Abstract
We report the case of a 27‐year‐old patient with subacute anti‐neurofascin‐155 neuropathy with bifacial palsy, who showed excellent response to rituximab. We provide longitudinal data of established clinical scores, nerve conduction studies, antibody titers, and novel imaging methods (nerve ultrasonography and corneal confocal microscopy). Clinical and electrophysiological improvement followed the reduction of serum antibody titer and correlated with a reduction of corneal inflammatory cellular infiltrates whereas the increase in the cross‐sectional area of the peripheral nerves remained 12 months after first manifestation. Our findings suggest that novel techniques provide useful follow‐up parameters in paranodopathies.
Introduction
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a rare relapsing autoimmune neuropathy with a multifaceted presentation. 1 , 2 , 3 Recently, the description of pathogenic antibodies – predominantly of the IgG4 class – against nodal and paranodal proteins such as neurofascin‐186, neurofascin‐155, contactin‐1, and contactin‐associated protein‐1 has shed new light to a small subset of CIDP cases. 4 , 5 , 6 , 7
More specifically, antibodies against neurofascin‐155 (NF155) correlate with a distinct clinical phenotype. 8 , 9 , 10 , 11 , 12 , 13 , 14 The response to first‐line treatment with intravenous immunoglobulins (IVIg) is poor 9 , 10 , 11 , 13 , 14 but patients show a good response to anti‐CD20 targeted therapy. 13 , 14 , 15 , 16
Here, we report the case of a 27‐year‐old male with anti‐NF155 antibody‐positive neuropathy with poor response to IVIg but a good response to rituximab (RTX). We provide for the first time in the literature longitudinal findings of high‐resolution nerve ultrasound (HRUS) and corneal confocal microscopy (CCM).
Case Description and Results
A 27‐year‐old male patient was admitted to the clinic due to a subacute, progressive sensorimotor affection of all four extremities. Onset was reported approximately 3 weeks before admission beginning with ascending numbness and prickling of the feet and hands, which rapidly involved the lower extremities up to the groin, followed by a progressive, predominantly distal muscle weakness with concomitant gait disorder. He also reported distal leg pain. No difficulty of speech or swallowing was reported. Mild diarrhea occurred a few weeks before. The past medical and family history was unremarkable, except from a history of 8 pack‐years of cigarette consume and penicillin allergy.
On clinical examination, hypoesthesia of the hands and the lower body with pallanesthesia and areflexia of the legs, minor flaccid symmetric distal tetraparesis as well as limb and gait ataxia were noted. Distally, finger flexion and foot dorsiflexion were grade 4 of the Medical Research Council (MRC) sum score on both sides. The remainder of the examination was normal. No autonomic dysfunction was noted.
Cerebrospinal fluid (CSF) analysis revealed an albuminocytologic dissociation with marked increase in protein of 319 mg/dl (normal 15‐45 mg/dl) and normal cell count. Oligoclonal bands were absent. Further laboratory testing for other causes of polyneuropathy revealed no abnormalities. Anti‐neurofascin‐155 IgG (of the IgG4 subclass) antibodies were positive in a high titer of 1:3200 (detected with cell‐based immunofluorescence and confirmed with teased‐nerve method in two different laboratories). No further antibodies were detected.
NCS revealed marked prolongation of distal motor latency (DML) and F‐wave‐latencies symmetrically affecting the nerves of the upper extremities and a less symmetric reduction of motor conduction velocity (mCV). The compound muscle action potential (cMAP) amplitudes were normal. The nerves of the lower extremities were not excitable (Figure 1, Table S1). The EFNS/PNS criteria for definite CIDP were fulfilled. MRI studies of the brain and the complete spinal axis were unremarkable.
Figure 1.
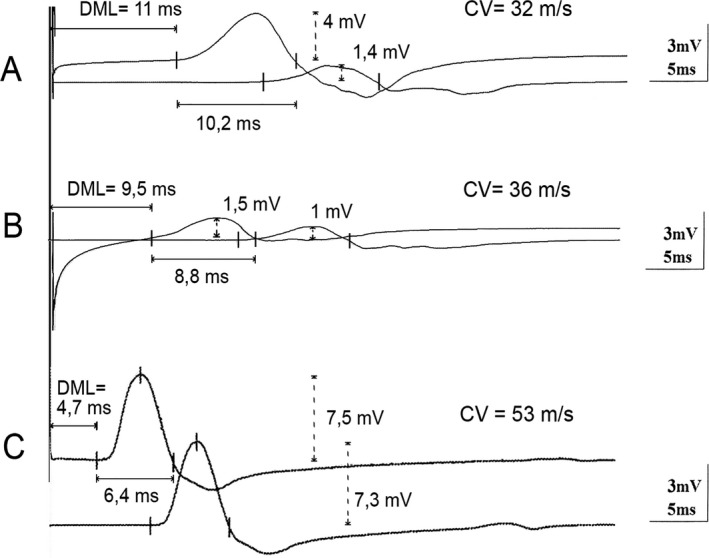
Electroneurography traces of the left median nerve at 2 (Panel A), 5 (Panel B), and 12 months (Panel C). cMAPs after stimulation at the wrist and the elbow. At 2 months a marked prolongation of DML and distal cMAP duration as well as CV slowing were observed. Furthermore a conduction block, with reduction of the cMAP amplitudes >50% after proximal stimulation, is noted. At 5 months the distal cMAP amplitudes are also diminished, while DML, CV, and distal cMAP duration are slightly improved. At 12 months an almost full recovery is observed. The distal cMAP amplitude also recovers to normal values, indicating that the previous marked reduction was mainly due to a distal conduction block. DML, distal motor latency; CV, conduction velocity; cMAP, compound muscle action potential.
The initial treatment, pending results of the serology panel, was five cycles of plasma exchange (PE). A brief improvement was followed by a deterioration, including bifacial palsy in approximately 1 week. In view of the in the meantime available positive results for NF155 antibodies the therapy was immediately escalated to RTX (cumulative 2g). Due to a further relapse with deterioration of muscle weakness 1 month later, the patient was treated with IVIg (2 g/kg of body weight) without significant improvement. Therefore, a course of intravenous corticosteroids (4.5 g prednisolone over 5 days) followed by a further PE session (six cycles) were applied. This led to clinical stabilization. However, improvement occurred only 5 months after disease onset (approximately 3 months after Rituximab application). During the following months symptoms improved steadily. At 12 months, we noted a complete recovery of muscle strength, facial weakness, and subjective sensory deficits. Minor gait instability and sensory ataxia persisted, but the patient could walk without aids (Table 1). Repeat treatment with RTX was planned.
Table 1.
Evolution of clinical scores, NF155 antibodies titer and CCM parameters
| Onset | 1 month | 2 months | 3 months | 5 months | 12 months | |
|---|---|---|---|---|---|---|
| MRCss | 73 | 65 | 64.5 | 52.5 | 51 | 80 |
| INCAT/ODSS | 3 | 5 | 5 | 6 | 6 | 3 |
| NF155 Antibody Titer | 1:3200 | 1:1000 | 1:320 | 1:100 | 1:32 | 1:32 |
| CCM (cells/mm2) | 121 | – | – | – | – | 21 |
INCAT/ODSS, Inflammatory Neuropathy Cause and Treatment Overall Disability Sum Score; MRCss, modified Medical Research Council sum score (max. of 80 with lower scores corresponding to lower muscle strength).
The titer of anti‐NF155 antibodies started to diminish promptly after initiation of treatment already at first month. This decrease preceded the clinical and electrophysiological improvement (Table 1).
The nerves of the lower extremities remained not excitable after 12 months despite clinical improvement. At the nerves of the upper extremities DML, F‐wave‐latencies and cMAP‐duration prolongation was detected with a peak at 2 months, cMAP amplitudes decreased with a maximum at 5 months. The mCV reduction plateaued at 2 to 5 months. At 12 months mCV, F‐wave‐latency and cMAP‐duration were mostly normalized, cMAP amplitudes and DML also significantly recovered (Figure 1, Table S1).
Needle electromyography initially showed abnormal spontaneous activity which persisted after 12 months. Chronic neurogenic changes were noted at month 2 and also persisted thereafter.
HRUS was performed with the use of an 18 MHz ultrasound transducer. The median, ulnar, common radial, tibial, fibular and sural nerves were studied bilaterally throughout their visualizable courses. The brachial plexus was also assessed in the supraclavicular and interscalene spaces. Standardized CSA measurements were performed at 30 predefined locations according to the protocol of our group. 17 , 18 At 2 months after initial diagnosis multifocal peripheral nerve enlargement (median, ulnar, and tibial nerve) was noted. The brachial plexus was enlarged bilaterally. In the following months more nerves showed a cross‐sectional area (CSA) enlargement (radial nerve). The Bochum ultrasound score 19 (BUS, ranging from 0 to 4 points, with 1 point given for increased CSA at each of the four following sites: ulnar nerve in Guyons' canal, ulnar nerve in the upper arm, radial nerve in the spiral groove, and sural nerve between the gastrocnemius muscle), which was developed from our group as a marker of CIDP (if more than two sites show increased CSA, score of 2 or more), reached the maximum of 3 points 12 months after initial presentation. A further HRUS marker for clinical progression is the intranerve CSA variability 20 (max CSA/min CSA of one nerve), which increased for the majority of the nerves 2–5 months after the initial presentation and stabilized at high values 12 months after. (Figure 2, Table 2).
Figure 2.
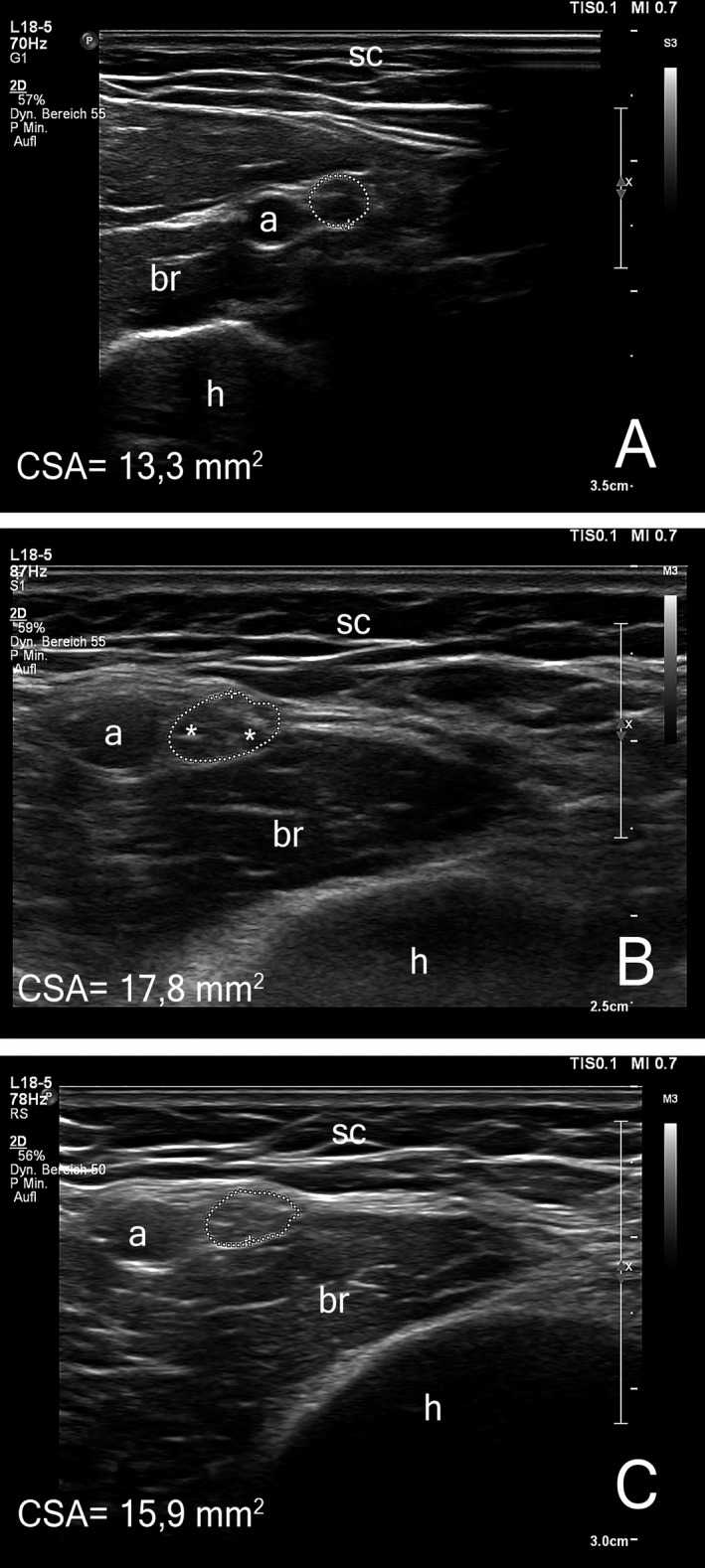
Representative images of high‐resolution nerve ultrasound. Transverse images of the left median nerve at the distal upper arm are shown at 2 (Panel A), 5 (Panel B), and 12 months (Panel C). The cross section of the median nerve is outlined with a doted circle, (h) denotes the humerus, (a) the brachial artery, (sc) the subcutaneous fat. Asterisks in panel B mark individual nerve fascicles. The initially normal CSA increases significantly between months 2 and 5 in the active disease phase (reference range 2,6‐14,1 mm2). After clinical stabilization nerve swelling shows a small reduction. Intranerve CSA variability shows a corresponding course. (s. Table 2). CSA, cross‐sectional area.
Table 2.
Sonographic mean cross‐sectional area values in mm2.
| Nerve | Site | 2 Month | 5 Month | 12 Month | Ref. range | |||
|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | |||
| Median | Carpal tunnel | 15,1 | 13,4 | 13,7 | 13,7 | 10,9 | 11,5 | 1,3–12,6 |
| Forearm | 8 | 8,2 | 7,5 | 7,9 | 6,73 | 6,75 | 3,4–12,6 | |
| Upper arm | 14,5 | 13,3 | 20,0 | 17,8 | 21,9 | 15,9 | 2,6–14,1 | |
| intranerve CSA variability | 1,8 | 1,61 | 2,66 | 2,25 | 3,25 | 2,35 | <1,5 | |
| Ulnar | Gyuon canal | 6,6 | 5,5 | 5,03 | 7,59 | 9,76 | 6,16 | 3,1–7,2 |
| Forearm | 7,6 | 8,5 | 6,3 | 6,79 | 6,83 | 5,89 | 2,9–8,0 | |
| Elbow | 7,7 | 9,3 | 7,99 | 8,78 | 8,57 | 11 | 2,5–8,1 | |
| Upper arm | 7,7 | 7,7 | 9,94 | 9,43 | 12,3 | 9,92 | 2,9–10,2 | |
| intranerve CSA variability | 1,16 | 1,69 | 1,97 | 1,39 | 1,80 | 1,86 | <1,5 | |
| Radial | Spiral groove | 6,2 | 8,7 | 12,8 | 7,1 | 5,69 | 8,19 | 0,2‐6,3 |
| Brachial Plexus | Intrascalene space | 65,1 | n/a | 20,4 | 20,1 | 48,4 | 35,1 | 9,3–52,6 |
| Supraclavicular space | 144 | 145 | 117 | 99 | 96,5 | 91,8 | 9,6–82,7 | |
| Fibular | Fibular Head | 9,9 | 11,6 | 9,35 | 8,72 | 17,2 | 10,7 | 2,5–11,7 |
| Popliteal fossa | 5,2 | 8,5 | 11,6 | 7,69 | 14,7 | 10,4 | 4,0–13,2 | |
| intranerve CSA variability | 1,9 | 1,36 | 1,24 | 1,13 | 1,17 | 1,03 | <1,5 | |
| Tibial | Popliteal fossa | 23 | 25,3 | 44,1 | 36,8 | 39,3 | 47,9 | 3,0–13,8 |
| Ankle | 14,4 | 13,6 | 13,2 | 15,8 | 14,5 | 15,2 | 3,4–9,2 | |
| intranerve CSA variability | 1,59 | 1,86 | 3,34 | 2,33 | 2,71 | 3,15 | <1,5 | |
| Sural | Between heads of gastrocnemius | 2,2 | 2,5 | 1,4 | 1,61 | 1,79 | 1,53 | 0,5–3 |
CSA, cross‐sectional area.
CCM was performed with a Heidelberg Retina Tomograph III with a Rostock cornea module, the images were analyzed with ACC‐Metrics software, version 2.0 (Xin Chen and Mohammad Dabbah, Manchester, UK), the average of five images was calculated. Two months after initial diagnosis CCM showed an increased number of corneal cell infiltrates (121/mm2), but normal corneal nerve fiber density and corneal nerve branch density and only a minor reduction in corneal nerve fiber length. After 12 months the corneal cell infiltrates were markedly reduced (21/mm2). However, there was a progressive reduction in the corneal nerve fiber length and density (Figure 3).
Figure 3.
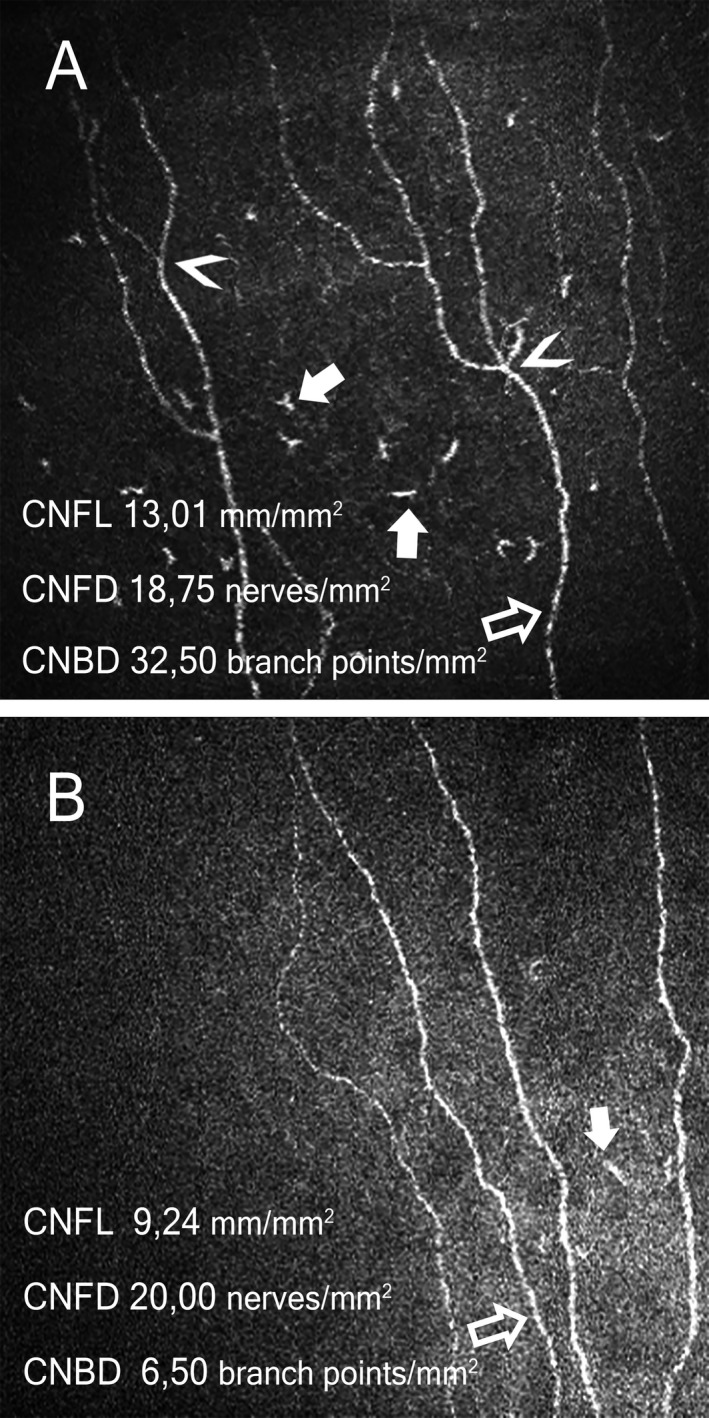
Representative images of corneal confocal microscopy at 2 (Panel A) and 12 months (Panel B). Nerve fibers indicated with open arrows, corneal immunological cells with solid arrows, nerve branch points with arrowheads. At 2 months increased cellular infiltrates of 121/mm2 were observed, which were reduced to 21/mm2 after treatment. The CNFL and CNBD decreased over the period of time. CNFL, corneal nerve fiber length (normal value> 14,05 mm/mm2), CNFD, corneal nerve fiber density (normal value> 14,54 nerves/mm2, CNBD, corneal nerve branch density (normal value> 16,49 branching points/mm2), normal values from Tavakoli et al. 2015.
Discussion
This case demonstrates a thorough longitudinal study of a paranodal neuropathy. Acute presentation and progression over 8 weeks in combination with the nerve conduction studies and CSF findings led to the clinical diagnosis of a chronic demyelinating inflammatory polyradiculoneuropathy. Prompt testing for anti‐NF155 antibodies helped to diagnose an anti‐NF155 immune neuropathy and enabled early treatment with RTX, based on prior small case series. 13 , 14 , 15 , 16 , 21
Bifacial palsy was a noteworthy feature in our case. This is a common manifestation of Guillain–Barré syndrome but rare in CIDP. Cases of NF155 neuropathy with cranial nerve involvement have been reported, 22 , 23 the exact incidence remains unknown. In the case of Miller Fisher syndrome, Chiba et al 24 showed that GQ1b expression is proportionally higher in oculomotor nerves. Further studies are required to determine the specific expression of NF155 in cranial nerves.
As previously reported 9 , 10 , 11 , 13 , 14 treatment response after IVIg was poor in our patient. Corticosteroid treatment did not infer any relevant improvement either, which is why we escalated to PE after the second relapse. Although at initial presentation PE lead to a minor and only temporary improvement, disease progression was stopped after the second PE treatment. Although PE was less effective regarding improvement, it led to a decrease in the antibody titer (1 month later). However, the timing of clinical stabilization and the following robust remission (5 months after initial presentation and 4 months after Rituximab) without need for further interventions argue that the anti‐CD20 treatment was mainly responsible.
As previously shown 25 , 26 the improvement of electrophysiological parameters correlated with the clinical improvement in a lagged fashion, demonstrating that new methods with better temporal correlation to the clinical development are needed.
The anti‐NF155 antibodies titer fell promptly after treatment initiation (PE and Rituximab). This trend preceded clinical improvement. Further studies are needed to show if anti‐NF155 antibody titers are a useful prognostic and disease monitoring tool. 27
To our knowledge, this is the second report 13 of HRUS findings in anti‐NF155 antibody‐positive neuropathy, but a longitudinal study has never reported before yet. We saw a multifocal and asymmetric swelling of the nerves distally as well as proximally. The nerves were hypoechogenic, which could imply an inflammatory affection according to previous data. 28 The increased CSA of multiple peripheral nerves correlated with previous reports 10 , 13 and in accordance with the expected changes in inflammatory neuropathies. 19 , 29 , 30 Bochum ultrasound score increased successively from 1 to 3 points in the course of 12 months indicating that acute paranodopathies do not present with typical sonographical BUS characteristics at the beginning of the disease. An increase in intranerve CSA variability for the majority of nerves coincided temporally with the active disease phase between months 2 and 5. We have previously shown that the increase in CSA variability and BUS correspond with disease progression in typical CIDP. 20 , 31 , 32 At 12 months we did not observe a relevant improvement of CSA or its intranerve variability despite clinical improvement. This could be attributed to the follow‐up interval, as previous studies 20 , 33 show an improvement over a period of more than 12 months. However, there are no previous longitudinal ultrasound studies on paranodopathies.
To our knowledge, this is the first report of the use of CCM in a case of anti‐NF155 antibody‐positive autoimmune neuropathy. CCM is a noninvasive technique which has shown its capacity to detect small fiber damage in several types of neuropathies 34 and it is used in inflammatory neuropathies recently. 32 , 35 , 36 Patients with autonomic neuropathies and CIDP patients with symptoms of small fiber damage, such as painful neuropathy, show a greater loss of corneal nerve fibers. However, classical CIDP patients still show significant abnormalities. 35 , 36 The small fiber damage in the cornea may thus mirror an axonal damage of the peripheral nerves. In our patient a marked decrease in corneal nerve parameters correlated with a remaining reduction of the amplitudes of the nerves in the lower extremities over the course of time. It is noteworthy that no signs or symptoms of small fiber neuropathy were noted at 12 months and the paraesthesias the patient had at presentation had resolved. We have recently shown that an increased number of inflammatory corneal cell infiltrates correlates with disease progression in immunoneuropathies. 32 Conversely, in this case the reduction in inflammatory cell infiltrates correlated with clinical remission.
Concluding, we investigated for the first time further CCM and HRUS markers of disease activity in a case of paranodopathy. Surely, longitudinal testing in a larger number of patients is needed to better understand the temporal relation of these alterations but these methods seem to be promising to determine axonal degeneration and inflammatory activity in paranodopathies.
Conflicts of Interest
Athanasopoulos Diamantis has no conflict of interest to report. Jeremias Motte received travel grants from Biogen idec, Novartis AG, Teva and Eisai GmbH, his research is funded by Klaus Tschira Foundation and Ruhr‐University, Bochum (FoRUM‐program), none related to this work. Anna Lena Fisse received research funding by Georgius Agricola Stiftung Ruhr, received honoraria and travel grants from Novartis AG, Sanofi and Eisai GmbH, none related to this work. Owns shares of Fresenius SE & Co., Gilead Sciences, Medtronic PLC and Novartis AG. Thomas Grueter received travel reimbursement from Sanofi Genzyme and Biogen Idec, none related to this work. Dietrich Sturm received research funding from the Ruhr‐University, Bochum (FoRUM‐program), none related to this work. Martin Tegenthoff has received speaker honoraria from Pfizer, Novartis, and Mundipharma. He also acknowledges a grant support from Genzyme, none related to this work. Nadine Trampe, Melissa Sgodzai, and Rafael Klimas have no conflict of interest to report. Luis Querol has provided expert testimony for Grifols, SanofiGenzyme, Novartis, UCB, Roche, CSL Behring, Johnson and Johnson, Alexion and Annexon, has received research funds from Novartis Spain, Sanofi‐Genzyme, and Grifols, research support from the GBS‐CIDP Foundation and the Department of Health of the Government of Catalonia, as well as travel funding and speaker honoraria from CSL Behring, Novartis and Biogen. R. Gold has received consultation fees and speaker honoraria from Bayer Schering, Biogen idec, Merck Serono, Novartis, Sanofi‐Aventis and TEVA. He also acknowledges grant support from Bayer Schering, Biogen idec, Merck Serono, Sanofi‐Aventis and TEVA, none related to this work. Kalliopi Pitarokoili received travel funding and speaker honoraria from Biogen Idec, Novartis and Bayer Schering Pharma and funding from the Ruhr‐University, Bochum (FORUM‐Program), none related to this work.
Author Contributions
All authors have read and approved the manuscript. Athanasopoulos Diamantis and Jeremias Motte were involved in patient treatment, acquisition, analysis and interpretation of data, and drafting/revising the manuscript for content. Anna Lena Fisse, Thomas Grueter, and Dietrich Sturm were involved in acquisition, analysis and interpretation of data, and drafting/revising the manuscript for content. Nadine Trampe was involved in patient treatment, acquisition, analysis and interpretation of data, and revising the manuscript for content. Martin Tegenthoff, Luis Querol, and Ralf Gold were involved in critical comments during data collection, drafting and manuscript revision. Melissa Sgodzai and Rafael Klimas were involved in acquisition, analysis and interpretation of data, revising the manuscript for content. Kalliopi Pitarokoili was involved in first idea, patient treatment, acquisition, analysis and interpretation of data, drafting and manuscript revision, and study supervision.
Ethical Standards
The patient gave written informed consent.
Supporting information
Table S1. Selected longitudinal nerve conduction studies findings. Abbreviations: ampl.= amplitudes, dur.= duration, cMAP = compound motor action potential, DML = distal motor latency, lat.= latency, mCV = motor conduction velocity, n/a = not available, x = nonexcitable
Funding information
There was no funding source.
References
- 1. Broers MC, Bunschoten C, Nieboer D, et al. Incidence and prevalence of chronic inflammatory demyelinating polyradiculoneuropathy: a systematic review and meta‐analysis. Neuroepidemiology 2019;52:161–172. 10.1159/000494291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bunschoten C, Jacobs BC, Van den Bergh PYK, et al. Progress in diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Lancet Neurol 2019;18:784–794. 10.1016/S1474-4422(19)30144-9. Epub 2019 May 7. [DOI] [PubMed] [Google Scholar]
- 3. Lehmann HC, Burke D, Kuwabara S. Chronic inflammatory demyelinating polyneuropathy: update on diagnosis, immunopathogenesis and treatment. J Neurol Neurosurg Psychiatry 2019;90:981‐987. Published online 2019 Apr 16. 10.1136/jnnp-2019-320314. [DOI] [PubMed] [Google Scholar]
- 4. Querol L, Illa I. Paranodal and other autoantibodies in chronic inflammatory neuropathies. Curr Opin Neurol 2015;28:474–479. 10.1097/WCO.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 5. Querol L, Devaux J, Rojas‐Garcia R, Illa I. Autoantibodies in chronic inflammatory neuropathies: diagnostic and therapeutic implications. Nat Rev Neurol 2017;13:533 10.1038/nrneurol.2017.84. [DOI] [PubMed] [Google Scholar]
- 6. Vural A, Doppler K, Meinl E. Autoantibodies against the node of ranvier in seropositive chronic inflammatory demyelinating polyneuropathy: diagnostic, pathogenic, and therapeutic relevance. Front Immunol 2018; 9:1029 Published online 2018 May 14. 10.3389/fimmu.2018.01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uncini A, Vallat JM. Autoimmune nodo‐paranodopathies of peripheral nerve: the concept is gaining ground. J Neurol Neurosurg Psychiatry 2017. Published online 2017 Dec 16. 10.1136/jnnp-2017-317192. [DOI] [PubMed] [Google Scholar]
- 8. Ng JK, Malotka J, Kawakami N, et al. Neurofascin as a target for autoantibodies in peripheral neuropathies. Neurology 2012;79:2241–2248. 10.1212/WNL.0b013e31827689ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Querol L, Nogales‐Gadea G, Rojas‐Garcia R, et al. Neurofascin IgG4 antibodies in CIDP associate with disabling tremor and poor response to IVIg. Neurology 2014;82:879–886. 10.1212/WNL.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogata H, Yamasaki R, Hiwatashi A, et al. Characterization of IgG4 anti‐neurofascin 155 antibody‐positive polyneuropathy. Ann Clin Transl Neurol 2015; 2: 960–971. Published online 2015 Sep 11. 10.1002/acn3.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devaux JJ, Miura Y, Fukami Y, et al. Neurofascin‐155 IgG4 in chronic inflammatory demyelinating polyneuropathy. Neurology 2016;86:800–807. 10.1212/WNL.0000000000002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu W, Xin Y, He Z, Zhao Y. Association of neurofascin IgG4 and atypical chronic inflammatory demyelinating polyneuropathy: A systematic review and meta‐analysis. Brain Behav 2018; 8: e01115 Published online 2018 Sep 21. 10.1002/brb3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garg N, Park SB, Yiannikas C, et al. Neurofascin‐155 IgG4 Neuropathy: Pathophysiological insights, spectrum of clinical severity and response to treatment. Muscle Nerve 2017. Published online 2017 Nov 11. 10.1002/mus.26010 [DOI] [PubMed] [Google Scholar]
- 14. Burnor B, Yang L, Zhou H, et al. Neurofascin antibodies in autoimmune, genetic, and idiopathic neuropathies. Neurology 2018;90:e31–e38. 10.1212/WNL.0000000000004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Querol L, Rojas‐García R, Diaz‐Manera J, et al. Rituximab in treatment‐resistant CIDP with antibodies against paranodal proteins. Neurol Neuroimmunol Neuroinflamm 2015; 2: e149 Published online 2015 Sep 3. 10.1212/NXI.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Painous C, López‐Pérez MA, Illa I, Querol L. Head and voice tremor improving with immunotherapy in an anti‐NF155 positive CIDP patient. Ann Clin Transl Neurol 2018; 5: 499–501. Published online 2018 Mar 7. 10.1002/acn3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kerasnoudis A, Pitarokoili K, Behrendt V, et al. Cross sectional area reference values for sonography of peripheral nerves and brachial plexus. Clin Neurophysiol 2013;124:1881–1888. Published online 2013 Apr 10. 10.1016/j.clinph.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 18. Kerasnoudis A. Nerve ultrasound in a case of chronic inflammatory demyelinating neuropathy. Muscle Nerve 2013;47:443–446. [DOI] [PubMed] [Google Scholar]
- 19. Kerasnoudis A, Pitarokoili K, Behrendt V, et al. Nerve ultrasound score in distinguishing chronic from acute inflammatory demyelinating polyneuropathy. Clin Neurophysiol 2014;125: 635–641. Published online 2013 Sep 23. 10.1016/j.clinph.2013.08.014 [DOI] [PubMed] [Google Scholar]
- 20. Fisse AL, Pitarokoili K, Trampe N, et al. Clinical Sonographic and electrophysiologic longitudinal features of chronic inflammatory demyelinating polyneuropathy. J Neuroimaging. 2018. Published online 2018 Nov 8. 10.1111/jon.12579. [DOI] [PubMed] [Google Scholar]
- 21. Dionne A, Nicolle MW, Hahn AF. Clinical and electrophysiological parameters distinguishing acute‐onset chronic inflammatory demyelinating polyneuropathy from acute inflammatory demyelinating polyneuropathy. Muscle Nerve 2010;41:202–207. 10.1002/mus.21480. [DOI] [PubMed] [Google Scholar]
- 22. Caetano A, Ladeira F, Fernandes M, et al. Acute‐onset chronic inflammatory demyelinating polyneuropathy with anti‐neurofascin‐155 antibodies and bilateral facial nerve enhancement. J Neuroimmunol. 2019;336:577026 10.1016/j.jneuroim.2019.577026. [DOI] [PubMed] [Google Scholar]
- 23. Franques J, Chapon F, Devaux J, Mathis S. Cranial nerve hypertrophy in IgG4 anti‐neurofascin 155 antibody‐positive polyneuropathy. Neurology 2017;88:e52 10.1212/WNL.0000000000003616. [DOI] [PubMed] [Google Scholar]
- 24. Chiba A, Kusunoki S, Obata H, et al. Ganglioside composition of the human cranial nerves, with special reference to pathophysiology of Miller Fisher syndrome. Brain Res 1997;745:32–36. [DOI] [PubMed] [Google Scholar]
- 25. Bril V, Banach M, Dalakas MC, et al. Electrophysiologic correlations with clinical outcomes in CIDP. Muscle Nerve 2010;42:492–497. 10.1002/mus.21733. [DOI] [PubMed] [Google Scholar]
- 26. Cirillo G, Todisco V, Ricciardi D, Tedeschi G. Clinical‐neurophysiological correlations in chronic inflammatory demyelinating polyradiculoneuropathy patients treated with subcutaneous immunoglobulin. Muscle Nerve 2019;60:662–667. 10.1002/mus.26669. Epub 2019 Aug 23. [DOI] [PubMed] [Google Scholar]
- 27. Fujita A, Ogata H, Yamasaki R, et al. Parallel fluctuation of anti‐neurofascin 155 antibody levels with clinico‐electrophysiological findings in patients with chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Sci 2018; 384: 107–112. Published online 2017 Nov 27. 10.1016/j.jns.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 28. Fisse AL, Pitarokoili K, Motte J, et al. Nerve echogenicity and intranerve CSA variability in high‐resolution nerve ultrasound (HRUS) in chronic inflammatory demyelinating polyneuropathy (CIDP). J Neurol 2019;266:468–475. 10.1007/s00415-018-9158-3. [DOI] [PubMed] [Google Scholar]
- 29. Kerasnoudis A, Pitarokoili K, Haghikia A, et al. Nerve ultrasound protocol in differentiating chronic immune‐mediated neuropathies. Muscle Nerve 2016; 54: 864–871. Published online 2016 Sep 21. 10.1002/mus.25138. [DOI] [PubMed] [Google Scholar]
- 30. Grimm A, Décard BF, Axer H, The FP. Ultrasound pattern sum score ‐ UPSS. A new method to differentiate acute and subacute neuropathies using ultrasound of the peripheral nerves. Clin Neurophysiol. 2015; 126: 2216–2225. Published online 2015 Feb 3. 10.1016/j.clinph.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 31. Kerasnoudis A, Pitarokoili K, Gold R, Yoon MS. Nerve Ultrasound and Electrophysiology for Therapy Monitoring in Chronic Inflammatory Demyelinating Polyneuropathy. J Neuroimaging 2015; 25: 931–939. Published online 2015 Aug 3. 10.1111/jon.12279. [DOI] [PubMed] [Google Scholar]
- 32. Pitarokoili K, Sturm D, Labedi A, et al. Neuroimaging markers of clinical progression in chronic inflammatory demyelinating polyradiculoneuropathy. Ther Adv Neurol Disord 2019;12: 1756286419855485. Published 2019 Jun:18. 10.1177/1756286419855485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Härtig F, Ross M, Dammeier NM, et al. Nerve ultrasound predicts treatment response in chronic inflammatory demyelinating polyradiculoneuropathy‐a prospective follow‐up. Neurotherapeutics. 2018;15:439–451. 10.1007/s13311-018-0609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cruzat A, Qazi Y, Hamrah P. In vivo confocal microscopy of corneal nerves in health and disease. Ocul Surf 2017;15:15–47. 10.1016/j.jtos.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schneider C, Bucher F, Cursiefen C, et al. Corneal confocal microscopy detects small fiber damage in chronic inflammatory demyelinating polyneuropathy (CIDP). J Peripher Nerv Syst 2014;19:322–327. 10.1111/jns.12098. [DOI] [PubMed] [Google Scholar]
- 36. Stettner M, Hinrichs L, Guthoff R, et al. Corneal confocal microscopy in chronic inflammatory demyelinating polyneuropathy. Ann Clin Transl Neurol 2016; 3:88–100. Published online 2015 Dec 28. 10.1002/acn3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Selected longitudinal nerve conduction studies findings. Abbreviations: ampl.= amplitudes, dur.= duration, cMAP = compound motor action potential, DML = distal motor latency, lat.= latency, mCV = motor conduction velocity, n/a = not available, x = nonexcitable


