Attention has been paid to H5N6 highly pathogenic avian influenza virus (HPAIV) because of its heavy burden on the poultry industry and human mortality. Since an influenza A virus carrying N6 neuraminidase (NA) has never spread in humans, the potential for H5N6 HPAIV to cause disease in humans and the efficacy of antiviral drugs against the virus need to be urgently assessed. We used nonhuman primates to elucidate the pathogenesis of H5N6 HPAIV as well as to determine the efficacy of antiviral drugs against the virus.
KEYWORDS: H5N6, NA inhibitor, avian influenza viruses, highly pathogenic, nonhuman primate
ABSTRACT
Attention has been paid to H5N6 highly pathogenic avian influenza virus (HPAIV) because of its heavy burden on the poultry industry and human mortality. Since an influenza A virus carrying N6 neuraminidase (NA) has never spread in humans, the potential for H5N6 HPAIV to cause disease in humans and the efficacy of antiviral drugs against the virus need to be urgently assessed. We used nonhuman primates to elucidate the pathogenesis of H5N6 HPAIV as well as to determine the efficacy of antiviral drugs against the virus. H5N6 HPAIV infection led to high fever in cynomolgus macaques. The lung injury caused by the virus was severe, with diffuse alveolar damage and neutrophil infiltration. In addition, an increase in interferon alpha (IFN-α) showed an inverse correlation with virus titers during the infection process. Oseltamivir was effective for reducing H5N6 HPAIV propagation, and continuous treatment with peramivir reduced virus propagation and the severity of symptoms in the early stage. This study also showed pathologically severe lung injury states in cynomolgus macaques infected with H5N6 HPAIV, even in those that received early antiviral drug treatments, indicating the need for close monitoring and further studies on virus pathogenicity and new antiviral therapies.
INTRODUCTION
Global epidemics of highly pathogenic avian influenza viruses (HPAIVs) have been continuing. The number of subtypes detected in domestic birds and the number of countries affected by HPAIVs are larger than those in past epidemics (2005 to 2012) (1). By genetic reassortment, new gene combinations of influenza viruses may create a high risk to human health due to an increase in transmission ability and antiviral drug resistance (2–4). The novel HPAIV of subtype H5N6 that has been detected since 2013 is associated with human mortality and has caused a great burden on the poultry industry (5–7). Nineteen humans were infected with H5N6 HPAIV, and 13 of them died (fatality rate of 68.4%) (7). The hemagglutinin (HA) proteins of reported H5N6 HPAIVs have affinity for both human-like (α2,6) and avian-like (α2,3) sialic acid receptors, suggesting that H5N6 HPAIV has a high potential for avian-to-human transmission (8–10). In addition, this subtype of virus was transmitted among mammalians by a direct-contact route and was found in wild birds, especially migratory waterfowl that transverse long distances, posing the potential threat of a wide dissemination of this virus (1, 10).
The pathogenesis of H5N6 HPAIV is controversial and remains to be elucidated. One study in mice and ferrets showed that H5N6 HPAIV was less pathogenic than the other H5 HPAIVs (8). On the other hand, it was shown that H5N6 HPAIV caused more severe disease in ferrets than did other H5 clade 2.3.4.4 viruses (11). Another study in ferrets also showed different pathogenicities among H5N6 HPAIVs (10). The pathogenic characteristics of H5N6 HPAIV must be determined more clearly, especially in models for which pathogenicity can be extrapolated to humans.
The state of antiviral drug resistance increases with the evolution of an influenza virus. Neuraminidase (NA) inhibitors (NAIs) are currently recommended for the treatment of most influenza A viruses, but some NAI resistance-conferring mutations have been reported (12–14). The majority of seasonal influenza A viruses are resistant to M2 ion channel inhibitors, but the frequency and distribution of amantadine (AMT)-resistant influenza virus variants depend on HA subtypes, host species, years of isolation, and geographical areas (12, 14, 15). The efficacy of antiviral drugs against H5N6 HPAIV, the first influenza A virus carrying N6 NA found in humans, is unknown. Therefore, the efficacy of available and easily accessible antiviral drugs such as NAIs and M2 ion channel inhibitors should be clarified in in vivo studies.
In the present study, we used the cynomolgus macaque model to investigate the pathogenicity and antiviral susceptibility of H5N6 HPAIV A/black swan/Akita/1/2016 (H5N6). Cynomolgus macaques were used because of their high genetic similarity to humans as well as their symptoms and histopathological findings, which are similar to those in humans infected with influenza viruses (16–18). The present study showed that H5N6 HPAIV caused severe pneumonia in macaques, even in those that received early treatments with NAIs. Oseltamivir (OTV) was effective for reducing H5N6 HPAIV propagation, and continuous treatment with peramivir (PRV) reduced virus propagation and symptoms effectively in the early stage. However, AMT had no effect on the early reduction of virus titers.
RESULTS
Virus replication in the respiratory tracts of cynomolgus macaques infected with H5N6 HPAIV.
First, we investigated the replication of A/black swan/Akita/1/2016 (H5N6) virus in the macaques’ respiratory tracts (19, 20). Virus was detected in the control group intragastrically and intravenously treated with saline until day 7 in swab samples from the nasal cavity and trachea and until day 6 in bronchial samples (Table 1; see also Table S1 in the supplemental material). In the nasal cavity, the virus titer increased, with a peak on day 6. In the groups treated with intragastric oseltamivir phosphate (30 mg/kg of body weight) or intravenous peramivir hydrate (30 mg/kg) for 5 days, no virus was detected on day 7 in the swab samples. The virus titers in the NAI treatment groups were lower than those in the control group after day 5. Meanwhile, in the group treated with intragastric AMT (10 mg/kg) continuously for 5 days, the virus titers were comparable to those in the control group, although the M2 gene of the inoculum virus (GenBank accession number LC198539.1) indicated sensitivity to AMT (19). Viruses were found in macaque A2 (nasal cavity) and macaque A3 (trachea and bronchus) on day 7 (Table 1). The virus titer areas under the concentration-time curve (AUCs) (the summation of virus titers from day 1 to day 7 and from day 2 to day 7) in the nasal swabs of the groups treated with PRV and OTV were significantly lower than those in the control group (Fig. S1). The AUC in the AMT-treated group was comparable to that in the control group. Thus, H5N6 HPAIV propagated in the macaques, and NAIs, but not AMT, were effective in the early reduction of virus titers.
TABLE 1.
Virus titers in swab samples of cynomolgus macaques infected with H5N6 and treated with antiviral drugs
| Sample typea | Treatment | Animalb | Virus titer (log10 TCID50/ml) on day after virus inoculationc
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Nasal swab | Saline | S1 | 3.50 | 2.67 | < | ≤0.83e | 2.50 | 2.67 | ≤1.33h |
| S2 | 3.83 | 3.23 | 1.83 | 2.00 | 3.44 | 4.83 | 3.00 | ||
| S3 | ≤0.67d | 1.50 | 2.33 | 3.50 | 3.50 | 3.67 | 3.23 | ||
| Oseltamivir | O1 | 3.50 | 1.50 | ≤0.67 | < | < | < | < | |
| O2 | 3.83 | ≤0.67 | 2.00 | ≤1.50j | ≤1.23g | < | < | ||
| O3 | < | ≤1.33 | < | < | < | < | < | ||
| Peramivir | P1 | ≤1.33 | < | < | < | < | < | < | |
| P2 | 3.50 | ≤1.00f | ≤1.00 | < | < | ≤0.67 | < | ||
| P3 | 4.00 | <0.67 | <1.00 | < | < | < | < | ||
| Amantadine | A1 | 3.50 | 1.67 | 2.23 | 2.50 | 2.23 | ≤1.77l | < | |
| A2 | ≤1.33 | < | ≤1.50 | ≤1.77 | 2.50 | 2.33 | ≤1.00 | ||
| A3 | ≤1.33 | < | < | < | < | < | < | ||
| Trachea swab | Saline | S1 | 4.67 | 3.33 | < | 1.67 | 2.00 | ≤0.67 | < |
| S2 | 3.67 | ≤1.00 | < | < | ≤0.67 | ≤0.67 | < | ||
| S3 | < | 2.50 | 2.50 | ≤0.75 | 1.83 | ≤0.67 | ≤0.67 | ||
| Oseltamivir | O1 | 2.67 | 2.83 | ≤1.83m | ≤0.83 | < | < | < | |
| O2 | 3.50 | 2.00 | 2.00 | 2.50 | < | < | < | ||
| O3 | 4.33 | 3.77 | 2.33 | < | < | < | < | ||
| Peramivir | P1 | 2.50 | 2.67 | ≤1.50 | ≤1.44i | <1.00 | < | < | |
| P2 | 4.00 | ≤1.00 | ≤1.44 | < | < | < | < | ||
| P3 | 4.50 | 2.77 | 2.00 | 1.83 | < | < | < | ||
| Amantadine | A1 | 5.67 | 2.50 | ≤2.17n | 3.33 | 2.67 | ≤1.23 | < | |
| A2 | 4.50 | ≤1.67k | ≤1.00 | ≤1.50 | 2.63 | ≤1.50 | < | ||
| A3 | 4.67 | 3.50 | < | 2.33 | 3.50 | < | 2.23 | ||
| Bronchial swab | Saline | S1 | 3.67 | 4.00 | 1.67 | 2.83 | 1.50 | 2.67 | < |
| S2 | 5.00 | 3.00 | ≤1.00 | ≤1.00 | 1.67 | ≤1.50 | < | ||
| S3 | 2.00 | < | 2.50 | 2.67 | 2.23 | 1.50 | < | ||
| Oseltamivir | O1 | 1.83 | 2.50 | 2.00 | < | ≤0.67 | < | < | |
| O2 | 4.00 | ≤1.00 | < | 2.23 | ≤0.83 | < | < | ||
| O3 | 3.33 | 2.50 | ≤1.23 | < | ≤0.67 | < | < | ||
| Peramivir | P1 | 3.00 | 2.50 | 2.00 | ≤1.50 | ≤1.00 | < | < | |
| P2 | 4.33 | 3.50 | ≤0.67 | < | < | < | < | ||
| P3 | 4.67 | 3.00 | < | 1.38 | < | < | < | ||
| Amantadine | A1 | 3.67 | 3.00 | ≤1.00 | ≤1.00 | 2.23 | < | < | |
| A2 | 4.50 | ≤1.23 | ≤1.77 | <1.50 | 3.50 | ≤2.25o | < | ||
| A3 | 4.00 | 2.33 | ≤1.33 | 3.23 | 3.00 | 2.25 | ≤1.00 | ||
Sampling organs.
Macaque identification.
<, no CPE-positive well in quadruplicate culture. The detection limit was 0.67 log10 TCID50/ml.
≤0.67, one CPE-positive well in a quadruplicate culture with an undiluted sample was observed.
≤0.83, two CPE-positive wells were observed in a quadruplicate culture, one with an undiluted sample and one with a 10-fold-diluted sample.
≤1.00, two CPE-positive wells in a quadruplicate culture with an undiluted sample were observed.
≤1.23, three CPE-positive wells were observed in quadruplicate culture, two with an undiluted sample and one with a 10-fold-diluted sample.
≤1.33, three CPE-positive wells in a quadruplicate culture with an undiluted sample were observed.
≤1.44, four CPE-positive wells were observed in a quadruplicate culture, two with an undiluted sample, one with a 10-fold-diluted sample, and one with a 100-fold-diluted sample.
≤1.50, four CPE-positive wells were observed in a quadruplicate culture, two with an undiluted sample and two with a 10-fold-diluted sample.
≤1.67, five CPE-positive wells were observed in a quadruplicate culture, three with an undiluted sample, one with a 10-fold-diluted sample, and one with a 1,000-fold-diluted sample.
≤1.77, five CPE-positive wells were observed in a quadruplicate culture, three with an undiluted sample and two with a 10-fold-diluted sample.
≤1.83, six CPE-positive wells were observed in a quadruplicate culture, three with an undiluted sample, one with a 10-fold-diluted sample, and two with a 100-fold-diluted sample.
≤2.17, six CPE-positive wells were observed in a quadruplicate culture, three with an undiluted sample and three with a 10-fold-diluted sample.
≤2.25, seven CPE-positive wells were observed in a quadruplicate culture, three with an undiluted sample, two with a 10-fold-diluted sample, and two with a 100-fold-diluted sample.
Tissues of the respiratory tract were used to determine the presence of H5N6 HPAIV on day 7 after virus infection (Table S2). We detected the virus in both upper respiratory tissues and lower respiratory tissues in the control group. A very small amount of virus was detected in the group treated with OTV. The AMT-treated group had much lower virus titers than those in the control group in the upper respiratory tracts on day 7. Meanwhile, more virus was detected in tonsils and lower respiratory samples in the PRV-treated group, and we did not find any NAI-resistant mutation that has been reported previously, such as E119V, I222L, R292K, and R371K (data not shown) (13, 21). No mutation associated with AMT resistance was detected in the genes of the virus recovered on day 7 in samples from the macaques treated with AMT (both tissues and swab samples) (data not shown). No virus was detected in other organs, including the mediastinal lymph nodes, heart, spleen, kidney, liver, conjunctiva, and brain (data not shown).
Clinical signs in cynomolgus macaques infected with H5N6 HPAIV.
To examine the clinical signs caused by infection by H5N6 HPAIV, body temperatures in the macaques were monitored. After H5N6 HPAIV infection, body temperatures in the macaques were very high (Fig. 1a). Body temperatures in 12 macaques increased by 3.0°C on average on the first night after virus inoculation. After that, body temperatures decreased by about 1.5°C on day 2, remained unchanged until day 5, and decreased on day 7. The body temperatures in the group treated with PRV were lower than those in the control group on day 1, day 3, and day 4 after virus inoculation, although no significant difference was detected after treatment. OTV and AMT did not reduce body temperature after H5N6 infection compared to saline. After stopping treatment, the body temperatures of the groups treated with PRV and AMT increased by about 0.7°C and then decreased on day 7 (Fig. 1a).
FIG 1.
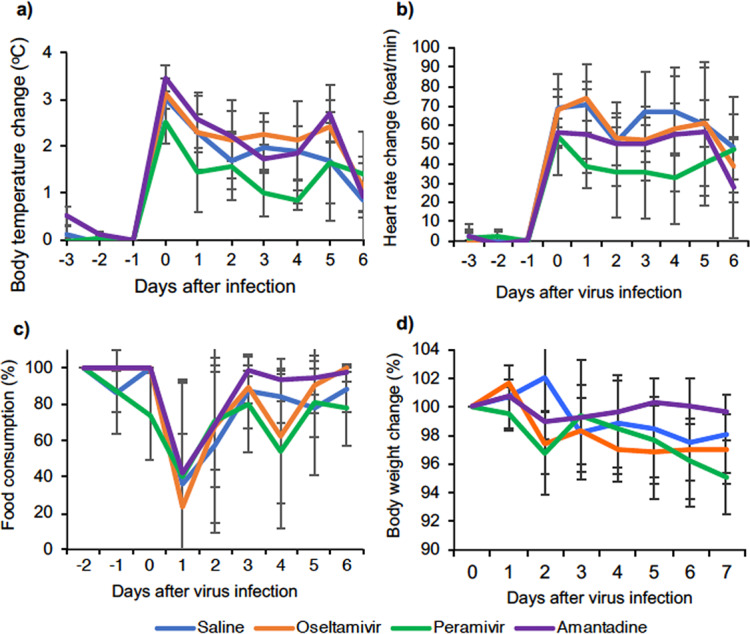
Symptoms in cynomolgus macaques challenged with A/black swan/Akita/1/2016 (H5N6). Cynomolgus macaques (n = 3) were inoculated with the virus on day 0. (a and b) Averages and standard deviations of body temperature (a) and heart rate (b) were determined by using a telemetry probe system during the night (from 8:00 p.m. to 8:00 a.m.), and data were adjusted to day −1. (c and d) Averages and standard deviations of food consumption and body weight. (c) Food consumption was estimated by using the following formula: food consumption = [(number of pellets given in the morning − number of pellets left at night)/number of pellets given in the morning] × 100 (%). (d) Body weight was monitored every day. Statistical differences among groups were calculated by a multicomparison ANOVA.
Using the same telemetry system as that used for measuring body temperature, we recorded the heart rates of the macaques throughout the experiment. Heart rate has been one of the criteria for estimating the efficacy of antiviral treatment in clinical trials (22). After H5N6 HPAIV infection, the heart rate increased from 84.4 ± 15.6 beats/min (average ± standard deviation) at night before infection to 145.7 ± 24 beats/min at night on day 0 after virus infection and did not recover completely until day 6 in all groups (Fig. 1b). PRV treatment rapidly decreased the heart rate, but the heart rate increased on days 5 and 6. The heart rate decreased in the OTV-treated group after day 3 and in the AMT-treated group only on day 6.
We also observed changes in appetite and body weight in the period of infection (Fig. 1c and d). On the day after infection (day 1), all of the macaques left more than 50% of the food pellets. Appetite started to recover after day 3 in all groups, but complete recovery on day 3 was seen only in macaques treated with AMT. Appetite in the OTV group was completely recovered on day 7. Food consumption had still not returned to normal on the last day in the control group and the PRV group. Body weights of all macaques decreased after day 2 or 3 (Fig. 1d). We did not find any significant difference in weight loss or change of appetite among the groups on each day.
Pathological characteristics in the lungs of cynomolgus macaques 7 days after infection with H5N6 HPAIV.
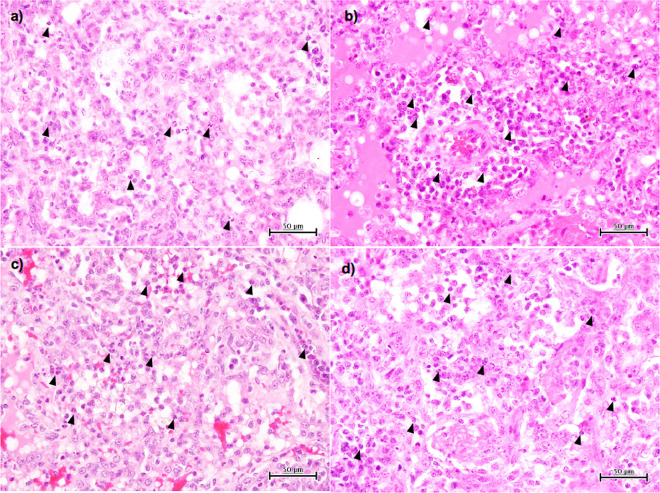
Viral pneumonia was histologically examined 7 days after virus inoculation. All of the macaques survived and were autopsied on day 7. Macroscopically, dark-red areas indicating lung congestion were observed in the control macaques as well as in the antiviral drug-treated macaques (data not shown). Microscopically, many CD163-positive macrophages and neutrophils were present in the alveoli of the lungs of all cynomolgus macaques, and the levels of lung injury appeared to be similar among the four groups (Fig. 2 and Fig. S2a). There was no significant difference in acute lung injury scores among the four groups (Fig. S2b). We also found a larger number of bronchus-associated lymphoid tissues (BALTs) in the group treated with AMT than in the other treated groups, although the difference was not significant (Fig. S2c). Thus, H5N6 HPAIV caused severe pneumonia and lymphocyte responses in the lungs of cynomolgus macaques.
FIG 2.
Viral pneumonia in cynomolgus macaques challenged with A/black swan/Akita/1/2016 (H5N6). Shown are images of H&E staining of lung tissues collected 7 days after virus infection. Representative photos of cynomolgus macaques treated with saline (a), oseltamivir (b), peramivir (c), and amantadine (d) are shown. Black arrowheads point to neutrophils.
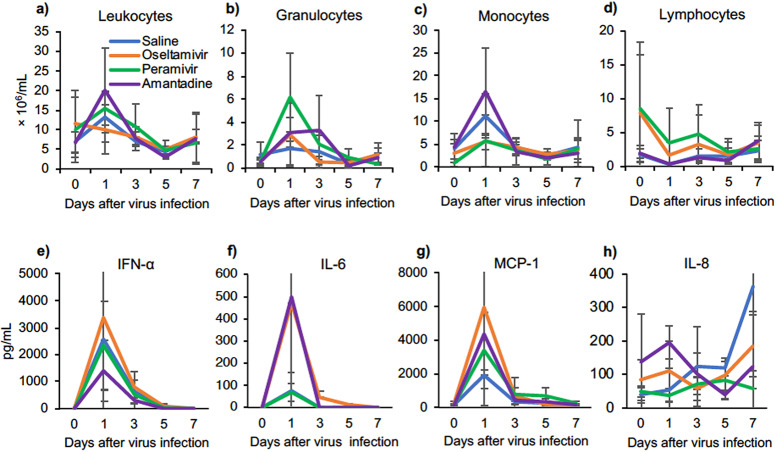
Changes in peripheral blood cells and cytokine responses in cynomolgus macaques infected with H5N6 HPAIV.
Inflammatory responses in peripheral blood after virus infection were examined. Increases in the numbers of total leukocytes, monocytes, and granulocytes (Fig. 3a to c) and a decrease in the number of lymphocytes (Fig. 3d) were detected on day 1 before treatment, and they then gradually returned to normal levels. In plasma, levels of interferon alpha (IFN-α), interleukin-6 (IL-6), and monocyte chemoattractant protein 1 (MCP-1) were significantly increased on day 1 and then decreased on day 3 (Fig. 3e to g). Levels of IL-8 did not increase on day 1 but tended to increase after day 3 (Fig. 3h). Levels of IFN-γ, tumor necrosis factor alpha (TNF-α), and IL-4 in plasma were increased slightly after infection (Fig. S3a to c). The increase in IL-6 had no relation to virus titers (data not shown), and we found that the increase in IFN-α was inversely correlated with virus titers in the trachea and bronchus on day 1 (Fig. 3e and Fig. S4). Cytokine responses in lung tissues were examined since severe pneumonia was observed histologically. Compared to the levels in the other three groups, high levels of IFN-γ, IL-6, MCP-1, and IL-8 were found in lung homogenates in the PRV group on day 7 (Fig. S3d to g), but there were no statistically significant differences. Thus, H5N6 HPAIV induced significant cytokine responses in peripheral blood on day 1, followed by inflammatory cytokine responses in the lung on day 7.
FIG 3.
Peripheral blood cell populations and cytokine/chemokine responses in cynomolgus macaques challenged with A/black swan/Akita/1/2016 (H5N6). (a to d) Concentrations in peripheral blood cells collected on the indicated days. (a) total leukocytes; (b) granulocytes; (c) monocytes; (d) lymphocytes. (e to h) Levels of cytokines/chemokines in plasma after virus infection. The average values and standard deviations are shown.
Efficacy of antiviral drugs against H5N6 HPAIV in vitro.
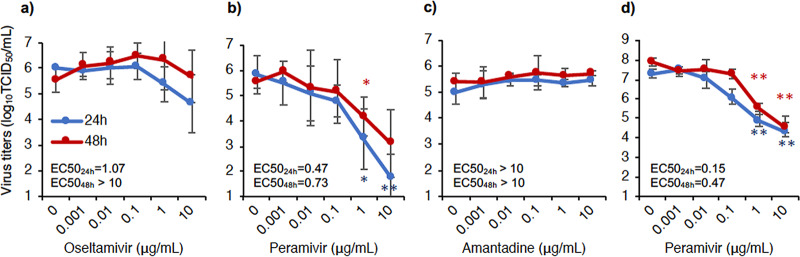
The efficacy of the antiviral drugs was investigated in an in vitro study using Madin-Darby canine kidney (MDCK) cells. A/black swan/Akita/1/2016 (H5N6) was sensitive to OTV and PRV, as indicated by a decrease in virus titers when the drug concentrations were increased. At concentrations of 1 and 10 μg/ml (24 h), virus titers in the presence of PRV were significantly lower than those in the absence of PRV. Half-maximal effective concentrations (EC50s) at 24 h of culture were 1.07 μg/ml and 0.47 μg/ml for OTV and PRV, respectively (Fig. 4a and b). AMT had no inhibitory effect on the propagation of H5N6 HPAIV, even at 10 μg/ml (Fig. 4c), whereas AMT showed an inhibitory effect on the propagation of A/Aichi/2/1968 (H3N2) (Fig. S5). These results are consistent with virus titers in swab samples of treated macaques. Furthermore, the virus isolated from a tonsil of macaque P2 on day 7 was sensitive to PRV in vitro (Fig. 4d), as indicated by the finding that the virus titers were significantly lower than those without treatment at a PRV concentration of 1 μg/ml (both 24 h and 48 h).
FIG 4.
Efficacy of antiviral drugs against A/black swan/Akita/1/2016 (H5N6) virus in vitro. MDCK cells were infected with the virus at a multiplicity of infection (MOI) of 0.01 and cultured with antiviral drugs at various concentrations. (a) Oseltamivir; (b and d) peramivir; (c) amantadine. Sensitivities of the inoculum virus (a to c) and the virus recovered from a tonsil of macaque P2 on day 7 (d) were examined. The supernatant of each well was collected 24 h and 48 h after virus infection. Next, virus titers in the supernatants were determined by the Reed-Muench method. Averages and standard deviations of data from three independent experiments are shown in panels a to c. Averages and standard deviations of data from triplicate cultures are shown in panel d. EC50 values were calculated by using the Quest Graph EC50 calculator (3 February 2020; AAT Bioquest, Inc. [https://www.aatbio.com/tools/ec50-calculator]). The asterisks show significant differences in virus titers with treatment at each antiviral drug concentration and without treatment (0 μg/ml) (*, P < 0.05; **, P < 0.01 [by Student’s t test]).
DISCUSSION
In the present study, we found that HPAIV A/black swan/Akita/1/2016 (H5N6) propagated in both the upper and lower respiratory tracts of macaques and caused severe inflammation with pneumonia and cytokine responses in the macaque model, comparable to the severe states in humans infected with H5N6 HPAIV (7). NAIs (OTV and PRV), but not an M2 inhibitor (AMT), showed inhibitory effects on virus replication in in vivo and in vitro studies.
The macaques infected with A/black swan/Akita/1/2016 (H5N6) showed fever of up to 40°C on average and lymphopenia on day 1, similar to the symptoms in H5N6 HPAIV-infected patients (7, 23). The prominent pathological characteristic of diffuse lung inflammation in the macaques was the same as that found in postmortem studies of human cases of infection with H5N6 HPAIV and other influenza virus infections (24–27). Thus, the macaques represented human patients in clinical signs and pneumonia. However, this strain, which preferentially bound to α2,3-linked sialic acid receptors (M. Okamatsu et al., unpublished data), propagated in both the upper and lower respiratory tracts, whereas the other H5N6 HPAIVs possessed binding affinity for both α2,6-linked sialic acid-bearing receptors and α2,3-linked receptors (8–10). Therefore, receptor-binding preference is not the only factor that determines the pathogenicity of this strain in macaques.
Cytokines/chemokines are associated with pathogenicity, relating to the disease severity of influenza virus infection and also the potential for new therapy development (28, 29). In the present study, the levels of most of the cytokines increased in plasma of the macaques on day 1 and then decreased to normal ranges, which is the same as the findings for macaques infected with H5N1 HPAIV (30). IL-8 increased later (after day 3) and continued to increase until day 7. Previous studies showed that increases in IL-6 were correlated with high virus loads in the respiratory tracts and symptoms that appeared in macaques and humans after H5N1 HPAIV infection (30, 31), but the increase in IL-6 did not show a correlation with virus titers in the present study. Differences in genes and proteins other than HA between the H5N6 HPAIV used in the present study and the H5N1 HPAIV used in our previous study might affect cytokine responses, although further studies are required to identify amino acids responsible for these differences in the future. IFN-α increased significantly and was inversely correlated with virus titers, indicating that IFN-α may be a protective factor against H5N6 HPAIV infection in macaques. Together with NAIs, early treatment with IFN-α might be a potential therapy for H5N6 HPAIV infection as for H5N1 HPAIV and H7N9 virus (32, 33).
NAIs were effective against H5N6 HPAIV infection in the present study. The virus titers in swab samples were reduced on day 5 in the groups treated with both NAIs in vivo. NAIs at higher concentrations also inhibited virus propagation 24 h and/or 48 h after infection in vitro. Treatment with PRV, which resulted in rapid reductions in body temperature and heart rate, seemed to be more effective than OTV in the early stage after virus infection. On day 7, the virus was detected in tonsils and lung tissues of cynomolgus macaques that had been treated with PRV, and the symptoms (high body temperature, high heart rate, and decreased body weight) in this group did not recover well after day 5. A recent study on humans with seasonal influenza virus infections showed a rebound of the virus load after stopping PRV treatment (30, 34). However, no NAI-resistant mutation was found in the present and previous studies (30, 34). These results suggest that additional administration of PRV is required for the treatment of H5N6 HPAIV infection.
AMT, a drug that has not been used widely for a long time due to the rapid emergence of drug resistance in seasonal influenza virus infection, did not show any effect on the early reduction of virus shedding compared to that in the control group in the present study, although no AMT-resistant mutation was found in the M2 gene before and after virus inoculation. Ilyushina et al. reported that AMT resistance of H7N7 HPAIVs without any M2 gene mutation was associated with the contribution of HA to viral fusion activity (35). Therefore, the efficacy of AMT against H5N6 HPAIV might be dependent on a gene constellation and/or high pathogenicity of the virus since the susceptibility of H5 HPAIV to amantadine varied among strains without amino acid residues associated with resistance to amantadine in the M2 protein (36, 37).
AMT showed no direct antiviral effects on the reduction of virus titers from day 2 to day 5 during administration. However, we found lower virus titers in respiratory tissues of macaques treated with AMT than in control macaques on day 7, and this difference might be related to the slight increase of BALTs in the lungs compared with other groups. AMT is a dopamine agonist that has potent effects on T cells, and a disabled function of regulatory T cells leads to BALT development (38, 39). Therefore, it is possible that the late efficacy of AMT for the reduction of tissue virus titers is dependent on the immune response of BALT formation after viral infection instead of direct antiviral effects of AMT.
Despite the symptomatic and virological improvements due to the antiviral treatment, the pathological findings of severe alveolar damage were not greatly different among the three treatment groups and the control group. Therefore, antiviral treatment with both NAIs and an M2 inhibitor may have limited effectiveness pathologically until day 7. This study showed the need for close monitoring and further studies on virus pathogenicity and the development of new antiviral therapies.
MATERIALS AND METHODS
Ethnics statement.
This study was done in strict accordance with guidelines for the husbandry and management of laboratory animals of the Research Center for Animal Life Science at the Shiga University of Medical Science (http://www.rcals.jp/node/135) and standards relating to the care and fundamental guidelines for proper conduct of animal experiments and related activities in academic research institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science, and Technology, Japan. The protocols were approved by the Shiga University of Medical Science Animal Experiment Committee [permit number 2017-3-15(H1)]. The Research Center for Animal Life Science at the Shiga University of Medical Science has permission to import cynomolgus macaques and provides regular veterinary care, monitoring, balanced nutrition, and environmental enrichment. At the endpoint of 7 days after virus inoculation, the macaques were euthanized with ketamine and then intravenously injected with pentobarbital (200 mg/kg of body weight). The animals were monitored every day with the clinical score system shown in Table S3 in the supplemental material, and veterinary examinations were also performed to alleviate suffering. It was decided that the animals would be euthanized if the scores reached 15 (a humane endpoint). Ten-day-old embryonated chicken eggs were used to propagate an inoculum virus (obtained from Sasaki Chemical Co., Ltd., Kyoto, Japan).
Animals.
Twelve healthy adult female cynomolgus macaques (Macaca fascicularis) (4 to 13 years old) from China, Indonesia, and Vietnam were used in this study. The study schedule is shown in Table S1. To reduce suffering, ketamine (5 mg/kg) and xylazine (1 mg/kg) were used to anesthetize the animals before the collection of samples and virus inoculation. The animals were provided food pellets of CMK-2 (CLEA Japan, Inc., Tokyo, Japan) once a day after recovery from anesthesia and drinking water ad libitum. The appetite percentage was calculated as follows: % appetite = [(number of pellets given in the morning − number of pellets left at night)/number of pellets given in the morning] × 100. Each macaque was housed individually with controlled humidity (71% to 82%), temperature (23.8°C to 27.4°C), and light (12-h light/12-h dark cycle; dark from 8:00 p.m. to 8:00 a.m.). Three weeks before virus inoculation, we implanted telemetry probes (catalog number TA10CTA-D70; Data Sciences International, St. Paul, MN) into the macaques’ peritoneal cavities under anesthetic conditions with ketamine-xylazine and isoflurane inhalation in order to monitor mainly body temperature and heart rate. The macaques used in the present study were negative for herpes B virus, hepatitis E virus, Mycobacterium tuberculosis, Shigella spp., Salmonella spp., and Entamoeba histolytica. Twelve macaques were divided into four groups: macaques S1, S2, and S3 (animal identification) were treated with saline as controls; O1, O2, and O3 were treated with OTV; P1, P2, and P3 were treated with PRV; and A1, A2, and A3 were treated with AMT. Under anesthetic conditions, swab samples were collected from the eyes, nasal cavity, oral cavity, and trachea in about 30 s using cotton sticks from day 1 to day 7 after virus inoculation. Bronchial swab samples were collected using a bronchoscope (catalog number MEV-2560; Machida Endoscope Co., Ltd., Tokyo, Japan) and cytology brushes (catalog number BC-203D-2006; Olympus Co., Tokyo, Japan). Each of the samples (from cotton sticks and brushes) was put into 1 ml Eagle’s minimal essential medium (EMEM) containing 0.1% bovine serum albumin (BSA) and antibiotics (penicillin G and streptomycin). On day 7, macaques were autopsied, and tissue samples were sectioned into small pieces and stored at −80°C. On the day of virus titration or tissue cytokine measurement, tissue samples were homogenized. The homogenate was adjusted with EMEM (0.1% BSA, penicillin, and streptomycin) to 10% (wt/vol) and centrifuged at 8,000 rpm for 3 min at 4°C. The supernatants were collected and used for virus titration and cytokine measurements.
Viruses.
The highly pathogenic avian influenza virus A/black swan/Akita/1/2016 (H5N6) (NCBI taxonomy identifier 1921521) was isolated from a dead black swan in a zoo (19). The virus was propagated in 10-day-old embryonated chicken eggs at 35°C for 24 h once at Hokkaido University and once at the Shiga University of Medical Science and was titrated with MDCK cells (American Type Culture Collection, Manassas, VA). The macaques were challenged with A/black swan/Akita/1/2016 (H5N6) (3 × 106 TCID50 [50% tissue culture infective doses]) in 7 ml Hanks’ buffered saline solution (HBSS). The virus solution (0.05 ml for each conjunctiva, 0.5 ml for each nostril, 0.9 ml for the oral cavity, and 5 ml for the trachea) was inoculated on day 0. Influenza virus A/Aichi/2/1968 (H3N2) was propagated in MDCK cells. Virus titers in samples were determined as described previously (40). Briefly, the MDCK cells were cultured in EMEM with 10% fetal bovine serum (FBS), penicillin G (50,000 U/ml), and streptomycin (50 mg/ml) in a humidified incubator (5% CO2 at 37°C). MDCK cells (in cell-confluent wells) were washed twice and incubated with 100 μl of the sample in multiple 10-fold dilutions (quadruplicate) for 1 h in 5% CO2 at 35°C. Next, the cells were washed with HBSS once and cultured in EMEM with 0.1% BSA, penicillin G (50,000 U/ml), and streptomycin (50 mg/ml) in a humidified incubator (5% CO2 at 35°C) for 3 days. Cytopathic effect (CPE) was observed with a microscope. The level of detection was 0.67 log10 TCID50/ml, which means that there was one CPE-positive well in quadruplicate cultures with undiluted samples. All experiments were done under the conditions of biosafety level 3 containment at the Research Center for Animal Life Science at the Shiga University of Medical Science.
Compounds.
Oseltamivir phosphate (Chugai Pharmaceutical Co., Ltd., Tokyo, Japan), peramivir hydrate (Shionogi Co., Ltd., Osaka, Japan), and amantadine hydrochloride (Symmetrel; Tanabe Mitsubishi Co., Ltd., Osaka, Japan) were used in the in vivo study. Oseltamivir phosphate and amantadine phosphate were dissolved in distilled water and administered into the stomach with a catheter (30 mg/kg and 10 mg/kg, respectively) once a day for 5 days. Peramivir phosphate (30 mg/kg) was intravenously injected into the macaques once a day for 5 days (30). Saline was administered in cynomolgus macaques by both the intragastric and intravenous routes with volumes adjusted to the administration of oseltamivir (intragastric route) and peramivir (intravenous route). The doses of oseltamivir phosphate and peramivir hydrate used in the present study are doses that induce higher areas under the concentration-time curve than those standardly indicated in humans (oseltamivir phosphate at 75 mg twice a day and peramivir hydrate at 600 mg once a day) (16, 30). The dose of amantadine phosphate was higher than that recommended for pediatric patients (from 4.4 to 8.8 mg/kg/day). In in vitro experiments, oseltamivir carboxylate (ChemScene, LLC, Monmouth Junction, NJ), peramivir hydrate (Shionogi Co.), and amantadine hydrochloride (LKT Laboratories, Inc., MN) were used with MDCK cells. The compounds were diluted to the indicated concentrations with EMEM (0.1% BSA and antibiotics) and then added to the culture of virus-infected MDCK cells for 24 h or 48 h.
Blood cell and cytokine measurements.
Peripheral blood was collected before virus infection or before antiviral drug/saline administration on the days indicated in Table S1. Plasma and peripheral blood mononuclear cells were separated by using Leucosep (Greiner Bio-One) according to the manufacturer’s instructions and stored at −80°C. The cell components of peripheral blood were counted by using a hemocytometer (Vetscan HMII; Abaxis, Union City, CA). Levels of cytokines/chemokines in plasma or lung homogenates (10%, wt/vol) were measured using the Milliplex MAP nonhuman primate cytokine panel and Luminex 200 (Millipore Corp., Billerica, MA) according to the manufacturer’s instructions.
Histopathological examination.
Immediately after autopsy, lung tissues were fixed with 10% neutral buffered formalin. The fixed tissues were embedded in paraffin. They were then cut into 3-μm-thick sections and stained with hematoxylin and eosin (H&E). Acute lung injuries were estimated by two pathologists according to a four-parameter scoring system: alveolar capillary congestion, hemorrhage, infiltration or aggregation of neutrophils in the airspace or vessel wall, and thickness of the alveolar wall (41). Each parameter was scored from 0 to 4, with 0 for no or little damage, 1 for <25% damage, 2 for 25% to 50% damage, 3 for 50% to 75% damage, and 4 for >75% damage. In total, 8 H&E-stained sections for each macaque’s lung were examined (1 section from the upper and middle lobes and 2 sections from the lower lobes in bilateral lungs). Averages of data from three macaques were used to compare the acute lung injury levels among the four groups.
Statistical analysis.
Statistical differences of the values (virus titers, symptoms, pathological features, and cytokines) among the four groups were analyzed by multicomparison analysis of variance (ANOVA). Statistical analysis was performed with R software version 3.6.2. Student’s t test was used for comparisons in the neuraminidase inhibition tests. P values of <0.05 were considered a statistically significant difference.
Supplementary Material
ACKNOWLEDGMENTS
This research is partly supported by grants-in-aid for scientific research (B), JSPS KAKENHI grant number 15H04720; by a grant from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, for a joint research program of the Research Center for Zoonosis Control, Hokkaido University; and by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) of the Japan Agency for Medical Research and Development (AMED) under grant number JP 19fm0108008. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We thank Naoko Kitagawa, Hideaki Ishida, Takako Sasamura, and Chikako Kinoshita for their assistance in the experiment and Hideaki Tsuchiya, Shinichiro Nakamura, Takahiro Nakagawa, and Ikuo Kawamoto for animal care.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.OIE. 2018. OIE situation report for avian influenza. OIE, Paris, France. [Google Scholar]
- 2.Li C, Chen H. 2014. Enhancement of influenza virus transmission by gene reassortment. Curr Top Microbiol Immunol 385:185–204. doi: 10.1007/82_2014_389. [DOI] [PubMed] [Google Scholar]
- 3.Ottmann M, Duchamp MB, Casalegno JS, Frobert E, Moules V, Ferraris O, Valette M, Escuret V, Lina B. 2010. Novel influenza A(H1N1) 2009 in vitro reassortant viruses with oseltamivir resistance. Antivir Ther 15:721–726. doi: 10.3851/IMP1576. [DOI] [PubMed] [Google Scholar]
- 4.Neumann G, Noda T, Kawaoka Y. 2009. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.OIE. 2017. OIE situation report for avian influenza. OIE, Paris, France. [Google Scholar]
- 6.Jiang H, Wu P, Uyeki TM, He J, Deng Z, Xu W, Lv Q, Zhang J, Wu Y, Tsang TK, Kang M, Zheng J, Wang L, Yang B, Qin Y, Feng L, Fang VJ, Gao GF, Leung GM, Yu H, Cowling BJ. 2017. Preliminary epidemiologic assessment of human infections with highly pathogenic avian influenza A(H5N6) virus, China. Clin Infect Dis 65:383–388. doi: 10.1093/cid/cix334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi Y, Tan S, Yang Y, Wong G, Zhao M, Zhang Q, Wang Q, Zhao X, Li L, Yuan J, Li H, Li H, Xu W, Shi W, Quan C, Zou R, Li J, Zheng H, Yang L, Liu WJ, Liu D, Wang H, Qin Y, Liu L, Jiang C, Liu W, Lu L, Gao GF, Liu Y. 2019. Clinical and immunological characteristics of human infections with H5N6 avian influenza virus. Clin Infect Dis 68:1100–1109. doi: 10.1093/cid/ciy681. [DOI] [PubMed] [Google Scholar]
- 8.Sun H, Pu J, Wei Y, Sun Y, Hu J, Liu L, Xu G, Gao W, Li C, Zhang X, Huang Y, Chang KC, Liu X, Liu J. 2016. Highly pathogenic avian influenza H5N6 viruses exhibit enhanced affinity for human type sialic acid receptor and in-contact transmission in model ferrets. J Virol 90:6235–6243. doi: 10.1128/JVI.00127-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui KP, Chan LL, Kuok DI, Mok CK, Yang ZF, Li RF, Luk GS, Lee EF, Lai JC, Yen HL, Zhu H, Guan Y, Nicholls JM, Peiris JS, Chan MC. 2017. Tropism and innate host responses of influenza A/H5N6 virus: an analysis of ex vivo and in vitro cultures of the human respiratory tract. Eur Respir J 49:1601710. doi: 10.1183/13993003.01710-2016. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Z, Guo Z, Zhang C, Liu L, Chen L, Zhang C, Wang Z, Fu Y, Li J, Shao H, Luo Q, Qian J, Liu L. 2017. Avian influenza H5N6 viruses exhibit differing pathogenicities and transmissibilities in mammals. Sci Rep 7:16280. doi: 10.1038/s41598-017-16139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herfst S, Mok CKP, van den Brand JMA, van der Vliet S, Rosu ME, Spronken MI, Yang Z, de Meulder D, Lexmond P, Bestebroer TM, Peiris JSM, Fouchier RAM, Richard M. 2018. Human clade 2.3.4.4 A/H5N6 influenza virus lacks mammalian adaptation markers and does not transmit via the airborne route between ferrets. mSphere 3:e00405-17. doi: 10.1128/mSphere.00405-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain M, Galvin HD, Haw TY, Nutsford AN, Husain M. 2017. Drug resistance in influenza A virus: the epidemiology and management. Infect Drug Resist 10:121–134. doi: 10.2147/IDR.S105473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaymard A, Charles-Dufant A, Sabatier M, Cortay JC, Frobert E, Picard C, Casalegno JS, Rosa-Calatrava M, Ferraris O, Valette M, Ottmann M, Lina B, Escuret V. 2016. Impact on antiviral resistance of E119V, I222L and R292K substitutions in influenza A viruses bearing a group 2 neuraminidase (N2, N3, N6, N7 and N9). J Antimicrob Chemother 71:3036–3045. doi: 10.1093/jac/dkw275. [DOI] [PubMed] [Google Scholar]
- 14.Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM, Centers for Disease Control and Prevention . 2011. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend Rep 60:1–24. [PubMed] [Google Scholar]
- 15.Dong G, Peng C, Luo J, Wang C, Han L, Wu B, Ji G, He H. 2015. Adamantane-resistant influenza A viruses in the world (1902-2013): frequency and distribution of M2 gene mutations. PLoS One 10:e0119115. doi: 10.1371/journal.pone.0119115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoh Y, Shichinohe S, Nakayama M, Igarashi M, Ishii A, Ishigaki H, Ishida H, Kitagawa N, Sasamura T, Shiohara M, Doi M, Tsuchiya H, Nakamura S, Okamatsu M, Sakoda Y, Kida H, Ogasawara K. 2015. Emergence of H7N9 influenza A virus resistant to neuraminidase inhibitors in nonhuman primates. Antimicrob Agents Chemother 59:4962–4973. doi: 10.1128/AAC.00793-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arikata M, Itoh Y, Okamatsu M, Maeda T, Shiina T, Tanaka K, Suzuki S, Nakayama M, Sakoda Y, Ishigaki H, Takada A, Ishida H, Soda K, Pham VL, Tsuchiya H, Nakamura S, Torii R, Shimizu T, Inoko H, Ohkubo I, Kida H, Ogasawara K. 2012. Memory immune responses against pandemic (H1N1) 2009 influenza virus induced by a whole particle vaccine in cynomolgus monkeys carrying Mafa-A1*052:02. PLoS One 7:e37220. doi: 10.1371/journal.pone.0037220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pham VL, Nakayama M, Itoh Y, Ishigaki H, Kitano M, Arikata M, Ishida H, Kitagawa N, Shichinohe S, Okamatsu M, Sakoda Y, Tsuchiya H, Nakamura S, Kida H, Ogasawara K. 2013. Pathogenicity of pandemic H1N1 influenza A virus in immunocompromised cynomolgus macaques. PLoS One 8:e75910. doi: 10.1371/journal.pone.0075910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiono T, Okamatsu M, Matsuno K, Haga A, Iwata R, Nguyen LT, Suzuki M, Kikutani Y, Kida H, Onuma M, Sakoda Y. 2017. Characterization of H5N6 highly pathogenic avian influenza viruses isolated from wild and captive birds in the winter season of 2016-2017 in Northern Japan. Microbiol Immunol 61:387–397. doi: 10.1111/1348-0421.12506. [DOI] [PubMed] [Google Scholar]
- 20.Okamatsu M, Ozawa M, Soda K, Takakuwa H, Haga A, Hiono T, Matsuu A, Uchida Y, Iwata R, Matsuno K, Kuwahara M, Yabuta T, Usui T, Ito H, Onuma M, Sakoda Y, Saito T, Otsuki K, Ito T, Kida H. 2017. Characterization of highly pathogenic avian influenza virus A(H5N6), Japan, November 2016. Emerg Infect Dis 23:691–695. doi: 10.3201/eid2304.161957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi WS, Jeong JH, Kwon JJ, Ahn SJ, Lloren KKS, Kwon HI, Chae HB, Hwang J, Kim MH, Kim CJ, Webby RJ, Govorkova EA, Choi YK, Baek YH, Song MS. 2018. Screening for neuraminidase inhibitor resistance markers among avian influenza viruses of the N4, N5, N6, and N8 neuraminidase subtypes. J Virol 92:e01580-17. doi: 10.1128/JVI.01580-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Jong MD, Ison MG, Monto AS, Metev H, Clark C, O’Neil B, Elder J, McCullough A, Collis P, Sheridan WP. 2014. Evaluation of intravenous peramivir for treatment of influenza in hospitalized patients. Clin Infect Dis 59:e172–e185. doi: 10.1093/cid/ciu632. [DOI] [PubMed] [Google Scholar]
- 23.Yang Z-F, Mok CKP, Peiris JSM, Zhong N-S. 2015. Human infection with a novel avian influenza A(H5N6) virus. N Engl J Med 373:487–489. doi: 10.1056/NEJMc1502983. [DOI] [PubMed] [Google Scholar]
- 24.Shieh WJ, Blau DM, Denison AM, Deleon-Carnes M, Adem P, Bhatnagar J, Sumner J, Liu L, Patel M, Batten B, Greer P, Jones T, Smith C, Bartlett J, Montague J, White E, Rollin D, Gao R, Seales C, Jost H, Metcalfe M, Goldsmith CS, Humphrey C, Schmitz A, Drew C, Paddock C, Uyeki TM, Zaki SR. 2010. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am J Pathol 177:166–175. doi: 10.2353/ajpath.2010.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liem NT, Nakajima N, Phat LP, Sato Y, Thach HN, Hung PV, San LT, Katano H, Kumasaka T, Oka T, Kawachi S, Matsushita T, Sata T, Kudo K, Suzuki K. 2008. H5N1-infected cells in lung with diffuse alveolar damage in exudative phase from a fatal case in Vietnam. Jpn J Infect Dis 61:157–160. [PubMed] [Google Scholar]
- 26.Feng Y, Hu L, Lu S, Chen Q, Zheng Y, Zeng D, Zhang J, Zhang A, Chen L, Hu Y, Zhang Z. 2015. Molecular pathology analyses of two fatal human infections of avian influenza A(H7N9) virus. J Clin Pathol 68:57–63. doi: 10.1136/jclinpath-2014-202441. [DOI] [PubMed] [Google Scholar]
- 27.Gao R, Pan M, Li X, Zou X, Zhao X, Li T, Yang H, Zou S, Bo H, Xu J, Li S, Zhang M, Li Z, Wang D, Zaki SR, Shu Y. 2016. Post-mortem findings in a patient with avian influenza A (H5N6) virus infection. Clin Microbiol Infect 22:574.e1–574.e5. doi: 10.1016/j.cmi.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Betakova T, Kostrabova A, Lachova V, Turianova L. 2017. Cytokines induced during influenza virus infection. Curr Pharm Des 23:2616–2622. doi: 10.2174/1381612823666170316123736. [DOI] [PubMed] [Google Scholar]
- 29.Van Reeth K. 2000. Cytokines in the pathogenesis of influenza. Vet Microbiol 74:109–116. doi: 10.1016/s0378-1135(00)00171-1. [DOI] [PubMed] [Google Scholar]
- 30.Kitano M, Itoh Y, Ishigaki H, Nakayama M, Ishida H, Pham VL, Arikata M, Shichinohe S, Tsuchiya H, Kitagawa N, Kobayashi M, Yoshida R, Sato A, Le QM, Kawaoka Y, Ogasawara K. 2014. Efficacy of repeated intravenous administration of peramivir against highly pathogenic avian influenza A (H5N1) virus in cynomolgus macaques. Antimicrob Agents Chemother 58:4795–4803. doi: 10.1128/AAC.02817-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha DQ, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szretter KJ, Gangappa S, Belser JA, Zeng H, Chen H, Matsuoka Y, Sambhara S, Swayne DE, Tumpey TM, Katz JM. 2009. Early control of H5N1 influenza virus replication by the type I interferon response in mice. J Virol 83:5825–5834. doi: 10.1128/JVI.02144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q, Ma J, Strayer DR, Mitchell WM, Carter WA, Ma W, Richt JA. 2014. Emergence of a novel drug resistant H7N9 influenza virus: evidence based clinical potential of a natural IFN-alpha for infection control and treatment. Expert Rev Anti Infect Ther 12:165–169. doi: 10.1586/14787210.2014.870885. [DOI] [PubMed] [Google Scholar]
- 34.Sato M, Hashimoto K, Kawasaki Y, Hosoya M. 2018. Immune response after a single intravenous peramivir administration in children with influenza. Antivir Ther 23:435–441. doi: 10.3851/IMP3222. [DOI] [PubMed] [Google Scholar]
- 35.Ilyushina NA, Govorkova EA, Russell CJ, Hoffmann E, Webster RG. 2007. Contribution of H7 haemagglutinin to amantadine resistance and infectivity of influenza virus. J Gen Virol 88:1266–1274. doi: 10.1099/vir.0.82256-0. [DOI] [PubMed] [Google Scholar]
- 36.Togo Y, Hornick RB, Felitti VJ, Kaufman ML, Dawkins AT Jr, Kilpe VE, Claghorn JL. 1970. Evaluation of therapeutic efficacy of amantadine in patients with naturally occurring A2 influenza. JAMA 211:1149–1156. doi: 10.1001/jama.1970.03170070019004. [DOI] [PubMed] [Google Scholar]
- 37.Kandeil A, Kayed A, Moatasim Y, Webby RJ, McKenzie PP, Kayali G, Ali MA. 2017. Genetic characterization of highly pathogenic avian influenza A H5N8 viruses isolated from wild birds in Egypt. J Gen Virol 98:1573–1586. doi: 10.1099/jgv.0.000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levite M. 2016. Dopamine and T cells: dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol (Oxf) 216:42–89. doi: 10.1111/apha.12476. [DOI] [PubMed] [Google Scholar]
- 39.Kocks JR, Davalos-Misslitz ACM, Hintzen G, Ohl L, Förster R. 2007. Regulatory T cells interfere with the development of bronchus-associated lymphoid tissue. J Exp Med 204:723–734. doi: 10.1084/jem.20061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitano M, Itoh Y, Kodama M, Ishigaki H, Nakayama M, Nagata T, Ishida H, Tsuchiya H, Torii R, Baba K, Yoshida R, Sato A, Ogasawara K. 2010. Establishment of a cynomolgus macaque model of influenza B virus infection. Virology 407:178–184. doi: 10.1016/j.virol.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF Jr, Wynn TA, Gause WC. 2012. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med 18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.