Chagas disease, caused by the protozoan Trypanosoma cruzi, is one of the main causes of death due to cardiomyopathy and heart failure in Latin American countries. The treatment of Chagas disease is directed at eliminating the parasite, decreasing the probability of cardiomyopathy and disrupting the disease transmission cycle. Benznidazole (BZ) and nifurtimox (Nfx) are recognized as effective drugs for the treatment of Chagas disease by the World Health Organization, but both have high toxicity and limited efficacy, especially in the chronic disease phase.
KEYWORDS: Chagas disease, benznidazole, aspirin, therapy, cardioprotective effects, lipoxin
ABSTRACT
Chagas disease, caused by the protozoan Trypanosoma cruzi, is one of the main causes of death due to cardiomyopathy and heart failure in Latin American countries. The treatment of Chagas disease is directed at eliminating the parasite, decreasing the probability of cardiomyopathy and disrupting the disease transmission cycle. Benznidazole (BZ) and nifurtimox (Nfx) are recognized as effective drugs for the treatment of Chagas disease by the World Health Organization, but both have high toxicity and limited efficacy, especially in the chronic disease phase. At low doses, aspirin (ASA) has been reported to protect against T. cruzi infection. We evaluated the effectiveness of BZ in combination with ASA at low doses during the acute disease phase and evaluated cardiovascular aspects and cardiac lesions in the chronic phase. ASA treatment prevented the cardiovascular dysfunction (hypertension and tachycardia) and typical cardiac lesions. Moreover, BZ+ASA-treated mice had a smaller cardiac fibrotic area than BZ-treated mice. These results were associated with an increase in numbers of eosinophils and reticulocytes and levels of nitric oxide in the plasma and cardiac tissue of ASA-treated mice relative to respective controls. These effects of ASA and BZ+ASA in chronically infected mice were inhibited by pretreatment with the lipoxin A4 (LXA4) receptor antagonist Boc-2, indicating that the protective effects of ASA are mediated by ASA-triggered lipoxin. These results emphasize the importance of exploring new drug combinations for treatments of the acute phase of Chagas disease that are beneficial for patients with chronic disease.
INTRODUCTION
Chagas disease, caused by the protozoan Trypanosoma cruzi, is the main cause of death due to cardiomyopathy and heart failure in Latin American countries (1). Currently, the treatment for Chagas disease involves the administration of benznidazole (BZ) or nifurtimox (Nfx) (2). Both are very toxic and have limited efficacy, especially in the chronic phase of the disease (3). Benznidazole therapy during the acute phase of Chagas disease reduces the parasite load but does not prevent chronic cardiac lesions (4). Recently, a new strategy of combination drug therapy has emerged to improve BZ-based treatment (5–7). This approach could improve BZ treatment efficacy and decrease drug resistance and toxicity (8, 9).
Studies have shown that eicosanoids produced during acute infection may act as immunomodulators that aid in the transition to and maintenance of the chronic phase of Chagas disease (10). Treatment with aspirin (ASA), which is a nonselective and irreversible inhibitor of cyclooxygenases COX-1 and COX-2, before T. cruzi infection (11) or during the chronic phase of Chagas disease (12, 13) alters the natural course of the disease, which suggests that ASA administration could serve as a new therapeutic approach for Chagas disease (14–18).
However, to the best of our knowledge, there are no experimental studies on the combination of ASA and BZ in vivo. Therefore, in this study, we evaluated the efficacy of the combination of BZ and ASA in mice chronically infected with a partially BZ-resistant (Y) T. cruzi strain to further support the clinical evaluation of combination therapies in the early stage of T. cruzi infection.
RESULTS
Infection course and therapy response in T. cruzi-infected mice.
The experimental design is presented in Fig. 1. Infected untreated (I-NT) mice presented high parasitemia, which peaked at 9 days postinfection (dpi) (Fig. 2A). As expected, BZ treatment reduced parasitemia, whereas ASA reduced the load of parasites in the blood after 9 days, and parasitemia levels among the ASA, BZ-alone, and BZ+ASA treatment groups and the NT group were not significantly different at 13 to 31 dpi (Fig. 2A). Interestingly, the peak parasitemia level in mice treated with BZ or BZ+ASA (compared with that in the I-NT mice) was consistently lower than that in mice treated with ASA alone, confirming the efficiency of BZ therapy in the acute phase of infection. All mice treated with BZ alone or in combination with ASA survived for 31 days after infection and chronification. Mice that were untreated and those treated with ASA alone exhibited 76% and 84% survival rates during the acute phase, respectively (Fig. 2C). Parasites were not detected in the blood of untreated or treated chronically infected mice (data not shown).
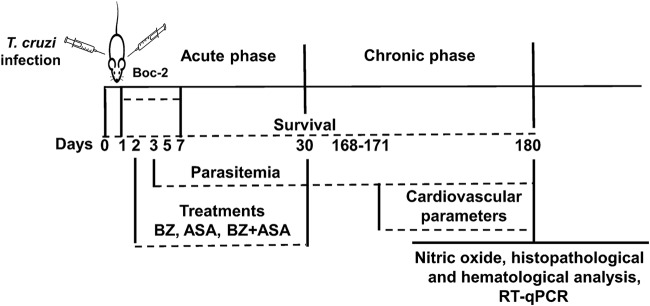
FIG 1.
Experimental design. BALB/c mice were treated daily with 25 mg/kg benznidazole (BZ), aspirin (ASA), or a combination of BZ and ASA by oral gavage at 2 days postinfection for 30 days. The antagonist Boc-2 (10 μM/kg) was intraperitoneally injected from 1 day postinfection every 48 h for 7 days before ASA treatment. Parasitemia during the acute phase was monitored by counting blood-borne trypomastigotes. Survival was observed daily, until the end of the experiment. Cardiovascular parameters (heart rate [HR] and mean arterial pressures [MAP]) were measured using CODA (a mouse tail cuff blood pressure system) between 171 and 180 days postinfection. At 180 days postinfection, nitrite levels in the plasma and cardiac tissue were quantified. All blood analyses and cell counts were performed using standard methods. Parasite loads in the hearts of treated and untreated mice were quantified by RT-qPCR.
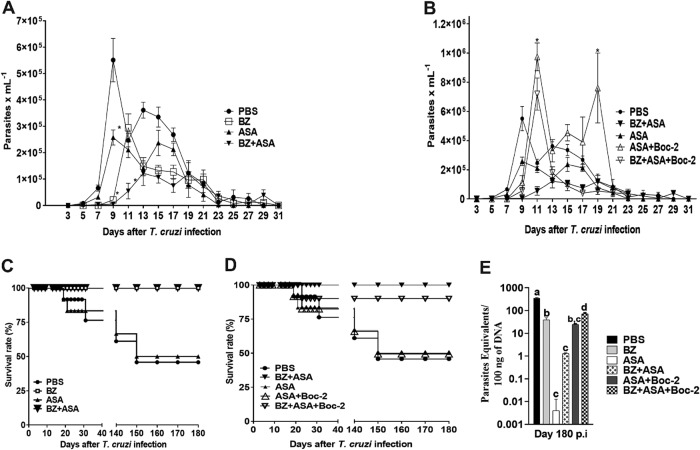
FIG 2.
Infection course and response to treatment in BALB/c mice infected with 500 trypomastigotes of T. cruzi. The mice were treated daily with 25 mg/kg benznidazole (BZ), aspirin (ASA), or a combination of BZ and ASA by oral gavage at 2 days postinfection for 30 days. The antagonist Boc-2 (10 μM/kg) was intraperitoneally injected from 1 day postinfection every 48 h for 7 days before ASA treatment. At 180 days postinfection, T. cruzi parasite loads in the hearts of treated and untreated mice were quantified by RT-qPCR. (A and B) Parasitemia levels in the acute phase of infection; (C and D) mouse survival rates; (E) cardiac parasite loads measured by RT-qPCR. *, P < 0.05 compared to the untreated infected group (PBS). Means with different letters are significantly different (P < 0.05, two-way ANOVA with Dunnett’s multiple-comparison posttest).
The levels of parasite DNA (parasite equivalents per 100 ng of total DNA) in hearts of T. cruzi-infected mice are shown in Fig. 2E. By day 180 postinfection (p.i.), the group of infected mice treated with BZ presented a reduction in the parasite load in the cardiac tissue compared to the untreated group (38.94 ± 9.57 versus 355.8 ± 17.4; Dunnett’s multiple-comparison test, P < 0.05). The I-BZ+ASA group showed a significant reduction in cardiac parasite burden shown by real-time quantitative PCR (RT-qPCR) compared with both the I-NT and I-BZ groups (1.28 ± 0.1 versus 355.8 ± 17.4 and 38.94 ± 9.57, respectively; Dunnett’s multiple-comparison test, P < 0.05). Surprisingly, infected mice treated with ASA showed the lowest parasite load in cardiac tissue compared to the untreated group (0.004 ± 0.008 versus 355.8 ± 17.4, respectively) and the BZ treatment group but were not statistically distinguishable from the BZ+ASA group (Fig. 2E).
We treated the mice with a lipoxin A4 (LXA4) receptor antagonist (Boc-2) from 1 day postinfection every 48 h for 7 days before initiating the ASA treatment (Fig. 1). Then, the effects of ASA+Boc-2 and BZ+ASA+Boc-2 treatments were assessed by comparing these mice with those without Boc-2 pretreatment. We found that the protective effects of ASA and BZ+ASA (Fig. 2A and B) in infected mice were inhibited by Boc-2 pretreatment, which was confirmed by an increase in parasitemia (Fig. 2C), a decrease in mouse survival rate (Fig. 2D), and an increase in cardiac parasitism (Fig. 2E) (0.004 ± 0.008 and 1.28 ± 0.1 versus 24.58 ± 1.66 and 73.2 ± 6.73, respectively; Dunnett’s multiple-comparison test, P < 0.05).
Cardiovascular and hematological parameters in mice chronically infected with T. cruzi in response to combination therapy using benznidazole and aspirin during the acute phase of experimental Chagas disease.
The I-NT mice (n = 6 mice/group) exhibited tachycardia (Table 1) compared with uninfected (NI) mice (I-NT [539 ± 1 beats per min {bpm}] versus NI [503 ± 1 bpm], P < 0.0001). Benznidazole treatment increased the heart rate (HR) in NI mice (Table 1) compared with that in the NI mice not treated with BZ (NI-BZ [538 ± 3 bpm] versus NI [503 ± 1 bpm], P < 0.001). However, neither ASA alone nor BZ+ASA changed the HR in these mice (Table 1). Treatment of infected mice with BZ increased the HR compared with that in NT and infected mice (I-BZ [602 ± 6 bpm] versus I-NT [539 ± 1 bpm], P < 0.0001). Aspirin treatment reduced the HR in infected mice compared with that in the I-NT (I-ASA [490 ± 3 bpm] versus I-NT [539 ± 1 bpm], P < 0.0001) and I-BZ (I-ASA [490 ± 3 bpm] versus I-BZ [602 ± 6 bpm], P < 0.0001) groups. The BZ+ASA treatment exerted an intermediate effect with respect to increasing the HR in infected mice, which was similar to that in the I-NT group (Table 1).
TABLE 1.
HR and MAP in mice chronically infected with T. cruzi in response to treatment with BZ or combination treatment with ASA in the acute phase of infection or left untreateda
| Exptl group | HR (bpm) | MAP (mm Hg) |
|---|---|---|
| NI | 500 ± 1.4 A | 103 ± 0.6 A |
| NI-BZ | 538 ± 3.2 B | 103 ± 0.6 A |
| NI-ASA | 505 ± 1.1 A | 94 ± 0.5 B |
| NI-BZ+ASA | 504 ± 2.4 A | 96 ± 0.2 B,C |
| I-NT | 539 ± 1.4 B | 117 ± 0.5 D |
| I-BZ | 602 ± 5.8 D | 115 ± 0.4 D |
| I-ASA | 490 ± 2.5 A | 103 ± 0.2 A |
| I-BZ+ASA | 537 ± 4.1 B | 100 ± 0.9 A,C |
| I-ASA+Boc-2 | 544 ± 5.2 B | 123 ± 0.8 E |
| I-BZ+ASA+Boc-2 | 538 ± 3.1 B | 119 ± 0.8 D,E |
Mice were treated daily with 25 mg/kg benznidazole (BZ), aspirin (ASA), or a combination of BZ and ASA by oral gavage at 2 days postinfection for 30 days. The antagonist Boc-2 (10 μM/kg) was intraperitoneally injected from 1 day postinfection every 48 h for 7 days before ASA treatment. Heart rate (HR) and mean arterial pressure (MAP) were measured using CODA (a mouse tail cuff blood pressure system) at 180 days postinfection. Values are means ± standard errors of the means. Values followed by the same letter are not significantly different (P < 0.05; two-way ANOVA with Tukey posttest). NI, uninfected group; I, infected group.
The mean arterial pressure (MAP) in the I-NT mice was increased compared with that in the NI mice (117 ± 1 mm Hg versus 103 ± 1 mm Hg, P < 0.0001) (Table 1), and treatment with BZ did not significantly change the MAP (103 ± 1 mm Hg versus 103 ± 1 mm Hg, P > 0.9999). In contrast, treatment of NI mice with ASA alone or BZ+ASA decreased the MAP compared with that in the NI and NI-BZ mice (NI-ASA [95 ± 0.375 mm Hg] versus NI [103 ± 1 mm Hg], P < 0.0001; NI-ASA versus NI-BZ [103 ± 1 mm Hg], P < 0.001; NI-BZ+ASA [96 ± 2 mm Hg] versus NI, P < 0.0001; NI-BZ+ASA versus NI-BZ, P < 0.01) (Table 1). Treatment of infected mice with BZ did not alter the MAP compared with that in the I-NT mice (116 ± 0.213 mm Hg versus 117 ± 1 mm Hg, P > 0.05); however, ASA or BZ+ASA treatment of infected mice decreased the MAP compared with that in the I-NT or I-BZ mice (P < 0.0001 for I-ASA [104 ± 0.222 mm Hg] versus I-NT [117 ± 1 mm Hg], I-ASA versus I-BZ [116 ± 0.213 mm Hg], I-BZ+ASA [100 ± 1 mm Hg] versus I-NT, and I-BZ+ASA versus I-BZ) (Table 1).
Infection and treatment significantly affected eosinophils and neutrophils, as indicated by a significant increase in these cell populations (P < 0.05) at 180 dpi in BZ- and BZ+ASA-treated mice compared with that in the NI mice (Table 2). Surprisingly, we observed a significant increase in reticulocytes (P < 0.001) and eosinophils (P < 0.001) but a reduction in neutrophils in the I-ASA mice (Table 2). No significant differences were observed in the hematocrit level, platelet count, or leukocyte count at 180 dpi (data not shown).
TABLE 2.
Hematological parameters in mice chronically infected with T. cruzi and treated with BZ or BZ+ASA in the acute phase of infection or left untreateda
| Exptl group | Reticulocytes (%) | Eosinophils (μl) | Neutrophils (mm3) |
|---|---|---|---|
| NI | 7 ± 0.2 A | 68 ± 6.1 A | 1,673 ± 87.6 A |
| I-NT | 9 ± 0.4 A | 138 ± 4.5 B | 2,715 ± 146.2 B |
| I-BZ | 9 ± 0.4 A | 134 ± 0.9 B | 2,684 ± 309.7 B |
| I-ASA | 13 ± 0.9 B | 200 ± 5.4 C | 1,497 ± 135.4 A |
| I-BZ+ASA | 10 ± 0.9 A | 149 ± 22.6 B | 1,760 ± 24.8 A,C |
| I-ASA+Boc-2 | 8 ± 0.5 A | 95 ± 31.5 A,B | 2,530 ± 170.2 B,C |
| I-BZ+ASA+Boc-2 | 9 ± 0.4 A | 130 ± 16.3 B | 2,763 ± 244.6 B |
Mice were treated daily with 25 mg/kg benznidazole (BZ), aspirin (ASA), or a combination of BZ and ASA by oral gavage at 2 days postinfection for 30 days. The antagonist Boc-2 (10 μM/kg) was intraperitoneally injected from 1 day postinfection every 48 h for 7 days before ASA treatment. Heart rate (HR) and mean arterial pressure (MAP) were measured using CODA (a mouse tail cuff blood pressure system) at 180 days postinfection. Values are means ± standard errors of the means. Means followed by the same letter are not significantly different (P < 0.05; two-way ANOVA with Tukey posttest). NI, uninfected group; I, infected group.
Next, we evaluated the cardiovascular and hematological parameters in infected mice treated with Boc-2 before monotherapy or combination therapy. As shown in Table 1, the HR in the I-ASA mice decreased relative to that of the I-NT mice (I-ASA [490 ± 3 bpm] versus I-NT [539 ± 1 bpm], P < 0.0001).
This effect was inhibited by Boc-2 pretreatment of mice (I-ASA [490 ± 3 bpm] versus I-ASA+Boc-2 [544 ± 5 bpm], P < 0.0001) (Table 1). The ASA+BZ, ASA+Boc-2, and ASA+BZ+Boc-2 combination treatments did not change the HR compared with that of I-NT mice (Table 1). The MAP was elevated in I-NT mice and decreased by treatment with ASA alone (117 ± 1 mm Hg versus 104 ± 0.2 mm Hg, P < 0.01) or BZ+ASA (117 ± 1 mm Hg versus100 ± 1 mm Hg, P < 0.05) (Table 1). Notably, Boc-2 pretreatment reversed the hypotensive effect of ASA alone and BZ+ASA (Table 1).
Hematological evaluation revealed that pretreatment with Boc-2 did not alter the platelet and leukocyte counts or the hematocrit level (data not shown), but it reversed the increase in reticulocytes (Table 2) and eosinophils provoked by ASA (Table 2). Furthermore, it reduced the effect of ASA on neutrophil count (Table 2).
Cardioprotective effects and nitrite level in the chronic phase in response to therapy.
The effects of the combination treatment in infected mice were evaluated and compared with those in infected mice treated with BZ or ASA alone and NI mice. The fibrotic areas in the hearts of chronically infected mice were larger than those in NI mice (Fig. 3). Benznidazole treatment during the acute phase reduced the fibrotic area in chronically infected mice, whereas ASA treatment decreased typical cardiac tissue injuries (extensive destruction of muscle fibers followed by fibrosis and adipose replacement) that are associated with chronic T. cruzi infection, which were similar to those in the heart muscle of NI mice. Moreover, the BZ+ASA-treated mice had smaller fibrotic areas than the BZ-treated mice.
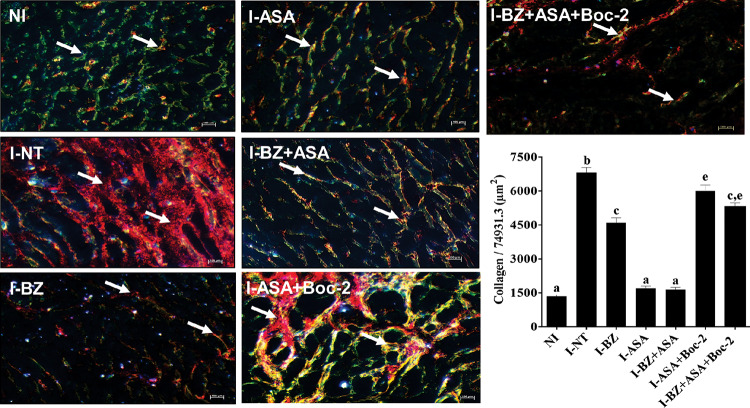
FIG 3.
Effect of combination treatment with benznidazole plus aspirin (BZ+ASA) on chronic cardiac lesions. BALB/c mice were inoculated with 500 trypomastigotes of T. cruzi by intraperitoneal injection, treated with daily doses of 25 mg/kg BZ or ASA alone or in combination for 30 consecutive days, and euthanized at 180 days postinfection. The antagonist Boc-2 (10 μM/kg) was administered via intraperitoneal injection from 1 day postinfection every 48 h for 7 days before ASA treatment. After 180 days postinjection, the mice were euthanized. Uninfected control (NI) mice were also evaluated. Fibrotic areas in the heart muscles of treated and uninfected mice can be observed in histological sections of the hearts imaged at a magnification of ×40 that are representative of picrosirius red staining patterns. I-NT, infected and untreated; I-BZ, infected and treated with BZ; I-ASA, infected and treated with ASA; I-BZ+ASA, infected and treated with BZ+ASA. Polarized-light images show the fibrotic areas (arrows) in yellow-orange (type I collagen) and green (type III collagen). In the graph, means with different letters are significantly different (P < 0.05; two-way ANOVA with Tukey posttest).
At 180 dpi, hearts of infected mice treated previously with Boc-2 showed more fibrotic areas than those of mice treated only with ASA (Fig. 3). Thus, we determined that the intensity of heart damage was consistent with increased levels of neutrophils (Table 2).
As expected, T. cruzi infection triggered an elevation in circulating nitric oxide (NO) (Fig. 4A) and NO in heart tissue (Fig. 4B). Notably, compared to the untreated infected mice, BZ-treated mice were not statistically distinguishable in terms of both plasma and cardiac tissue (Fig. 4A and B) (P > 0.05).
FIG 4.
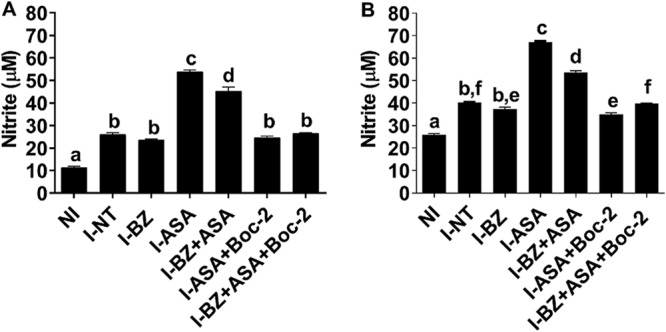
Nitrite level in response to treatment with benznidazole (BZ) or combined treatment with aspirin (ASA) in mice infected with T. cruzi. The antagonist Boc-2 (10 μM/kg) was administered via intraperitoneal injection from 1 day postinfection every 48 h for 7 days before ASA treatment. After 180 days postinjection, the mice were euthanized. Uninfected control (NI) mice were also evaluated. NO was determined by measuring nitrite levels in plasma (A) and cardiac tissue (B) using the cadmium-copper system followed by the Griess reaction. The results are expressed as means ± standard errors of the means from 5 animals per group and are representative of 2 independent experiments. Means not sharing a letter are significantly different (P < 0.05; 2-way ANOVA with Tukey posttest).
On the other hand, a significant increase in circulating NO in cardiac tissue was observed in ASA-treated mice compared to the untreated infected and BZ-treated infected mice (Fig. 4A and B) (P < 0.05). Similarly, a significant increase of NO was observed in plasma and hearts of BZ+ASA treated mice compared to untreated infected and BZ-treated infected mice. Moreover, Boc-2 diminished NO levels in plasma and cardiac tissue when it was combined with ASA or BZ (Fig. 4A and B, respectively).
DISCUSSION
Treatment of chronic T. cruzi infection is challenging because of several limitations of the available drugs, such as BZ and Nfx, including low efficacy and toxicity. Recent studies have shown that new treatment strategies, including combinations of drugs with different mechanisms of action, may improve therapeutic efficacy and reduce adverse effects (6–8, 19–22). Moreover, T. cruzi infection is restricted to selected tissues, where very low levels of intracellular parasitic forms hinder the direct action of BZ on the parasite (23, 24).
The results of the present study showed that the administration of 25 mg BZ/kg of body weight/day during the acute disease phase was effective in alleviating the symptoms of T. cruzi infection in susceptible mice (7, 25). This was reflected in the rate of survival, decrease in parasitemia during the acute phase, and reduction of cardiac parasitism and heart damage in chronically infected mice compared to the untreated group after monotherapy with BZ. Cardiovascular parameters such as HR and MAP in Swiss mice were described previously, when we investigated the effect of treadmill training on the acute phase of T. cruzi infection (26). In the present study, our data show that in the chronic phase, the animals presented hypertension and tachycardia compared to the uninfected animals and that treatment during the acute phase, specifically with BZ, elevated the heart rate and did not change the high blood pressure found in the animals.
Treatment with BZ did not alter reticulocyte, eosinophil, and neutrophil counts and plasma and cardiac nitrite levels compared with those in the NI mice. However, we observed a decrease in parasite load in the cardiac tissue in mice treated with BZ. To the best of our knowledge, this is the first study to evaluate hematological and cardiovascular parameters such as HR and MAP in chronically infected BALB/c mice treated with BZ, and the results reinforce the toxicity and efficacy of BZ (24).
The acute phase of Chagas disease is characterized by immunosuppression induced by T. cruzi parasites to evade the host immune response. This immunosuppressive state is mediated in part by prostaglandins (27, 28), NO, and cytokines (27, 29). In fact, increased circulating levels of prostaglandin E2 (PGE2), thromboxane A2 (TXA2), and prostaglandin F2a (PGF2a) have been observed in mice infected with T. cruzi (30), and during the acute phase, macrophages and spleen cells from T. cruzi-infected mice produce high levels of PGE2 and NO (27, 31). Thus, the inhibition of the host cyclooxygenase (COX) emerged naturally as a possible therapeutic in Chagas disease (10, 14–16). It has been previously reported that COX-2 acetylation by ASA modifies its activity, promoting the synthesis of 15-epi-LXA4, a lipid involved in the resolution of inflammation acting as an anti-inflammatory molecule in the acute phase of T. cruzi infection (25). Therefore, we determined whether the effects observed in our experimental model were mediated by LXA4.
In the present study, the administration of ASA at a low dose (25 mg/kg) in the acute phase of infection decreased cardiac parasitism and heart damage in chronically infected mice compared with I-NT or BZ-treated mice. However, the ASA group could not be distinguished from the BZ+ASA group. In addition, ASA treatment decreased the HR and hypertension in infected animals. These findings help clarify the relationship between the chagasic heart and cardiovascular functions.
In the present study, in mice treated with ASA, there was an increase in the percentage of reticulocytes. This can be partially explained by the upregulation of nitric oxide production and the consequent increase in oxidative stress during bone marrow activity (32). The increase in eosinophil count with ASA treatment further explains the decrease in parasites in the hearts of chronically infected mice (33–35).
We also found that ASA exhibited a therapeutic effect at 25 mg/kg/day and that this effect was inhibited by pretreatment with Boc-2. Moreover, the number of neutrophils increased with Boc-2 pretreatment, which was associated with higher fibrosis in the cardiac tissue. In fact, neutrophils may regulate T. cruzi experimental infection and determine susceptibility and resistance to infection (36). Furthermore, neutrophils and monocytes contribute to inflammation and fibrosis associated with Chagas cardiomyopathy via the production of metalloproteinases and cytokines, respectively (37). This phenomenon possibly correlates with the increased level of 15-epi-LXA4—known as “aspirin-triggered lipoxin”—upon treatment with low-dose ASA, which is consistent with the results of Molina-Berríos et al. (25). In addition, studies about BZ treatment using lower doses in prevention of heart lesions have shown contradictory results (38–40). BZ therapy with optimal doses (100 mg/kg) during the acute phase of Chagas disease reduced parasite load but did not prevent chronic cardiac lesions (4). Combinations of benznidazole and other drugs at suboptimal doses have efficacy equivalent or superior to that of the drugs given at their optimal doses (6, 21). Our data agree with these reports in that treatment of infected animals with BZ using lower doses (25 mg/kg) than those indicated above are unable to induce complete parasitological cures or provide full cardiac protection. In fact, BZ chemotherapy does not always ensure a better long-term prognosis in patients with Chagas cardiomyopathy, and heart morphofunctional deterioration may progress as it does in untreated patients (41).
In summary, our results demonstrate that it is possible to achieve the same or better therapeutic effect using lower dosages of benznidazole and aspirin in the acute phase of Chagas disease and could be crucial for defining the course of pathological process in T. cruzi infection, which might help predict future damage to the heart in chronically infected animals. Since both drugs are commercially available, their use in combination should be considered for evaluation in the treatment of Chagas disease patients, aiming to reduce the potential toxicity of benznidazole.
MATERIALS AND METHODS
Ethics statement.
Animal handling and experimental procedures were carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals proposed by the Brazilian National Council of Animal Experimentation (COBEA) (52). The study protocol was approved by the Internal Scientific Commission and the Ethics in Animal Experimentation Committee of Londrina State University (CEUA approval number 4628.2016.40). Every effort was made to minimize pain and suffering to the animals used in these experiments.
Animals.
Six- to 8-week-old, 20- to 25-g male BALB/c mice were purchased from the Multidisciplinary Center for Biological Research, University of Campinas (Campinas, Brazil). Swiss male mice were obtained from breeding colonies in the animal facility of the Center for Biological Sciences, Londrina State University (Paraná, Brazil). The mice were maintained in an animal house in the Department of Pathological Sciences, Center for Biological Sciences, State University of Londrina, with a controlled environment temperature (21 to 23°C) and a 12-h day-night cycle. The mice were fed a commercial rodent diet (Nuvilab-CR1; Quimtia-Nuvital, Colombo, Paraná, Brazil) and sterilized water ad libitum. All the animals were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and euthanized by cervical dislocation.
Compounds.
Benznidazole (N-benzyl-2-nitro-1H-imidazole-1-acetamide) tablets were purchased from Laboratório Farmacêutico do Estado de Pernambuco (LAFEPE, Brazil). ASA and Direct Red 80 were purchased from Sigma (St. Louis, MO, USA). Boc-2 was purchased from MP Biomedicals (Solon, OH, USA). Benznidazole tablets were crushed and suspended in distilled water, whereas ASA was directly dissolved in dimethyl sulfoxide (DMSO; Labsynth, Diadema, São Paulo, Brazil) at a final DMSO concentration of less than 0.5% (vol/vol). Boc-2 was suspended in sterile saline.
Parasites and infection protocol.
Trypanosoma cruzi strain Y of the T. cruzi I lineage (42) was maintained by weekly intraperitoneal (i.p.) inoculation of blood trypomastigotes into Swiss mice. When required for analysis, parasite-rich blood was collected from these inoculated Swiss mice and appropriately diluted with phosphate-buffered saline (PBS; pH 7.2). BALB/c mice were infected via an i.p. injection of 500 blood-derived trypomastigotes. The control group was injected with the same volume of sterile PBS (pH 7.2). Parasitemia was monitored by counting blood-borne trypomastigotes in 5 μl of fresh blood collected from the tail vein (43). At 3 days postinfection (dpi), direct microscopic visualization of circulating trypomastigotes in the peripheral blood of mice confirmed T. cruzi infection. Survival was observed daily until the end of the experiment (Fig. 1).
Treatment schemes.
The mice were divided into the following 10 groups: uninfected (NI), uninfected and treated with BZ (NI-BZ), uninfected and treated with ASA (NI-ASA), uninfected and treated with BZ+ASA (NI-BZ+ASA), infected and untreated (I-NT), infected and treated with BZ (I-BZ), infected and treated with ASA (I-ASA), infected and treated with BZ and ASA (I-BZ+ASA), infected and treated with ASA and Boc-2 (I-ASA+Boc-2), and infected and treated with BZ, ASA, and Boc-2 (I-BZ+ASA+Boc-2). In all assays, only mice with positive parasitemia were used in the infected groups.
The treatments with BZ, ASA, and the combination were initiated at 2 dpi (25); six mice per treatment group were administered 25 mg/kg BZ, ASA, or a combination of BZ and ASA by oral gavage once per day for 30 days. The LXA4 receptor antagonist Boc-2 (10 μM/kg/mouse) (16, 44) was injected i.p. at 1 dpi every 48 h for 7 days before aspirin-containing therapies (Fig. 1). The drugs were prepared as described above, and each mouse was administered 0.1 ml of each drug. Control groups received PBS following the same regimen as animals receiving other treatments.
Noninvasive cardiovascular measurements.
Heart rate (HR) and mean arterial blood pressure (MAP) of conscious mice in all treatment groups were measured using the CODA mouse and rat tail cuff blood pressure system (Kent Scientific Co., CT, USA). The measurements were taken at 171, 173, 175, 177, and 180 dpi (Fig. 1), and the mean value was calculated for each mouse (26).
Hematological analysis.
Whole blood was collected from mice under ketamine (100 mg/kg) and xylazine (10 mg/kg) anesthesia using intracardiac puncture and aliquoted into tubes containing 1 mg/ml sodium EDTA as the anticoagulant. Platelets, leukocytes, reticulocytes, eosinophils, and neutrophils were counted using standard methods (45–47). Hematocrits were determined by spinning down capillary tubes filled with blood using a microcentrifuge (Novatecnica, Piracicaba, São Paulo, Brazil). The total number of nucleated cells collected was determined by manual hemocytometer count. All blood analyses and cell counts were performed at 180 dpi.
Quantification of nitrite levels in plasma and cardiac tissue.
At 180 dpi, the anesthetized mice were euthanized, and the heart tissue was sampled for immune and histopathological assays. The heart was halved; one half was fixed in 10% formaldehyde prepared in 0.1 M PBS (pH 7.3) for histopathological analysis, and the other half was used for determination of nitrite levels and quantification of T. cruzi parasite load by qPCR. Nitrite levels in the plasma and cardiac tissue were determined using the cadmium-copper system followed by the Griess reaction, as described previously (48) with some modifications proposed by Panis et al. (49).
RT-qPCR.
Real-time quantitative PCR (RT-qPCR) was performed to determine the tissue parasite burden in the control and T. cruzi-infected and treated mice. The heart tissue was weighed and washed with PBS. Genomic DNA was isolated using a lysis buffer (50 mM Tris-HCl [pH 7.6], 10 mM EDTA, 0.5% sodium dodecyl sulfate [SDS], 0.2 mg/ml proteinase K [Invitrogen, Carlsbad, CA]), followed by phenol-chloroform extraction. The samples were mechanically homogenized (Ultra Stirrer; Scientific SDN BHD, Seri Kembangan, Malaysia), heated at 55°C for 12 h, and extracted twice with phenol-chloroform-isoamyl alcohol solution (25:24:1). Cold absolute ethanol (Merck; twice the volume of the aqueous phase) was added to the extracted sample, which was stored at −20°C for 12 h. Thereafter, the sample was centrifuged for 10 min at 10,000 × g, washed with 70% ethanol, dried at room temperature, and resuspended in ultrapure water. Total DNA concentration was determined using a spectrophotometer (Synergy HT; Biotek, USA). The RT-qPCR assay was performed on a Rotor-Gene Q 5-Plex apparatus (Qiagen, Germany) using a 20-μl reaction mixture containing 100 ng total DNA, 1 μM concentrations of the oligonucleotides TFZ-F (5′-GCTCTTGCCCACAMGGGTGC-3′) and TFZ-R (5′-CCAAGCGGATAGTTCAGG-3′) (50), and Platinum SYBR green qPCR Super Mix UDG with ROX reagent (Invitrogen Corporation, New York, USA), according to the manufacturer’s recommendations. The amplification conditions were as follows: an initial step at 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 30 s, 57°C for 30 s, and 72°C for 30 s, followed by dissociation curve construction at 60 to 95°C (0.5°C/s). The cardiac tissue parasite burden was calculated using a standard curve constructed with DNA obtained from culture samples of T. cruzi epimastigotes (51). The parasite load was expressed as parasite equivalents/100 ng of total DNA obtained from cardiac tissue.
Polarized microscopy of picrosirius red-stained collagen.
To examine cardiac fibrosis, the heart tissue samples were fixed with 10% buffered formalin solution, dehydrated, cleared, and embedded in Paraplast (Sigma, St. Louis, MO, USA). The embedded tissue blocks were cut into 3-μm-thick sections and stained with Direct Red 80 (Sigma) to visualize collagen fibers, and sections were chosen at random and evaluated at a magnification of ×40, resulting in the analysis of a total area of 74,931 μm2 of myocardium (19). Polarized light images were obtained using an Axio Zeiss photon microscope (Oberkochen, Germany). The fibrotic area was defined in the polarized light images considering the sum of areas occupied by types I (yellow-orange) and III (green) collagen using Image-Pro Plus 6.0 (Media Cybernetics, Silver Spring, USA).
Statistical analysis.
Data were assessed using analysis of variance (ANOVA), and differences between mean values were determined using Tukey or Dunnett’s multiple-comparison posttests. A log-rank (Mantel-Cox) test was used to compare the survival curves. Differences were considered significant at a P value of <0.05. Statistical analyses were performed using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA).
ACKNOWLEDGMENTS
We thank Andréia Carla Eugénio Pupim for technical support in histological analyses.
This study was supported by grants from Fundação Araucária (Chamada de projeto 09/2016 Programa Institucional de Pesquisa Básica e Aplicada, Conv. 001/2017, Protocolo 47.396, SIT. 31675) and by Conselho Nacional Desenvolvimento Científico e Tecnológico (CNPq), CAPES. P.P.-F., M.C.M.-P., M.I.L.-M., W.A.V., L.M.Y., and S.F.Y.-O. are research fellows of CNPq. B.F.C.L. and E.R.T. are research fellows of CAPES. R.S.P. was supported by Ministério da Ciência e Tecnologia, Ensino Superior e Técnico Profissional de Moçambique.
Conception and design: P.P.-F., M.C.M.-P., R.S.P., and A.D.M.; acquisition of data: R.S.P., A.D.M., M.I.L.-M., B.F.C.L., J.P.S., E.R.T., E.J.D.A.A., L.M.Y., and S.F.Y.-O.; analysis of data: P.P.F., M.C.M.-P., R.S.P., B.F.C.L., E.R.T., and E.J.D.A.A.; materials and reagents: W.A.V., P.P.F., S.F.Y.-O., L.M.Y., and M.C.M.-P.; drafting or revising and final approval of the manuscript: P.P.F., M.C.M.-P., R.S.P., A.D.M., W.A.V., E.J.D.A.A., S.F.Y.-O., and L.M.Y.
REFERENCES
- 1.Garcia S, Ramos CO, Senra JF, Vilas-Boas F, Rodrigues MM, Campos-de-Carvalho AC, Ribeiro-Dos-Santos R, Soares MB. 2005. Treatment with benznidazole during the chronic phase of experimental Chagas’ disease decreases cardiac alterations. Antimicrob Agents Chemother 49:1521–1528. doi: 10.1128/AAC.49.4.1521-1528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva ACC, Brelaz-de-Castro MCA, Leite ACL, Pereira VRA, Hernandes MZ. 2019. Chagas disease treatment and rational drug discovery: a challenge that remains. Front Pharmacol 10:873. doi: 10.3389/fphar.2019.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morilla MJ, Romero EL. 2015. Nanomedicines against Chagas disease: an update on therapeutics, prophylaxis and diagnosis. Nanomedicine (Lond) 10:465–481. doi: 10.2217/nnm.14.185. [DOI] [PubMed] [Google Scholar]
- 4.Caldas IS, Talvani A, Caldas S, Carneiro CM, de Lana M, da Matta Guedes PM, Bahia MT. 2008. Benznidazole therapy during acute phase of Chagas disease reduces parasite load but does not prevent chronic cardiac lesions. Parasitol Res 103:413–421. doi: 10.1007/s00436-008-0992-6. [DOI] [PubMed] [Google Scholar]
- 5.Puente V, Demaria A, Frank FM, Batlle A, Lombardo ME. 2018. Anti-parasitic effect of vitamin C alone and in combination with benznidazole against Trypanosoma cruzi. PLoS Negl Trop Dis 12:e0006764. doi: 10.1371/journal.pntd.0006764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diniz LDF, Urbina JA, de Andrade IM, Mazzeti AL, Martins TAF, Caldas IS, Talvani A, Ribeiro I, Bahia MT. 2013. Benznidazole and posaconazole in experimental Chagas disease: positive interaction in concomitant and sequential treatments. PLoS Negl Trop Dis 7:e2367. doi: 10.1371/journal.pntd.0002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cevey AC, Mirkin GA, Penas FN, Goren NB. 2016. Low-dose benznidazole treatment results in parasite clearance and attenuates heart inflammatory reaction in an experimental model of infection with a highly virulent Trypanosoma cruzi strain. Int J Parasitol Drugs Drug Resist 6:12–22. doi: 10.1016/j.ijpddr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batista DDGJ, Batista MM, de Oliveira GM, Britto CC, Rodrigues ACM, Stephens CE, Boykin DW, Soeiro MDNC. 2011. Combined treatment of heterocyclic analogues and benznidazole upon Trypanosoma cruzi in vivo. PLoS One 6:e22155. doi: 10.1371/journal.pone.0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribeiro I, Sevcsik AM, Alves F, Diap G, Don R, Harhay MO, Chang S, Pecoul B. 2009. New, improved treatments for Chagas disease: from the R&D pipeline to the patients. PLoS Negl Trop Dis 3:e484. doi: 10.1371/journal.pntd.0000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machado FS, Mukherjee S, Weiss LM, Tanowitz HB, Ashton AW. 2011. Bioactive lipids in Trypanosoma cruzi infection. Adv Parasitol 76:1–31. doi: 10.1016/B978-0-12-385895-5.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silvero-Isidre A, Morínigo-Guayuán S, Meza-Ojeda A, Mongelós-Cardozo M, Centurión-Wenninger C, Figueredo-Thiel S, Sanchez DF, Acosta N. 2018. Protective effect of aspirin treatment on mouse behavior in the acute phase of experimental infection with Trypanosoma cruzi. Parasitol Res 117:189–200. doi: 10.1007/s00436-017-5693-6. [DOI] [PubMed] [Google Scholar]
- 12.Massocatto CL, Martins Moreira N, Muniz E, Marques de Araújo S, Pinge-Filho P, Rossi RM, de Almeida Araújo EJ, de Mello Gonçales Sant'ana D. 2017. Treatment with low doses of aspirin during chronic phase of experimental Chagas’ disease increases oesophageal nitrergic neuronal subpopulation in mice. Int J Exp Pathol 98:356–362. doi: 10.1111/iep.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massocatto CL, Moreira NM, Muniz E, Pinge-Filho P, Rossi RM, Araújo EJDA, Sant'Ana DDMG. 2017. Aspirin prevents atrophy of esophageal nitrergic myenteric neurons in a mouse model of chronic Chagas disease. Dis Esophagus 30:1–8. doi: 10.1111/dote.12449. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee S, Machado FS, Huang H, Oz HS, Jelicks LA, Prado CM, Koba W, Fine EJ, Zhao D, Factor SM, Collado JE, Weiss LM, Tanowitz HB, Ashton AW. 2011. Aspirin treatment of mice infected with Trypanosoma cruzi and implications for the pathogenesis of Chagas disease. PLoS One 6:e16959. doi: 10.1371/journal.pone.0016959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hideko Tatakihara VL, Cecchini R, Borges CL, Malvezi AD, Graca-de Souza VK, Yamada-Ogatta SF, Rizzo LV, Pinge-Filho P. 2008. Effects of cyclooxygenase inhibitors on parasite burden, anemia and oxidative stress in murine Trypanosoma cruzi infection. FEMS Immunol Med Microbiol 52:47–58. doi: 10.1111/j.1574-695X.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- 16.Malvezi AD, da Silva RV, Panis C, Yamauchi LM, Lovo-Martins MI, Zanluqui NG, Tatakihara VL, Rizzo LV, Verri WA Jr, Martins-Pinge MC, Yamada-Ogatta SF, Pinge-Filho P. 2014. Aspirin modulates innate inflammatory response and inhibits the entry of Trypanosoma cruzi in mouse peritoneal macrophages. Mediators Inflamm 2014:580919. doi: 10.1155/2014/580919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malvezi AD, Panis C, da Silva RV, de Freitas RC, Lovo-Martins MI, Tatakihara VL, Zanluqui NG, Neto EC, Goldenberg S, Bordignon J, Yamada-Ogatta SF, Martins-Pinge MC, Cecchini R, Pinge-Filho P. 2014. Inhibition of cyclooxygenase-1 and cyclooxygenase-2 impairs Trypanosoma cruzi entry into cardiac cells and promotes differential modulation of the inflammatory response. Antimicrob Agents Chemother 58:6157–6164. doi: 10.1128/AAC.02752-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carvalho de Freitas R, Lonien SCH, Malvezi AD, Silveira GF, Wowk PF, da Silva RV, Yamauchi LM, Yamada-Ogatta SF, Rizzo LV, Bordignon J, Pinge-Filho P. 2017. Trypanosoma cruzi: inhibition of infection of human monocytes by aspirin. Exp Parasitol 182:26–33. doi: 10.1016/j.exppara.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Assíria Fontes Martins T, de Figueiredo Diniz L, Mazzeti AL, da Silva do Nascimento ÁF, Caldas S, Caldas IS, de Andrade IM, Ribeiro I, Bahia MT. 2015. Benznidazole/itraconazole combination treatment enhances anti-Trypanosoma cruzi activity in experimental Chagas disease. PLoS One 10:e0128707. doi: 10.1371/journal.pone.0128707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araujo MS, Martins-Filho OA, Pereira ME, Brener Z. 2000. A combination of benznidazole and ketoconazole enhances efficacy of chemotherapy of experimental Chagas’ disease. J Antimicrob Chemother 45:819–824. doi: 10.1093/jac/45.6.819. [DOI] [PubMed] [Google Scholar]
- 21.Penitente AR, Leite AL, de Paula Costa G, Shrestha D, Horta AL, Natali AJ, Neves CA, Talvani A. 2015. Enalapril in combination with benznidazole reduces cardiac inflammation and creatine kinases in mice chronically infected with Trypanosoma cruzi. Am J Trop Med Hyg 93:976–982. doi: 10.4269/ajtmh.15-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guedes-da-Silva FH, Batista D, Da Silva CF, Pavao BP, Batista MM, Moreira OC, Souza LRQ, Britto C, Rachakonda G, Villalta F, Lepesheva GI, Soeiro M. 2019. Successful aspects of the coadministration of sterol 14α-demethylase inhibitor VFV and benznidazole in experimental mouse models of Chagas disease caused by the drug-resistant strain of Trypanosoma cruzi. ACS Infect Dis 5:365–371. doi: 10.1021/acsinfecdis.8b00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urbina JA, Docampo R. 2003. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol 19:495–501. doi: 10.1016/j.pt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Perin L, Moreira da Silva R, Fonseca KD, Cardoso JM, Mathias FA, Reis LE, Molina I, Correa-Oliveira R, Vieira PM, Carneiro CM. 2017. Pharmacokinetics and tissue distribution of benznidazole after oral administration in mice. Antimicrob Agents Chemother 61:e02410-16. doi: 10.1128/AAC.02410-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molina-Berríos A, Campos-Estrada C, Henriquez N, Faúndez M, Torres G, Castillo C, Escanilla S, Kemmerling U, Morello A, López-Muñoz RA, Maya JD. 2013. Protective role of acetylsalicylic acid in experimental Trypanosoma cruzi infection: evidence of a 15-epi-lipoxin A(4)-mediated effect. PLoS Negl Trop Dis 7:e2173. doi: 10.1371/journal.pntd.0002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucchetti BFC, Zanluqui NG, de Ataides Raquel H, Lovo-Martins MI, Tatakihara VLH, de Oliveira Belem M, Michelini LC, de Almeida Araujo EJ, Pinge-Filho P, Martins-Pinge MC. 2017. Moderate treadmill exercise training improves cardiovascular and nitrergic response and resistance to Trypanosoma cruzi infection in mice. Front Physiol 8:315. doi: 10.3389/fphys.2017.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinge-Filho P, Tadokoro CE, Abrahamsohn IA. 1999. Prostaglandins mediate suppression of lymphocyte proliferation and cytokine synthesis in acute Trypanosoma cruzi infection. Cell Immunol 193:90–98. doi: 10.1006/cimm.1999.1463. [DOI] [PubMed] [Google Scholar]
- 28.Michelin MA, Silva JS, Cunha FQ. 2005. Inducible cyclooxygenase released prostaglandin mediates immunosuppression in acute phase of experimental Trypanosoma cruzi infection. Exp Parasitol 111:71–79. doi: 10.1016/j.exppara.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Abrahamsohn IA, Coffman RL. 1995. Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection. J Immunol 155:3955–3963. [PubMed] [Google Scholar]
- 30.Cardoni RL, Antunez MI. 2004. Circulating levels of cyclooxygenase metabolites in experimental Trypanosoma cruzi infections. Mediators Inflamm 13:235–240. doi: 10.1080/09637480400003022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borges MM, Kloetzel JK, Andrade HF Jr, Tadokoro CE, Pinge-Filho P, Abrahamsohn I. 1998. Prostaglandin and nitric oxide regulate TNF-alpha production during Trypanosoma cruzi infection. Immunol Lett 63:1–8. doi: 10.1016/S0165-2478(98)00034-0. [DOI] [PubMed] [Google Scholar]
- 32.Donizette Malvezi A, Cecchini R, Souza F, Eduardo Tadokoro C, Vicente Rizzo L, Pinge-Filho P. 2004. Involvement of nitric oxide (NO) and TNF-alpha in the oxidative stress associated with anemia in experimental Trypanosoma cruzi infection. FEMS Immunol Med Microbiol 41:69–77. doi: 10.1016/j.femsim.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Molina HA, Kierszenbaum F, Hamann KJ, Gleich GJ. 1988. Toxic effects produced or mediated by human eosinophil granule components on Trypanosoma cruzi. Am J Trop Med Hyg 38:327–334. doi: 10.4269/ajtmh.1988.38.327. [DOI] [PubMed] [Google Scholar]
- 34.Nakhle MC, de Menezes MDC, Irulegui I. 1989. Eosinophil levels in the acute phase of experimental Chagas’ disease. Rev Inst Med Trop Sao Paulo 31:384–391. doi: 10.1590/s0036-46651989000600004. [DOI] [PubMed] [Google Scholar]
- 35.Nascentes GAN, Meira WSF, Lages-Silva E, Ramírez LE. 2010. Immunization of mice with a Trypanosoma cruzi-like strain isolated from a bat: predictive factors for involvement of eosinophiles in tissue damage. Vector Borne Zoonotic Dis 10:989–997. doi: 10.1089/vbz.2009.0185. [DOI] [PubMed] [Google Scholar]
- 36.Luna-Gomes T, Filardy AA, Rocha JDB, Decote-Ricardo D, LaRocque-de-Freitas IF, Morrot A, Bozza PT, Castro-Faria-Neto HC, DosReis GA, Nunes MP, Freire-de-Lima CG. 2014. Neutrophils increase or reduce parasite burden in Trypanosoma cruzi-infected macrophages, depending on host strain: role of neutrophil elastase. PLoS One 9:e90582. doi: 10.1371/journal.pone.0090582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medeiros NI, Fares RC, Franco EP, Sousa GR, Mattos RT, Chaves AT, Nunes MD, Dutra WO, Correa-Oliveira R, Rocha MO, Gomes JA. 2017. Differential expression of matrix metalloproteinases 2, 9 and cytokines by neutrophils and monocytes in the clinical forms of Chagas disease. PLoS Negl Trop Dis 11:e0005284. doi: 10.1371/journal.pntd.0005284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segura M, Molina de Raspi E, Basombrio M. 1994. Reversibility of muscle and heart lesions in chronic, Trypanosoma cruzi infected mice after late trypanomicidal treatment. Mem Inst Oswaldo Cruz 89:213–216. doi: 10.1590/s0074-02761994000200017. [DOI] [PubMed] [Google Scholar]
- 39.Andrade S, Magalhães J, Pontes A. 1989. Therapy of the chronic phase of the experimental infection by Trypanosoma cruzi with benzonidazole and nifurtimox. Rev Soc Bras Med Trop 22:113–118. doi: 10.1590/s0037-86821989000300001. [DOI] [PubMed] [Google Scholar]
- 40.Bustamante JM, Presti MSL, Rivarola HW, Fernández AR, Enders JE, Fretes RE, Paglini-Oliva P. 2007. Treatment with benznidazole or thioridazine in the chronic phase of experimental Chagas disease improves cardiopathy. Int J Antimicrobial Agents 29:733–737. doi: 10.1016/j.ijantimicag.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Caldas IS, Santos EG, Novaes RD. 2019. An evaluation of benznidazole as a Chagas disease therapeutic. Expert Opin Pharmacother 20:1797–1807. doi: 10.1080/14656566.2019.1650915. [DOI] [PubMed] [Google Scholar]
- 42.Zingales B, Second Satellite Meeting, Andrade SG, Briones MR, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG, Second Satellite M. 2009. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz 104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 43.Brener Z. 1962. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo 4:389–396. [PubMed] [Google Scholar]
- 44.Pamplona FA, Menezes-de-Lima O Jr, Takahashi RN. 2010. Aspirin-triggered lipoxin induces CB1-dependent catalepsy in mice. Neurosci Lett 470:33–37. doi: 10.1016/j.neulet.2009.12.050. [DOI] [PubMed] [Google Scholar]
- 45.Marcondes MC, Borelli P, Yoshida N, Russo M. 2000. Acute Trypanosoma cruzi infection is associated with anemia, thrombocytopenia, leukopenia, and bone marrow hypoplasia: reversal by nifurtimox treatment. Microbes Infect 2:347–352. doi: 10.1016/s1286-4579(00)00333-6. [DOI] [PubMed] [Google Scholar]
- 46.Lovo-Martins MI, Malvezi AD, da Silva RV, Zanluqui NG, Tatakihara VLH, Camara NOS, de Oliveira APL, Peron JPS, Martins-Pinge MC, Fritsche KL, Pinge-Filho P. 2017. Fish oil supplementation benefits the murine host during the acute phase of a parasitic infection from Trypanosoma cruzi. Nutr Res 41:73–85. doi: 10.1016/j.nutres.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 47.da Silva RV, Malvezi AD, da Silva Augusto L, Kian D, Tatakihara VLH, Yamauchi LM, Yamada-Ogatta SF, Rizzo LV, Schenkman S, Pinge-Filho P. 2013. Oral exposure to Phytomonas serpens attenuates thrombocytopenia and leukopenia during acute infection with Trypanosoma cruzi. PLoS One 8:e68299. doi: 10.1371/journal.pone.0068299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Navarro-Gonzálvez JA, García-Benayas C, Arenas J. 1998. Semiautomated measurement of nitrate in biological fluids. Clin Chem 44:679–681. doi: 10.1093/clinchem/44.3.679. [DOI] [PubMed] [Google Scholar]
- 49.Panis C, Mazzuco TL, Costa CZ, Victorino VJ, Tatakihara VL, Yamauchi LM, Yamada-Ogatta SF, Cecchini R, Rizzo LV, Pinge-Filho P. 2011. Trypanosoma cruzi: effect of the absence of 5-lipoxygenase (5-LO)-derived leukotrienes on levels of cytokines, nitric oxide and iNOS expression in cardiac tissue in the acute phase of infection in mice. Exp Parasitol 127:58–65. doi: 10.1016/j.exppara.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 50.Cummings KL, Tarleton RL. 2003. Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol Biochem Parasitol 129:53–59. doi: 10.1016/s0166-6851(03)00093-8. [DOI] [PubMed] [Google Scholar]
- 51.Valdez RH, Tonin LTD, Ueda-Nakamura T, Silva SO, Dias Filho BP, Kaneshima EN, Yamada-Ogatta SF, Yamauchi LM, Sarragiotto MH, Nakamura CV. 2012. In vitro and in vivo trypanocidal synergistic activity of N-butyl-1-(4-dimethylamino) phenyl-1, 2, 3, 4-tetrahydro-β-carboline-3-carboxamide associated with benznidazole. Antimicrob Agents Chemother 56:507–512. doi: 10.1128/AAC.05575-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.CEUA. 2020. Comissão de Ética no Uso de Animais. Diretriz brasileira para o cuidado e a utilização de animais em atividades de ensino ou de pesquisa científica. http://www.uel.br/comites/ceua/pages/arquivos/DBCA%20-%202016.pdf. Accessed 10 May 2020.