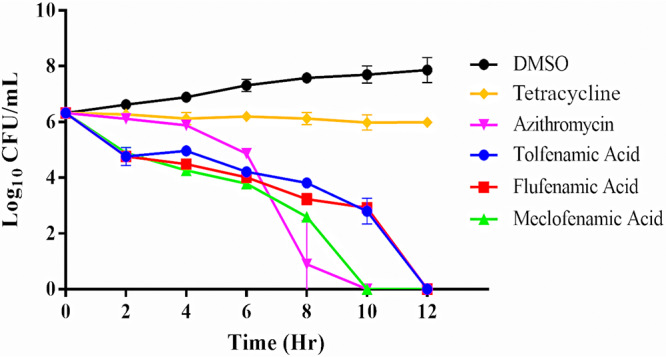
Time-kill assay of fenamic acids (tolfenamic acid, flufenamic acid, and meclofenamic acid) and bacteriostatic (tetracycline) and bactericidal (azithromycin) control antibiotics (all tested at 5× MIC) against a clinical isolate of N. gonorrhoeae (strain 178). Each agent was tested in triplicate, and error bars represent standard deviations.
Evaluation of dual therapy of fenamic acids in combination with azithromycin or ceftriaxone.
The current CDC-recommended treatment consists of dual therapy using an oral dose of azithromycin and an injectable dose of ceftriaxone. This strategy for treating gonococcal infections is an effort to prevent resistance from developing against last-resort therapeutics. To this end, we investigated the combination of the most potent fenamic acid (tolfenamic acid) with the current drugs of choice (azithromycin and ceftriaxone) against four strains of N. gonorrhoeae. In addition, the combination of azithromycin and ceftriaxone was evaluated as a comparison control. As presented in Table 4, tolfenamic acid exhibited an indifferent effect when combined with either ceftriaxone or azithromycin against all four strains of N. gonorrhoeae, with a fractional inhibitory concentration (FIC) index that ranged from 0.75 to 1.25. Interestingly, azithromycin and ceftriaxone exhibited an indifferent relationship, with an FIC index that ranged from 1.5 to 2 against all four strains of N. gonorrhoeae tested.
TABLE 4.
Fractional inhibitory concentration index range of tolfenamic acid in combination with either azithromycin or ceftriaxone against N. gonorrhoeae clinical isolatesa
| Drug and treatment | MIC (μg/ml) for Neisseria gonorrhoeae strain |
|||
|---|---|---|---|---|
| 175 | 167 | 214 | 194 | |
| Tolfenamic acid | ||||
| Alone | 2 | 4 | 8 | 4 |
| Combination | 1 | 1 | 1 | 2 |
| Azithromycin | ||||
| Alone | 8 | 8 | 8 | 0.5 |
| Combination | 4 | 4 | 8 | 0.25 |
| FIC index | 1 | 0.75 | 1.125 | 1 |
| Tolfenamic acid | ||||
| Alone | 2 | 4 | 8 | 4 |
| Combination | 1 | 1 | 4 | 2 |
| Ceftriaxone | ||||
| Alone | 0.015 | 0.007 | 0.03 | 0.5 |
| Combination | 0.007 | 0.007 | 0.015 | 0.25 |
| FIC index | 1 | 1.25 | 1.25 | 1 |
| Ceftriaxone | ||||
| Alone | 0.015 | 0.007 | 0.03 | 0.5 |
| Combination | 0.015 | 0.007 | 0.03 | 0.5 |
| Azithromycin | ||||
| Alone | 8 | 8 | 8 | 0.5 |
| Combination | 8 | 4 | 8 | 0.5 |
| FIC index | 2 | 1.5 | 2 | 2 |
The FIC (fractional inhibitory concentration) index data showed an indifferent relationship of the fenamic acids with azithromycin and ceftriaxone.
Intracellular clearance activity of fenamic acids in N. gonorrhoeae-infected endocervical cells.
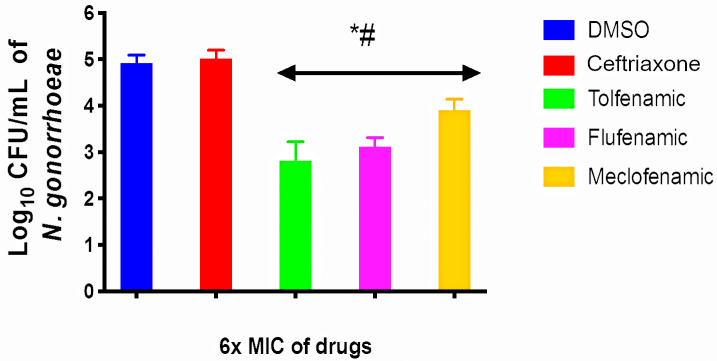
N. gonorrhoeae can invade and replicate inside endocervical cells through membrane ruffling, which is mediated by effector proteins secreted through a type IV secretion system (18). This ability is often associated with persistent gonococcal infections. Antigonococcal drugs with the ability to penetrate endocervical cells at a concentration that is high enough to clear infection are a major advantage. Although ceftriaxone is used clinically to treat gonococcal infections, it is unable to clear intracellular N. gonorrhoeae due to its high polarity and poor cellular permeability. Fenamic acids possess a small structure relative to ceftriaxone with proper hydrophilic/hydrophobic properties that prompted us to investigate if they could clear intracellular N. gonorrhoeae inside infected endocervical cells. As presented in Fig. 2, the exposure of infected endocervical cells to each fenamic acid (at 6× MIC) for 24 h resulted in a significant reduction in intracellular N. gonorrhoeae. Tolfenamic acid was the most potent molecule, as it produced a 2.1-log10 reduction in intracellular N. gonorrhoeae. Flufenamic acid exhibited similar potency, as it reduced the burden of intracellular bacteria by 1.83 log10 units. Meclofenamic acid was the least effective fenamic acid but still generated a 1-log10 reduction of intracellular N. gonorrhoeae. As expected, ceftriaxone did not significantly reduce the burden of intracellular N. gonorrhoeae. These results indicate that fenamic acids are able to penetrate infected endocervical cells at a concentration that is high enough to effectively reduce the burden of intracellular N. gonorrhoeae.
FIG 2.
Intracellular clearance assay of tolfenamic, flufenamic, and meclofenamic acids and ceftriaxone (all tested at 6× MIC) against N. gonorrhoeae in infected human endocervical cells (End1/E6E7). End1/E6E7 cells were infected with N. gonorrhoeae strain 194 for 6 h and then treated with either fenamic acids or ceftriaxone for 24 h. End1/E6E7 cells were subsequently lysed, and intracellular bacterial CFU were determined. Error bars represent standard deviations from triplicate samples used for each test agent. Cells treated with fenamic acids were compared to both cells treated with the negative control (DMSO) (*) and cells treated with ceftriaxone (#) (P < 0.01 analyzed via an unpaired t test).
Fenamic acids reduce cytokine production by endocervical cells infected with N. gonorrhoeae.
The upregulation of several important proinflammatory cytokines is a hallmark of gonococcal infections. Both piliated and nonpiliated gonococci can induce a marked increase in the production of interleukin-8 (IL-8), IL-6, and IL-1β (19). We hypothesized that since fenamic acids are a class of nonsteroidal anti-inflammatory drugs (NSAIDs), they would have an anti-inflammatory effect on endocervical cells infected with N. gonorrhoeae. To test this hypothesis, we measured cytokine production in the supernatant of endocervical cells (End1/E6E7), after establishing infection with N. gonorrhoeae, in the presence or absence of fenamic acids or ceftriaxone (all tested at 1/2× MIC). As depicted in Fig. 3, tolfenamic acid, flufenamic acid, and meclofenamic acid significantly reduced IL-8 production by infected endocervical cells by 85%, 82%, and 67%, respectively. All three fenamic acids had moderate activity on IL-6 and reduced its production by 41%, 35%, and 20%, respectively (Fig. 4). Fenamic acids displayed no significant reduction in the production of IL-1β (data not shown). As expected, ceftriaxone did not exhibit significant reductions in the tested cytokines (4.5% reduction of IL-8 and 7% reduction of IL-6).
FIG 3.
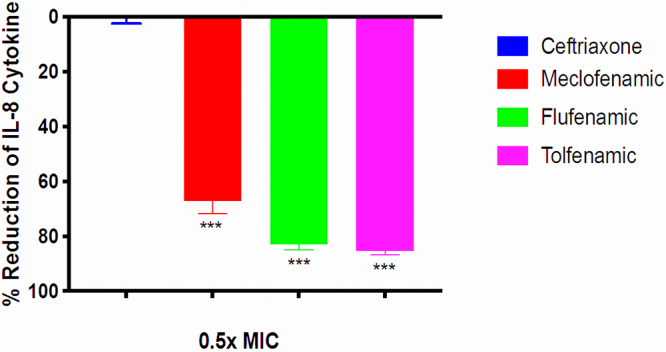
Effects of fenamic acids and ceftriaxone on IL-8 expression by endocervical cells. IL-8 levels were assessed in End1/E6E7 endocervical cells infected with N. gonorrhoeae strain 194 in the presence and absence of 1/2× MIC of fenamic compounds or ceftriaxone. The optical density at 450 nm (OD450) coincides with the level of IL-8 in the cell supernatant. Error bars represent standard deviations from triplicate samples used for each test agent. The experiment was conducted twice, and asterisks denote a significant difference between cells treated with flufenamic acid, meclofenamic acid, or tolfenamic acid and those treated with ceftriaxone (P < 0.01 analyzed via an unpaired t test).
FIG 4.
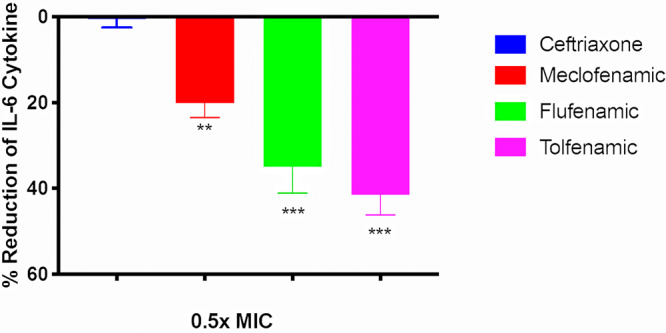
Effects of fenamic acids and ceftriaxone on IL-6 expression by endocervical cells. IL-6 levels were assessed in End1/E6E7 endocervical cells infected with N. gonorrhoeae strain 194 in the presence and absence of 1/2× MIC of fenamic compounds or ceftriaxone. The OD450 coincides with the level of IL-6 in the cell supernatant. Error bars represent standard deviations from triplicate samples used for each test agent. The experiment was conducted twice, and asterisks denote a significant difference between cells treated with flufenamic acid, meclofenamic acid, or tolfenamic acid and those treated with ceftriaxone (P < 0.01 analyzed via an unpaired t test).
Postantibiotic effect of fenamic acids against N. gonorrhoeae.
In order to investigate the ability of the fenamic acids to exhibit a prolonged inhibitory effect against N. gonorrhoeae following a brief exposure period, a postantibiotic effect (PAE) experiment was performed. As presented in Table 5, all three fenamic drugs (when tested at 10× MIC) displayed a PAE that ranged from 4 to 8 h against four clinical strains of N. gonorrhoeae. This effect was slightly less than that of azithromycin, which exhibited a PAE of 8 h against all four N. gonorrhoeae strains tested.
TABLE 5.
In vitro postantibiotic effects of fenamic acids and azithromycin against strains of N. gonorrhoeae
| Strain | Postantibiotic effect (h) for drug |
|||
|---|---|---|---|---|
| Tolfenamic acid | Flufenamic acid | Meclofenamic acid | Azithromycin | |
| N. gonorrhoeae 181 | 8 | 4 | 4 | 8 |
| N. gonorrhoeae 194 | 6 | 6 | 6 | 8 |
| N. gonorrhoeae 186 | 6 | 4 | 6 | 8 |
| N. gonorrhoeae 198 | 6 | 4 | 6 | 8 |
Frequency of spontaneous mutation against fenamic acids.
Given the promising in vitro results for the three fenamic acids against N. gonorrhoeae, we strove to investigate if N. gonorrhoeae is able to develop resistance rapidly to these drugs. We utilized a single-step resistance assay to determine the frequency of spontaneous mutation to each fenamic acid tested against three different clinical N. gonorrhoeae strains, as previously described (20). No mutants were isolated when N. gonorrhoeae was exposed to either tolfenamic acid or flufenamic acid at a concentration of 10× MIC, resulting in a low frequency of mutation of <2.4 × 10−10 (Tables 6 and 7). Meclofenamic acid exhibited a frequency of mutation of 5.1 × 10−8. N. gonorrhoeae developed resistance rapidly to the positive control, rifampin, which exhibited a high frequency of resistance that ranged from 1.2 × 10−6 to 4.17 × 10−6. Tolfenamic acid was further tested for mutant formation using additional concentrations of 4×, 8×, and 16× MIC. No colonies were formed at 8× or 16× MIC. However, at 4× MIC, tolfenamic acid exhibited a frequency of mutation of 1.5 × 10−8 to 3.07 × 10−8.
TABLE 6.
Single-step resistance assay of fenamic acids (tolfenamic acid, flufenamic acid, and meclofenamic acid) and rifampin at 10× MIC against three strains of N. gonorrhoeae
| Strain | Frequency of spontaneous mutation for drug |
|||
|---|---|---|---|---|
| Tolfenamic acid | Flufenamic acid | Meclofenamic acid | Rifampin | |
| N. gonorrhoeae 197 | <2.4 × 10−10 | <2.4 × 10−10 | 5.1 × 10−8 | 1.8 × 10−6 |
| N. gonorrhoeae 202 | <2.4 × 10−10 | <2.4 × 10−10 | 1.2 × 10−9 | 4.17 × 10−6 |
| N. gonorrhoeae 206 | <2.4× 10−10 | <2.4 × 10−10 | 6.12 × 10−8 | 1.2 × 10−6 |
TABLE 7.
Frequency of spontaneous mutation against tolfenamic acid at 4×, 8×, and 16× MIC
| Strain | Frequency of spontaneous mutation against tolfenamic acid at: |
||
|---|---|---|---|
| 4× MIC | 8× MIC | 16× MIC | |
| N. gonorrhoeae 197 | 3.07 × 10−8 | <2.4 × 10−10 | <2.4 × 10−10 |
| N. gonorrhoeae 202 | 1.5 × 10−8 | <2.4 × 10−10 | <2.4 × 10−10 |
| N. gonorrhoeae 206 | 1.12 × 10−8 | <2.4 × 10−10 | <2.4 × 10−10 |
Evaluation of the structure-activity relationship of fenamic acids.
We studied the structure-activity relationship (SAR) of fenamic acids in an effort to help medicinal chemists design and develop more potent analogues in the future. We noticed that the three fenamic acids evaluated against N. gonorrhoeae (tolfenamic acid, flufenamic acid, and meclofenamic acid) are all N-phenylanthranilic acid derivatives, and hence, we hypothesized that N-phenylanthranilic acid is a novel antigonorrheal scaffold. To investigate this hypothesis and study the SAR of fenamic acids further, we tested the antigonorrheal activities of commercially available N-phenylanthranilic acid derivatives (Table 8). Three derivatives [dichlorophenyl-ABA, N-(2-amino-4-chlorophenyl) anthranilic acid, and N-phenylanthranilic acid] inhibited the growth of 12 different isolates of N. gonorrhoeae and 8 isolates of lactobacilli. Specifically, dichlorophenyl-ABA exhibited a noticeably lower MIC value than meclofenamic acid against all tested isolates (up to an 8-fold difference) and had MIC values similar to those of tolfenamic acid and flufenamic acid (no more than a 2-fold difference except against strain 184). N-(2-Amino-4-chlorophenyl) anthranilic acid and N-phenylanthranilic acid were considerably less potent than dichlorophenyl-ABA. Both molecules exhibited moderate activity against N. gonorrhoeae, with MIC50 and MIC90 values of 32 μg/ml. Nevertheless, the MIC values across the tested isolates were consistent for both analogues, similar to what was observed with the fenamic acids and dichlorophenyl-ABA. On the other hand, 3-amino-2-(3,5-dimethyl-phenylamino) benzoic acid, 3-amino-2-p-tolylamino benzoic acid, and 3-amino-2-phenylamino benzoic acid were inactive against N. gonorrhoeae, with an MIC90 value that exceeded 128 μg/ml. All derivatives exhibited a good selectivity profile, as they were inactive against all species of Lactobacillus tested, with MIC values exceeding 128 μg/ml (Table 3).
TABLE 8.
MICs of fenamic acids (tolfenamic acid, flufenamic acid, and meclofenamic acid), their analogues [dichlorophenyl-ABA, N-(2-amino-4-chlorophenyl) anthranilic acid, N-phenylanthranilic acid, 3-amino-2-(3,5-dimethyl-phenylamino) benzoic acid, 3-amino-2-p-tolylamino benzoic acid, and 3-amino-2-phenylamino benzoic acid], and a control antibiotic (azithromycin) against 12 clinical isolates of N. gonorrhoeae
| N. gonorrhoeae strain | MIC (μg/ml) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tolfenamic acid | Flufenamic acid | Meclofenamic acid | Dichlorophenyl-ABA | N-(2-Amino-4-chlorophenyl) anthranilic acid | N-Phenylanthranilic acid | 3-Amino-2-(3,5-dimethyl-phenylamino) benzoic acid | 3-Amino-2-p-tolylamino benzoic acid | 3-Amino-2-phenylamino benzoic acid | Azithromycin | |
| 167 | 2 | 2 | 8 | 2 | 16 | 16 | 128 | >128 | >128 | 16 |
| 175 | 4 | 4 | 8 | 4 | 32 | 16 | 128 | >128 | >128 | 16 |
| 179 | 2 | 4 | 4 | 2 | 16 | 64 | 128 | >128 | >128 | 16 |
| 181 | 2 | 2 | 8 | 4 | 32 | 32 | 128 | >128 | >128 | 512 |
| 182 | 4 | 4 | 16 | 4 | 16 | 16 | >128 | >128 | >128 | 1 |
| 184 | 2 | 4 | 8 | 1 | 64 | 16 | 128 | >128 | >128 | 0.5 |
| 187 | 4 | 8 | 8 | 4 | 32 | 32 | 128 | >128 | >128 | 2 |
| 192 | 4 | 8 | 16 | 4 | 32 | 32 | 128 | >128 | >128 | 2 |
| 198 | 4 | 8 | 16 | 4 | 32 | 32 | 128 | >128 | >128 | 0.5 |
| 202 | 8 | 8 | 16 | 4 | 32 | 32 | 128 | >128 | >128 | 8 |
| 204 | 4 | 8 | 16 | 8 | 32 | 32 | >128 | >128 | >128 | 0.5 |
| 207 | 8 | 8 | 32 | 8 | 32 | 32 | >128 | >128 | >128 | 1 |
| MIC50 | 4 | 8 | 16 | 4 | 32 | 32 | 128 | >128 | >128 | 2 |
| MIC90 | 8 | 8 | 16 | 8 | 32 | 32 | >128 | >128 | >128 | 16 |
DISCUSSION
Recent reports of gonococcal isolates that are unresponsive to last-resort drugs demonstrate the urgent need to find new therapeutic options. Drug repurposing has emerged as a valid method to circumvent the lengthy and traditional method of de novo drug discovery. Therefore, our group adopted a drug-repurposing strategy to identify new candidates to treat multidrug-resistant N. gonorrhoeae.
Three fenamic acids (tolfenamic acid, flufenamic acid, and meclofenamic acid) that possessed potent in vitro antibacterial activity against N. gonorrhoeae were identified. Fenamic acids are derivatives of anthranilic acid, a class of nonsteroidal anti-inflammatory drugs (NSAIDs) that inhibit mammalian cyclooxygenase activity and prostaglandin H2 synthesis (21, 22). These NSAIDs are FDA-approved, over-the-counter medications that are often used in tandem with antibiotics to treat inflammation and various symptoms that are associated with bacterial infections.
In the present study, tolfenamic acid, flufenamic acid, and meclofenamic acid were evaluated against a panel of multidrug-resistant N. gonorrhoeae clinical isolates. Tolfenamic acid was the most potent drug identified, with an MIC50 of 4 μg/ml against 45 clinical isolates of N. gonorrhoeae, while meclofenamic acid exhibited the weakest activity against N. gonorrhoeae (MIC50 = 16 μg/ml). The MIC values of all three fenamic acids were within a 2-fold difference between strains. This suggests that cross-resistance between fenamic acids and current antibiotics used for the treatment of N. gonorrhoeae, including cephalosporins and azithromycin, is unlikely, although further investigation is needed to confirm this point.
After confirming the antibacterial activity of the three fenamic acids in vitro against multidrug-resistant N. gonorrhoeae, we next assessed their killing kinetics. All three fenamic acids exhibited bactericidal activity similar to that of azithromycin, as they reduced the high starting inoculum of N. gonorrhoeae (∼1 × 106 CFU/ml) to below the limit of detection within 12 h. This result indicates that fenamic acids are bactericidal agents against N. gonorrhoeae in vitro. Drugs with bactericidal activity have been postulated to possess several advantages over their bacteriostatic counterparts, including improving the outcome of the disease, limiting the spread of infection, shortening the duration of treatment, and potentially reducing the emergence of bacterial resistance (23).
In an era where antibiotic resistance has become a public health crisis, it is important to assess knowledge on the frequency of resistance against novel antibacterial agents early in preclinical development. This prompted us to investigate the potential emergence of spontaneous resistance of N. gonorrhoeae to fenamic acids. For both tolfenamic acid and flufenamic acid, no mutants were isolated when tested at 10× MIC, resulting in a frequency of mutation of <2.4 × 10−10. Mutants to meclofenamic acid were isolated at a frequency of 5.1 × 10−8; however, the mutants were unstable, and the MIC reverted to its original value. Overall, these results indicate a low likelihood of resistance emerging against all three flufenamic acids tested.
As noted above, due to the rapid manner in which N. gonorrhoeae develops resistance to antibiotics, monotherapy is no longer recommended. Thus, we investigated the relationship between each of the fenamic acids in combination with either ceftriaxone or azithromycin against four strains of N. gonorrhoeae. Utilizing a standard checkerboard assay, tolfenamic acid, flufenamic acid, and meclofenamic acid exhibited an indifferent relationship with both azithromycin and ceftriaxone, with an FIC index that ranged from 0.75 to 1.25. This finding is of interest because the FIC indices for the flufenamic acids in combination with azithromycin or ceftriaxone were lower than that for ceftriaxone and azithromycin together (FIC index ranging from 1.5 to 2).
The fenamic acids all exhibited antibacterial activity against extracellular N. gonorrhoeae. However, N. gonorrhoeae is capable of invading both the endocervical and ectocervical epithelia of the female genital tract, resulting in intracellular infections that are challenging to treat (18). Intracellular N. gonorrhoeae is often associated with the persistent nature of the disease that presents as an asymptomatic infection, often leading to ascending infection of the uterus and fallopian tubes and, in some rare cases, disseminated infection. Given that all three fenamic acids have a smaller size and structure than ceftriaxone and exhibit bactericidal activity against extracellular N. gonorrhoeae, we hypothesized that they would have the ability to gain entry into endocervical cells infected with N. gonorrhoeae and exert their antibacterial activity within. Subsequently, End1/E6E7 cells were infected with N. gonorrhoeae and treated with either tolfenamic acid, flufenamic acid, meclofenamic acid, or ceftriaxone, all at 6× MIC, for 24 h. All three fenamic acids generated a significant reduction in the burden of intracellular N. gonorrhoeae. Tolfenamic acid was the most potent drug and generated a 2.1-log10 reduction in the N. gonorrhoeae burden within infected endocervical cells. Ceftriaxone was ineffective and did not reduce the bacterial count inside infected endocervical cells. All three fenamic acids, particularly tolfenamic acid, outperformed ceftriaxone in the ability to reduce the burden of intracellular N. gonorrhoeae.
A challenge with the entry of N. gonorrhoeae into host epithelial cells is the strong inflammatory response that results in the production of a series of proinflammatory cytokines, specifically including IL-6, IL-1β, and IL-8. Inflammation in the urogenital tract can become severe and is associated with the development of chronic inflammatory disorders (24). An advantage of using NSAIDs to combat bacterial infections is that they can exert both anti-inflammatory and antibacterial activity in tandem, reducing the severity of disease symptoms as well as clearing the bacterial infection. We hypothesized that fenamic acids, as members of the NSAID drug class, would be able to reduce the cytokine production that is associated with gonococcal infection. Using endocervical cells, we established infections and treated the cells with subinhibitory concentrations of the three fenamic acids or ceftriaxone. As expected, all three fenamic acid drugs significantly reduced IL-8 and IL-6 expression. However, no notable decrease was observed in the level of IL-β. Interestingly, tolfenamic acid, which was the most potent drug that reduced the burden of intracellular N. gonorrhoeae in infected endocervical cells, was the most potent drug with anti-inflammatory activity, as it reduced the expression of IL-8 by 85% and of IL-6 by 48%. Ceftriaxone, in contrast, was ineffective, as it reduced IL-8 expression by infected endocervical cells by only 4.5%.
Determining the postantibiotic effect of drugs is an important step in finding effective dosing regimens. Drugs that are capable of inducing long PAEs are associated with longer dosing intervals. This is considered an advantage in terms of both cost and patient compliance. PAE studies performed (after bacterial exposure to 10× MIC of each drug for 1 h) found that all three fenamic acid drugs were able to suppress N. gonorrhoeae growth for 4 to 8 h postexposure.
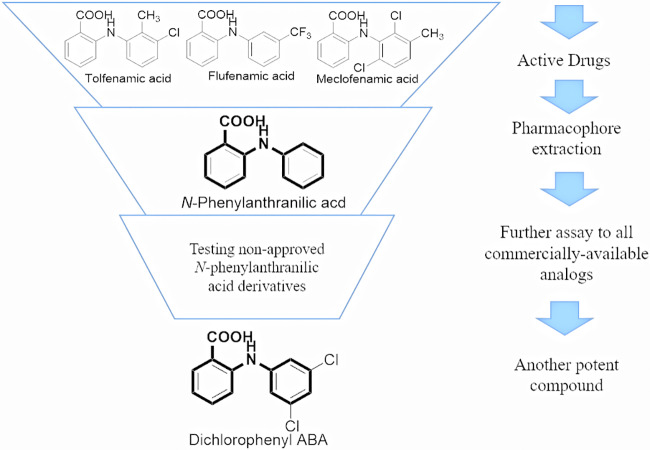
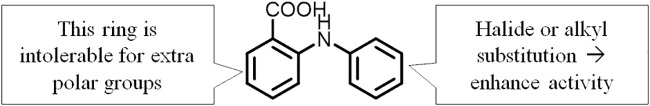
Structurally, the three fenamic acids identified from the initial screening are all N-phenylanthranilic acid derivatives (Fig. 5). Therefore, we examined the antigonorrheal activities of commercially available drugs bearing the N-phenylanthranilate core. Interestingly, unsubstituted N-phenylanthranilic acid possessed moderate antigonorrheal activity, with MIC values that ranged between 16 and 32 μg/ml. Taking into account the higher MIC values of all other derivatives in which an extra amine group was attached meta to the carboxylate group, this observation highlights the importance of the substitution pattern on the main pharmacophore of the N-phenylanthranilic acid scaffold. In this vein, this study sheds light on a preliminary structure-activity relationship (SAR) of N-phenylanthranilic acid as a novel antigonorrheal scaffold. Briefly, the ring attached to the carboxylate appears intolerable to an additional polar group. On the other hand, the ring attached to the amino group can accommodate many different substituents (Fig. 6). This preliminary SAR study paves the road to developing more potent N-phenylanthranilic acid analogues against N. gonorrhoeae. Careful modification of these drugs may result in an analogue with far greater potency than tolfenamic acid and dichlorophenyl-ABA against N. gonorrhoeae.
FIG 5.
Overview of the SAR in this study.
FIG 6.
Novel antigonorrheal activity scaffold with a preliminary SAR.
Broad-spectrum antimicrobials are known to disrupt the healthy microbiome, which provides the human body with various functions, including preventing colonization by pathogens (25–28). Identifying new treatments that protect healthy microflora while selectively killing the target pathogen would ideally result in a better posttreatment outcome (29–31). Studies have shown that a vaginal microbiome dominated by Lactobacillus spp. is less susceptible to N. gonorrhoeae infection (32, 33). Thus, we evaluated the fenamic acids and N-phenylanthranilic acid derivatives against various species of lactobacilli to determine whether fenamic acids will have a deleterious effect on vaginal commensals. The fenamic acids did not inhibit the growth of different vaginal Lactobacillus species examined (MIC50 > 128 μg/ml). In addition, potent analogues against N. gonorrhoeae, such as dichlorophenyl-ABA, N-(2-amino-4-chlorophenyl) anthranilic acid, and N-phenylanthranilic acids, were also ineffective against the Lactobacillus species tested. Azithromycin, in contrast, exhibited potent inhibitory activity against all species of lactobacilli tested (MIC < 1 μg/ml). Given that the fenamic acids preferentially target N. gonorrhoeae over Lactobacillus spp., this highlights a potential advantage of these drugs over current antigonococcal agents like azithromycin.
An important consideration for the development of agents to treat gonorrhea is the ability to reach the site of infection, the urogenital tract. In this regard, fenamic acids have been proven to be effective in the treatment of female genital tract disorders such as pelvic endometriosis and dysmenorrhea (34, 35). Additionally, tolfenamic acid possesses good oral bioavailability, as ∼60% of the dose reaches systemic circulation, with a peak concentration (above the MIC50 against N. gonorrhoeae) achieved within 1.3 to 1.9 h (36–39). This suggests that oral dosing of tolfenamic acid is plausible, which could enhance patient compliance in treating gonorrhea. The good oral bioavailability and ability to treat female genital tract disorders suggest that fenamic acids have the potential to treat N. gonorrhoeae infections, although further in vivo investigation is needed.
In conclusion, we have reported the selective bactericidal activity of fenamic acids against N. gonorrhoeae in vitro as part of an effort to respond to the global need to discover new antigonococcal agents. The fact that fenamic acids are off-patent, clinically approved, and active against N. gonorrhoeae could provide a more rapid and cost-effective pathway to clinical trials either as a single agent or as a part of combination therapy with existing antibiotics. Also, the structural versatility of the fenamic acids allows for further optimization to develop more potent analogues against N. gonorrhoeae. Further investigation of fenamic acids as novel antigonococcal agents is warranted to clearly elucidate their antibacterial mechanism and to evaluate their activity in suitable animal models of N. gonorrhoeae infection.
MATERIALS AND METHODS
Bacterial strains, media, and reagents.
Clinical isolates of N. gonorrhoeae were obtained from the Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA) Antibiotic Resistant Isolate Bank (Table 1). Lactobacillus vaginal isolates (L. gasseri, L. jensenii, L. johnsonii, L. crispatus, and L. rhamnosus) were obtained from the Biodefense and Emerging Infections Research Resources Repository (BEI Resources) (Table 2). Tolfenamic acid, flufenamic acid, meclofenamic acid, N-phenylanthranilic acid (Alfa Aesar, MA), dichlorophenyl-ABA, N-(2-amino-4-chlorophenyl) anthranilic acid, 3-amino-2-(3,5-dimethyl-phenylamino) benzoic acid, 3-amino-2-p-tolylamino benzoic acid, 3-amino-2-phenylamino benzoic acid (Sigma-Aldrich, MA), and azithromycin (TCI America, OR) were purchased from commercial vendors. Lactobacillus de Man, Rogosa, and Sharpe (MRS) medium and brucella-supplemented medium (BSM) were commercially purchased (Becton, Dickinson-Difco, TX). For BSM, dextrose (d-glucose) (Fisher Chemicals, PA), yeast extract (Fisher Bioreagents, PA), protease peptone, agarose (Sigma-Aldrich, MA), 10% Tween 80 (Amresco, OH), 0.05% hematin in NaOH, a 0.1% pyridoxal solution, and a 1% NAD solution (Chem-Impex International Inc., IL) were used as supplements, as previously described (40). Chocolate II agar (GC II agar with hemoglobin and IsoVitaleX; Becton, Dickinson, TX) was used to culture N. gonorrhoeae strains, and agar-supplemented MRS medium was used to culture Lactobacillus species.
Susceptibility analysis of fenamic acids against clinical isolates of N. gonorrhoeae.
The broth dilution assay was initially used to determine the MICs of all test agents against N. gonorrhoeae and Lactobacillus strains used in this study. Briefly, a culture of N. gonorrhoeae was grown overnight on chocolate II agar supplemented with hemoglobin and IsoVitaleX, swabbed, and suspended in phosphate-buffered saline (PBS) to achieve a turbidity equivalent to a 1.0 McFarland standard. This inoculum was diluted in brucella broth (1:100, vol/vol) supplemented with 5 g of dextrose and yeast extract, 2 g of protease peptone, 0.75 g of agarose, 30 ml of a 0.05% hematin solution in NaOH, 5 ml of 10% Tween 80 in distilled water, 6 ml of a 0.1% pyridoxal solution, and 1.5 ml of a 1% NAD solution per liter of medium. Stock solutions of each drug and control antibiotic were added to microtiter plates and serially diluted 2-fold. Plates were incubated for 24 h at 37°C with 5% CO2 prior to recording the MIC. A confirmatory test was conducted for five N. gonorrhoeae strains using the CLSI-recommended agar dilution method (41) to confirm MIC values obtained from the broth dilution assay. Each drug was tested in triplicates at least twice.
Specificity of fenamic acids against species of Lactobacillus present in the vaginal microbiome.
The fenamic acids and N-phenylanthranilic acid derivatives were evaluated for antibacterial activity against commensal bacteria present in the lower female reproductive tract of healthy individuals (L. gasseri, L. jensenii, L. rhamnosus, and L. crispatus). Lactobacillus strains were grown in MRS agar, diluted in PBS to achieve a 0.5 McFarland standard, and diluted 1:300 in MRS broth. The culture was incubated with test agents for 72 h anaerobically at 37°C prior to recording MIC values.
Time-kill kinetics of fenamic acids and control drugs.
N. gonorrhoeae strain 178 was grown overnight on chocolate II agar supplemented with hemoglobin and IsoVitaleX. A single colony was then suspended in 10 ml BSM broth and incubated for 18 h in a shaking incubator at 37°C. Next, the culture was diluted again 1:100 in BSM broth for 8 h under the same conditions to facilitate growth at logarithmic phase. Thereafter, the inoculum was diluted 1:100 in fresh BSM broth, distributed into Eppendorf tubes containing 5× MIC of the test agents (tolfenamic acid, flufenamic acid, meclofenamic acid, azithromycin, and tetracycline) in triplicate, and evaluated against N. gonorrhoeae via a standard time-kill assay. The tubes were placed in a shaking incubator at 37°C, and aliquots were collected after 0, 2, 4, 6, 8, 10, 12, and 24 h; serially diluted in PBS; and plated on chocolate II agar. Plates were incubated at 37°C with 5% CO2 for 18 h prior to counting the colonies.
Evaluation of dual therapy of fenamic acids in combination with azithromycin or ceftriaxone.
The relationship of tolfenamic acid in combination with the currently recommended therapeutics to treat gonorrhea (azithromycin and ceftriaxone) as well as the combinatorial activity of the drugs of choice together against N. gonorrhoeae were assessed through a standard checkerboard assay, as described previously (42, 43). The medium used for this assay is the same as the one described above for the susceptibility analysis.
Intracellular clearance assay.
The gentamicin protection assay was utilized to explore the ability of the fenamic acids to penetrate N. gonorrhoeae-infected endocervical cells and exert their antigonococcal activity, as described previously (19, 44).
Cytokine production in infected endocervical cells.
To investigate whether fenamic acids have any anti-inflammatory activity on cytokine production, IL-8, IL-6, and IL-β (key cytokines that are associated with gonococcal infection) were detected in the supernatants of N. gonorrhoeae-infected human endocervical cells that were exposed to either meclofenamic acid, flufenamic acid, tolfenamic acid, or ceftriaxone, as described in a previous study (20). Briefly, endocervical cells were infected with N. gonorrhoeae strain 194 for 2 h, followed by treatment with 0.5× MIC of each fenamic acid or ceftriaxone (in quadruplicates) for 8 h at 37°C with 5% CO2. After 8 h of exposure, the supernatants were collected, and cytokine concentrations were measured using enzyme-linked immunosorbent assay (ELISA) kits (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions.
Postantibiotic effect of fenamic acids against N. gonorrhoeae.
In order to investigate the ability of fenamic acids to exhibit a prolonged inhibitory effect against N. gonorrhoeae following a brief exposure period, a postantibiotic effect (PAE) experiment was performed. The experiment was conducted as described previously (45). Briefly, a culture of N. gonorrhoeae grown overnight was prepared and diluted to an initial concentration of 1.0 × 106 CFU/ml. The bacterial culture was then aliquoted in Eppendorf tubes containing 10× MIC of either tolfenamic acid, flufenamic acid, meclofenamic acid, or azithromycin. Dimethyl sulfoxide (DMSO) served as a negative control. Tubes were incubated for 1 h in a shaking incubator at 37°C. At the end of the exposure period, cultures were diluted 1:1,000 in Brucella supplemented broth base (BSB) to wash out the drugs, and samples were collected and plated every 2 h for 12 h. The PAE was calculated as described previously (45), as PAE = T − C, where T is the time for the viable count of an antibiotic-exposed culture to increase by 1 log10 unit above the count observed immediately after dilution and C is the corresponding time for the negative control to increase by 1 log10 unit.
N. gonorrhoeae frequency of spontaneous mutation against fenamic acids.
The frequency of spontaneous mutations of important clinical isolates of N. gonorrhoeae was investigated after a single passage with meclofenamic acid, flufenamic acid, tolfenamic acid, or rifampin, all tested at 10× MIC, according to a previously described procedure (46). Next, tolfenamic acid was tested further at 4 ×, 8×, and 16× MIC against the same 3 strains.
Evaluation of the structure-activity relationship of fenamic acids against N. gonorrhoeae.
To study the structure-activity relationship (SAR) of fenamic acids, commercially available N-phenylanthranilic acid derivatives were evaluated via the broth dilution assay against N. gonorrhoeae. Additionally, anthranilic acids that are chemically related to the fenamic acids [dichlorophenyl-ABA, N-(2-amino-4-chlorophenyl) anthranilic acid, N-phenylanthranilic acid, 3-amino-2-(3,5-dimethyl-phenylamino) benzoic acid, 3-amino-2-p-tolylamino benzoic acid, and 3-amino-2-phenylamino benzoic acid] were also evaluated for activity against N. gonorrhoeae.
ACKNOWLEDGMENTS
We thank the CDC and the FDA Antibiotic Resistance Isolate Bank, Atlanta, GA, for providing the clinical isolates for this study.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.World Health Organization. 2016. WHO guidelines for the treatment of Neisseria gonorrhoeae. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 2.AbdelKhalek A, Abutaleb NS, Mohammad H, Seleem MN. 2018. Repurposing ebselen for decolonization of vancomycin-resistant enterococci (VRE). PLoS One 13:e0199710. doi: 10.1371/journal.pone.0199710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thangamani S, Mohammad H, Younis W, Seleem MN. 2015. Drug repurposing for the treatment of staphylococcal infections. Curr Pharm Des 21:2089–2100. doi: 10.2174/1381612821666150310104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thangamani S, Mohammad H, Abushahba MF, Sobreira TJ, Seleem MN. 2016. Repurposing auranofin for the treatment of cutaneous staphylococcal infections. Int J Antimicrob Agents 47:195–201. doi: 10.1016/j.ijantimicag.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammad H, AbdelKhalek A, Abutaleb NS, Seleem MN. 2018. Repurposing niclosamide for intestinal decolonization of vancomycin-resistant enterococci. Int J Antimicrob Agents 51:897–904. doi: 10.1016/j.ijantimicag.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AbdelKhalek A, Abutaleb NS, Elmagarmid KA, Seleem MN. 2018. Repurposing auranofin as an intestinal decolonizing agent for vancomycin-resistant enterococci. Sci Rep 8:8353. doi: 10.1038/s41598-018-26674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thangamani S, Maland M, Mohammad H, Pascuzzi PE, Avramova L, Koehler CM, Hazbun TR, Seleem MN. 2017. Repurposing approach identifies auranofin with broad spectrum antifungal activity that targets Mia40-Erv1 pathway. Front Cell Infect Microbiol 7:4. doi: 10.3389/fcimb.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thangamani S, Younis W, Seleem MN. 2015. Repurposing celecoxib as a topical antimicrobial agent. Front Microbiol 6:750. doi: 10.3389/fmicb.2015.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thangamani S, Younis W, Seleem MN. 2015. Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections. Sci Rep 5:11596. doi: 10.1038/srep11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younis W, AbdelKhalek A, Mayhoub AS, Seleem MN. 2017. In vitro screening of an FDA-approved library against ESKAPE pathogens. Curr Pharm Des 23:2147–2157. doi: 10.2174/1381612823666170209154745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Younis W, Thangamani S, Seleem MN. 2015. Repurposing non-antimicrobial drugs and clinical molecules to treat bacterial infections. Curr Pharm Des 21:4106–4111. doi: 10.2174/1381612821666150506154434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashburn TT, Thor KB. 2004. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 13.Scannell JW, Blanckley A, Boldon H, Warrington B. 2012. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov 11:191–200. doi: 10.1038/nrd3681. [DOI] [PubMed] [Google Scholar]
- 14.Jin G, Wong ST. 2014. Toward better drug repositioning: prioritizing and integrating existing methods into efficient pipelines. Drug Discov Today 19:637–644. doi: 10.1016/j.drudis.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong CR, Sullivan DJ Jr.. 2007. New uses for old drugs. Nature 448:645–646. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]
- 16.Foschi C, Salvo M, Cevenini R, Parolin C, Vitali B, Marangoni A. 2017. Vaginal lactobacilli reduce Neisseria gonorrhoeae viability through multiple strategies: an in vitro study. Front Cell Infect Microbiol 7:502. doi: 10.3389/fcimb.2017.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spurbeck RR, Arvidson CG. 2008. Inhibition of Neisseria gonorrhoeae epithelial cell interactions by vaginal Lactobacillus species. Infect Immun 76:3124–3130. doi: 10.1128/IAI.00101-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards JL, Shao JQ, Ault KA, Apicella MA. 2000. Neisseria gonorrhoeae elicits membrane ruffling and cytoskeletal rearrangements upon infection of primary human endocervical and ectocervical cells. Infect Immun 68:5354–5363. doi: 10.1128/iai.68.9.5354-5363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fichorova RN, Desai PJ, Gibson FC III, Genco CA. 2001. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun 69:5840–5848. doi: 10.1128/iai.69.9.5840-5848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenz JD, Shirk KA, Jolicoeur A, Dillard JP. 2018. Selective inhibition of Neisseria gonorrhoeae by a dithiazoline in mixed infections with Lactobacillus gasseri. Antimicrob Agents Chemother 62:e00826-18. doi: 10.1128/AAC.00826-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orlando BJ, Malkowski MG. 2016. Substrate-selective inhibition of cyclooxygeanse-2 by fenamic acid derivatives is dependent on peroxide tone. J Biol Chem 291:15069–15081. doi: 10.1074/jbc.M116.725713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinarello CA. 2010. Anti-inflammatory agents: present and future. Cell 140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohamed MF, Abdelkhalek A, Seleem MN. 2016. Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Sci Rep 6:29707. doi: 10.1038/srep29707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chateau A, Seifert HS. 2016. Neisseria gonorrhoeae survives within and modulates apoptosis and inflammatory cytokine production of human macrophages. Cell Microbiol 18:546–560. doi: 10.1111/cmi.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaser MJ. 2016. Antibiotic use and its consequences for the normal microbiome. Science 352:544–545. doi: 10.1126/science.aad9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ. 2012. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis JD, Chen EZ, Baldassano RN, Otley AR, Griffiths AM, Lee D, Bittinger K, Bailey A, Friedman ES, Hoffmann C, Albenberg L, Sinha R, Compher C, Gilroy E, Nessel L, Grant A, Chehoud C, Li H, Wu GD, Bushman FD. 2015. Inflammation, antibiotics, and diet as environmental stressors of the gut microbiome in pediatric Crohn’s disease. Cell Host Microbe 18:489–500. doi: 10.1016/j.chom.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, Subbarao P, Mandhane P, Becker A, McNagny KM, Sears MR, Kollmann T, CHILD Study Investigators, Mohn WW, Turvey SE, Finlay BB. 2015. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 7:307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 29.Mondhe M, Chessher A, Goh S, Good L, Stach JE. 2014. Species-selective killing of bacteria by antimicrobial peptide-PNAs. PLoS One 9:e89082. doi: 10.1371/journal.pone.0089082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao J, Carter RA, Vuagniaux G, Barbier M, Rosch JW, Rock CO. 2016. A pathogen-selective antibiotic minimizes disturbance to the microbiome. Antimicrob Agents Chemother 60:4264–4273. doi: 10.1128/AAC.00535-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clausen TD, Bergholt T, Bouaziz O, Arpi M, Eriksson F, Rasmussen S, Keiding N, Lokkegaard EC. 2016. Broad-spectrum antibiotic treatment and subsequent childhood type 1 diabetes: a nationwide Danish cohort study. PLoS One 11:e0161654. doi: 10.1371/journal.pone.0161654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breshears LM, Edwards VL, Ravel J, Peterson ML. 2015. Lactobacillus crispatus inhibits growth of Gardnerella vaginalis and Neisseria gonorrhoeae on a porcine vaginal mucosa model. BMC Microbiol 15:276. doi: 10.1186/s12866-015-0608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonio MA, Hawes SE, Hillier SL. 1999. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis 180:1950–1956. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- 34.Kauppila A, Puolakka J, Ylikorkala O. 1979. Prostaglandin biosynthesis inhibitors and endometriosis. Prostaglandins 18:655–661. doi: 10.1016/0090-6980(79)90033-9. [DOI] [PubMed] [Google Scholar]
- 35.Delgado J, Simonin G, Servier C, Garcia R, Yoma J. 1994. Tolfenamic acid and mefenamic acid in the treatment of primary dysmenorrhoea. Pharmacol Toxicol 75(Suppl 2):89–91. doi: 10.1111/j.1600-0773.1994.tb02008.x. [DOI] [PubMed] [Google Scholar]
- 36.McKellar QA, Lees P, Gettinby G. 1994. Pharmacodynamics of tolfenamic acid in dogs. Evaluation of dose response relationships. Eur J Pharmacol 253:191–200. doi: 10.1016/0014-2999(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 37.Lentjes EG, van Ginneken CA. 1987. Pharmacokinetics of flufenamic acid in man. Int J Clin Pharmacol Ther Toxicol 25:185–187. [PubMed] [Google Scholar]
- 38.Pedersen SB. 1994. Biopharmaceutical aspects of tolfenamic acid. Pharmacol Toxicol 75(Suppl 2):22–32. doi: 10.1111/j.1600-0773.1994.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 39.Pentikainen PJ, Neuvonen PJ, Backman C. 1981. Human pharmacokinetics of tolfenamic acid, a new anti-inflammatory agent. Eur J Clin Pharmacol 19:359–365. doi: 10.1007/BF00544587. [DOI] [PubMed] [Google Scholar]
- 40.Cartwright CP, Stock F, Gill VJ. 1994. Improved enrichment broth for cultivation of fastidious organisms. J Clin Microbiol 32:1825–1826. doi: 10.1128/JCM.32.7.1825-1826.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CLSI. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. CLSI, Wayne, PA. [Google Scholar]
- 42.Mohammad H, Mayhoub AS, Cushman M, Seleem MN. 2015. Anti-biofilm activity and synergism of novel thiazole compounds with glycopeptide antibiotics against multidrug-resistant staphylococci. J Antibiot (Tokyo) 68:259–266. doi: 10.1038/ja.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohamed MF, Hammac GK, Guptill L, Seleem MN. 2014. Antibacterial activity of novel cationic peptides against clinical isolates of multi-drug resistant Staphylococcus pseudintermedius from infected dogs. PLoS One 9:e116259. doi: 10.1371/journal.pone.0116259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alhashimi M, Mayhoub A, Seleem MN. 2019. Repurposing salicylamide for combating multidrug-resistant Neisseria gonorrhoeae. Antimicrob Agents Chemother 63:e01225-19. doi: 10.1128/AAC.01225-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pankuch G, Jacobs M, Appelbaum P. 2003. Postantibiotic effects of garenoxacin (BMS-284756) against 12 gram-positive or-negative organisms. Antimicrob Agents Chemother 47:1140–1142. doi: 10.1128/aac.47.3.1140-1142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thangamani S, Mohammad H, Abushahba MF, Sobreira TJ, Hedrick VE, Paul LN, Seleem MN. 2016. Antibacterial activity and mechanism of action of auranofin against multi-drug resistant bacterial pathogens. Sci Rep 6:22571. doi: 10.1038/srep22571. [DOI] [PMC free article] [PubMed] [Google Scholar]