Drug repositioning is the only feasible option to immediately address the COVID-19 global challenge. We screened a panel of 48 FDA-approved drugs against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which were preselected by an assay of SARS-CoV. We identified 24 potential antiviral drug candidates against SARS-CoV-2 infection. Some drug candidates showed very low 50% inhibitory concentrations (IC50s), and in particular, two FDA-approved drugs—niclosamide and ciclesonide—were notable in some respects.
KEYWORDS: COVID-19, FDA-approved drug, SARS-CoV-2
ABSTRACT
Drug repositioning is the only feasible option to immediately address the COVID-19 global challenge. We screened a panel of 48 FDA-approved drugs against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which were preselected by an assay of SARS-CoV. We identified 24 potential antiviral drug candidates against SARS-CoV-2 infection. Some drug candidates showed very low 50% inhibitory concentrations (IC50s), and in particular, two FDA-approved drugs—niclosamide and ciclesonide—were notable in some respects.
INTRODUCTION
COVID-19 is an emerging infectious disease caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). Although the case fatality rate due to this viral infection varies from 1% to 12% (2), the transmission rate is relatively high (3), and recently, the WHO declared the COVID-19 outbreak a pandemic. Currently, there are no vaccines or therapeutics available, and the patients with COVID-19 are being treated with supportive care.
Drug repositioning could be an effective strategy to respond immediately to emerging infectious diseases since new drug development usually takes more than 10 years (4). FDA-approved drugs provide safe alternatives only in the case where at least modest antiviral activity can be achieved. Accordingly, several drugs are being tested in numerous clinical trials (5), including remdesivir, lopinavir, and chloroquine (6).
We screened approximately 3,000 FDA- and Investigational New Drug (IND)-approved drugs against SARS-CoV to identify antiviral drug candidates (unpublished data). Since SARS-CoV and SARS-CoV-2 are very similar (79.5% sequence identity) (1), the drugs which show antiviral activity against SARS-CoV are expected to show a similar extent of antiviral activity against SARS-CoV-2.
A total of 35 drugs were selected from the earlier SARS-CoV screening results. In addition, 13 drugs were included based on recommendations from infectious diseases specialists (Table 1). For screening experiments, Vero cells were used and each drug was added to the cells prior to the virus infection. At 24 h after the infection, the infected cells were scored by immunofluorescence analysis with an antibody specific for the viral N protein of SARS-CoV-2. The confocal microscope images of both the viral N protein and cell nuclei were analyzed using our in-house Image Mining (IM) software, and the dose-response curve (DRC) for each drug was generated (Fig. 1).
TABLE 1.
Pharmacological actions and registration status of drugs
| Drug name | Pharmacological action | Drugs@FDA labela | WHO essential medicine statusb | Organization(s)c |
|---|---|---|---|---|
| Abemaciclib | Antineoplastic agents | NDA #208855 | NAd | USAN, INN |
| Amodiaquine dihydrochloride | Antimalarials | NDA #006441 | Essential | USP, INN, BAN |
| Anidulafungin | Antifungal agents | NDA #021948 | NA | USAN, INN, BAN |
| Bazedoxifene | Antiestrogen | NDA #22247 | NA | INN, USAN, JAN |
| Berbamine hydrochloride | Natural products | NA | NA | NA |
| Camostat | Protease inhibitor | NA | NA | JAN, INN |
| Cepharanthine | Anti-inflammatory agents | NA | NA | JAN |
| Chloroquine diphosphate | Antimalarials | ANDA #091621 | Essential | USP, BAN |
| Ciclesonide | Antiallergic agents | NDA #021658 | NA | USAN, INN |
| Clomiphene citrate | Fertility agents | ANDA #075528 | Essential | USAN, USP |
| Cyclosporine | Antifungal agents | ANDA #065017 | NA | USAN, USP |
| Digitoxin | Cardiovascular agents | ANDA #084100 | NA | USP, INN, BAN, JAN |
| Digoxin | Cardiovascular agents | NDA #021648 | Essential | USP, INN, BAN, JAN |
| Dihydrogambogic acid | Natural products | NA | NA | NA |
| Droloxifene | Antineoplastic agents | NA | NA | USAN, INN |
| Dronedarone HCl | Cardiovascular agents | ANDA #205903 | NA | USAN |
| Ebastine | Antihistaminic agents | NA | NA | USAN, INN, BAN |
| Eltrombopag | Treatment of thrombocytopenia | ANDA #209938 | NA | INN |
| Gilteritinib | Antineoplastic agents | NDA #211349 | NA | USAN, INN |
| Hexachlorophene | Anti-infective agents | NA | NA | USP, INN, BAN |
| Hydroxyprogesterone caproate | Hormones | ANDA #211777 | NA | USP, INN, JAN |
| Isoosajin | Natural products | NA | NA | NA |
| Isopomiferin | Antioxidant | NA | NA | NA |
| Ivacaftor | Treatment of cystic fibrosis | NDA #203188 | NA | USAN, INN |
| Lanatoside C | Cardiovascular agents | NA | NA | INN, BAN, DCF, JAN, NF |
| LDK378 | Antineoplastic agents | NDA #211225 | NA | USAN, INN |
| Loperamide hydrochloride | Antidiarrheals | NDA #021855 | Essential | USAN, USP, JAN |
| Lopinavir | Antiviral agents | NDA #021906 | Essential | USAN, USP, INN, BAN |
| Lusutrombopag | Treatment of thrombocytopenia | NDA #210923 | NA | USAN, INN |
| Mefloquine | Antimalarials | ANDA #076392 | Essential | USAN, INN, BAN |
| Mequitazine | Histamine antagonists | NA | NA | INN, BAN, DCF, JAN |
| Niclosamide | Antiparasitic agents | NDA #018669 | Essential | USAN, INN, BAN |
| Osajin | Natural products | NA | NA | NA |
| Osimertinib mesylate | Antineoplastic agents | NDA #208065 | NA | USAN |
| Ouabain | Cardiovascular agents | NA | NA | USP |
| Oxyclozanide | Antiparasitic agents | NA | NA | INN, BAN |
| Penfluridol | Antipsychotic | NA | NA | NA |
| Perhexiline maleate | Cardiovascular agents | NA | NA | USAN |
| Phenazopyridine hydrochloride | Analgesic | NDA #021105 | Essential | USAN, USP |
| Proscillaridin | Cardiovascular agents | NA | NA | USAN, INN, BAN, JAN |
| Quinacrine hydrochloride | Antimalarials/antiparasitic agents | NA | NA | INN, BAN |
| Remdesivir (GS-5734) | Antiviral agents | NA | NA | USAN |
| Salinomycin sodium | Antibacterial agents | NA | NA | INN, BAN |
| Tetrandrine | Antiviral agents | NA | NA | NA |
| Thioridazine hydrochloride | Antipsychotic | ANDA #088004 | NA | USP, JAN |
| Tilorone | Antiviral agents | NA | NA | INN |
| Toremifene citrate | Antineoplastic agents | ANDA #208813 | NA | USAN |
| Triparanol | Hypolipidemic agents | NA | NA | INN, BAN |
Latest New Drug Application (NDA) and Abbreviated New Drug Application (ANDA) information retrieved from Drugs@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/; accessed March 2020).
According to the WHO Model List of Essential Medicines, 21st List (2019).
BAN, British Approved Name; DCF, Data Clarification Form; INN, International Nonproprietary Names; JAN, Japanese Accepted Name; USAN, United States Adopted Names; USP, The United States Pharmacopeial Convention; NF, USP-National Formulary.
NA, not available.
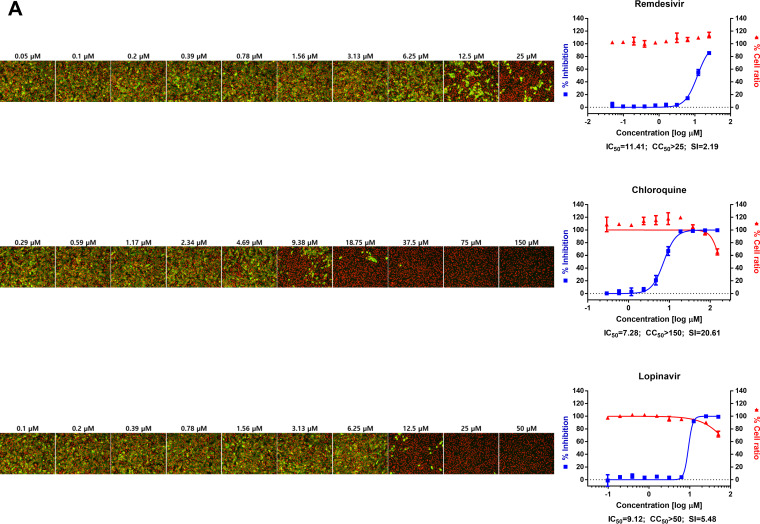
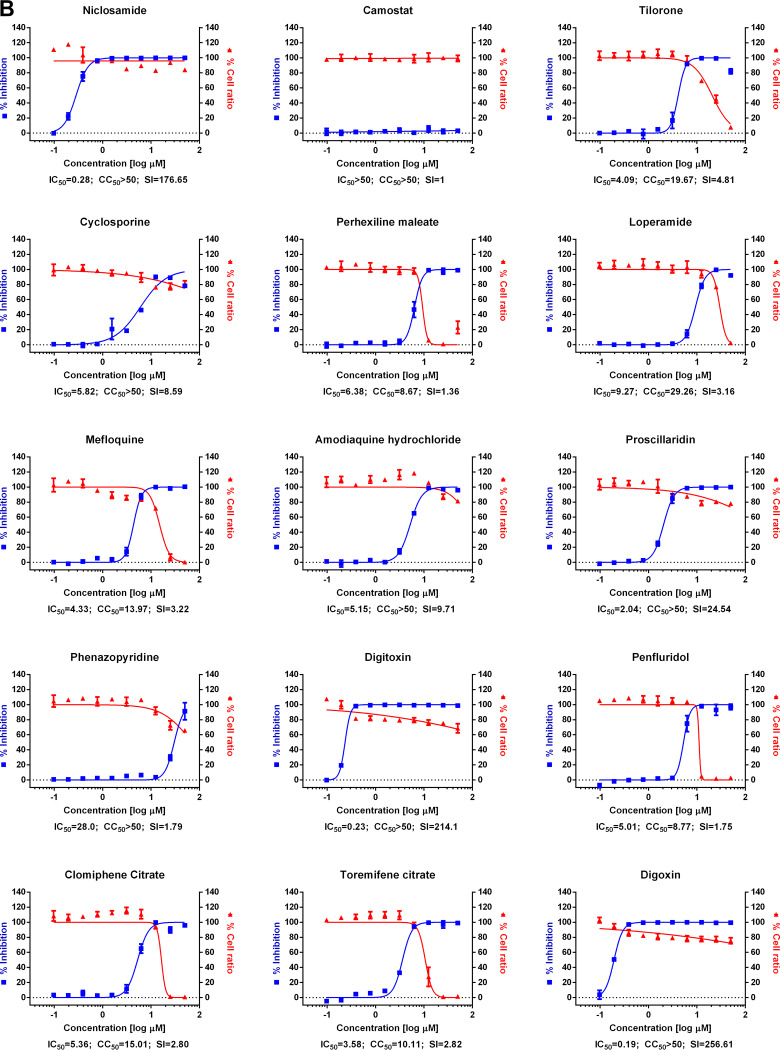
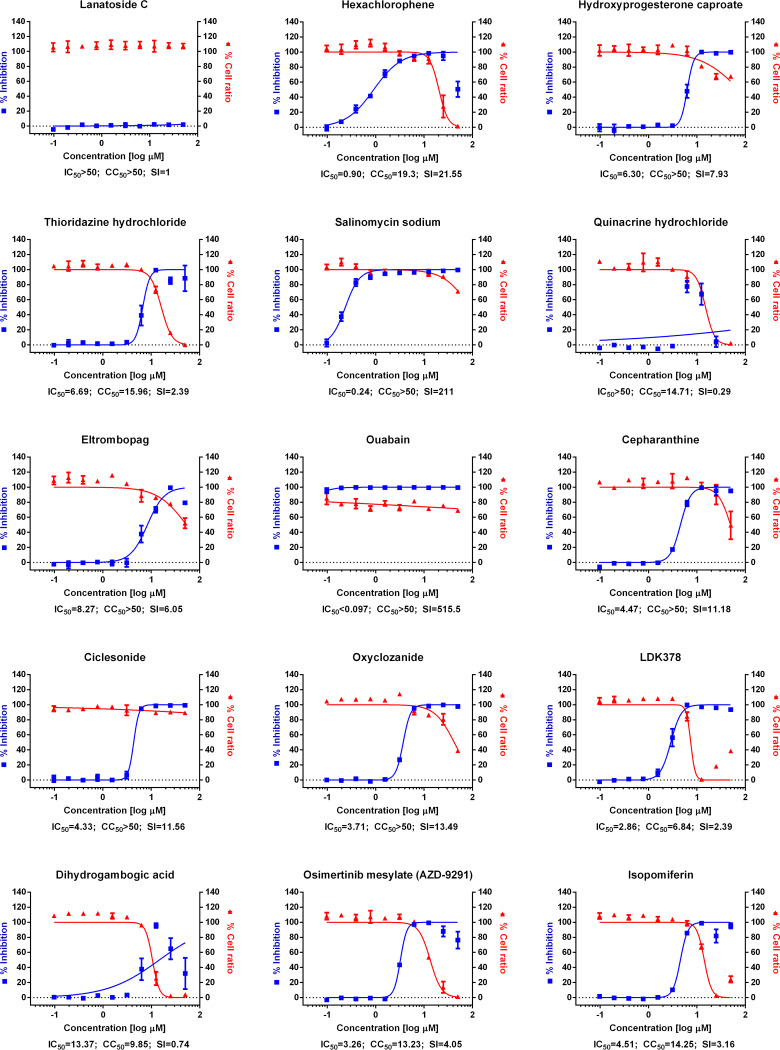
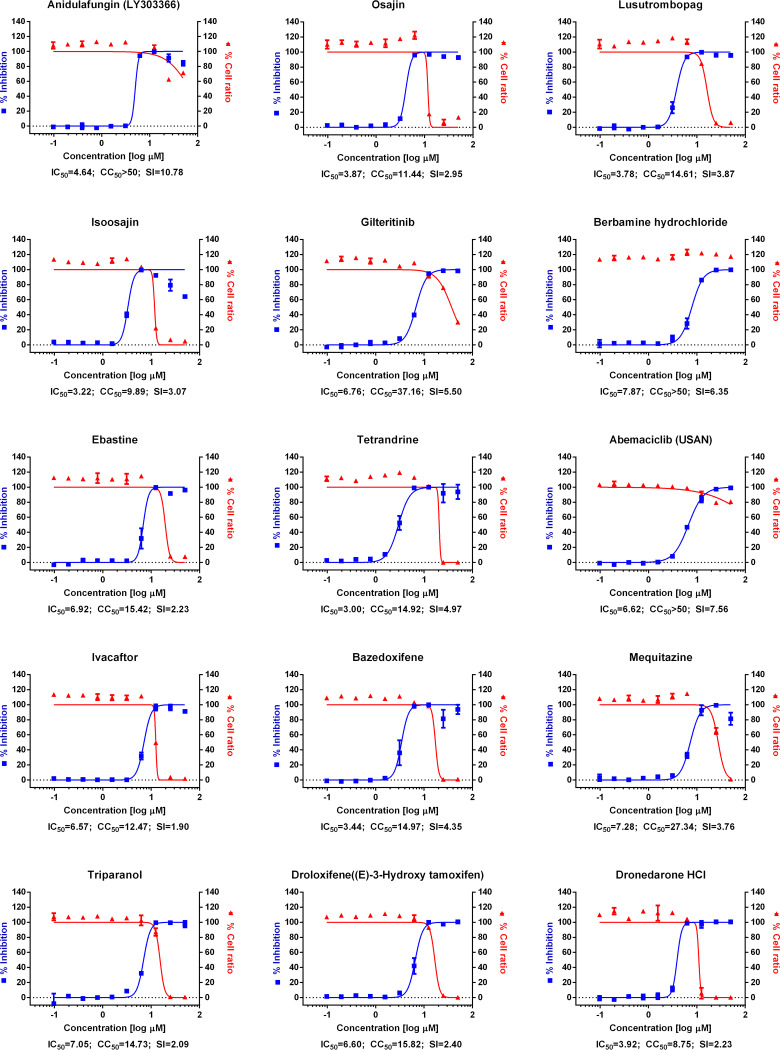
FIG 1.
(A) Dose-response curve analysis by immunofluorescence for reference drugs. The blue squares represent inhibition of SARS-CoV-2 infection (%), and the red triangles represent cell viability (%). The confocal microscope images show cell nuclei (red) and viral N protein (green) at each drug concentration. Means ± SD were calculated from duplicate experiments. (B) Dose-response curve analysis by immunofluorescence for 45 drugs that were tested in this study. The blue squares represent inhibition of SARS-CoV-2 infection (%), and the red triangles represent cell viability (%). Means ± SD were calculated from duplicate experiments.
Chloroquine, lopinavir, and remdesivir were used as reference drugs with 50% inhibitory concentration (IC50) values of 7.28, 9.12, and 11.41 μM, respectively (Fig. 1A). Among the 48 drugs that were evaluated in our study, 24 drugs showed potential antiviral activities against SARS-CoV-2, with IC50 values in between 0.1 and 10 μM, namely, tilorone, cyclosporine, loperamide, mefloquine, amodiaquine, proscillaridin, digitoxin, digoxin, hexachlorophene, hydroxyprogesterone caproate, salinomycin, ouabain, cepharanthine, ciclesonide, oxyclozanide, anidulafungin, gilteritinib, berbamine, tetrandrine, abemaciclib, ivacaftor, bazedoxifene, niclosamide, and eltrombopag.
Among these 24 drugs, 2 FDA-approved drugs drew our attention. First, niclosamide, an anthelminthic drug, exhibited very potent antiviral activity against SARS-CoV-2 (IC50, 0.28 μM). Not surprisingly, its broad-spectrum antiviral effect has been well documented in the literature (7), including antiviral properties against SARS-CoV and Middle East respiratory syndrome (MERS)-CoV (8, 9). Recently, Gassen et al. demonstrated that niclosamide inhibits SKP2 activity, which enhances autophagy and reduces MERS-CoV replication (9). A similar mechanism might be attributable to the inhibition of SARS-CoV-2 infection by niclosamide. Although niclosamide has a pharmacokinetic flaw of low absorption, further development or drug formulation could enable an effective delivery of this drug to the target tissue (10).
Second, ciclesonide is another interesting drug candidate for further development, although its antiviral potency was much lower (IC50, 4.33 μM) than niclosamide. It is an inhaled corticosteroid used to treat asthma and allergic rhinitis (11). A recent report by Matsuyama et al. corroborated our finding of ciclesonide as a potential antiviral drug against SARS-CoV-2 (12). A treatment report of three patients who were infected by SARS-CoV-2 in Japan (13) warrants further clinical investigation of this drug in patients with COVID-19. Intriguingly, an underlying mechanism for the suppression of viral infection by ciclesonide has been revealed by the isolation of a drug-resistant mutant (12). The isolation of the drug-resistant mutant indicated that NSP15, a viral endoribonuclease, is the molecular target of ciclesonide. Together, these findings suggest that it is not unreasonable to consider that ciclesonide exhibits direct-acting antiviral activity in addition to its intrinsic anti-inflammatory function. In the future, small interfering RNA (siRNA) targeting the hormone receptor will allow for an assessment of the extent of this direct-acting antiviral activity. With its proven anti-inflammatory activity, ciclesonide may represent a potent drug which can manifest dual roles (antiviral and anti-inflammatory) for the control of SARS-CoV-2 infection.
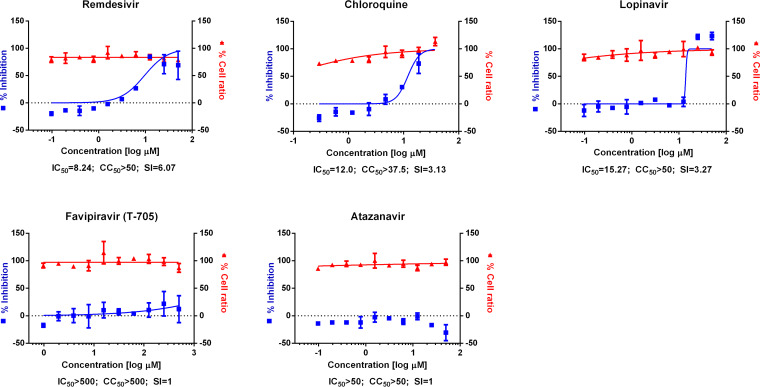
Prior to our evaluation of 48 drugs against SARS-CoV-2 infection, we also tested the antiviral activity of several other drugs based on the cytopathic effect of the virus in the presence of each drug (Fig. 2). In particular, the effects of favipiravir and atazanavir on SARS-CoV-2 were compared with those of the reference drugs (chloroquine, lopinavir, and remdesivir) because favipiravir is considered a drug candidate for clinical trials and atazanavir was recently predicted as the most potent antiviral drug by artificial intelligence (AI)-inference modeling (14). However, in the current work, we did not observe any antiviral activity of either favipiravir or atazanavir.
FIG 2.
Dose-response curve analysis by cytopathic effect. The blue squares represent inhibition of SARS-CoV-2 infection (%), and the red triangles represent cell viability (%). Means ± SD were calculated from duplicate experiments.
In summary, we selected and screened 48 FDA-approved drugs based on our SARS-CoV screening, and our screening campaign revealed 24 potential antiviral drug candidates against SARS-CoV-2. Our findings could be further validated in an appropriate animal model and, hopefully, developed through subsequent clinical trials in order to provide additional therapeutic options for patients with COVID-19.
Virus and cells.
Vero cells were obtained from the American Type Culture Collection (ATCC CCL-81) and maintained at 37°C with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM; Welgene), supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1× antibiotic-antimycotic solution (Gibco). SARS-CoV-2 (βCoV/KOR/KCDC03/2020) was provided by Korea Centers for Disease Control and Prevention (KCDC) and was propagated in Vero cells. Viral titers were determined by plaque assays in Vero cells. All experiments using SARS-CoV-2 were performed at Institut Pasteur Korea in compliance with the guidelines of the Korea National Institute of Health (KNIH), using enhanced biosafety level 3 (BSL3) containment procedures in laboratories approved for use by the KCDC.
Reagents.
Chloroquine diphosphate (CQ; C6628) was purchased from Sigma-Aldrich (St. Louis, MO), lopinavir (LPV; S1380) was purchased from SelleckChem (Houston, TX), and remdesivir (HY-104077) was purchased from MedChemExpress (Monmouth Junction, NJ). Chloroquine was dissolved in Dulbecco’s phosphate-buffered saline (DPBS; Welgene), and all other reagents were dissolved in dimethyl sulfoxide (DMSO) for the screening. Anti-SARS-CoV-2 N protein antibody was purchased from Sino Biological Inc. (Beijing, China). Alexa Fluor 488 goat anti-rabbit IgG (H+L) secondary antibody and Hoechst 33342 were purchased from Molecular Probes. Paraformaldehyde (PFA) (32% aqueous solution) and normal goat serum were purchased from Electron Microscopy Sciences (Hatfield, PA) and Vector Laboratories, Inc. (Burlingame, CA), respectively.
DRC analysis by immunofluorescence.
Ten-point DRCs were generated for each drug. Vero cells were seeded at 1.2 × 104 cells per well in DMEM, supplemented with 2% FBS and 1× antibiotic-antimycotic solution (Gibco), in black, 384-well μClear plates (Greiner Bio-One) 24 h prior to the experiment. Ten-point DRCs were generated, with compound concentrations ranging from 0.1 to 50 μM. For the viral infections, plates were transferred into the BSL3 containment facility and SARS-CoV-2 was added at a multiplicity of infection (MOI) of 0.0125. The cells were fixed at 24 hours postinfection (hpi) with 4% PFA and analyzed by immunofluorescence. The acquired images were analyzed using in-house software to quantify cell numbers and infection ratios, and antiviral activity was normalized to positive (mock) and negative (0.5% DMSO) controls in each assay plate. DRCs were fitted by sigmoidal dose-response models, with the following equation: Y = bottom + (top − bottom)/[1 + (IC50/X)Hillslope], using XLfit 4 software or Prism7. IC50 values were calculated from the normalized activity data set-fitted curves. All IC50 and 50% cytotoxic concentration (CC50) values were measured in duplicate, and the quality of each assay was controlled by Z’-factor and the coefficient of variation in percent (%CV).
DRC analysis by CPE.
Ten-point DRCs were generated for each drug. Vero cells were seeded at 1.2 × 104 cells per well in DMEM, supplemented with 2% FBS and 1× antibiotic-antimycotic solution (Gibco) in white, 384-well μClear plates (Greiner Bio-One) 24 h prior to the experiment. Ten-point DRCs were generated, with compound concentrations ranging from 0.1 to 50 μM. For the viral infections, plates were transferred into the BSL3 containment facility and SARS-CoV-2 was added at a multiplicity of infection (MOI) of 0.05 and incubated at 37°C for 72 h. Cell viability was measured using the CellTiter-Glo luminescent cell viability assay (Promega), according to the manufacturer’s instructions. Antiviral activity was determined by the degree of inhibition of viral cytopathic effect (CPE). The results were normalized to positive (mock) and negative (0.5% DMSO) controls in each assay plate. DRCs were fitted by sigmoidal dose-response models, with the following equation: Y = bottom + (top − bottom)/[1 + (IC50/X)Hillslope], using XLfit 4 software or Prism7. IC50 values were calculated from the normalized activity data set-fitted curves. All IC50 and CC50 values were measured in duplicate, and the quality of each assay was controlled by Z’-factor and the coefficient of variation in percent (%CV).
ACKNOWLEDGMENTS
We thank Wang-Shick Ryu and Spencer Shorte for their helpful discussion and review of the manuscript. The pathogen resource (NCCP43326) for this study was provided by the National Culture Collection for Pathogens.
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2017M3A9G6068245 and NRF-2020M3E9A1041756).
REFERENCES
- 1.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizumoto K, Chowell G. 2020. Estimating the risk of 2019 novel coronavirus death during the course of the outbreak in China, 2020. medRxiv doi: 10.1101/2020.02.19.20025163. [DOI] [Google Scholar]
- 3.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. 2020. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashburn TT, Thor KB. 2004. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 5.Maxmen A. 2020. More than 80 clinical trials launch to test coronavirus treatments. Nature 578:347–348. doi: 10.1038/d41586-020-00444-3. [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. 2020. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J, Shi P-Y, Li H, Zhou J. 2020. Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect Dis doi: 10.1021/acsinfecdis.0c00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu C-J, Jan J-T, Chen C-M, Hsieh H-P, Hwang D-R, Liu H-W, Liu C-Y, Huang H-W, Chen S-C, Hong C-F, Lin R-K, Chao Y-S, Hsu J. 2004. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob Agents Chemother 48:2693–2696. doi: 10.1128/AAC.48.7.2693-2696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gassen NC, Niemeyer D, Muth D, Corman VM, Martinelli S, Gassen A, Hafner K, Papies J, Mösbauer K, Zellner A, Zannas AS, Herrmann A, Holsboer F, Brack-Werner R, Boshart M, Müller-Myhsok B, Drosten C, Müller MA, Rein T. 2019. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-coronavirus infection. Nat Commun 10:5770. doi: 10.1038/s41467-019-13659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith TC, Kinkel AW, Gryczko CM, Goulet JR. 1976. Absorption of pyrvinium pamoate. Clin Pharmacol Ther 19:802–806. doi: 10.1002/cpt1976196802. [DOI] [PubMed] [Google Scholar]
- 11.Schaffner TJ, Skoner DP. 2009. Ciclesonide: a safe and effective inhaled corticosteroid for the treatment of asthma. J Asthma Allergy 2:25–32. doi: 10.2147/jaa.s4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuyama S, Kawase M, Nao N, Shirato K, Ujike M, Kamitani W, Shimojima M, Fukushi S. 2020. The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15. bioRxiv. doi: 10.1101/2020.03.11.987016. [DOI] [PMC free article] [PubMed]
- 13.Iwabuchi K, Yoshie K, Kurakami Y, Takahashi K, Kato Y, Morishima T. 2020. Therapeutic potential of ciclesonide inhalation for COVID-19 pneumonia: report of three cases. J Infect Chemother 26:625–632. doi: 10.1016/j.jiac.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck BR, Shin B, Choi Y, Park S, Kang K. 2020. Predicting commercially available antiviral drugs that may act on the novel coronavirus (2019-nCoV), Wuhan, China through a drug-target interaction deep learning model. bioRxiv. doi: 10.1101/2020.01.31.929547. [DOI] [PMC free article] [PubMed]