Abstract
Objective
The objective of this study was to investigate the safety and efficacy of remote ischemic conditioning (RIC) combined with intravenous thrombolysis (IVT) in the treatment of acute ischemic stroke (AIS).
Methods
Patients with AIS who underwent IVT were enrolled and 1:1 randomized to the RIC group and sham‐RIC group in this study. RIC (or sham‐RIC) was performed twice within 6–24 h of IVT. The subjects in the two groups were followed up for 90 days. The safety outcome included the ratio of hemorrhagic transformation (HT), adverse events during the follow‐up, blood pressure within the first 24 h after IVT, and laboratory tests 24 h after IVT. The efficacy outcome included the modified Rankin Scale (mRS) score, National Institute of Health Stroke Scale (NIHSS) score during the follow‐up, and level of high‐sensitivity C‐reactive protein (hs‐CRP) tested 24 h after IVT.
Results
Forty‐nine patients (24 in the RIC group and 25 in the sham‐RIC group) were recruited. No significant difference was observed in the ratio of HT, adverse events, blood pressure, coagulation function or liver function between groups. In addition, there was no significant difference in mRS score and NIHSS score during the follow‐up between groups. However, patients in the RIC group exhibited a significant lower level of hs‐CRP compared with the control group (P = 0.048).
Interpretation
RIC combined with IVT is safe in the treatment of AIS. The neuroprotective and anti‐inflammatory effects of this therapy warrant further study on a larger scale.
Introduction
Acute ischemic stroke (AIS) is one of the primary causes of death and disability worldwide. 1 , 2 Intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator (rt‐PA) has been demonstrated to be the most effective medication to treat AIS within 4.5 h from onset and is recommended by current guidelines in different countries. 3 , 4 , 5 Nevertheless, there remains a considerable proportion of patients who cannot achieve a good clinical outcome after IVT. In addition, IVT increases the odds of fatal intracranial hemorrhage (ICH) by approximately 7‐fold. 6 Therefore, a treatment that can be combined with rt‐PA to enhance the benefit and minimize the risk of thrombolysis is needed.
Remote ischemic conditioning (RIC), which refers to several transient cycles of deliberate ischemia and reperfusion in limbs, has been reported to be effective in the treatment of patients with AIS as a complementary therapy. 7 The mechanism of the neuroprotective effect of RIC is speculated to include the protection of endothelial cells, release of nitric oxide from vessels, and suppression of inflammation via the regulation of gene expression. 8 The combination of RIC and rt‐PA has shown an additive effect compared with rt‐PA alone in murine models. 9 However, evidence regarding whether RIC is safe and effective for application to patients with AIS who receive intravenous rt‐PA remains scarce. A single‐center randomized study suggested that RIC is safe and reduces the risk of infarction when applied to patients with AIS prior to the initiation of thrombolysis. 10 A more recent study recruited 30 patients with AIS and performed RIC for seven consecutive days after thrombolysis. Their results suggest that their methodology of RIC is well‐tolerated and feasible, although the clinical outcomes do not differ between groups. 11 Nonetheless, considering the numerous episodes of RIC after thrombolysis in that study, the combination of RIC and IVT was not highlighted. To date, no agreement has been reached on how many episodes of RIC should be conducted or the timing of intervention, when combining RIC with rt‐PA to treat AIS.
In this pilot randomized study, 50 patients who were diagnosed as AIS and underwent IVT were enrolled and RIC (or sham‐RIC) was conducted twice within the first 24 h after IVT. We aimed to demonstrate the safety of RIC combined with rt‐PA IVT in the treatment of AIS and investigate the potential positive effect of this treatment approach to direct future clinical trials on a larger scale.
Materials and Methods
This SERICT‐AIS study (Safety and Effectiveness of Remote Ischemic Conditioning Combined with Intravenous Thrombolysis in Treating Acute Ischemic Stroke) was a single‐center, randomized, outcome observer‐blinded clinical trial (NCT04027621) performed at the First Hospital of Jilin University. The Ethics Committee of the First Hospital of Jilin University approved the study design. Written informed consent was obtained from all participants or their legal representatives.
Participants
We prospectively enrolled patients with AIS who underwent rt‐PA IVT at the Department of Neurology of the First Hospital of Jilin University. The inclusion criteria of eligible participants are as follows: age ≥18 and <80 years; male or female; diagnosed with AIS and received rt‐PA IVT at a dose of 0.9 mg/kg within 4.5 h from onset; prestroke modified Rankin Scale (mRS) score of ≤0 to 1; pre‐thrombolysis National Institute of Health Stroke Scale (NIHSS) score of >4 and <25; Glasgow coma scale (GCS) score of ≥8; patients or their legal representatives agreed to treatment and signed the informed consent.
The exclusion criteria of this study are as follows: patients who had contraindications for RIC, e.g., severe soft tissue injury, fracture, subclavian steal syndrome, or peripheral vascular disease in the upper limbs; Patients who underwent endovascular treatment; patients who had a history of atrial fibrillation, or electrocardiogram suggested atrial fibrillation; life expectancy <3 months; pregnant or breast‐feeding women; unwilling to be followed up or poor compliance for treatment; patients enrolled or having been enrolled in another clinical trial within 3 months of this clinical trial.
Randomization
The randomization code was computer‐generated and put into a sealed opaque envelop. Once written informed consent was obtained, the on‐call physicians who were not involved in data analysis or clinical ratings would number the participant and open the corresponding envelop. The treatment allocation was determined based on the randomization code in the envelop. The outcome observers were blinded to the group information.
Sample size
No formal sample size calculation was performed in this study. As an exploratory study, 50 patients were deemed to be an appropriate number to explore the potential effect of RIC and to provide pilot data for future trials.
Study design
Once written informed consent was obtained, the baseline demographic, clinical characteristics, and results of laboratory tests were collected. Patients in the RIC group received RIC two times within 6–24 h from thrombolysis. For avoiding severe erythema or petechia at local skin, the first RIC was performed 6 h after IVT and the second RIC was performed 18 h after thrombolysis. Patients in the control group received sham‐RIC at the same timepoints. Subjects in both groups received standard medical treatment according to the Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. 5 Blood pressure was recorded every 15 min within 2 h after IVT, every 30 min within 2 to 6 h after IVT, and every 1 h until 24 h. The coagulation routine, including thrombin time (TT), activated partial thromboplastin time (APTT), prothrombin time (PT), international normalized ratio (INR), prothrombin activity (PTA), fibrinogen (FBG), liver function including aspartate transaminase (AST) and alanine aminotransferase (ALT), high‐sensitivity C‐reactive protein (hs‐CRP), and homocysteine (Hcy) were tested 24 h after IVT. Head computed tomography (CT) scans were performed for all the subjects 24 h after IVT to diagnose hemorrhagic transformation (HT) according to radiographic criteria (hemorrhagic infarction [HI] type 1, HI‐2, parenchymal hematoma [PH] type 1, and PH‐2). 12 Any HT that led to an increase in NIHSS score of 4 points and more or death was defined as a symptomatic intracranial hemorrhage (sICH). Head magnetic resonance imaging (MRI), cerebrovascular Doppler ultrasound and other risk factors of AIS were screened within 24–72 h of admission to determine the etiological classification of AIS according to China ischemic stroke subclassification (CISS). 13 All patients were followed up for 90 days to evaluate the efficacy and safety of RIC combined with IVT in the treatment of AIS.
RIC
RIC was performed by an automatic device (BB‐RIC‐D1/LAPUL Medical Devices Co, Ltd. China). One entire intervention episode was composed of four cycles of 5 min of ischemia followed by 5 min of re‐perfusion on the healthy upper limb. Limb ischemia was induced by the inflation of a blood pressure cuff to 200 mm Hg for the RIC group. The sham‐RIC was performed in the same way except that the blood pressure cuff was inflated to 60 mmHg.
Outcome assessment
The safety outcome of this study are: ratio of patients who experienced HT within 7 days or before discharge (whichever was earlier); ratio of patients who experienced any adverse events (such as recurrent stroke, heart failure, or myocardial infarction) within the 90‐day follow‐up; laboratory tests (including coagulation routine and liver function) 24 h after IVT; blood pressure between 6–24 h after IVT.
The efficacy outcome of this study are: distribution of mRS score and ratio of patients with mRS score of 0–1 at the 90‐day follow‐up; NIHSS scores 24 h, 7 days or discharge (whichever was earlier), and 1 month after baseline; concentration of Hs‐CRP and Hcy 24 h after IVT.
Statistical analysis
The Statistical Package for the Social Sciences Version 17.0 (SPSS, IMB, West Grove, PA) was used to perform the statistical analyses. Continuous data that complied with normal distribution were presented as mean and standard deviation and were compared using Student’s t‐tests. Continuous data that did not comply with normal distribution were presented as median and quartiles and Mann–Whitney U tests were performed for comparison. Discrete variables were expressed as the rate (percentage) and are analyzed using chi‐square tests. The fluctuation of blood pressure within the first 24 h after IVT between groups was compared using a repeated measures ANOVA. Statistical significance was declared if a calculated two‐tailed P value was <0.05.
Results
Fifty patients with AIS (25 in the RIC group and 25 in the sham‐RIC group) consented to participate in this study and completed all required cycles of RIC (or sham‐RIC) between July 25, 2019 and October 1, 2019 at the First Hospital of Jilin University. However, one patient in the RIC group presented with no acute infarct on his head MRI and was diagnosed with stroke mimics; thus, was excluded from the final analysis. In total, 49 patients (24 in the RIC group and 25 in the sham‐RIC group) was analyzed (Fig. 1). All patients have completed the 90‐day follow‐up. The baseline demographic and clinical characteristics of patients in the RIC group and sham‐RIC group are shown in Table 1. No significant difference was observed between groups.
Figure 1.
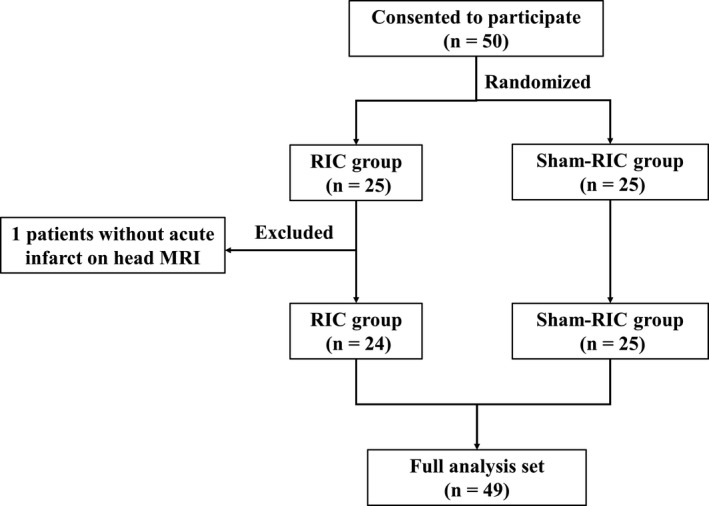
Trial profile. RIC, remote ischemic conditioning; MRI, magnetic resonance imaging.
Table 1.
Baseline demographic and clinical characteristics for all patients.
| RIC group (n = 24) | Sham‐RIC group (n = 25) | |
|---|---|---|
| Age (years) | 59.5 ± 8.5 | 61.3 ± 11.0 |
| Males patients | 20 (83.3%) | 18 (72.0%) |
| Blood pressure (mmHg) | ||
| Systolic pressure | 151.1 ± 20.3 | 154.0 ± 18.7 |
| Diastolic pressure | 92.1 ± 10.5 | 86.1 ± 10.4 |
| Blood glucose (mmol/L) | 8.25 (6.63, 8.88) | 7.70 (6.65, 9.25) |
| OTT (min) | 177.8 ± 44.4 | 192.2 ± 48.7 |
| NIHSS before thrombolysis | 7.0 (6.0, 11.0) | 9.0 (5.5, 11.5) |
| NIHSS before RIC | 6.5 (2.3, 9.8) | 5.0 (4.0, 9.0) |
| Past medical history | ||
| Hypertension | 11 (45.8%) | 16 (64.0%) |
| Diabetes mellitus | 6 (25.0%) | 5 (20.0%) |
| Smoking | 13 (54.2%) | 16 (64.0%) |
| Drinking | 11 (45.8%) | 15 (60.0%) |
| TIA | 0 (0.0%) | 1 (4.0%) |
| Stroke | 11 (45.8%) | 6 (24.0%) |
| CHD | 2 (8.3%) | 2 (8.0%) |
| Current use of antiplatelet agents | 1 (4.2%) | 0 (0.0%) |
| CISS | ||
| LAA | 8 (33.3%) | 9 (36.0%) |
| CS | 1 (4.2%) | 0 (0.0%) |
| PAD | 11 (45.8%) | 11 (44.0%) |
| OE | 2 (8.3%) | 3 (12.0%) |
| UE | 2 (8.3%) | 2 (8.0%) |
Blood pressure and blood glucose were tested prior to the administration of rt‐PA.
RIC, remote ischemic conditioning; OTT, onset‐to‐treatment time; NIHSS, National Institute of Health Stroke Scale; TIA, transient ischemic attack; CHD, coronary artery heart disease; CISS, China ischemic stroke subclassification; LAA, large artery atherosclerosis; CS, cardiogenic stroke; PAD, penetrating artery disease; OE, other etiology; UE, undetermined etiology.
HT and other adverse events
In the RIC group, one patient (4.2%) presented with HT in the head CT performed 24 h after IVT, which was classified as PH‐1. In the sham‐RIC group, four patients (16%) presented with HT, which was composed of two HI‐1, one HI‐2, and one PH‐1. There was no significant difference in the ratio of HT between groups (P = 0.171). None of the HT was symptomatic. During the 90‐day follow‐up, one (4.2%) patient in the RIC group died of pulmonary infection, one (4.2%) patient in the RIC group had recurrent ischemic stroke, and 1 (4%) patient in the sham‐RIC group developed vascular dementia. No other adverse event was reported. The ratio of adverse events during the follow‐up was not significantly different between groups (2/24 (8.3%) in the RIC group versus 1/25 (4.0%) in the sham‐RIC group, P = 0.609).
Blood pressure and laboratory tests
Blood pressure within the first 24 h after IVT was compared between groups (Fig. 2). Prior to the first RIC 6 h after IVT, there was no significant difference in systolic blood pressure (SBP, P = 0.732) or diastolic blood pressure (DBP, P = 0.385) between groups. After the completion of the first RIC (within 6–24 h after IVT), no significant difference was observed in SBP (P = 0.960) or DBP (P = 0.244) between groups. In addition, there was no significant difference between groups in the level of coagulation function (TT, APTT, PT, INR, PTA, and FBG) or liver function (AST and ALT) tested 24 h after IVT. The details of the laboratory tests were showed in Table 2.
Figure 2.
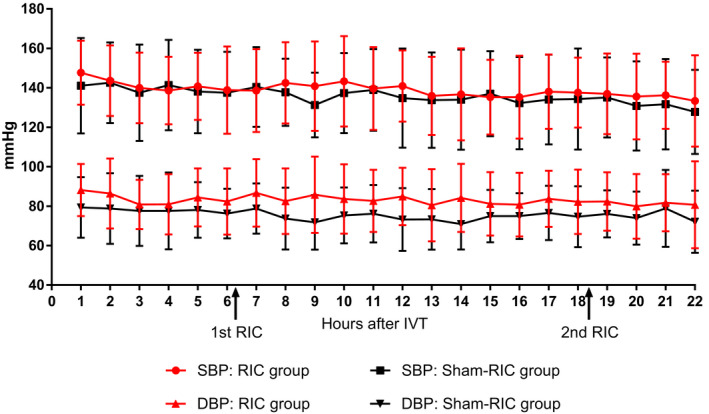
Fluctuation of blood pressure within the first 24 h after IVT. IVT, intravenous thrombolysis; SBP, systolic blood pressure; DBP, diastolic blood pressure; RIC, remote ischemic conditioning.
Table 2.
Laboratory tests at 24 h after thrombolysis between the two groups.
| Laboratory tests | RIC group (n = 24) | Sham‐RIC group (n = 25) | P | References |
|---|---|---|---|---|
| Coagulation routine | ||||
| TT, s | 15.3 ± 2.2 | 15.7 ± 2.6 | 0.634 | 11.0–17.8 |
| APTT, s | 30.0 ± 2.6 | 30.4 ± 3.6 | 0.731 | 20.0–40.0 |
| PT, s | 12.0 ± 0.6 | 11.8 ± 0.8 | 0.361 | 9.0–13.0 |
| INR | 1.03 ± 0.05 | 1.01 ± 0.07 | 0.357 | 0.80–1.20 |
| PTA, % | 95.3 ± 8.0 | 98.1 ± 9.9 | 0.283 | 80–120 |
| FBG, g/L | 2.73 ± 0.72 | 2.45 ± 0.48 | 0.118 | 2.00–4.00 |
| Liver function | ||||
| AST, U/L | 22.3 ± 5.3 | 23.2 ± 6.4 | 0.548 | 15.0–40.0 |
| ALT, U/L | 17.3 ± 7.0 | 17.9 ± 8.5 | 0.788 | 9.0–50 |
| Hs‐CRP, mg/L | 3.13 (3.02, 3.23) | 4.85 (3.02, 6.85) | 0.048 | 0–3.50 |
RIC, remote ischemic conditioning; TT, thrombin time; APTT, activated partial thromboplastin time; PT, prothrombin time; INR, international normalized ratio; PTA, prothrombin activity; FBG, fibrinogen; AST, aspartate transaminase; ALT, alanine aminotransferase; Hs‐CRP, high sensitive C‐reactive protein.
Efficacy outcome
At the 90‐day follow‐up, no significant difference was observed in the ratio of patients who achieved good clinical outcome (mRS score ≤ 1) between groups (15/24 (62.5%) in the RIC group versus 17/25 (68.0%) in the sham‐RIC group, P = 0.686) (Table 3). The value of mRS score on day −90 was not significantly different (P = 0.350). Figure 3 shows the distribution of mRS score on day −90. The mRS scores of all patients on day −90 were within 0–4 except for one patient in the RIC group who died during the follow‐up and accordingly had an mRS score of 6. There was no significant difference in the NIHSS scores 24 h, 7 days, and 30 days after baseline between groups (P = 1.000, 0.513, and 0.910, respectively). The level of hs‐CRP tested 24 h after IVT in the RIC group was significantly lower than that in the sham‐RIC group (3.13 (3.02–3.23) mg/L in the RIC group versus 4.85 (3.02–6.85) mg/L in the sham‐RIC group (P = 0.048); however, no significant difference was observed in the level of Hcy at the same timepoint between groups (P = 0.503).
Table 3.
Efficacy outcome between the two groups.
| RIC group (n = 24) | Sham‐RIC group (n = 25) | P | |
|---|---|---|---|
| mRS 0–2, n (%) | 15 (62.5) | 17 (68.0) | 0.686 |
| mRS‐90 days | 1.0 (1.0, 2.0) | 1.0 (0.0, 2.5) | 0.350 |
| NIHSS | |||
| 24 h | 4.5 (2.0, 7.8) | 5.0 (2.0, 7.5) | 1.000 |
| 7 days | 3.0 (1.3, 6.5) | 2.0 (1.0, 6.0) | 0.513 |
| 30 days | 1.5 (1.0, 2.0) | 2.0 (0.5, 3.5) | 0.910 |
RIC, remote ischemic conditioning; mRS, modified Rankin Scale; NIHSS, National Institute of Health stroke scale.
Figure 3.
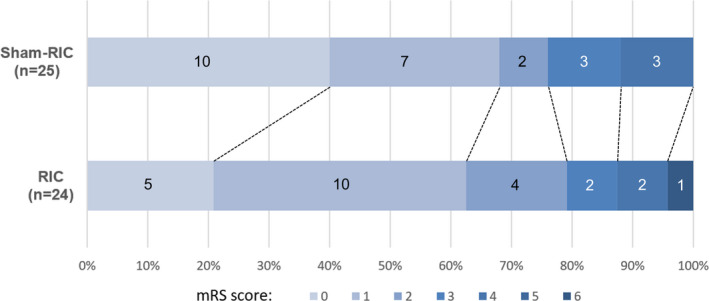
Distribution of mRS score at 90 days. mRS, modified Rankin Scale; RIC, remote ischemic conditioning. Figure on the bar indicates the number of patients who have corresponding mRS score at 90‐day follow‐up.
Discussion
Our results suggested that the combination of RIC and intravenous rt‐PA was safe for the treatment of AIS as no significant difference was observed between groups in the ratio of HT, the ratio of adverse events during the follow‐up, or other clinical parameters. However, the patients in the RIC group exhibited a significantly lower concentration of hs ‐CRP 24 h after IVT than those in the sham‐RIC group, which implied that RIC had an anti‐inflammatory effect in patients with AIS who underwent IVT.
Several clinical trials have studied the neuroprotective effect of RIC in patients with cerebrovascular diseases. They reported that long‐term RIC (up to 1 year) initiated in the nonacute phase of stroke may reduce the risk of recurrent stroke in patients with intracranial artery stenosis and slow down cognition decline in patients with cerebral small‐vessel disease. 14 , 15 Another study performed RIC for 14 consecutive days on nonthrombolysis patients with stoke (within 72 h of ictus) and found that RIC might improve neurological outcome. 16 In addition, Remote Ischemic Conditioning After Stroke Trial 2 (RECAST‐2) recruited patients with stroke within 6 h from onset (55% thrombolyzed) and performed RIC twice daily for up to 4 days. Their results demonstrate that RIC is safe and feasible in hyperacute phase of stroke. 17 The present study adds to the growing body of evidence regarding this topic. Particularly, we focus on the application of RIC in patients undergoing IVT.
To date, there have been three published clinical trials that investigate the effect of RIC on patients with AIS who received IVT. Hougaard et al. performed a single episode of RIC on patients suspected of AIS when they were transported to the hospital (where they received IVT if the diagnosis of AIS was confirmed). Although their major outcomes were neutral, a tissue survival analysis suggested that prehospital RIC might have immediate neuroprotective effects. 10 However, the baseline NIHSS score in the intervention group of that study was significantly lower than that in the controls. Moreover, 45 of 247 patients (18.2%) failed to complete the entire four cycles of RIC because of insufficient transportation time. Generally, Hougaard et al’s study demonstrated the feasibility of applying RIC prior to IVT, but clinical trials with a more well‐rounded design to explore the role of RIC in the treatment of AIS after IVT are still needed. In another study, conducted by Che et al, 30 patients with AIS were 1:1 randomized to the RIC group and control group. They performed RIC immediately after IVT (the median time from completion of IVT to the first cycle of RIC was 66 min) and twice daily for the following 6 days. Although the major outcomes were negative, they discovered a significant reduction in NIHSS score on day −30 in the RIC group. 11 However, considering the methodology of RIC in Che et al’s study, it is difficult to determine whether this neuroprotective effect is from the combination of RIC and IVT or from the RIC performed in the following 6 days, because a number of studies have already reported the neuroprotective effect of repeated RIC for consecutive days in AIS without IVT. 7 , 14 , 15 , 16 RECAST‐2 also performed post hoc analysis on the data of participants undergoing IVT (55%). This high‐quality randomized trial demonstrated the feasibility of RIC in this cohort, whereas other outcomes (e.g., laboratory tests) were not compared in thrombolysis patients. Future studies should focus on the efficacy of RIC combined with IVT. 17 Our study only performed RIC twice within the first 24 h after IVT; thus, we placed greater emphasis on the combination of RIC and IVT in the interpretation of our results. Additionally, the baseline NIHSS score in patients in Che et al.’s study was 6.5 (4.0–10.0), whereas in ours this was 8.0 (6.0–11.0). Taken together, our study provides further insight into the application of RIC combined with IVT to patients with more severe AIS.
Our study also included the laboratory tests to investigate safety outcome. Multiple markers of coagulation are reported to be associated with HT. 18 Our results showed no significant difference in the ratio of HT or coagulation function between groups, further demonstrating the safety of RIC combined with intravenous rt‐PA.
Our study has clinical implications on the anti‐inflammatory effect of RIC. The level of hs‐CRP has been reported to be negatively associated with successful recanalization and good clinical outcomes in patients with AIS treated by rt‐PA. 19 , 20 Although conclusions remain controversial, the CRP‐lowering effect of RIC in cardio‐cerebrovascular disease has been studied recently. A single‐center randomized control study conducted on patients with myocardial infarction observed a significantly lower level of hs‐CRP 24 h after percutaneous coronary intervention in patients who received RIC 1 h before surgery than the control patients. 21 RECAST recruited 26 patients with AIS and found that RIC had no effect on the level of CRP, although an elevation of the level of heat shock protein 27 (HSP 27) was found, which was considered a neuroprotective biomarker. 22 In this study, patients in the RIC group exhibited a significantly lower level of hs‐CRP tested 24 h after thrombolysis (P = 0.048), which suggested that RIC was effective in reducing hs‐CRP in patients with AIS who underwent IVT. However, we did not test hs‐CRP prior to the first RIC. Additionally, the baseline NIHSS score in the RIC group was slightly lower than that in the sham‐RIC group (although this was not significant). Consequently, it cannot be ascertained whether the difference in the level of hs‐CRP is related to the intervention in our study. Hyper‐homocysteinemia (HHcy) is a risk factor of cerebrovascular diseases. It is believed that HHcy leads to endothelial dysfunction via the increase of ROS and deactivation of nitric oxide. 23 Nevertheless, the level of Hcy in the RIC group was not significantly different from that in the control group in our study. Given the small sample size of current studies (including ours), the effect of RIC on hs‐CRP as well as on other biomarkers still needs to be investigated in future studies.
There are several limitations to our study. First, as a pilot study, only 50 subjects were recruited. The small sample size in our study was not enough to detect the efficacy of RIC and may have led to statistical bias. Second, the pathogenesis of AIS was not considered during the recruitment of participants. The severity of blood‐brain barrier disruption and inflammation caused by large artery occlusion, small vessel disease, or embolic stroke can be different, and might have caused a different response to RIC. The application of RIC combined with IVT in different subtypes of AIS should also be studied in future trials.
Overall, our study suggests that RIC combined with intravenous rt‐PA is safe in the treatment of AIS. The neuroprotective and anti‐inflammatory effect of this therapy warrants further study on a larger scale.
Conflict of Interest
None.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (Grant No. 81971105) to Zhen‐Ni Guo, the National Key R&D Program of China (2016YFC1301600), JLUSTIRT (2017TD‐12) and Jilin Provincial Key Laboratory (20190901005JC) to Yi Yang.
Funding Information
This project was supported by the National Natural Science Foundation of China (Grant No. 81971105) to Zhen‐Ni Guo, the National Key R&D Program of China (2016YFC1301600), JLUSTIRT (2017TD‐12) and Jilin Provincial Key Laboratory (20190901005JC) to Yi Yang.
Funding Statement
This work was funded by JLUSTIRT grant 2017TD‐12; National Natural Science Foundation of China grant 81971105; National Key R&D Program of China grant 2016YFC1301600; Jilin Provincial Key Laboratory grant 20190901005JC.
References
- 1. GBD 2017 Causes of Death Collaborators . Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol 2019;18:394–405. [DOI] [PubMed] [Google Scholar]
- 3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019;50:e344–e418. [DOI] [PubMed] [Google Scholar]
- 4. Committee ESOEE, Committee EW. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008;25:457–507. [DOI] [PubMed] [Google Scholar]
- 5. Neurology CSo , Society CS . Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin J Neurol 2018;51:666–682. [Google Scholar]
- 6. Emberson J, Lees K, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta‐analysis of individual patient data from randomised trials. Lancet 2014;384:1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Landman TRJ, Schoon Y, Warle MC, et al. Remote ischemic conditioning as an additional treatment for acute ischemic stroke. Stroke 2019;50:1934–1939. [DOI] [PubMed] [Google Scholar]
- 8. Zhou G, Li MH, Tudor G, et al. Remote ischemic conditioning in cerebral diseases and neurointerventional procedures: recent research progress. Front Neurol 2018;9:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoda MN, Siddiqui S, Herberg S, et al. Remote ischemic perconditioning is effective alone and in combination with intravenous tissue‐type plasminogen activator in murine model of embolic stroke. Stroke 2012;43:2794–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hougaard KD, Hjort N, Zeidler D, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke. Stroke 2014;45:159–167. [DOI] [PubMed] [Google Scholar]
- 11. Che R, Zhao W, Ma Q, et al. rt‐PA with remote ischemic postconditioning for acute ischemic stroke. Ann Clin Transl Neurol 2019;6:364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yaghi S, Willey JZ, Cucchiara B, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017;48:e343–e361. [DOI] [PubMed] [Google Scholar]
- 13. Gao S, Wang YJ, Xu AD, et al. Chinese ischemic stroke subclassification. Front Neurol 2011;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meng R, Asmaro K, Meng L, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology 2012;79:1853–1861. [DOI] [PubMed] [Google Scholar]
- 15. Wang Y, Meng R, Song H, et al. Remote ischemic conditioning may improve outcomes of patients with cerebral small‐vessel disease. Stroke 2017;48:3064–3072. [DOI] [PubMed] [Google Scholar]
- 16. Li Y, Liang K, Zhang L, et al. Upper limb ischemic postconditioning as adjunct therapy in acute stroke patients: a randomized pilot. J Stroke Cerebrovasc Dis 2018;27:3328–3335. [DOI] [PubMed] [Google Scholar]
- 17. England TJ, Hedstrom A, O'Sullivan SE, et al. Remote ischemic conditioning after stroke trial 2: a phase IIb randomized controlled trial in hyperacute stroke. J Am Heart Assoc 2019;3:e013572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bagoly Z, Szegedi I, Kalmandi R, et al. Markers of coagulation and fibrinolysis predicting the outcome of acute ischemic stroke thrombolysis treatment: a review of the literature. Front Neurol 2019;10:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bivard A, Lincz LF, Maquire J, et al. Platelet microparticles: a biomarker for recanalization in rtPA‐treated ischemic stroke patients. Ann Clin Transl Neurol 2017;4:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yue Y, Li Z, Hu L, et al. Clinical characteristics and risk score for poor clinical outcome of acute ischemic stroke patients treated with intravenous thrombolysis therapy. Brain Behav 2019;9:e01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou F, Song W, Yin L, et al. Effects of remote ischemic preconditioning on myocardial injury and endothelial function and prognosis after percutaneous coronary intervention in patients with acute coronary syndrome. Eur Rev Med Pharmacol Sci 2017;21:4642–4648. [PubMed] [Google Scholar]
- 22. England T, Hedstrom A, O'Sullivan S, et al. RECAST (Remote Ischemic Conditioning After Stroke Trial): a pilot randomized placebo controlled phase ii trial in acute ischemic stroke. Stroke 2017;48:1412–1415. [DOI] [PubMed] [Google Scholar]
- 23. Moretti R, Caruso P. The controversial role of homocysteine in neurology: from labs to clinical practice. Int J Mol Sci 2019;20:231. [DOI] [PMC free article] [PubMed] [Google Scholar]


