Abstract
Objective
Deficiencies and excess of essential elements and toxic metals are implicated in amyotrophic lateral sclerosis (ALS), but the age when metal dysregulation appears remains unknown. This study aims to determine whether metal uptake is dysregulated during childhood in individuals eventually diagnosed with ALS.
Methods
Laser ablation‐inductively coupled plasma‐mass spectrometry was used to obtain time series data of metal uptake using biomarkers in teeth from autopsies or dental extractions of ALS (n = 36) and control (n = 31) participants. Covariate data included sex, smoking, occupational exposures, and ALS family history. Case–control differences were identified in temporal profiles of metal uptake for individual metals using distributed lag models. Weighted quantile sum (WQS) regression was used for metals mixture analyses. Similar analyses were performed on an ALS mouse model to further verify the relevance of dysregulation of metals in ALS.
Results
Metal levels were higher in cases than in controls: 1.49 times for chromium (1.11–1.82; at 15 years), 1.82 times for manganese (1.34–2.46; at birth), 1.65 times for nickel (1.22–2.01; at 8 years), 2.46 times for tin (1.65–3.30; at 2 years), and 2.46 times for zinc (1.49–3.67; at 6 years). Co‐exposure to 11 elements indicated that childhood metal dysregulation was associated with ALS. The mixture contribution of metals to disease outcome was likewise apparent in tooth biomarkers of an ALS mouse model, and differences in metal distribution were evident in ALS mouse brains compared to brains from littermate controls.
Interpretation
Overall, our study reveals direct evidence that altered metal uptake during specific early life time windows is associated with adult‐onset ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal disease characterized by motor neuron degeneration and complex pathogenesis.1 The gene‐time‐environment hypothesis of ALS suggests that environmental exposures, superimposed on genetic risk and aging, underlie the pathogenesis of ALS.2 Metals are plausible risk factors for ALS due to their role in biology and widespread exposure.3 The extent to which metal toxicity promotes late‐onset neurodegeneration, however, depends on exposure duration, developmental stage, metal toxicokinetics, and interactive toxic mechanisms in metal mixtures.4, 5
While there is an urgent need to identify metal exposures associated with ALS to improve our pathophysiologic understanding and modify disease risk, there are limitations in quantifying early life exposure to metals using surveys or biofluids after disease onset. The teeth are an attractive tissue to overcome these limitations because of their unique developmental properties. Deciduous (baby) teeth commence development prenatally, and permanent (adult) teeth around birth, growing in an incremental manner similar to growth rings in trees.6 During mineralization, essential nutrient elements and toxic metals that enter the blood circulation after exposure or as part of normal metabolism are captured in these chronological rings. The growth rate of teeth is well known because teeth undergo limited remodeling, except in the case of dental caries or attrition, trace element deposits in teeth are stable. Therefore, retrospective and accurate early life metal uptake assessment is reliable.7, 8
We have previously used laser ablation‐inductively coupled plasma‐mass spectrometry (LA‐ICP‐MS) to develop temporal profiles of metal exposure during early development associated with mental health conditions using deciduous teeth.9, 10, 11, 12, 13 This technique uses a laser beam to scan along the growth trajectory of the tooth and the ejected metal deposits are transferred to a mass‐spectrometer for quantitative assessment of metal content. Here, we refined this technique to analyze permanent teeth of ALS participants and neurologically healthy controls. We uncovered metal uptake differences between groups and discrete developmental windows – postnatally to approximately 15 years – when metals, individually and in combination, are significantly associated with ALS onset decades later. We further verified the relevance of metal dysregulation in a transgenic mouse model of ALS. This is the first study to retrospectively link early life metal dyshomeostasis to ALS risk using tooth biomarkers.
Participants and Methods
Study participants
ALS participants diagnosed in accordance with the revised EI Escorial criteria14 were recruited from the University of Michigan ALS Clinic. C9orf72 genotyping was performed as previously reported15 and Cu2+/Zn2+ superoxide dismutase‐1 (SOD1) genotyping was performed in a CLIA‐certified laboratory. Control participants undergoing multiple teeth extractions were recruited at the University of Michigan School of Dentistry. Adult participants and/or next of kin provided informed consent. This research was approved by the University of Michigan Institutional Review Board (#HUM00028826).
Sample collection and analyses for human samples
Human permanent teeth were obtained at autopsy or routine dental extractions. Metal concentrations were determined by LA‐ICP‐MS.10 A New Wave Research NWR‐193 laser ablation unit (Electro Scientific Industries) with a 193 nm ArF excimer laser connected to an Agilent Technologies 8800 triple‐quad ICP‐MS (Agilent Technologies) was used. Helium was the carrier gas from the laser ablation cell and was mixed with argon before entering the ICP‐MS via a Y‐piece. Sensitivity (maximum analyte ion counts), oxide formation (232Th16O+/232Th+, < 0.3%), and fractionation (232Th+/238U+, 100 ± 5%) were monitored daily using NIST SRM 612 (trace elements in glass). The laser was scanned in dentine parallel to the dentine‐enamel junction from the dentine horn tip toward the tooth cervix. A pre‐ablation scan removed surface contamination. Data were analyzed as metal (metal studied) to calcium (internal standard) ratios to control for variations in mineral content within a tooth and between samples. Each tooth was sampled, on average, at over 5000 locations (Table 1). We performed an untargeted scan and 11 metals were reliably detected in our analysis – barium, chromium, copper, lead, lithium, magnesium, manganese, nickel, strontium, tin, and zinc. All are either essential elements or neurotoxic metals we have previously analyzed.13 Other metals were not measured because they were either below detection limits (e.g., aluminum and mercury) or because they are not reliably detected by LA‐ICP‐MS (e.g., fluoride and iron).
Table 1.
LA‐ICP‐MS operating conditions for human and murine teeth and brain analysis.
| Teeth | Brain | ||||||
|---|---|---|---|---|---|---|---|
| NWR‐193 Laser (Human/Mouse) | Agilent 8800 ICP‐MS (Human/Mouse) | Cetac LSX − 213G2 + (Mouse) | Agilent 8900 ICP‐MS (Mouse) | ||||
| Wavelength (nm) | 193/193 | RF power (W) | 1350/1350 | Wavelength (nm) | 213 | RF power (W) | 1350 |
| Helium carrier flow (Lmin–1) | 0.8/0.8 | Argon carrier flow (Lmin–1) | 0.6/0.6 | Helium carrier flow (Lmin–1) | 0.75 | Argon carrier flow (Lmin–1) | 0.55 |
| Fluence (Jcm2) | 5/3 | Plasma gas flow (Lmin–1) | 15/15 | Fluence (mJ) | 0.05 | Plasma gas flow (Lmin–1) | 15 |
| Repetition rate (Hz) | 10/20 | Sample Depth (mm) | 4/4 | Repetition rate (Hz) | 20 | Sample Depth (mm) | 4 |
| Spot size (μm) | 35/25 | Scan mode | Peak hopping | Spot size (μm) | 30 | Scan mode | Peak hopping |
| Scan speed (μm s–1) | 35/25 | Integration time (ms) | 50–55/22‐100 | Scan speed (μms–1) | 75 | Integration time (ms) | 27‐32 |
Demographic differences in case and control participants were determined via chi‐squared, Fisher's exact, or Student’s t‐test. We applied a variant of distributed lag models (DLMs) to identify specific time periods (birth to 15 years) when the above 11 individual metals were differentially absorbed. DLMs adjust for past (lagged) exposures to identify differences in temporal profiles of metal exposure between groups.16 Confidence intervals (CIs), projected on the case–control divergence in metal concentrations over time, identified critical periods by the separation of model CIs from a zero boundary (critical susceptibility window).17
Developmental windows associated with ALS and the relative contribution to health outcome from exposure to all metals jointly or individually were determined by weighted quantile sum (WQS) regression. WQS is a penalized regression technique that uses a nonnegative, unit‐sum constraint grouping variables into a unidimensional index. Each variable of the index (metals) contributes to the index value (0 and 1 or −1 and 0). The index emerges as a weighted sum of the component variables, where the weights are estimated empirically. Because the WQS regression estimates the individual and combined effects of metals on ALS at a prespecified time window, rather than the entire time period studied, we used lagged WQS. Incorporating features of DLMs, the WQS index was calculated over time while adjusting for past lagged values, resulting in a “lagged” WQS.4, 18, 19 Lagged WQS identified time periods at which metals in a mixture were significantly different between groups. We calculated 95% CIs to identify statistically significant periods of case–control differences and applied Holm–Bonferroni corrections to adjust for multiple comparison effects.16, 20 A sensitivity analysis was performed by removing subjects with a family history of ALS or known genetic mutations. The type of tooth does not impact the statistical models used.
ALS animal model, sample collection, and analysis
Eight male and eight female hemizygous mice expressing mutant human SOD1‐G93A [B6.Cg‐Tg(SOD1*G93A)1Gur/J]21 (SOD1G93A) and 16 sex‐matched wild‐type (WT) control littermates (JAX stock #004435; Jackson Laboratories, Bar Harbor, Maine) were fed with a 5L0D rodent diet, which contains chromium, cobalt, copper, magnesium, manganese, iron, selenium, and zinc, and maintained and housed at the University of Michigan following the Committee on the Care and Use of Animals guidelines (approval #PRO00008431).22 Four animals per group were sacrificed using pentobarbital (Vortech, Dearborn, MI) at the ages of 41, 73, 120 days, and end stage. End stage was determined based on weight lost, limb paralysis, or lack of righting reflexes.23 Age‐ and sex‐matched controls were sacrificed at the same time for each SOD1G93A mouse. Animals were genotyped by PCR as per the supplier’s instructions. Brains were frozen in liquid nitrogen and lower mandibles were dissected for LA‐ICP‐MS analysis.
Metal concentrations in mouse incisors were determined at specific locations by LA‐ICP‐MS (Table 1). A two‐tiered statistical approach was implemented to evaluate the discrete effects of individual elements, and to evaluate the combined mixture effect. To address the first goal, simple linear models were constructed to compare metal mean counts of SOD1G93A and littermate controls while controlling for age and sex. A logistic WQS regression model was constructed24 to evaluate if mixture effects predicted the SOD1G93A genotype.
For murine brain metal distribution analysis, 30‐µm frozen brain sections from transgenic and WT mice were qualitatively analyzed by LA‐ICP‐MS. A Cetac LSX − 213G2 + laser ablation unit (Teledyne Instruments, Inc.) with 213nm Nd:YAG laser connected to an 8900 triple‐quad ICP‐MS (Agilent Technologies) was used (Table 1).
Results
Participants and samples
Permanent teeth were analyzed from 36 ALS and 31 control participants (Table 2). Two cases were born in earlier decades compared to controls (Fig. S1). ALS onset was bulbar for 13 participants (36.1%) and limb for 23 participants (63.9%). Most (88.9%) had sporadic ALS, while four participants were having familial ALS (fALS) – two were positive for pathological C9orf72 GGGGCC expansions, one was positive for SOD1 A4V, and one had a SOD1 A4V family history of ALS. Samples were taken at autopsy for 35 of the ALS participants, and from routine dental extractions for all controls and one ALS participant. Sex distribution was similar for ALS and control participants. Except for one subject of unreported ethnicity, ALS participants were Caucasian, while the control group consisted of Caucasians (83.9%), Black/African Americans (12.9%), or other ethnicity (3.2%). Smoking was less common in ALS participants than in controls (50.0% of cases versus 77.4% of controls, P = 0.004). The samples consisted of first molars (43%), followed by second molars (24%), canines (13%), incisors (12%), premolars (4%), and third molars (3%).
Table 2.
Characteristics of study participants.
| Characteristics | ALS group (n = 36) | Control group (n = 31) | P‐value | |
|---|---|---|---|---|
| Age (years)(a,b,c) | 63.0, 62.1 ± 1.8, (27‐87) | 57.5, 55.5 ± 2.1 (25‐74) | 0.0180(f) | |
| Sex | Male | 17 (47.2%) | 16 (51.6%) | 0.808(g) |
| Female | 19 (52.8%) | 15 (48.4%) | – | |
| Onset | Bulbar | 13 (36.1%) | – | – |
| Limb | 23 (63.9%) | – | – | |
| Family history/known causative gene | Yes(d) | 4 (11.1%) | – | – |
| No(e) | 32 (88.9%) | – | – | |
| Ethnicity | Caucasian | 35 (97.2%) | 26 (83.9%) | 0.047(h) |
| Black/AA | – | 4 (12.9%) | – | |
| Other | – | 1 (3.2%) | – | |
| NA | 1 (2.8%) | 0 | ||
| Tobacco | Yes | 18 (50.0%) | 24 (77.4%) | 0.004(g) |
| No | 18 (50.0%) | 4 (12.9%) | – | |
| NA | – | 3 (9.7%) | – | |
(a) median; (b) mean ± standard error; (c) range; (d) familial ALS was determined by known family history or genetic alterations for SOD1 or C9orf 72; (e) one adopted participant was classified as sporadic ALS as it was negative for abnormal C9orf72 expansions; (f) Student’s t‐test; (g) Fisher's exact test: male and female for sex, Yes and No answers for smoking; (h) Chi‐squared test: Caucasian, black/AA, and other for ethnicity; AA, African American; NA, Data not available.
Critical windows of ALS case–control divergence for individual metals
DLMs revealed a significant increase in individual metals uptake in ALS cases versus controls and the developmental stages of maximal difference were distinct for each metal (Fig. 1A, arrows). Chromium uptake increased after age 10 in cases. In contrast, manganese was significantly higher in cases from birth until approximately 6 years and it was significantly lower between 12 and 15 years. Nickel and tin showed discrete windows of increased uptake in cases, from 6 to 10 years for nickel and from 0 to 2.5 years for tin. Zinc levels were significantly higher in cases throughout the study period. Although not significant, cases showed an increasing trend for copper uptake between birth and 10 years and for lead from 12 to 15 years, and a decreasing trend for lithium from birth to 15 years. At the point of maximal difference, ALS cases had higher uptake levels than controls by 1.49 times for chromium (95% CI, 1.11–1.82; at 15 years), 1.82 times for manganese (95% CI, 1.34–2.46; at birth), 1.65 times for nickel (95% CI, 1.22–2.01; at 8 years), 2.46 times for tin (95% CI, 1.65–3.30; at 2 years), and 2.46 times for zinc (95% CI, 1.49–3.67; at 6 years) (Fig. 1B).
Figure 1.
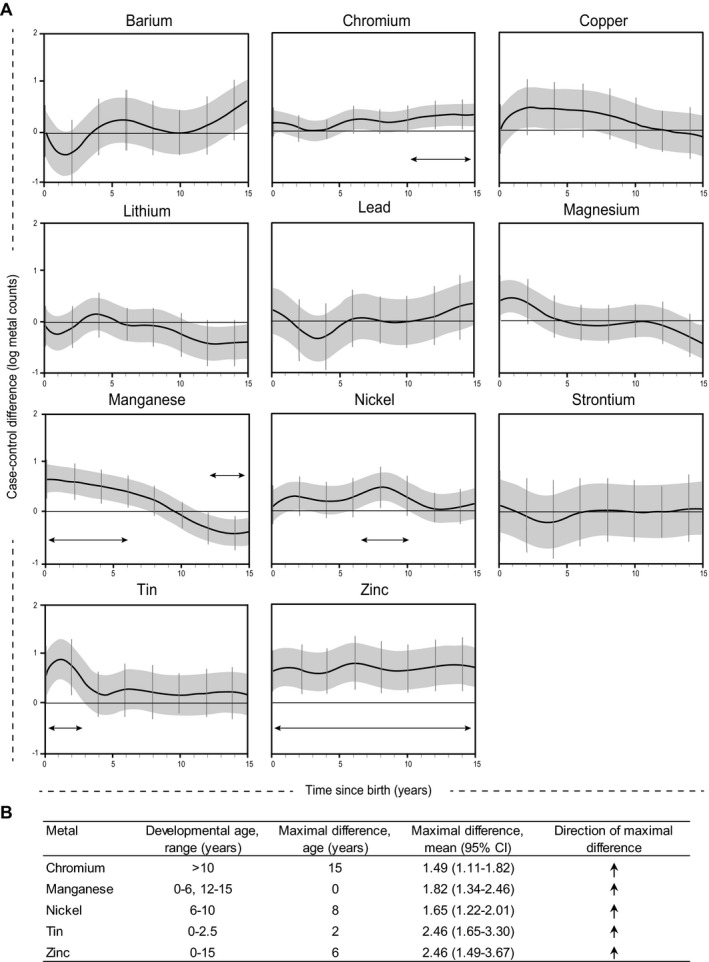
Early life developmental differences in metal uptake between ALS cases and controls. (A) Graphical representation of reverse DLMs. Results were adjusted for batch, sex, age at tooth extraction, smoking history, and intra‐subject correlated observations. Significant windows of exposure are defined as periods where the 95% piecewise CIs (grey bands) and the 95% Holm–Bonferroni family‐wise CIs (vertical bars) do not cross zero. ALS cases, n=36; controls, n=31. The developmental stages of maximal difference are indicated by arrows. (B) Critical windows of divergence for individual metals between cases and controls observed in (A).
Co‐exposure to metal mixtures in ALS
Metals generally exist as mixtures; therefore, we examined the contribution of multiple metals exposure during childhood on ALS development using lagged WQS. We found higher metal concentrations in the ALS cases during three exposure windows (Fig. 2A). In the first exposure window (0‐ <2 years), the 11 metals tested (barium, chromium, copper, lithium, magnesium, manganese, nickel, lead, tin, strontium, and zinc) showed higher concentrations in a mixture, each metal contributing similarly. In contrast, in the second exposure window (7‐9 years), barium, copper, lead, and zinc showed the greatest differences. In the third exposure window (13‐15 years), barium, copper, lithium, lead, magnesium, tin, and zinc showed the greatest differences between groups compared to the other three components at 15 years (Fig. 2B). These data indicate that: 1) overall metal dysregulation in ALS occurs in early childhood; however, this effect is primarily limited to a few metals in adolescence; 2) barium, copper, lead, strontium, and zinc are the strongest drivers associated with ALS when considered in a mixture in late childhood and adolescence; and 3) the effects of copper and lead are only significant with other metal exposures (Fig. 1).
Figure 2.
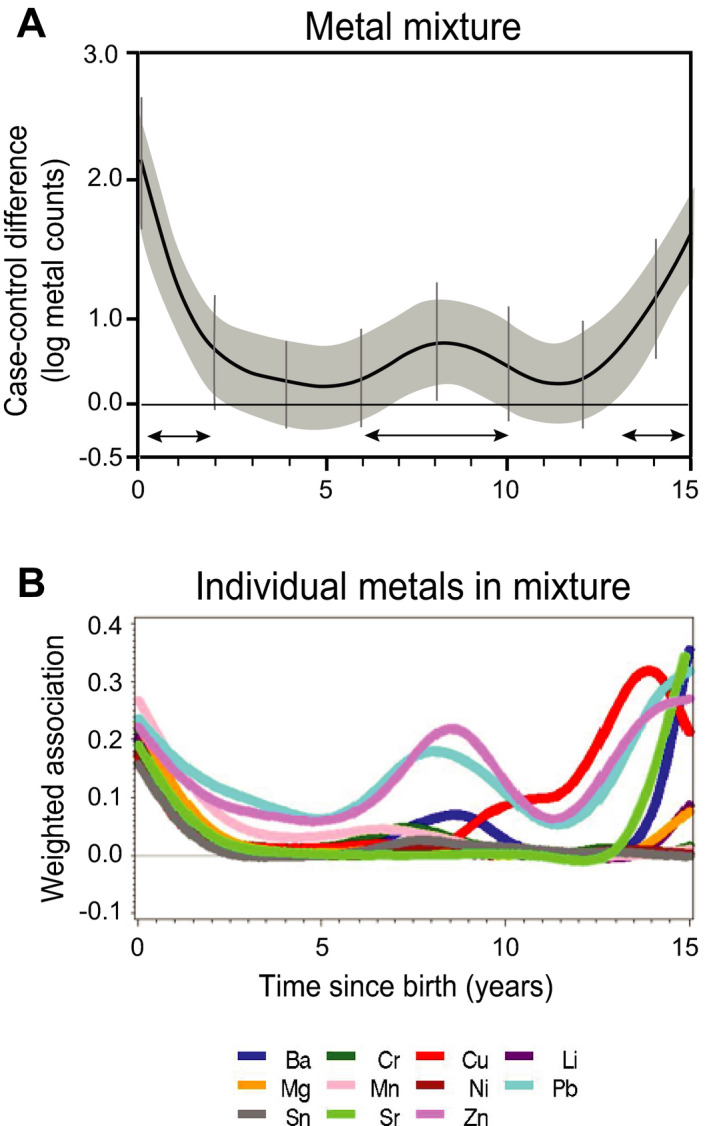
Co‐exposure of multiple metals during childhood is associated with ALS in adulthood. Lagged WQS analysis of 11 metals for ALS cases (n = 36) versus controls (n = 31). (A) Weighted DLM adjusted for batch, sex, age at tooth extraction, smoking history, and intra‐subject correlated observations, where 95% piecewise CIs of the index (grey bands) and 95% Holm–Bonferroni family‐wise CIs adjusted for multiple comparisons (vertical bars) represent the regions of the graph that are statistically significant. (B) Relative weights of each metal in the mixture over time. Barium, Ba; chromium, Cr; copper, Cu; lithium, Li; magnesium, Mg; manganese, Mn; nickel, Ni; lead, Pb; tin, Sn; strontium, Sr; and zinc, Zn. Three developmental exposure windows at which metal concentrations are higher in the ALS cases compared to controls are indicated by arrows (0– <2, 7–9 and 13–15 years).
A sensitivity analysis excluding the fALS cases did not show significant changes in the direction of metal uptake and the maximal differences between groups when the metals were studied individually or in a mixture (Figs. S2, S3A). However, lead and zinc have a larger contribution to the mixture effect between 5 and 12 years and no late contributions (13–15 years) of copper, strontium, and barium in mixtures were observed in the absence of the fALS cases (Fig. S3B).
Interactive effect of metal mixtures in an ALS mouse model
We examined tooth biomarkers in an ALS mouse model overexpressing human mutant SOD1G93A. Element analyses of murine incisors at specific ages or when studied as a group did not identify fluctuations in metal uptake associated with ALS, individually or in combination (Fig. 3A, Table S1). Marginal differences were observed for manganese (P < 0.08) and cobalt (P < 0.1). However, WQS analysis revealed that a metal mixture is significantly different between SOD1G93A versus WT mice (P < 0.037). This effect is driven by manganese, cobalt, chromium, calcium, strontium, and zinc, with smaller contributions from other metals (Fig. 3B). The mixture contribution of metals to disease outcome in an environmentally controlled animal model of ALS may be more meaningful than studying each metal individually.
Figure 3.
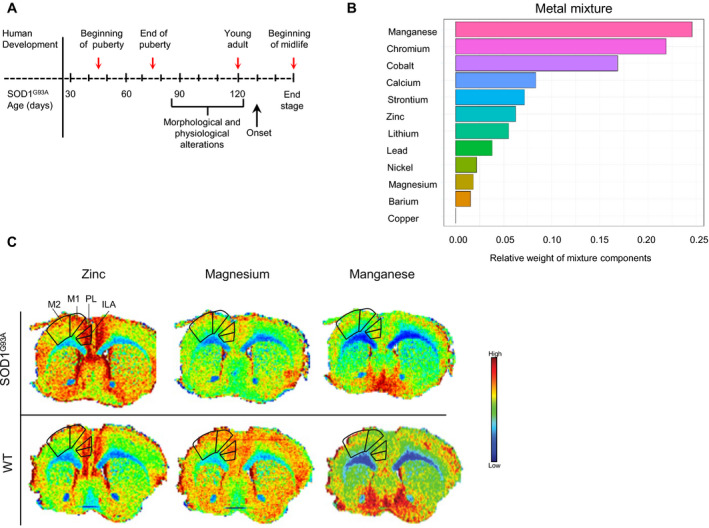
Metal dyshomeostasis in a mouse model of ALS. (A) Lower mandibles and whole brains were harvested from SOD1G93A and littermate control mice at 47, 71, 120, and ~160 days of age (red arrows). Corresponding developmental periods in humans are indicated (top). Morphological and physiological alterations such as neuromuscular junction dysfunction and motor skill deterioration in SOD1G93A mice develop before disease onset. (B) LA‐ICP‐MS was used to determine metal concentrations in teeth and mixture analyses were performed by WQS to identify the relative weights (importance) of each metal in the mixture when comparing transgenic to wild‐type (WT) mice by groups (P = 0.037; SOD1G93A n = 14; WT n = 15). (C) Brain distribution of biometals is altered in transgenic mice. Representative metal spatial concentration for zinc, magnesium, and manganese by LA‐ICP‐MS on 30 µm anterior sections of coronal mouse brain from SODG93A and WT animals (end‐of‐stage). Anatomical regions of interest are delineated. M1, primary motor cortex; M2, secondary motor cortex; PL, prelimbic area; ILA, infralimbic area. For elemental maps, pixel size is approximately 30 × 30 µm.
Metal distribution in brain is altered in an ALS mouse model
To further elucidate metal dysregulation in ALS, we visualized metal spatial distribution in mouse brain sections by LA‐ICP‐MS. Qualitative analysis of whole brain maps from SOD1G93A and WT mice showed clear differences of metal distribution. In particular, we observed increased zinc and decreased magnesium levels in transgenic mice compared to WT mice, especially in regions such as the motor cortex, the prelimbic, and infralimbic areas of the frontal cortex, and nucleus of the vertical limb of the diagonal band. No appreciable differences were observed with manganese between groups (Fig. 3C).
Discussion
Epidemiological and clinical evidence links metal exposure to ALS risk,25 and metal deposits in teeth are stable and reflect exposure and metal metabolism during development. Hence, we analyzed human permanent teeth to retrospectively determine whether early life metal dyshomeostasis associates with ALS. We detected increased uptake of metals in ALS cases, including chromium, manganese, nickel, tin, and zinc, all of which have been associated with ALS after diagnosis.3, 25 In a mixtures analysis, we identified critical developmental windows where increased uptake of 11 metals were associated with ALS. Finally, we analyzed tooth and brain biomarkers from a mouse model of fALS, which supported the mixture contribution of metals to disease outcome. This study represents the first direct evidence showing that early life metal dysregulation is associated with ALS using permanent teeth as metal uptake biomarkers.
Although the etiology of ALS is unknown, metal dyshomeostasis is linked to cellular mechanisms dysregulated in ALS, including energy metabolism, protein control, oxidative stress, glutamate excitotoxicity, neuroinflammation, and nucleocytoplasmic transport.26, 27, 28 We observed the uptake of essential metals that are involved in several biological processes and are important for the proper function of proteins encoded by ALS‐associated genes. For example, copper and zinc are cofactors for SOD1 while manganese is a cofactor for SOD2 and glutamine synthetase29, and altered levels and chronic exposure to these metals lead to aggregation and cellular mislocalization of SOD1 and transactive response DNA binding protein 43 KDa (TDP‐43).30, 31, 32 Moreover, the redox capacity of copper increases oxidative stress, while zinc and manganese accumulation in motor neurons and astrocytes contributes to excitotoxicity.31, 33, 34 In addition, overexposure to nickel results in damage to mitochondria and alterations in cognition and locomotion,35, 36 while high levels of barium compromises myelin sheath integrity and alters sodium–potassium ion pumps in neurodegeneration.37 Nonessential metals, including lead and tin, also induce cellular injury by increasing damaging reactive oxygen species and promoting mitochondrial dysfunction, altered energy metabolism, and cytoplasmic TDP‐43 aggregation.38, 39
Humans are exposed to multiple toxic metals and essential elements throughout life. Our results support the growing notion that in a complex mixture of environmental chemical exposures, toxicants have an additive or synergistic effect on health outcomes.4, 40, 41 Moreover, early life environmental insults are linked to predisposition to late‐onset neurodegeneration.42 Therefore, our analysis is unique as it retrospectively distinguishes developmental windows at which metal interactions may contribute to ALS onset.
The dynamic interplay between metals, aging, and genetics has been reported in fALS animal models and autopsy tissue. Each region of the central nervous system displays a unique normal metal pattern associated with its function. However, copper, iron, manganese, and zinc are redistributed and aberrantly accumulated in the central nervous system in an age‐dependent manner or in association with ALS.43, 44, 45, 46, 47 The metal accumulation rate in the motor cortex and the prefrontal cortex in ALS, for example, may exceed the ability of antioxidant defense and detoxification mechanisms of neurons in those regions to cope with metal‐dependent reactive oxygen species generation, and may thus contribute to impaired executive function and conditioned fear in ALS.48
Our sensitivity analysis yields interesting insights, especially when considered within the context of toxicant–gene interactions and the multistep ALS model.2, 49 Lead and zinc are strongly associated with sALS in a mixture analysis between the ages of 5 and 12 years, while barium, copper, and strontium are less so. Metals may also influence disease outcome differently based on an individual’s genetic background. A person with sALS may be more influenced by lead and zinc in mid‐childhood, whereas those with fALS may be impacted by other metals, such as barium, copper, and strontium in mid‐adolescence. In this study, our small sample size limited us from exploring metal‐by‐metal and metal‐by‐genotype interactions. Nonetheless, considering that our mouse model, contained within a highly controlled environment, also shows a contribution of metals to ALS risk, it does give credence to our findings in humans. Metal dyshomeostasis in tooth biomarkers from SOD1G93A mice provides a powerful tool to identify adverse environmental and genetic factors associated with molecular mechanisms triggering metal dyshomeostasis in ALS.
Although restoration of metal homeostasis provides promising results as ALS therapeutics,45, 50 early interventions may be more effective and preventative. It is becoming clear that metal–gene interactions are linked to predisposition to metal toxicity and disease; however, early life metal–gene interactions in ALS are still not completely understood. This is likely due to lack of early life biomarkers, complex and unknown disease etiology, and statistical methodology limitations.51 The present work also opens up further questions: (1) what are the mechanisms leading to early life metal dyshomeostasis and abnormal cellular distribution? and (2) how and when do early life metal–gene interactions associated with ALS risk take place? In other words, can we differentiate whether metal dyshomeostasis increases ALS risk or whether other factors leading to ALS predispose individuals to an aberrant metal accumulation?
As ALS is a late‐onset disease, and in many cases, teeth have been extensively restored or are absent at the time of death, obtaining preserved permanent teeth from ALS and control participants is challenging and limits sample size. Despite our small cohort size, our novel tooth‐matrix biomarkers and stringent statistical methods increased our power to detect statistically significant findings in multivariable‐adjusted models after controlling for any significant difference between groups. Of note, our cohort consisted of mostly Michigan residents and may restrict the generalizability of our findings, so validation in a larger cohort is needed. Furthermore, LA‐ICP‐MS is not the ideal method for measuring other elements in teeth, such as aluminum, fluoride, iron, and mercury, preventing us from studying their impact on ALS. As tooth‐matrix biomarkers represent a new methodology, future studies will yield insight into alterations from racial/ethnic differences, lifetime smoking exposure, and geographical locations. Our study’s observational design cannot confirm causation, and other interacting exposome and genetic factors, as well as metal uptake and metabolism may contribute to adverse health outcomes.40
Overall, identifying causative links between early life metal uptake and late‐onset neurodegeneration is challenging. However, our results support the gene‐time‐environment and multiple hit hypotheses by showing an association between early life metal dysregulation and ALS in different developmental windows, as well as joint effects of co‐exposure to multiple metals2, 42, 52 (Fig. 4). These effects may contribute to various pathologic alterations in cells that induce a fragile state in motor neurons during development and increase susceptibility to premature damage of neurons and supporting nonneuronal cells. Environmental and occupational exposures during early adulthood in combination with aging may then trigger ALS onset.52 Hence, while more studies are needed, the current data support the link between early life adverse systemic elemental dysregulation and adult‐onset neurodegeneration.
Figure 4.
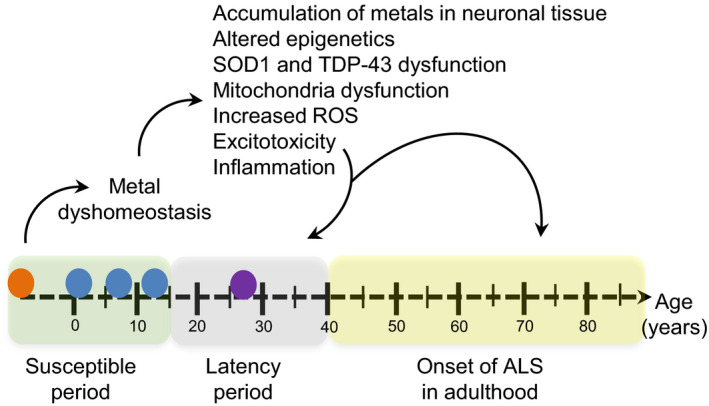
Proposed framework of metal exposure and the development of ALS. In ALS, metal dyshomeostasis may be influenced by genetic mutations (SOD1, TDP‐43, C9orf72, genetic modifiers) (orange circle) and/or exposure to environmental toxicants (blue circles) during the prenatal and childhood periods (susceptible period). Alterations in biological pathways and epigenetic mechanisms associated with ALS pathology in addition to exposure to other environmental factors (purple circle) in early adulthood (latency period) and aging may be pathological triggers or mediators of ALS onset and progression later in life.
Author Contributions
CFR and CA contributed equally to this work. CFR, TMB, ELF, and MA conceptualized and designed the study. CFR, CG, PC, GAB TMB, KAM, MA, and CA involved in acquisition and analysis of data. CFR, MA, SAG, and ELF involved in drafting and editing of the manuscript.
Conflict of Interest
All authors report no conflict of interest.
Supporting information
Figure S1. ALS cases and control participants were born in similar decades. Bar plot representing frequency distribution of birth decades for all cases versus controls was generated using Tableau. ALS cases, n = 36; controls, n = 31.
Figure S2. Early life developmental differences in metal uptake between sporadic ALS cases and controls. Reverse DLMs were adjusted for batch, sex, age at tooth extraction, smoking history, and intra‐subject correlated observations. Sensitivity analysis removing fALS samples indicated similar trends of metal uptake as when all the samples are included in the analysis (see Fig. 1). Sporadic ALS, n = 32 and controls, n = 31. Developmental exposure windows, arrows.
Figure S3. Sensitivity analysis of uptake and contribution of metals to sporadic ALS when analyzed as a mixture at specific developmental windows. Sensitivity analysis of lagged WQS of 11 metals for sporadic ALS cases (n = 32) versus controls (n = 31). (A) Weighted DLM adjusted for batch, sex, age at tooth extraction, smoking history, and intra‐subject correlated observations (95% piecewise CIs of the index, tan bands and 95% Holm–Bonferroni family‐wise CIs adjusted for multiple comparisons, vertical bars). (B) Relative weights of each metal in the mixture over time. Barium, Ba; chromium, Cr; copper, Cu; lithium, Li; magnesium, Mg; manganese, Mn; nickel, Ni; lead, Pb; tin, Sn; strontium, Sr; and zinc, Zn. Developmental exposure windows, arrows.
Acknowledgments
We thank the study participants and their families attending the University of Michigan ALS Clinic, the clinical arm of the ALS Center of Excellence; the Michigan Institute for Clinical and Health Research (MICHR, UL1TR000433); the Department of Oral and Maxilofacial Surgery, School of Dentistry, University of Michigan; Matthew D. Perkins, B.S. from the Michigan Brain Bank; Maegan A. Tabbey, B.S., Faye E. Mendelson, B.S., and Shayna Mason for assistance with autopsy and control teeth extractions; Andrea Smith, Jayna Duell, R.N., Blake Swihart, M.A., Elise Tuneff, B.S., and Drs. Jeffrey M. Jentzen, Roger L. Albin, and Vikram Shakkottai for facilitating sample collection; Dr. Fang He for assistance with C9orf72 genotyping; and Dr. Stacey Sakowski Jacoby for editorial assistance. At the University of Michigan, this work was supported in part by the National Institutes of Environmental Health Sciences (K23ES027221, Dr. Goutman), the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry (CDC/ATSDR Contract #200‐2013‐56856, Drs. Goutman and Feldman); the University of Michigan A. Alfred Taubman Medical Research Institute; the Katherine Rayner Fund; the Program for Neurology Research and Discovery (Drs. Feldman, Figueroa‐Romero, and Goutman); the Robert and Katherine Jacobs Environmental Health Initiative; the Target ALS Foundation (N021258‐00); and the Cariology Restorative Sciences and Endodontics Department Funds at University of Michigan School of Dentistry (Dr. Botero). At Mount Sinai, Dr. Austin was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K99HD087523) and Dr. Arora was supported by the National Institutes of Environmental Health Sciences (DP2ES025453, R01ES024674, U2C ES030859, U2C ES026561, and P30ES023515).
Funding information
At the University of Michigan, this work was supported in part by the National Institutes of Environmental Health Sciences (K23ES027221, Dr. Goutman), the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry (CDC/ATSDR Contract #200‐2013‐56856, Drs. Goutman and Feldman); the University of Michigan A. Alfred Taubman Medical Research Institute; the Katherine Rayner Fund; the Program for Neurology Research and Discovery (Drs. Feldman, Figueroa‐Romero, and Goutman); the Robert and Katherine Jacobs Environmental Health Initiative; the Target ALS Foundation (N021258‐00); and the Cariology Restorative Sciences and Endodontics Department Funds at University of Michigan School of Dentistry (Dr. Botero). At Mount Sinai, Dr. Austin was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K99HD087523) and Dr. Arora was supported by the National Institutes of Environmental Health Sciences (DP2ES025453, R01ES024674, U2C ES030859, U2C ES026561, and P30ES023515).
Funding Statement
This work was funded by The Target ALS Foundation grant N021258‐00; Agency for Toxic Substances and Disease Registry grant 200‐2013‐56856; National Institute of Environmental Health Sciences grants DP2ES025453, K23ES027221, P30ES023515, R01ES024674, U2C ES030859, and U2C ES026561; The University of Michigan A. Alfred Taubman Medical Research Institute grant ; The Eunice Kennedy Shriver National Institute of Child Health and Human Development grant K99HD087523; The Program for Neurology Research and Discovery grant ; The Robert and Katherine Jacobs Environmental Health Initiative grant ; The Cariology Restorative Sciences and Endodontics Department Funds at University of Michigan School of Dentistry grant .
References
- 1. Goutman SA. Diagnosis and clinical management of amyotrophic lateral sclerosis and other motor neuron disorders. Continuum (Minneap Minn) 2017;23:1332–1359. [DOI] [PubMed] [Google Scholar]
- 2. Al‐Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol 2013;9:617–628. [DOI] [PubMed] [Google Scholar]
- 3. Callaghan B, Feldman D, Gruis K, Feldman E. The association of exposure to lead, mercury, and selenium and the development of amyotrophic lateral sclerosis and the epigenetic implications. Neurodegener Dis 2011;8:1–8. [DOI] [PubMed] [Google Scholar]
- 4. Bello GA, Arora M, Austin C, et al. Extending the distributed lag model framework to handle chemical mixtures. Environ Res 2017;31:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flora SJS. Handbook of Arsenic Toxicology. Oxford, UK: Academic Press, 2015. [Google Scholar]
- 6. Gustafson G, Koch G. Age estimation up to 16 years of age based on dental development. Odontol Revy 1974;25:297–306. [PubMed] [Google Scholar]
- 7. Arora M, Austin C, Sarrafpour B, et al. Determining prenatal, early childhood and cumulative long‐term lead exposure using micro‐spatial deciduous dentine levels. PLoS ONE 2014;9:e97805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naik SB, Patil SN, Kamble SD, et al. Reliability of third molar development for age estimation by radiographic examination (Demirjian's Method). J Clin Diagn Res 2014;8:ZC25‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andra SS, Austin C, Arora M. The tooth exposome in children's health research. Curr Opin Pediatr 2016;28(2):221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arora M, Reichenberg A, Willfors C, et al. Fetal and postnatal metal dysregulation in autism. Nat Commun 2017;8:15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Modabbernia A, Velthorst E, Gennings C, et al. Early‐life metal exposure and schizophrenia: a proof‐of‐concept study using novel tooth‐matrix biomarkers. Eur Psychiatry 2016;36:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith MR, Yevoo P, Sadahiro M, et al. Integrative bioinformatics identifies postnatal lead (Pb) exposure disrupts developmental cortical plasticity. Sci Rep 2018;8:16388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Velthorst E, Smith L, Bello G, et al. New research strategy for measuring pre‐ and postnatal metal dysregulation in psychotic disorders. Schizophr Bull 2017;43:1153–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brooks BR, Miller RG, Swash M, et al. World Federation of Neurology Research Group on Motor Neuron Diseases . El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 15. Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21‐linked ALS‐FTD. Neuron 2011;72:257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen YH, Ferguson KK, Meeker JD, et al. Statistical methods for modeling repeated measures of maternal environmental exposure biomarkers during pregnancy in association with preterm birth. Environ Health 2015;26:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gasparrini A, Armstrong B, Kenward MG. Distributed lag non‐linear models. Stat Med 2010;29(21):2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gennings C, Sabo R, Carney E. Identifying subsets of complex mixtures most associated with complex diseases: polychlorinated biphenyls and endometriosis as a case study. Epidemiology 2010;21:S77–S84. [DOI] [PubMed] [Google Scholar]
- 19. Yorita Christensen KL, Carrico CK, Sanyal AJ, Gennings C. Multiple classes of environmental chemicals are associated with liver disease: NHANES 2003–2004. Int J Hyg Environ Health 2013;216:703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics 1979;6:65–70. [Google Scholar]
- 21. Gurney ME, Pu H, Chiu AY, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 1994;264:1772–1775. [DOI] [PubMed] [Google Scholar]
- 22. Sullivan KA, Hayes JM, Wiggin TD, et al. Mouse models of diabetic neuropathy. Neurobiol Dis 2007;28(3):276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Acevedo‐Arozena A, Kalmar B, Essa S, et al. A comprehensive assessment of the SOD1G93A low‐copy transgenic mouse, which models human amyotrophic lateral sclerosis. Dis Model Mech 2011;4:686–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carrico C, Gennings C, Wheeler DC, Factor‐Litvak P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat 2015;20:100–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cicero CE, Mostile G, Vasta R, et al. Metals and neurodegenerative diseases. A systematic review. Environ Res 2017;159:82–94. [DOI] [PubMed] [Google Scholar]
- 26. Mejzini R, Flynn LL, Pitout IL, et al. ALS genetics, mechanisms, and therapeutics: Where are we now? Front Neurosci 2019;13:1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sheykhansari S, Kozielski K, Bill J, et al. Redox metals homeostasis in multiple sclerosis and amyotrophic lateral sclerosis: a review. Cell Death Dis 2018;9(3):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith AP, Lee NM. Role of zinc in ALS. Amyotroph Lateral Scler 2007;8(3):131–143. [DOI] [PubMed] [Google Scholar]
- 29. Mezzaroba L, Alfieri DF, Colado Simao AN, Vissoci Reiche EM. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019;74:230–241. [DOI] [PubMed] [Google Scholar]
- 30. Caragounis A, Price KA, Soon CP, et al. Zinc induces depletion and aggregation of endogenous TDP‐43. Free Radic Biol Med 2010;48(9):1152–1161. [DOI] [PubMed] [Google Scholar]
- 31. Sirabella R, Valsecchi V, Anzilotti S, et al. Ionic homeostasis maintenance in ALS: focus on new therapeutic targets. Front Neurosci 2018;12:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sirangelo I, Iannuzzi C. The role of metal binding in the amyotrophic lateral sclerosis‐related aggregation of copper‐zinc superoxide dismutase. Molecules 2017;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karki P, Smith K, Johnson J Jr, et al. Role of transcription factor yin yang 1 in manganese‐induced reduction of astrocytic glutamate transporters: putative mechanism for manganese‐induced neurotoxicity. Neurochem Int 2015;88:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nutini M, Frazzini V, Marini C, et al. Zinc pre‐treatment enhances NMDAR‐mediated excitotoxicity in cultured cortical neurons from SOD1(G93A) mouse, a model of amyotrophic lateral sclerosis. Neuropharmacology 2011;60:1200–1208. [DOI] [PubMed] [Google Scholar]
- 35. Ijomone OM, Okori SO, Ijomone OK, Ebokaiwe AP. Sub‐acute nickel exposure impairs behavior, alters neuronal microarchitecture, and induces oxidative stress in rats' brain. Drug Chem Toxicol 2018;41:377–384. [DOI] [PubMed] [Google Scholar]
- 36. Ijomone OM, Olatunji SY, Owolabi JO, et al. Nickel‐induced neurodegeneration in the hippocampus, striatum and cortex; an ultrastructural insight, and the role of caspase‐3 and alpha‐synuclein. J Trace Elem Med Biol 2018;50:16–23. [DOI] [PubMed] [Google Scholar]
- 37. Purdey M. Chronic barium intoxication disrupts sulphated proteoglycan synthesis: a hypothesis for the origins of multiple sclerosis. Med Hypotheses 2004;62:746–754. [DOI] [PubMed] [Google Scholar]
- 38. Mitra J, Vasquez V, Hegde PM, et al. Revisiting metal toxicity in neurodegenerative diseases and stroke: therapeutic potential. Neurol Res Ther 2014;1:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ash PEA, Dhawan U, Boudeau S, et al. Heavy metal neurotoxicants induce ALS‐linked TDP‐43 pathology. Toxicol Sci 2019;167:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Silins I, Hogberg J. Combined toxic exposures and human health: biomarkers of exposure and effect. Int J Environ Res Public Health 2011;8:629–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goutman SA, Boss J, Patterson A, et al. High plasma concentrations of organic pollutants negatively impact survival in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2019;90:907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tartaglione AM, Venerosi A, Calamandrei G. Early‐life toxic insults and onset of sporadic neurodegenerative diseases‐an overview of experimental studies. Curr Top Behav Neurosci 2016;29:231–264. [DOI] [PubMed] [Google Scholar]
- 43. Dang TN, Lim NK, Grubman A, et al. Increased metal content in the TDP‐43(A315T) transgenic mouse model of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Front Aging Neurosci 2014;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tokuda E, Okawa E, Watanabe S, et al. Dysregulation of intracellular copper homeostasis is common to transgenic mice expressing human mutant superoxide dismutase‐1s regardless of their copper‐binding abilities. Neurobiol Dis 2013;54:308–319. [DOI] [PubMed] [Google Scholar]
- 45. Williams JR, Trias E, Beilby PR, et al. Copper delivery to the CNS by CuATSM effectively treats motor neuron disease in SOD(G93A) mice co‐expressing the copper‐chaperone‐for‐SOD. Neurobiol Dis 2016;89:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kasarskis EJ, Tandon L, Lovell MA, Ehmann WD. Aluminum, calcium, and iron in the spinal cord of patients with sporadic amyotrophic lateral sclerosis using laser microprobe mass spectroscopy: a preliminary study. J Neurol Sci 1995;130:203–208. [DOI] [PubMed] [Google Scholar]
- 47. Kihira T, Mukoyama M, Ando K, et al. Determination of manganese concentrations in the spinal cords from amyotrophic lateral sclerosis patients by inductively coupled plasma emission spectroscopy. J Neurol Sci 1990;98:251–258. [DOI] [PubMed] [Google Scholar]
- 48. Sgobio C, Trabalza A, Spalloni A, et al. Abnormal medial prefrontal cortex connectivity and defective fear extinction in the presymptomatic G93A SOD1 mouse model of ALS. Genes Brain Behav 2008;7:427–434. [DOI] [PubMed] [Google Scholar]
- 49. Sher RB. The interaction of genetics and environmental toxicants in amyotrophic lateral sclerosis: results from animal models. Neural Regen Res 2017;12:902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mangelsdorf I, Walach H, Mutter J. Healing of amyotrophic lateral sclerosis: a case report. Complement Med Res 2017;24:175–181. [DOI] [PubMed] [Google Scholar]
- 51. Broberg K, Engström K, Ameer S. Handbook of the Toxicology of Metals, 4th ed. Boston: Academic Press, 2015. [Google Scholar]
- 52. Yu B, Pamphlett R. Environmental insults: critical triggers for amyotrophic lateral sclerosis. Transl Neurodegener 2017;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. ALS cases and control participants were born in similar decades. Bar plot representing frequency distribution of birth decades for all cases versus controls was generated using Tableau. ALS cases, n = 36; controls, n = 31.
Figure S2. Early life developmental differences in metal uptake between sporadic ALS cases and controls. Reverse DLMs were adjusted for batch, sex, age at tooth extraction, smoking history, and intra‐subject correlated observations. Sensitivity analysis removing fALS samples indicated similar trends of metal uptake as when all the samples are included in the analysis (see Fig. 1). Sporadic ALS, n = 32 and controls, n = 31. Developmental exposure windows, arrows.
Figure S3. Sensitivity analysis of uptake and contribution of metals to sporadic ALS when analyzed as a mixture at specific developmental windows. Sensitivity analysis of lagged WQS of 11 metals for sporadic ALS cases (n = 32) versus controls (n = 31). (A) Weighted DLM adjusted for batch, sex, age at tooth extraction, smoking history, and intra‐subject correlated observations (95% piecewise CIs of the index, tan bands and 95% Holm–Bonferroni family‐wise CIs adjusted for multiple comparisons, vertical bars). (B) Relative weights of each metal in the mixture over time. Barium, Ba; chromium, Cr; copper, Cu; lithium, Li; magnesium, Mg; manganese, Mn; nickel, Ni; lead, Pb; tin, Sn; strontium, Sr; and zinc, Zn. Developmental exposure windows, arrows.


