Abstract
In the past 15 years, enormous progress has been made in cancer nanotechnology, and a several nanoparticles have entered clinical testing for cancer treatment. Among these nanoparticles are nanoscale metal–organic frameworks (nMOFs), a class of organic–inorganic hybrid nanomaterials constructed from metal binding sites and bridging ligands, which have attracted significant attention for their ability to integrate porosity, crystallinity, compositional and structural tunability, multifunctionality, and biocompatibility into a singular nanomaterial for cancer therapies. This Outlook article summarizes the progress on the design of nMOFs as nanosensitizers for photodynamic therapy (PDT), radiotherapy (RT), radiotherapy–radiodynamic therapy (RT-RDT), and chemodynamic therapy (CDT) via nMOF-mediated reactive oxygen species (ROS) generated under external energy stimuli or in the presence of endogenous chemical triggers. Inflammatory responses induced by nMOF-mediated ROS generation activate tumor microenvironments to potentiate cancer immunotherapy, extending the local treatment effects of nMOF-based ROS therapy to distant tumors via abscopal effects. Future research directions in nMOF-mediated ROS therapies and the prospect of clinical applications of nMOFs as cancer therapeutics are also discussed.
Short abstract
Upon stimulation, nMOFs generate reactive oxygen species to enhance immunogenic PDT, RT, RT-RDT, and CDT for local cancer treatment and potentiate cancer immunotherapy for systemic tumor rejection.
Introduction
Built from metal cluster secondary building units (SBUs) and bridging ligands, metal–organic frameworks (MOFs) integrate crystallinity, porosity, functionality, and modularity to afford a unique class of functional molecular materials.1−4 By scaling down MOFs to nanometer dimensions, nanoscale MOFs (nMOFs) were hypothesized to retain the synthetic flexibility, structural tunability, and multifunctionality of bulk MOFs to provide biocompatible molecular nanomaterials with potential in biomedical applications.5−7 The Lin laboratory’s exploration of nMOFs in biomedical applications started in 2005, with the support of the Cancer Nanotechnology initiative at the US National Cancer Institute.5−7 Lin and co-workers reported the design of nMOFs as imaging contrast agents in 2006,8 and followed up with several publications on the use of nMOFs in the delivery of exceptionally high payloads of diagnostic and therapeutic cargos.9−12
Nanoparticles have been used to elongate blood circulation times and enhance tumor uptake of chemotherapeutics via the enhanced permeability and retention (EPR) effect and active targeting strategies.13,14 Significant efforts have been devoted to developing nMOFs for anticancer drug delivery.15−17 As opposed to other nanoparticles (NPs), nMOFs can be rationally designed to possess multiple synergistic functions for cancer therapy without relying on cytotoxic agents, which often lead to severe general toxicity. In particular, nMOFs can be activated by either external energy stimuli or endogenous chemical triggers to generate cytotoxic reactive oxygen species (ROS) to kill cancer cells in an immunogenic fashion. Such nMOF-mediated immunogenic local treatment can further synergize with immunotherapies to afford systemic antitumor effects.
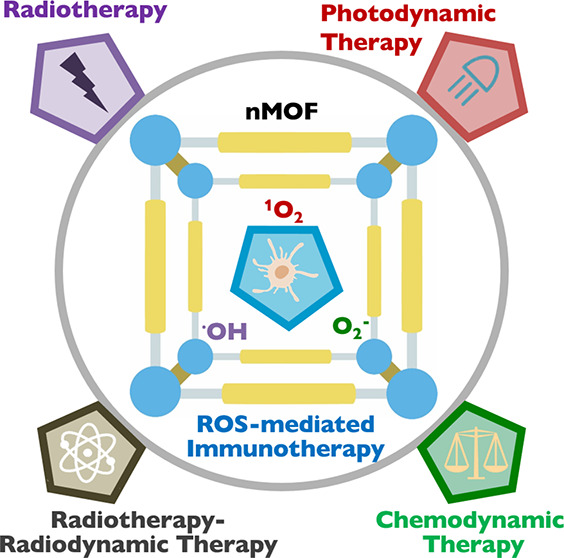
In this Outlook, we summarize the progress on the design of nMOFs as nanosensitizers for photodynamic therapy (PDT), radiotherapy (RT), radiotherapy–radiodynamic therapy (RT-RDT), and chemodynamic therapy (CDT). These therapies augment innate immunity via nMOF-mediated local inflammation to synergize with systemic immunotherapy, reinvigorating host antitumor immunity for systemic tumor rejection (Figure 1). We also discuss future research directions in nMOF-mediated ROS therapies and the prospect of using nMOFs as cancer therapeutics in clinical settings.
Figure 1.
Schematic showing local nMOF-mediated photodynamic therapy, radiotherapy, radiotherapy–radiodynamic therapy, and chemodynamic therapy promoting ROS generation to kill tumor cells and induce local inflammation, which augments innate and adaptive immunity to synergize with cancer immunotherapy.
Photodynamic Therapy
PDT provides highly effective local therapy against cancer using cytotoxic ROSs that are generated from a combination of three intrinsically nontoxic components: photosensitizers (PSs), light, and tissue oxygen (O2).18 However, the antitumor efficacy of PDT is limited by the poor solubility and inefficient cellular internalization of many conventional PSs, shallow tissue penetration of light, and often hypoxic tumor microenvironments.
Although liposomes and other nanoformulations have been used to enhance the delivery of PSs to tumors, it remains a challenge to simultaneously deliver high payloads and avoid self-quenching.19,20 Because of their short lifetimes, a large fraction of ROSs generated cannot diffuse out of the nanoformulations to attack subcellular compartments, limiting PDT efficacy of PS nanoformulations. A series of nMOFs have been designed to overcome aforementioned disadvantages of conventional PDT over the past six years.21
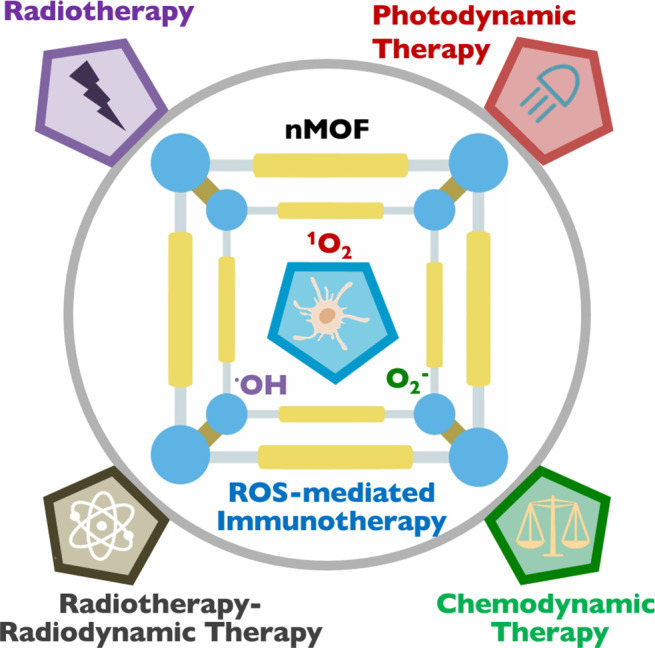
In 2014, Lin and co-workers first reported the use of nMOFs to address the limitations of PDT.22 The Hf-DBP nMOF was assembled from Hf-oxo clusters and 5,15-di(p-benzoato)porphyrin (H2DBP) as nanoplates 100 nm in diameter and 10 nm in thickness. The porous structure and nanoplate morphology of Hf-DBP facilitate the diffusion of 1O2 from the interior of the framework to cell cytoplasm to exert cytotoxic effects (Figure 2a). Hf-DBP carried an exceptionally high PS loading of 77 wt % and processed a 1O2 generation efficiency 2.8 times higher than that of H2DBP. Thus, nMOFs not only prevent aggregation of insoluble PSs but also alleviate self-quenching of PSs due to site isolation in the crystalline frameworks. PDT efficacy of Hf-DBP was tested in vitro and in vivo, using murine head and neck squamous cell SQ20B. Under light irradiation, Hf-DBP showed significantly enhanced cytotoxicity when compared to H2DBP or commercial PS Protoporphyrin IX (PpIX; see Figure 2b). When intratumorally injected into SQ20B tumor-bearing mice, a single Hf-DBP dose (3.5 mg/kg) and light irradiation eradicated tumors in half of the mice.
Figure 2.
(a) Scheme of nMOF-mediated PDT upon light irradiation.22 (b) Cytotoxicity of Hf-DBP, H2DBP, and commercial PpIX. (c) Illustration of electron transfer from porphyrin excited state to TiIV in Ti-TBP for Type I PDT.26 [Reprinted with permission from ref (25). Copyright 2019, American Chemical Society, Washington, DC.]
Light penetration is a major limitation for PDT. In 2015, Lin and co-workers improved photosensitizing performance of nMOFs by partially reducing the H2DBP ligand to 5,15-di(p-benzoato)chlorin (H2DBC). Hf-DBC nMOF showed enhanced photophysical properties and PDT efficacy, compared to Hf-DBP.23 Hf-DBC slightly red-shifted the lowest-energy Q-band and increased the extinction coefficient by a factor of 11. In colorectal cancer mouse models HT29 and CT26, Hf-DBC combined with irradiation suppressed tumor growth at a 1 mg/kg dose and completely eradicated tumors at a 3.5 mg/kg dose.
The Lin laboratory further improved the performance of nMOFs in PDT by reducing porphyrins to bacteriocholorins. Bacteriochlorins weakly absorb in the visible spectrum to minimize photosensitivity and strongly absorb in the near-infrared region (700–850 nm), but they are unstable toward oxygen and light. In 2020, we showed that nMOFs significantly stabilized bacteriochlorins for effective PDT.24 Experimental and computational studies showed that 5,10,15,20-tetra(p-benzoato)bacteriochlorin (TBB) ligands were stabilized by a factor of 14 toward oxygen and light in Zr-TBB nMOF because of geometrical constraints. Zr-TBB combined with irradiation at 740 nm regressed tumors of 4T1 and MC38 mouse models bearing breast and colon cancers to achieve cure rates of 40% and 60%, respectively.
Tumor hypoxia is another major obstacle to improving the anticancer efficacy of PDT. In 2018, Lin and co-workers reported the use of Fe-TBP, assembled from Fe3O clusters and 5,10,15,20-tetra(p-benzoato)porphyrin (TBP) ligands, to overcome tumor hypoxia.25 When irradiated with light under hypoxic conditions, Fe-TBP catalyzed a cascade reaction by decomposing intracellular hydrogen peroxide (H2O2) with the Fe3O clusters to produce ground-state O2 through a Fenton-like reaction and sensitizing the formation of cytotoxic 1O2 from generated O2 using photoexcited porphyrin moieties. After PDT treatment, Fe-TBP effectively regressed locally irradiated tumors of hypoxic CT26 colorectal adenocarcinoma.
Type I PDT is less O2 dependent than type II PDT and presents another strategy to overcome tumor hypoxia. In 2019, we designed Ti-TBP, composed of Ti-oxo chain SBUs and TBP ligands, for hypoxia-tolerant type I PDT.26 In addition to sensitizing 1O2 production from photoexcited TBP under light irradiation, Ti-TBP can also transfer electrons from excited TBP* species to TiIV-based SBUs to generate oxidized TBP• + ligands and reduced TiIII centers, propagating the generation of superoxide (O2–), H2O2, and hydroxyl radicals (•OH) as illustrated in Figure 2c. The generation of four distinct ROSs by Ti-TBP was probed in both test tubes and cells. Ti-TBP-mediated PDT elicited tumor regression of 98.4% (in volume) with a cure rate of 60% on hypoxic CT26 tumors.
Several other research groups have also made significant contributions to the development of nMOFs for PDT. Zhou and co-workers synthesized porphyrin-based nMOFs with diameters ranging from 30 nm to 190 nm and found the highest cellular uptake and cytotoxicity for 90 nm nMOF.27 Liu and co-workers PEGylated TBP-based nMOF through noncovalent interactions for PDT via intraveneous injection.28 Xie and co-workers incorporated BODIPY into UiO-66 nMOF via post-synthetic solvent-assisted ligand exchange to afford strong PDT efficacy.29 Li and co-workers recently attached porphyrin-based nMOFs to lanthanide nanoparticles to realize near-infrared (NIR)-excited PDT via an upconversion process.30
Radiotherapy
RT has been used for cancer treatment shortly after the discovery of X-rays in 1895. The objective of RT is to maximize the therapeutic effect of ionizing radiation on tumors while minimizing its side effects on adjacent healthy tissues. There have been many advances in the optimization of ionizing radiation sources and targeting tumor tissues with advanced imaging techniques in the last century, and as a result, RT is currently used to treat approximately half of all cancer patients. The therapeutic ratio of RT can be further enlarged with radiosensitizers (or radioenhancers) that, when accumulated in tumors, increase differential radiation absorption between healthy and tumor tissues. Although high-Z NPs including HfO2 and Au NPs have been extensively studied, no NP-based radiosensitizer has been approved by the FDA for clinical use. Research on Au NP radiosensitization revealed that ROS generation is inversely proportional to the diameter of Au NPs,31 which suggests that specific surface area may be an important design parameter for radiosensitization.
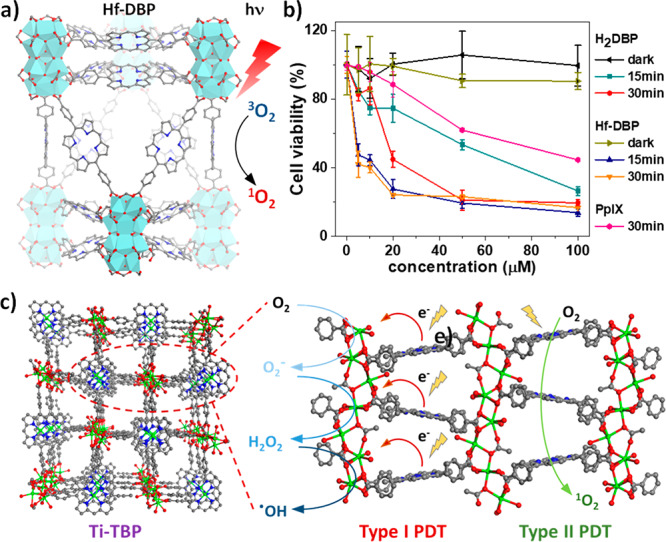
Based on this insight, in 2018, we reported the rational design of nMOFs for radiosensitization.32 By tuning the temperature and modulators, two Hf-based nMOFs with Hf6-oxo or Hf12-oxo SBUs, Hf6-DBA and Hf12-DBA, were synthesized via solvothermal reactions (Figure 3a). Both nMOFs possess high-Z elements and high specific surface areas, providing an excellent opportunity to determine the role of surface areas and SBUs on radiosensitization efficiency. Aminophenyl fluorescein (APF) assays showed that nMOFs produced significantly more •OH than HfO2 NPs (Figure 3b). It was reasoned that when an incident X-ray encounters Hf-oxo SBUs, it either directly elicits radiolysis to generate •OH, which can readily diffuse out of nMOFs through open channels, or generates lower-energy secondary radiation, which is absorbed by neighboring SBUs to further promote energy deposition in the periodic framework for enhanced radiosensitization (Figure 3c). In contrast, •OH can only be generated on the surface of solid HfO2 and the secondary radiation cannot be effectively used due to relatively low probability of encountering other NPs. The strong radiosensitization by nMOFs was supported by clonogenic assays, the gold standard to evaluate the effect of radiosensitization. Furthermore, Hf12-DBA outperformed Hf6-DBA in both APF and clonogenic assays, because of the higher X-ray energy absorption efficiency by each Hf12-oxo SBU over two Hf6-oxo SBUs, as indicated by X-ray radioluminescence study of related anthracene-based nMOFs. This study paves the way to design more-efficient nMOF radiosensitizers with electron-dense SBUs.
Figure 3.
(a) Structure models of Hf6-oxo, Hf12-oxo, Hf6-DBA, and Hf12-DBA. (b) •OH generated from HfO2, Hf6-DBA and Hf12-DBA upon irradiation probed by APF. (c) Schematic showing the radiosensitization process by Hf12-DBA.32 (d) Schematic illustration of radiosensitization by POM@Hf12-DBB-Ir with three different high-Z components for multifarious ROS generation.33 (e) •OH generation by POM@Hf12-DBB-Ir determined by APF assay. (f) O2– generation by POM@Hf12-DBB-Ir, as detected by ESR. [Reprinted with permission from ref (32). Copyright 2019, American Chemical Society, Washington, DC.]
To further enhance radiosensitization, we incorporated three different high-Z elements into a Hf12 nMOF in 2019.33 W18@Hf12-DBB-Ir was hierarchically assembled from Hf12-oxo clusters, Ir-based bridging ligands, and W-based polyoxometalates (POMs) in a two-step synthesis (Figure 3d). Upon X-ray irradiation, W18@Hf12-DBB-Ir significantly generated •OH from Hf12-oxo-mediated radiolysis, 1O2 from DBB-Ir-mediated radiodynamic effect, and O2– from W18 POM-mediated electron transfer, respectively. The synergistic effect of three different high-Z components in close proximity in W18@Hf12-DBB-Ir led to effective energy deposition and generation of three distinct ROSs, which was probed in both test tubes and in vitro studies. W18@Hf12-DBB-Ir showed stronger •OH generation than any other radiosensitizer, because of stronger X-ray absorption (Figure 3e). Electron spin resonance (ESR) studies showed that physically mixing W18 with Hf12-DBB-Ir did not enhance the generation of O2–, compared to W18 POMs (Figure 3f), indicating synergistic radiosensitization from the hierarchically assembled high-Z components via maximizing X-ray absorption and generating multifarious ROSs.
Radiotherapy–Radiodynamic Therapy
While PDT efficiently generates potent 1O2 from photoexcited PSs, limited light penetration depth has prevented its widespread use in treating deep-seated tumors. Ionizing radiations such as X-rays and γ-rays penetrate deeply into tissues but have relatively low efficiency in causing radiolysis and DNA damage. The Lin laboratory developed a new therapeutic modality termed RT-RDT by combining ionizing radiation and photosensitizing nMOFs.34 In the RT-RDT process, high-Z SBUs act as an energy absorber to not only enhance water radiolysis of X-rays or γ-rays to lead to RT effects but also transfer energy to photosensitizing linkers to generate 1O2 for RDT effects (Figure 4a). Lin and co-workers first published RT-RDT with Hf-DBP in 2018.35 Built from Hf12 SBUs and photosensitizing DBP linkers, Hf-DBP showed excellent antitumor effects when activated by ionizing radiations to effectively regress tumors in multiple cancer models. By generating •OH via Hf12-oxo mediated RT and 1O2 via energy transfer from Hf12-oxo SBUs to DBP linkers upon X-ray irradiation (Figure 4b), Hf-DBP effectively combines the advantages of PDT and RT to realize the new RT-RDT therapeutic modality.
Figure 4.
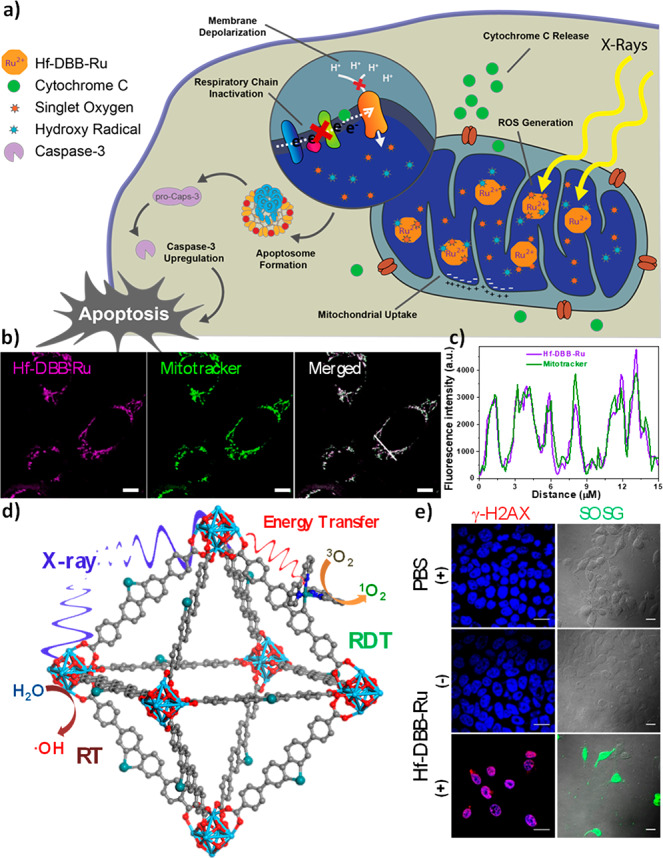
(a) Mitochondria-targeted RT-RDT by Hf-DBB-Ru.36 (b) Confocal images showing colocalization of Hf-DBB-Ru and mitochondria (scale bar = 50 μm). (c) Topographic profiles showing fluorescence intensities of straight white lines marked in panel (b). (d) Hf-DBB-Ru-mediated RT-RDT upon X-ray irradiation to generate both •OH via radiolysis and 1O2 via energy transfer to photosensitizing linkers. (e) DNA double strand breaks and 1O2 generation in vitro by Hf-DBB-Ru-mediated RT-RD, as probed by γ-H2AX and SOSG, respectively (scale bar = 10 μm).
Organelle-specific nMOFs can further enhance therapeutic efficacy of RT-RDT by generating ROSs at specific cellular compartments. We realized mitochondria-targeted RT-RDT with a tris(2,2′-bipyridyl)ruthenium(II) [Ru(bpy)32+]-based nMOF (Figure 4c).36 Hf-DBB-Ru was constructed from Hf6-oxo SBUs and Ru(bpy)32+ -based linkers with a dispersed positive charge on the surface for mitochondria targeting (Figures 4d and 4e). Upon irradiation with X-rays, Hf6-oxo SBUs in Hf-DBB-Ru efficiently absorb X-rays to enhance RT via •OH generation and enable RDT via exciting Ru(bpy)32+ to generate 1O2, eliciting strong cytotoxicity, as shown by clonogenic and MTS assays. Mitochondria-targeted RT-RDT depolarized the mitochondrial membrane potential, released cytochrome c, and disturbed the respiratory chain to initiate apoptotic pathways for programmable cell death.
Chemodynamic Therapy
CDT kills tumor cells with ROSs generated from endogenous chemical triggers, such as H2O2, hormonal metabolites, and glutathione. Many metal oxide NPs containing redox-active elements such as Mn, Fe, and Cu have been used to decompose intratumoral H2O2 to generate cytotoxic •OH through Fenton-like reactions to achieve CDT.37
Estradiol is overexpressed in many cancers. Bioavailable Cu2+ ions can catalyze estradiol metabolism to generate ROSs.38 In 2019, Lin and co-workers reported the use of Cu-TBP nMOF to mediate synergistic CDT and PDT for antitumor treatment.39 Cu-TBP decomposes in acidic tumor microenvironments to turn on the PDT effect of porphyrin and simultaneously release Cu2+ ions to hijack the estradiol metabolic pathway and promote cytotoxic ROS generation. Screening intracellular estradiol concentrations of different cell lines showed that melanoma cell B16F10 and ovarian cancer cell SKVO-3 expressed high levels of estradiol. They were chosen to test Cu-TBP-mediated CDT. Test tube and in vitro studies showed the generation of H2O2, •OH, and O2– when irradiating Cu-TBP with light via Cu-estradiol redox cycle and light-triggered, porphyrin-based PDT. Cu-TBP plus light treatment regressed B16F10 tumors with a tumor growth inhibition index (TGI) of 96.6% and completely eradicated SKOV-3 tumors with a TGI of 100%. Cu-TBP-mediated dual-triggered radical therapy also decreased intratumoral estradiol levels quantified by ELISA. This study shows the feasibility of using nMOFs to mediate CDT of hormonally dysregulated tumor phenotypes.
Cancer Immunotherapy
Advanced tumors escape immune surveillance by hijacking immunosuppressive cells, dysregulating cell signaling pathways, and deactivating effector cells/molecules. Cancer immunotherapy, particularly checkpoint blockade immunotherapy (CBI), has become an important treatment modality with acceptable side effects for some cancers by reactivating the host antitumor immunity. However, CBI and other immunotherapies typically do not work on nonimmunogenic (“cold”) tumors with immunosuppressive tumor microenvironments. ROSs generated by nMOFs can lead to highly inflammatory tumor microenvironments to synergize with immunotherapies to break immune tolerance and potentiate antitumor immunity.
Lin and co-workers reported the first use of nMOFs to synergize PDT with cancer immunotherapy in 2016 (Figure 5a).40 Hf-TBC was constructed from 5,10,15,20-tetra(p-benzoato)chlorin (TBC) and Hf6 SBUs and loaded with 4.7 wt % small molecule indoleamine 2,3-dioxygenase (IDO) inhibitor (IDOi) to afford IDOi@Hf-TBC. IDO is overexpressed in many tumors to convert tryptophan to kynurenine, leading to a hostile environment for cytotoxic T cells. IDOi@Hf-TBC exhibited superior in vivo efficacy and abscopal effects on bilateral CT26 and MC38 tumor models. Single local IDOi@Hf-TBC injection with light irradiation led to near elimination of treated primary tumors and significant regression of untreated distant tumors on both models. Mechanistic studies showed that Hf-TBC-mediated PDT caused immunogenic cell death (ICD) of cancer cells in the primary tumors, which activated the innate immune system and stimulated a tumor-specific T cell response. In the meanwhile, IDOi@Hf-TBC released IDOi into local tumor environment and blood circulation to systemically inhibit IDO activity to reverse immunosuppressive tumor microenvironments. This synergistic combination led to robust abscopal effects.
Figure 5.
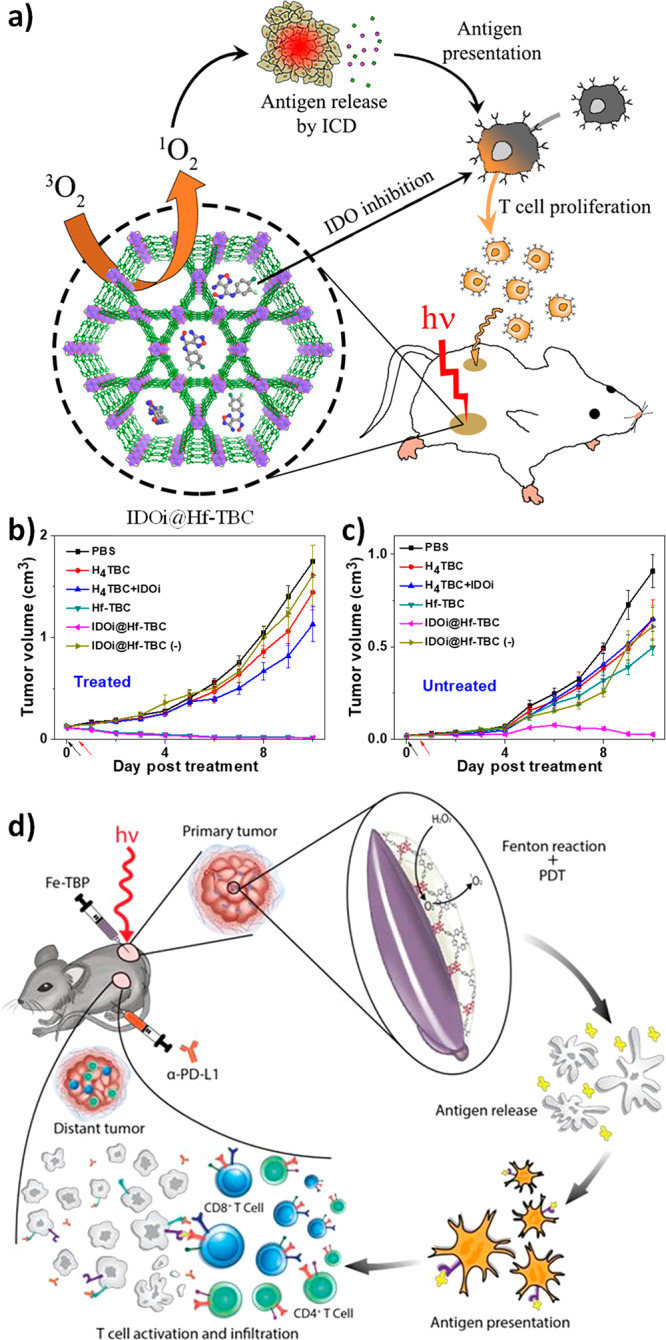
Scheme (a) and efficacy curves (b, c) to show abscopal effect of TBC-Hf-mediated local PDT synergized with IDO inhibition to attenuate immunosuppression to reactivate systemic antitumor immunity.40 [Reprinted with permission from ref (36). Copyright 2016, American Chemical Society, Washington, DC.] (d) Scheme of local nMOF-mediated hypoxic PDT on bilateral colorectal tumor model potentiated anti-PD-L1 checkpoint blockade immunotherapy to afford abscopal effect.25 [Reprinted with permission from ref (24). Copyright 2018, American Chemical Society, Washington, DC.]
ROSs generated by nMOFs have also been used to enhance therapeutic effects of CBI, which targets T cell inhibitory checkpoint signaling pathways, such as programmed cell death protein 1 (PD-1) and its ligand (PD-L1), to attenuate T cell exhaustion in immunosuppressive tumor microenvironments. Lin and co-workers showed that Fe-TBP mediated PDT significantly improved the efficacy of anti-PD-L1 treatment to elicit abscopal effects in a bilateral CT26 tumor model, leading to >90% regression of both treated primary tumors and untreated distant tumors via abscopal effects at a low Fe-TBP dose of 0.2 μmol, based on TBP and light dose of 45 J/cm2 (Figure 5d).25 Flow cytometry and immunostaining studies revealed significant tumor infiltration of cytotoxic T cells.
Zhang and co-workers designed tetrakis-(4-carboxyphenyl)tetrabenzoporphyrin (TTBP) based nMOF for PDT to enhance anti-PD-1 treatment in 2018.41 TTBP-nMOF exhibited stronger 1O2 generation than commercial PpIX and Hf-TBP. In vivo antitumor efficacy of TTBP-nMOF was tested on triple negative breast cancer 4T1 bearing mice via intravenous injection. TTBP-nMOF-mediated PDT showed strong cell killing effects and activated antitumor immune responses by increasing tumor-infiltrating T leukocytes and inflammatory cytokines. Combination therapy with anit-PD-1 was shown to suppress metastasis as well. Combination of nMOF-mediated PDT with an immune checkpoint inhibitor thus effectively extends local therapeutic effects of PDT to distant tumors via abscopal effects.
Besides inhibiting immunosuppressive pathways, nMOFs have also been used to activate immunostimulatory processes. In 2019, Lin and co-workers used cationic W-TBP nMOF to deliver anionic CpG and mediate immunogenic PDT.42 As an immunostimulatory oligodeoxynucleotide, CpG promotes antigen presentation via binding to toll-like receptor 9 in dendritic cells (DCs) but are susceptible to enzymatic degradation and cannot be efficiently internalized by cells due to their anionic nature. W-TBP allowed efficient loading of CpG and facile CpG internalization by DCs. W-TBP-mediated PDT induced ICD to release tumor antigens, whereas the delivered CpG-promoted DC maturation. Enhanced antigen presentation synergized with CBI to afford superb anticancer efficacy and robust abscopal effect with >97% tumor regression in a bilateral TUBO murine breast cancer model.
Outlook
Since the first paper on nMOF-mediated PDT in 2014,22 many research groups around the globe have developed a multitude of nMOFs with enhanced PDT efficacies by taking advantage of the molecular tunability of this unique class of nanomaterials. With the ability to integrate multiple functionalities into a single nMOF in a spatially controlled fashion, we envision several research directions of nMOF-mediated PDT aimed at addressing the issues facing conventional PSs: (1) incorporating O2-economizer and/or O2-generator into nMOFs for hypoxia-tolerant PDT; (2) optimizing dimensions of nMOFs to enhance the diffusion of ROSs for cell killing; (3) designing photosensitizing linkers to enhance absorption in the NIR spectrum while minimizing absorption in the visible spectrum; and (4) modifying the surface of nMOFs to enable systemic administration, endow biocompatibility, and enhance tumor uptake.
RT and RT-RDT overcome the limitation of shallow tissue penetration of light in PDT and are suited to treat deep-seated tumors. However, nMOF-mediated RT and RT-RDT processes are difficult to study, because of the limited understanding of ionizing radiation and matter interactions by most synthetic chemists. Several important issues must be addressed before the full potential of nMOF-mediated RT and RT-RDT can be assessed. First, the underlying physical processes of radioenhancement by nMOFs have not been elucidated. Although mathematical modeling such as Monte Carlo simulation can be used to assess enhanced utilization of secondary radiations in bulk phantoms, existing methods cannot determine amplification effects by high-Z SBUs in nMOFs. Second, the chemical processes responsible for radioenhancement are unclear. The factors that influence energy deposition, energy transfer, ROS generation and diffusion, and others are convoluted. Third, biological effects of nMOF-mediated RT and RT-RDT are extremely complicated and beyond the expertise of most synthetic chemists. It remains a great challenge to assemble committed teams with expertise in all of these areas to unravel the intricacies of nMOF-mediated RT and RT-RDT processes. From a chemical perspective, synthetic flexibility and structural tunability of nMOFs will aid the design of more potent nMOFs for radioenhancement. Two promising directions are increasing radiation energy deposition with higher Z elements, such as Pt, Au, and Bi, and incorporating small molecules as synergistic radiosensitizers.
CDT presents an interesting alternative to PDT, RT, and RT-RDT by harnessing endogenous chemical stimuli such as hormones, GSH, and H2O2 to generate cytotoxic ROS. Since CDT does not rely on external energy stimuli, in principle, it can be more tumor-specific, because metabolic abnormality likely only occurs in tumors. However, the information obtained from preclinical CDT studies on mouse models might not be translatable to human care, because of exaggerated metabolic abnormality in animal models. Redox activity of nMOF components for CDT processes can also cause general toxicity and side effects.
Cancer immunotherapy significantly broadens the utility of nMOFs in cancer treatment. On one hand, immunogenic local treatment with nMOFs generates immunostimulatory tumor microenvironments to potentiate cancer immunotherapy with tumor-infiltrating T cells. On the other hand, the systemic antitumor immunity generated by immunotherapeutic agents can extend local effects of nMOF treatment to distant tumors via abscopal effects. Recent publications have demonstrated the significant potential of nMOF-mediated PDT, RT, RT-RDT, and CDT in generating inflammatory responses and releasing tumor antigens to activate innate and adaptive immunity. The synergy observed in combining nMOF treatment with IDO inhibition or PD-1/PD-L1 blockade will inspire the extension of this strategy to other checkpoint inhibitors, immunosuppressive metabolism inhibitors, and immunostimulatory agonists. Significant research efforts on combining nMOF-mediated ROS generation and cancer immunotherapy are expected in the next few years.
Although several papers addressed nMOF toxicity in preclinical studies,35,43,44 toxicity remained a concern before nMOFs could be tested on humans. A milestone was reached when RiMO-301, which is an nMOF formulation for radioenhancement, entered the clinical trial stage in 2018 (NCT 03444714). Albeit still at an early stage, none of the patients dosed with RiMO-301 experienced treatment-related adverse events. Interestingly, one-third of the patients treated with RiMO-301 and X-ray radiation experienced durable partial responses. This first-in-human study should set the stage for many more nMOFs to be designed and tested for their therapeutic efficacy with the goal of down-selecting promising candidates to enter clinical trials. With synthetic tunability, future generations of nMOFs are expected to have enhanced efficacies for PDT, RT, RT-RDT, and CDT. Combinations of these local treatments with cancer immunotherapies can leverage strong local antitumor efficacy to afford systemic tumor rejection. The scientific community is marching closer to our vision of using nMOFs to treat cancer patients. The future of nMOF-based nanomedicines is bright and awaits talented multidisciplinary researchers to unlock their full potential in cancer therapy.
Author Contributions
The manuscript was written through contributions of all authors.
We acknowledge the National Cancer Institute (Nos. U54-CA151652, U01-CA151455, and U01-CA198989) and Department of Defense (No. PC170934P2), the University of Chicago Medicine Comprehensive Cancer Center (NIH CCSG: P30 CA014599), and the Ludwig Institute for Metastasis Research for funding support.
The authors declare the following competing financial interest(s): W.L. is founder and chairman of Coordination Pharmaceuticals, Inc., which licensed the nMOF technologies from the University of Chicago.
References
- Furukawa H.; Cordova K. E.; O’Keeffe M.; Yaghi O. M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341 (6149), 1230444. 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- Long J. R.; Yaghi O. M. The pervasive chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38 (5), 1213–1214. 10.1039/b903811f. [DOI] [PubMed] [Google Scholar]
- Horike S.; Shimomura S.; Kitagawa S. Soft porous crystals. Nat. Chem. 2009, 1 (9), 695. 10.1038/nchem.444. [DOI] [PubMed] [Google Scholar]
- Evans O. R.; Lin W. Crystal engineering of NLO materials based on metal– organic coordination networks. Acc. Chem. Res. 2002, 35 (7), 511–522. 10.1021/ar0001012. [DOI] [PubMed] [Google Scholar]
- Liu D.; Lu K.; Poon C.; Lin W. Metal–organic frameworks as sensory materials and imaging agents. Inorg. Chem. 2014, 53 (4), 1916–1924. 10.1021/ic402194c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Rocca J.; Liu D.; Lin W. Nanoscale metal–organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res. 2011, 44 (10), 957–968. 10.1021/ar200028a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C.; Liu D.; Lin W. Nanomedicine applications of hybrid nanomaterials built from metal–ligand coordination bonds: nanoscale metal–organic frameworks and nanoscale coordination polymers. Chem. Rev. 2015, 115 (19), 11079–11108. 10.1021/acs.chemrev.5b00125. [DOI] [PubMed] [Google Scholar]
- Rieter W. J.; Taylor K. M.; An H.; Lin W.; Lin W. Nanoscale metal– organic frameworks as potential multimodal contrast enhancing agents. J. Am. Chem. Soc. 2006, 128 (28), 9024–9025. 10.1021/ja0627444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K.; Aung T.; Guo N.; Weichselbaum R.; Lin W. Nanoscale metal–organic frameworks for therapeutic, imaging, and sensing applications. Adv. Mater. 2018, 30 (37), 1707634. 10.1002/adma.201707634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Zheng M.; Xie Z. Nanoscale metal–organic frameworks for drug delivery: a conventional platform with new promise. J. Mater. Chem. B 2018, 6 (5), 707–717. 10.1039/C7TB02970E. [DOI] [PubMed] [Google Scholar]
- Huxford R. C.; Della Rocca J.; Lin W. Metal–organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 2010, 14 (2), 262–268. 10.1016/j.cbpa.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C.; Lu K.; Liu D.; Lin W. Nanoscale metal–organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J. Am. Chem. Soc. 2014, 136 (14), 5181–5184. 10.1021/ja4098862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R. K.; Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7 (11), 653. 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X.; He C.; Kron S. J.; Lin W. Nanoparticle formulations of cisplatin for cancer therapy. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2016, 8 (5), 776–791. 10.1002/wnan.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Pashow K. M.; Della Rocca J.; Xie Z.; Tran S.; Lin W. Postsynthetic modifications of iron-carboxylate nanoscale metal–organic frameworks for imaging and drug delivery. J. Am. Chem. Soc. 2009, 131 (40), 14261–14263. 10.1021/ja906198y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris W.; Briley W. E.; Auyeung E.; Cabezas M. D.; Mirkin C. A. Nucleic acid–metal organic framework (MOF) nanoparticle conjugates. J. Am. Chem. Soc. 2014, 136 (20), 7261–7264. 10.1021/ja503215w. [DOI] [PubMed] [Google Scholar]
- Abanades Lazaro I.; Haddad S.; Sacca S.; Orellana-Tavra C.; Fairen-Jimenez D.; Forgan R. S. Selective surface PEGylation of UiO-66 nanoparticles for enhanced stability, cell uptake, and pH-responsive drug delivery. Chem. 2017, 2 (4), 561–578. 10.1016/j.chempr.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmans D. E.; Fukumura D.; Jain R. K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3 (5), 380–387. 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- Jin C. S.; Cui L.; Wang F.; Chen J.; Zheng G. Targeting-triggered porphysome nanostructure disruption for activatable photodynamic therapy. Adv. Healthcare Mater. 2014, 3 (8), 1240–1249. 10.1002/adhm.201300651. [DOI] [PubMed] [Google Scholar]
- Shao S.; Rajendiran V.; Lovell J. F. Metalloporphyrin nanoparticles: Coordinating diverse theranostic functions. Coord. Chem. Rev. 2019, 379, 99–120. 10.1016/j.ccr.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan G.; Ni K.; Lin W. Nanoscale metal–organic frameworks for phototherapy of cancer. Coord. Chem. Rev. 2019, 379, 65–81. 10.1016/j.ccr.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K.; He C.; Lin W. Nanoscale metal–organic framework for highly effective photodynamic therapy of resistant head and neck cancer. J. Am. Chem. Soc. 2014, 136 (48), 16712–16715. 10.1021/ja508679h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K.; He C.; Lin W. A chlorin-based nanoscale metal–organic framework for photodynamic therapy of colon cancers. J. Am. Chem. Soc. 2015, 137 (24), 7600–7603. 10.1021/jacs.5b04069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T.; Ni K.; Culbert A.; Lan G.; Li Z.; Jiang X.; Kaufmann M.; Lin W. Nanoscale Metal-Organic Frameworks Stabilize Bacteriochlorins for Type I and Type II Photodynamic Therapy. J. Am. Chem. Soc. 2020, 142 (16), 7334–7339. 10.1021/jacs.0c02129. [DOI] [PubMed] [Google Scholar]
- Lan G.; Ni K.; Xu Z.; Veroneau S. S.; Song Y.; Lin W. Nanoscale metal–organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J. Am. Chem. Soc. 2018, 140 (17), 5670–5673. 10.1021/jacs.8b01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan G.; Ni K.; Veroneau S. S.; Feng X.; Nash G. T.; Luo T.; Xu Z.; Lin W. Titanium-Based Nanoscale Metal–Organic Framework for Type I Photodynamic Therapy. J. Am. Chem. Soc. 2019, 141 (10), 4204–4208. 10.1021/jacs.8b13804. [DOI] [PubMed] [Google Scholar]
- Park J.; Jiang Q.; Feng D.; Mao L.; Zhou H.-C. Size-Controlled Synthesis of Porphyrinic Metal–Organic Framework and Functionalization for Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138 (10), 3518–3525. 10.1021/jacs.6b00007. [DOI] [PubMed] [Google Scholar]
- Liu J. F.; Guo Y. B.; Butler T. M.; Weaver M. L. Crystallography, compositions, and properties of white layer by wire electrical discharge machining of nitinol shape memory alloy. Mater. Des. 2016, 109, 1–9. 10.1016/j.matdes.2016.07.063. [DOI] [Google Scholar]
- Wang W.; Wang L.; Li Z.; Xie Z. BODIPY-containing nanoscale metal–organic frameworks for photodynamic therapy. Chem. Commun. 2016, 52 (31), 5402–5405. 10.1039/C6CC01048B. [DOI] [PubMed] [Google Scholar]
- Li Y.; Di Z.; Gao J.; Cheng P.; Di C.; Zhang G.; Liu B.; Shi X.; Sun L.-D.; Li L.; Yan C.-H. Heterodimers Made of Upconversion Nanoparticles and Metal–Organic Frameworks. J. Am. Chem. Soc. 2017, 139 (39), 13804–13810. 10.1021/jacs.7b07302. [DOI] [PubMed] [Google Scholar]
- Misawa M.; Takahashi J. Generation of reactive oxygen species induced by gold nanoparticles under x-ray and UV Irradiations. Nanomedicine 2011, 7 (5), 604–614. 10.1016/j.nano.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Ni K.; Lan G.; Chan C.; Quigley B.; Lu K.; Aung T.; Guo N.; La Riviere P.; Weichselbaum R. R.; Lin W. Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nat. Commun. 2018, 9 (1), 1–12. 10.1038/s41467-018-04703-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan G.; Ni K.; Veroneau S. S.; Luo T.; You E.; Lin W. Nanoscale Metal–Organic Framework Hierarchically Combines High-Z Components for Multifarious Radio-Enhancement. J. Am. Chem. Soc. 2019, 141 (17), 6859–6863. 10.1021/jacs.9b03029. [DOI] [PubMed] [Google Scholar]
- Lin W.; He C.; Lu K.; Ni K.; Lan G.. Nanoparticles for photodynamic therapy, x-ray induced photodynamic therapy, radiotherapy, chemotherapy, immunotherapy, and any combination thereof. U.S. Patent Application US20180153796A1, June 7, 2018.
- Lu K.; He C.; Guo N.; Chan C.; Ni K.; Lan G.; Tang H.; Pelizzari C.; Fu Y.-X.; Spiotto M. T.; Weichselbaum R. R.; Lin W. Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nat. Biomed. Eng. 2018, 2, 600–610. 10.1038/s41551-018-0203-4. [DOI] [PubMed] [Google Scholar]
- Ni K.; Lan G.; Veroneau S. S.; Duan X.; Song Y.; Lin W. Nanoscale metal-organic frameworks for mitochondria-targeted radiotherapy-radiodynamic therapy. Nat. Commun. 2018, 9 (1), 4321. 10.1038/s41467-018-06655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z.; Liu Y.; He M.; Bu W. Chemodynamic therapy: tumour microenvironment-mediated Fenton and Fenton-like reactions. Angew. Chem., Int. Ed. 2019, 58 (4), 946–956. 10.1002/anie.201805664. [DOI] [PubMed] [Google Scholar]
- Thibodeau P. A.; Paquette B. DNA damage induced by catecholestrogens in the presence of copper (II): generation of reactive oxygen species and enhancement by NADH. Free Radical Biol. Med. 1999, 27 (11–12), 1367–1377. 10.1016/S0891-5849(99)00183-5. [DOI] [PubMed] [Google Scholar]
- Ni K.; Aung T.; Li S.; Fatuzzo N.; Liang X.; Lin W. Nanoscale metal-Organic framework mediates radical therapy to enhance cancer immunotherapy. Chem. 2019, 5 (7), 1892–1913. 10.1016/j.chempr.2019.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lu K.; He C.; Guo N.; Chan C.; Ni K.; Weichselbaum R. R.; Lin W. Chlorin-based nanoscale metal–organic framework systemically rejects colorectal cancers via synergistic photodynamic therapy and checkpoint blockade immunotherapy. J. Am. Chem. Soc. 2016, 138 (38), 12502–12510. 10.1021/jacs.6b06663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J.-Y.; Zou M.-Z.; Zhang M.; Wang X.-S.; Zeng X.; Cong H.; Zhang X.-Z. π-extended benzoporphyrin-based metal–organic framework for inhibition of tumor metastasis. ACS Nano 2018, 12 (5), 4630–4640. 10.1021/acsnano.8b01186. [DOI] [PubMed] [Google Scholar]
- Ni K.; Luo T.; Lan G.; Culbert A.; Song Y.; Wu T.; Jiang X.; Lin W. A Nanoscale Metal–Organic Framework to Mediate Photodynamic Therapy and Deliver CpG Oligodeoxynucleotides to Enhance Antigen Presentation and Cancer Immunotherapy. Angew. Chem. 2020, 132 (3), 1124–1128. 10.1002/ange.201911429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamames-Tabar C.; Cunha D.; Imbuluzqueta E.; Ragon F.; Serre C.; Blanco-Prieto M. J.; Horcajada P. Cytotoxicity of nanoscaled metal–organic frameworks. J. Mater. Chem. B 2014, 2 (3), 262–271. 10.1039/C3TB20832J. [DOI] [PubMed] [Google Scholar]
- Baati T.; Njim L.; Neffati F.; Kerkeni A.; Bouttemi M.; Gref R.; Najjar M. F.; Zakhama A.; Couvreur P.; Serre C.; Horcajada P. In depth analysis of the in vivo toxicity of nanoparticles of porous iron (III) metal–organic frameworks. Chem. Sci. 2013, 4 (4), 1597–1607. 10.1039/c3sc22116d. [DOI] [Google Scholar]


