Cyclophilins play a key role in the life cycle of coronaviruses. Alisporivir (Debio 025) is a nonimmunosuppressive analogue of cyclosporine with potent cyclophilin inhibition properties. Alisporivir reduced SARS-CoV-2 RNA production in a dose-dependent manner in Vero E6 cells, with a 50% effective concentration (EC50) of 0.46 ± 0.04 μM. Alisporivir inhibited a postentry step of the SARS-CoV-2 life cycle. These results justify rapidly conducting a proof-of-concept phase 2 trial with alisporivir in patients with SARS-CoV-2 infection.
KEYWORDS: SARS-CoV-2, alisporivir, antiviral, cyclophilin
ABSTRACT
Cyclophilins play a key role in the life cycle of coronaviruses. Alisporivir (Debio 025) is a nonimmunosuppressive analogue of cyclosporine with potent cyclophilin inhibition properties. Alisporivir reduced SARS-CoV-2 RNA production in a dose-dependent manner in Vero E6 cells, with a 50% effective concentration (EC50) of 0.46 ± 0.04 μM. Alisporivir inhibited a postentry step of the SARS-CoV-2 life cycle. These results justify rapidly conducting a proof-of-concept phase 2 trial with alisporivir in patients with SARS-CoV-2 infection.
INTRODUCTION
In December 2019, an outbreak of pneumonia emerged in the Chinese city of Wuhan. A novel coronavirus was identified as the pathogen causing the disease, named COVID-19 (for coronavirus disease 2019). This new virus was called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) because of its genetic proximity to SARS-CoV. At the time of writing, over 3.5 million people have been diagnosed with COVID-19 worldwide, while over 250,000 of them have died from complications of the disease.
Currently, there are no vaccines or effective antiviral drugs targeting SARS-CoV-2. A pragmatic approach is to assess whether drugs that are approved for other indications or have reached late clinical developmental stages are effective against SARS-CoV-2 and could be rapidly repurposed for this indication. For instance, chloroquine has been shown to bear potent antiviral properties against SARS-CoV-2 in vitro, and several clinical trials are under way to assess its efficacy in patients with COVID-19. The nucleotide analogues remdesivir and favipiravir, as well as the antiretroviral drug lopinavir in combination with ritonavir, are also under clinical investigation.
Cyclophilins are cellular peptidyl-prolyl cis-trans isomerases that catalyze the interconversion of the two energetically preferred conformers of the planar peptide bond preceding an internal proline residue. Cyclophilins play a key role in the life cycle of many coronaviruses, including human coronaviruses 229E (HCoV-229E) and NL-63 (HCoV-NL63), feline infectious peritonitis coronavirus (FPIV), SARS-CoV, and Middle East respiratory syndrome coronavirus (MERS-CoV) (1–7). Cyclosporine A (CsA), a potent cyclophilin inhibitor, blocks the replication of various coronaviruses in vitro, including HCoV-229E, HCoV-NL63, FPIV, mouse hepatitis virus (MHV), avian infectious bronchitis virus, and SARS-CoV (5, 8–10). However, CsA cannot be used in patients with COVID-19 because of its strong immunosuppressive properties.
Alisporivir (Debio 025) is a nonimmunosuppressive analogue of CsA that potently inhibits cyclophilins. Alisporivir has been administered to more than 1,800 patients with chronic hepatitis C virus infection in phase 2 and 3 clinical trials, alone or in combination with pegylated interferon alpha and/or ribavirin. In vitro, alisporivir inhibits the replication of HCoV-229E, HCoV-NL63, MHV, SARS-CoV, and MERS-CoV at low-micromolar concentrations without cytotoxic effect (1, 10, 11).
The goal of this study was to assess the antiviral properties of alisporivir against SARS-CoV-2, with the objective of generating the preclinical proof of concept of antiviral effectiveness required to start a clinical trial in patients with COVID-19.
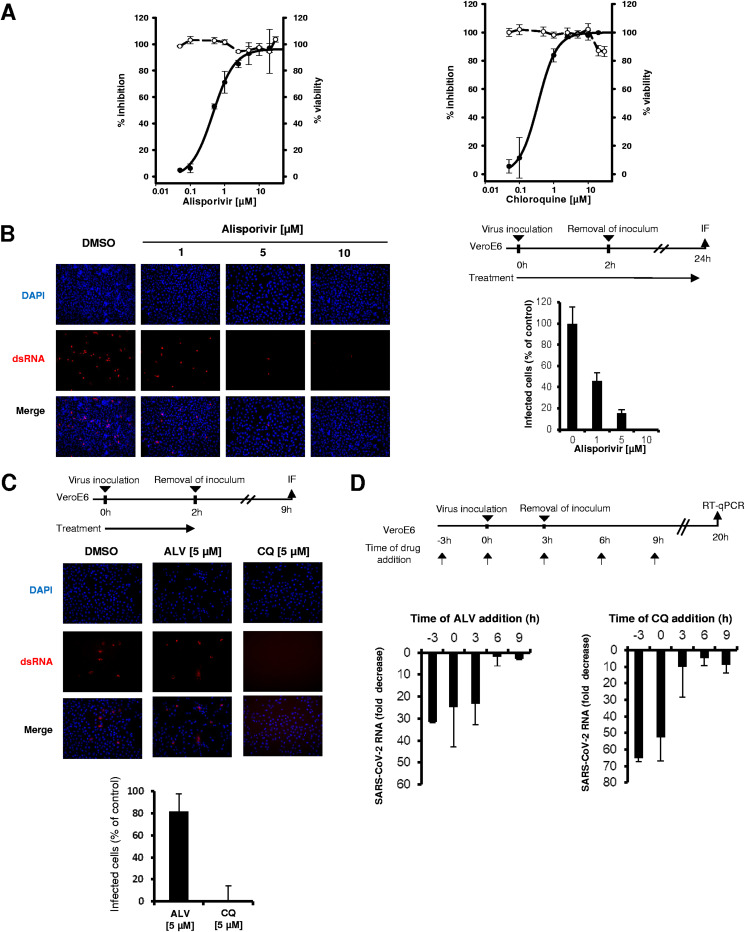
The antiviral effectiveness of increasing concentrations of alisporivir was measured in Vero E6 cells infected with a clinical isolate of SARS-CoV-2 at a multiplicity of infection (MOI) of 0.02 (Fig. 1A). Dimethyl sulfoxide (DMSO) was used as a negative control, while chloroquine was used as a positive control of antiviral inhibition. The compounds were added at the beginning of infection, and viral RNA was extracted from supernatants at 48 h postinfection and quantified by reverse transcriptase quantitative PCR (RT-qPCR). Alisporivir reduced SARS-CoV-2 RNA production in a dose-dependent manner: the 50% effective concentration (EC50) was 0.46 ± 0.04 μM, and the EC90 was 3.10 ± 1.40 μM. The maximum viral RNA reduction was 2 log10 at 5 μM. For comparison, the EC50 of chloroquine was 0.35 ± 0.02 μM (Fig. 1A). Neither alisporivir nor chloroquine was cytotoxic at the effective concentration, with 50% cytotoxic concentrations (CC50s) of >20 μM and therapeutic indexes of >43 and >57, respectively.
FIG 1.
Antiviral activity of alisporivir against SARS-CoV-2. The means ± standard deviations from 2 experiments performed in triplicate are shown. (A) Vero E6 cells were infected for 2 h with a SARS-CoV-2 clinical isolate at an MOI of 0.02 in the presence of increasing concentrations of alisporivir (left) or chloroquine (right). Cells were incubated for 48 h in the presence of the compounds, and SARS-CoV-2 RNA was quantified in cell supernatants by RT-qPCR (solid lines). Cell viability is shown with dashed lines. (B) SARS-CoV-2 infection of Vero E6 cells at an MOI of 0.4 assessed by immunofluorescence using anti-dsRNA antibodies in the presence of increasing concentrations of alisporivir. Infected cells were quantified using ImageJ software. (C) Effect of 5 μM alisporivir and 5 μM chloroquine on SARS-CoV-2 entry into Vero E6 cells, assessed by immunofluorescence using anti-dsRNA antibodies. (D) Time-of-addition experiments with alisporivir and chloroquine. Vero E6 cells were infected with SARS-CoV-2 at an MOI of 0.05 for 3 h; 10 μM alisporivir or 10 μM chloroquine was added at different time points and maintained until 20 h postinfection. SARS-CoV-2 RNA was quantified in cell supernatants by RT-qPCR. ALV, alisporivir; CQ, chloroquine.
We confirmed the anti-SARS-CoV-2 effectiveness of alisporivir by immunofluorescence. Vero E6 cells were infected at an MOI of 0.4 for 2 h in the presence of increasing concentrations of alisporivir. After virus removal, infected cells were incubated for 24 h in the presence of alisporivir and immunostained with an anti-double-stranded-RNA (dsRNA) antibody. Alisporivir reduced the number of SARS-CoV-2-infected cells in a dose-dependent manner, and complete inhibition was attained at 10 μM (Fig. 1B). Chloroquine also inhibited SARS-CoV-2 in this assay (data not shown).
The next experiment was aimed at identifying the step of the SARS-CoV-2 life cycle targeted by alisporivir. Chloroquine, which inhibits endosome-mediated viral entry, was used as a control. Vero E6 cells were infected at an MOI of 0.4 for 2 h in the presence of 5 μM alisporivir or chloroquine. After virus removal, cells were incubated for 7 h in the absence of the compounds, fixed, and immunostained with the anti-dsRNA antibody. No infected cells were detected in the presence of 5 μM chloroquine, confirming that chloroquine prevents SARS-CoV-2 entry into Vero E6 cells. In contrast, alisporivir did not inhibit SARS-CoV-2 entry into Vero E6 cells (Fig. 1C). This result was confirmed by a time-of-addition experiment showing that, in contrast to that of chloroquine, the effect of alisporivir was preserved when the compound was added 3 h postinfection. The antiviral effect of alisporivir was abolished when the compound was added 6 h postinfection (Fig. 1D). These results suggest that alisporivir inhibits a postentry step of the SARS-CoV-2 life cycle.
Taken together, our results demonstrate that the nonimmunosuppressive macrocyclic cyclophilin inhibitor alisporivir (Debio 025) exhibits strong, dose-dependent antiviral properties against SARS-CoV-2 in vitro. Alisporivir inhibits a postentry step of the SARS-CoV-2 life cycle through mechanisms that remain to be unraveled. These results justify rapidly conducting a proof-of-concept phase 2 trial to assess the antiviral properties and the effect of alisporivir on COVID-19 clinical outcomes in infected patients.
Alisporivir has been shown to be well tolerated when administered as a monotherapy (12). Preclinical pharmacology data indicate that, after oral administration, alisporivir is widely distributed in the whole body, including the lungs, and that its EC90 against SARS-CoV-2 in Vero E6 cells is clinically achievable in patients. In addition, because alisporivir inhibits all cellular cyclophilins, it also blocks mitochondrial cyclophilin D, a key regulator of mitochondrial permeability transition pore (mPTP) opening, a mechanism involved in triggering cell death. Therefore, besides its antiviral properties, alisporivir may also be effective in preventing lung tissue damage. A phase 2, proof-of-concept trial with alisporivir in patients with COVID-19 is planned to start very soon.
ACKNOWLEDGMENTS
This work was funded by the Fondation pour la Recherche Médicale and the Agence Nationale de la Recherche, Call ANR FLASH COVID-19.
Alisporivir was kindly provided by Debiopharm, Lausanne, Switzerland.
S.F. has served as an advisor and/or speaker for Abbvie; J.-M.P. has served as an advisor and/or speaker for Abbvie, Gilead, Merck, and Siemens Healthcare; A.A.-B. has served as a speaker for Abbvie.
REFERENCES
- 1.Carbajo-Lozoya J, Ma-Lauer Y, Malešević M, Theuerkorn M, Kahlert V, Prell E, von Brunn B, Muth D, Baumert TF, Drosten C, Fischer G, von Brunn A. 2014. Human coronavirus NL63 replication is cyclophilin A-dependent and inhibited by non-immunosuppressive cyclosporine A-derivatives including alisporivir. Virus Res 184:44–53. doi: 10.1016/j.virusres.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z, Mi L, Xu J, Yu J, Wang X, Jiang J, Xing J, Shang P, Qian A, Li Y, Shaw PX, Wang J, Duan S, Ding J, Fan C, Zhang Y, Yang Y, Yu X, Feng Q, Li B, Yao X, Zhang Z, Li L, Xue X, Zhu P. 2005. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J Infect Dis 191:755–760. doi: 10.1086/427811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Wilde AH, Pham U, Posthuma CC, Snijder EJ. 2018. Cyclophilins and cyclophilin inhibitors in nidovirus replication. Virology 522:46–55. doi: 10.1016/j.virol.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo C, Luo H, Zheng S, Gui C, Yue L, Yu C, Sun T, He P, Chen J, Shen J, Luo X, Li Y, Liu H, Bai D, Shen J, Yang Y, Li F, Zuo J, Hilgenfeld R, Pei G, Chen K, Shen X, Jiang H. 2004. Nucleocapsid protein of SARS coronavirus tightly binds to human cyclophilin A. Biochem Biophys Res Commun 321:557–565. doi: 10.1016/j.bbrc.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfefferle S, Schöpf J, Kögl M, Friedel CC, Müller MA, Carbajo-Lozoya J, Stellberger T, von Dall'Armi E, Herzog P, Kallies S, Niemeyer D, Ditt V, Kuri T, Züst R, Pumpor K, Hilgenfeld R, Schwarz F, Zimmer R, Steffen I, Weber F, Thiel V, Herrler G, Thiel H-J, Schwegmann-Wessels C, Pöhlmann S, Haas J, Drosten C, von Brunn A. 2011. The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog 7:e1002331. doi: 10.1371/journal.ppat.1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka Y, Sato Y, Sasaki T. 2017. Feline coronavirus replication is affected by both cyclophilin A and cyclophilin B. J Gen Virol 98:190–200. doi: 10.1099/jgv.0.000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Brunn A, Ciesek S, von Brunn B, Carbajo-Lozoya J. 2015. Genetic deficiency and polymorphisms of cyclophilin A reveal its essential role for human coronavirus 229E replication. Curr Opin Virol 14:56–61. doi: 10.1016/j.coviro.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Wilde AH, Zevenhoven-Dobbe JC, van der Meer Y, Thiel V, Narayanan K, Makino S, Snijder EJ, van Hemert MJ. 2011. Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol 92:2542–2548. doi: 10.1099/vir.0.034983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka Y, Sato Y, Osawa S, Inoue M, Tanaka S, Sasaki T. 2012. Suppression of feline coronavirus replication in vitro by cyclosporin A. Vet Res 43:41. doi: 10.1186/1297-9716-43-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma-Lauer Y, Zheng Y, Malešević M, von Brunn B, Fischer G, von Brunn A. 2020. Influences of cyclosporin A and non-immunosuppressive derivatives on cellular cyclophilins and viral nucleocapsid protein during human coronavirus 229E replication. Antiviral Res 173:104620. doi: 10.1016/j.antiviral.2019.104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Wilde AH, Falzarano D, Zevenhoven-Dobbe JC, Beugeling C, Fett C, Martellaro C, Posthuma CC, Feldmann H, Perlman S, Snijder EJ. 2017. Alisporivir inhibits MERS- and SARS-coronavirus replication in cell culture, but not SARS-coronavirus infection in a mouse model. Virus Res 228:7–13. doi: 10.1016/j.virusres.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawlotsky J-M, Flisiak R, Sarin SK, Rasenack J, Piratvisuth T, Chuang W-L, Peng C-Y, Foster GR, Shah S, Wedemeyer H, Hézode C, Zhang W, Wong KA, Li B, Avila C, Naoumov NV, VITAL-1 Study Team . 2015. Alisporivir plus ribavirin, interferon free or in combination with pegylated interferon, for hepatitis C virus genotype 2 or 3 infection. Hepatology 62:1013–1023. doi: 10.1002/hep.27960. [DOI] [PubMed] [Google Scholar]