Abstract
BACKGROUND AND AIMS:
In some states, liver transplantation (LT) for alcohol-associated liver disease (ALD) is covered by Medicaid only with documentation of abstinence and/or alcohol rehabilitation. Different Medicaid policies may affect the distribution of LT for ALD, particularly post-2011, as centers have adopted early (i.e., specific abstinence period not required) LT practices.
APPROACH AND RESULTS:
We surveyed Medicaid policies in all states actively performing LT and linked state policies to prospectively collected national registry data on LT recipients from 2002 to 2017 with ALD as the primary listing diagnosis. We categorized Medicaid policies for states as “restrictive” (requiring documentation of a specific abstinence period and/or rehabilitation) versus “unrestrictive” (deferring to center eligibility policies). Difference-of-differences analysis, comparing 2002–2011 versus 2012–2017, evaluated whether restrictive policies were associated with decreased proportion of LTs paid by Medicaid among patients with ALD post-2011. We performed sensitivity analyses to account for any differences by diagnosis of hepatocellular carcinoma, hepatitis C virus, nonalcoholic steatohepatitis, or Medicare insurance. We also performed a sensitivity analysis to account for any difference by prevalence of ALD among restrictive versus unrestrictive states. Of 10,836 LT recipients in 2002–2017, 7,091 were from 24 states in the restrictive group and 3,745 from 14 states in the unrestrictive group. The adjusted proportion (95% confidence interval) of LTs paid by Medicaid among restrictive versus unrestrictive states between 2002 and 2011 was 17.6% (15.4%−19.8%) versus 18.9% (15.4%−22.3%) (P = 0.54) and between 2012 and 2017, 17.2% (14.7%−19.7%) versus 23.2% (19.8%−26.6%) (P = 0.005). In difference-of-differences analysis, restrictive (versus unrestrictive) policies were associated with a 4.7% (0.8%−8.6%) (P = 0.02) absolute lower adjusted proportion of LTs for ALD paid by Medicaid post-2011.
CONCLUSIONS:
Restrictive Medicaid policies are present in most states with active LT centers and are associated with lower proportions of LTs for ALD paid by Medicaid post-2011 compared to states with unrestrictive Medicaid policies. Reevaluation of Medicaid alcohol use policies may be warranted, to align more closely with contemporary center-level practices.
Alcohol-associated liver disease (ALD) is implicated in 48% of liver-related deaths in the United States and has recently become the most common indication for liver transplantation (LT).(1,2) While many centers require patients with ALD to abstain from alcohol use for at least 6 months to be eligible for LT, the application of early LT for ALD (i.e., without a minimum period of abstinence) is rapidly increasing across the United States, particularly since the 2011 publication of a landmark European trial which showed significant survival benefit in early LT for patients with severe alcohol- associated hepatitis (AH).(2–4) While initially studied in a specific subpopulation (AH), early LT practices have since been generalized to the broader ALD population.(2–4)
The “6-month rule,” an arbitrary time frame, was informally suggested by an expert panel of transplant physicians after the first National Institutes of Health Consensus Development Conference on Liver Transplantation in 1983.(5,6) While widely adopted, there has never been an official United Network for Organ Sharing (UNOS) policy to restrict access to LT based upon duration of pre-LT abstinence, and LT centers are allowed to apply their own policies for LT eligibility.(5) Anecdotally, some Medicaid policies require specific durations of pre-LT abstinence and/or documentation of formal alcohol rehabilitation prior to LT for financial coverage of LT. There is no published literature summarizing these policies or their potential influence on the proportion of LTs for ALD paid by Medicaid.
In this study, we examined individual state Medicaid policies with regard to requirements of pre-LT abstinence and/or rehabilitation and their association with the proportion of LTs for ALD paid by Medicaid.
Methods
DETERMINING MEDICAID POLICY
We evaluated Medicaid organ transplant policies in all states with any active adult LT center in 2017, which corresponds to the available UNOS data at the time of this study. We systematically elicited state Medicaid policies for financial coverage of LT for ALD by directly contacting state Medicaid offices and practicing transplant physicians, first by e-mail and then telephone if necessary, and posing the following question: “conducting a study examining Medicaid policy for liver transplant in alcoholic liver disease between 2002 and 2017. In your experience, does (your state) Medicaid require specific documentation of pre-LT abstinence or rehab (e.g., 6 months sobriety) for financial coverage? … simple yes/no answer would suffice.” We obtained yes/no confirmation of Medicaid policies from all states. To obtain a response in all 38 states included in this study, we submitted official inquiries to the Medicaid offices in each of the individual states and obtained official responses from 21 of these states through this means. To obtain responses from the remaining 17 states, we contacted transplant physicians in each of the states—7 of 17 responses were from medical or surgical directors of an LT program, whereas the remaining 10 responses were from practicing transplant physicians at an active LT program in their respective state. We specifically asked if there were any changes in policy between 2002 and 2017—there were none. We then categorized these policies as restrictive versus unrestrictive; “restrictive” was defined as requiring documentation of a specific period of abstinence and/or rehabilitation. All others, including Medicaid policies that deferred to center eligibility policies, were categorized as “unrestrictive.” The interpretation of these policies was independently performed by two co-authors (B.P.L., N.A.T.), who agreed upon the categorization of all state policies as restrictive versus unrestrictive.
STUDY POPULATION
This study used prospectively collected UNOS registry data from LT recipients between 2002 and 2017 reflecting institution of the Model for End-Stage Liver Disease (MELD)–based allocation system and available UNOS data. We only included LT recipients with ALD as the primary listing diagnosis, defined as alcoholic cirrhosis or AH. Any patients aged <18 with human immunodeficiency virus, acute hepatic failure, MELD exception, prior LT, and unknown or unreported primary insurance status were excluded. Any patients with a non-ALD primary listing diagnosis (e.g., nonalcoholic steatohepatitis, hepatitis C virus, hepatitis B virus, autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, cryptogenic) were excluded. Given that hepatocellular carcinoma (HCC) is an exclusion criterion for early LT policies at some centers and that exception rules for HCC have changed over the course of this study period, patients with a listing or transplant diagnosis of HCC were excluded.(3,7–9) However, we performed a sensitivity analysis with patients with HCC included.
PREDICTOR VARIABLES AND INSURANCE STATUS
Patient characteristics, including insurance status, were captured at the time of LT. To account for regional differences in waiting times, regions were divided into low (3, 6, 10, 11), medium (2, 4, 7, 8), and high (1, 5, 9) groups by wait-list time. Type of medical insurance was categorized as Medicaid versus non-Medicaid by primary payment status at the time of LT. Non-Medicaid included those with private insurance, Medicare, Veterans Affairs (VA), self, donation, free care, foreign government, and state government agency listed as primary payment status. We also performed separate analyses with private versus nonprivate (Medicaid, Medicare, VA, self, donation, free care, foreign government, and state government agency) insurance by primary payment status at the time of LT.
To link patients to state policies, we linked each LT center to the corresponding state and Medicaid policy variables.
IMPACT OF EARLY LT FOR ALD
We hypothesized that the Franco-Belgian trial for early LT in AH published in 2011(4) prompted increased US acceptance of early LT not only for AH but also for the broader ALD population, with widening of disparities for LT for Medicaid ALD patients in states with restrictive Medicaid policies. Specifically, we hypothesized that the proportion of LT for ALD paid by Medicaid post-2011 would increase in unrestrictive states but not in restrictive states where financial eligibility for early LT was not available. While AH represents only a small subpopulation of ALD, there is evidence that centers have generalized early LT practices to the broad ALD population. (2) Thus, state policies were predicted to have a differential effect by era, even though state policies did not change between 2002 and 2017, due to center-level practices. To evaluate this “era” effect, we performed analyses dividing our study period into 2002–2011 and 2012–2017.
STATISTICAL ANALYSIS
Categorical variables were compared using the chi-squared test. Continuous variables were first assessed for normality, then compared using the t or Wilcoxon test as appropriate.
We conducted two difference-of-differences analyses, to assess the effect of restrictive state Medicaid policies with the adoption early LT center-level policies beginning after 2011 on the proportion of LT for ALD paid by Medicaid and to ensure that differences were not confounded by baseline population characteristics or secular trends in factors affecting rates of LT. First, we assessed the net effect of restriction in 2012–2017 compared to 2002–2011 on the proportion of Medicaid payment among LT recipients for ALD, using a logistic model including era, whether restrictive or nonrestrictive state, and their interaction and adjusting for individual-level characteristics affecting likelihood of LT (age, sex, race, college education, MELD score, portal vein thrombosis, receipt of simultaneous liver–kidney transplant, blood type) as well as region wait time and center clustering. The interaction of era and restrictive policies captures the effect of restriction in 2012–2017 and the net of any difference between restrictive and unrestrictive states in 2002–2011. We also used this approach to assess the net effect of restriction on private insurance payment for LT for ALD, on the hypothesis that restrictive Medicaid policies may have a reciprocal effect favoring patients with private insurance.
Second, to rule out reduced prevalence of ALD among all LT recipients in restrictive versus unrestrictive states as an explanation for reduced numbers of LT for ALD, we used the same difference-of-differences approach to assess the association of restriction in 2012–2017 with prevalence of ALD among all LT recipients, which included LT recipients with ALD and non-ALD (n = 46,113).
We performed a number of additional sensitivity analyses. To ensure that any differences by Medicaid alcohol use policies were isolated to ALD, we performed separate sensitivity analyses in cohorts restricted to hepatitis C virus and nonalcoholic steatohepatitis as primary listing diagnosis. We also used a similar approach to assess the net effect of restriction on Medicare insurance payment for LT for ALD – as a national policy, we hypothesized we would not observe differences by restrictive vs. unrestrictive state in this Medicare sensitivity analysis. To ensure that our findings were most associated with early LT practices (i.e. isolated to years after 2011), we also performed a sensitivity analysis which divided the cohort into three time periods (2002–2006, 2007–2011, 2012–2017).
Analyses were performed using Stata MP, version 14.2 (Stata Corp, College Station, TX). P values <0.05 were considered statistically significant.
The study was approved by the institutional review board at University of California, San Francisco.
Results
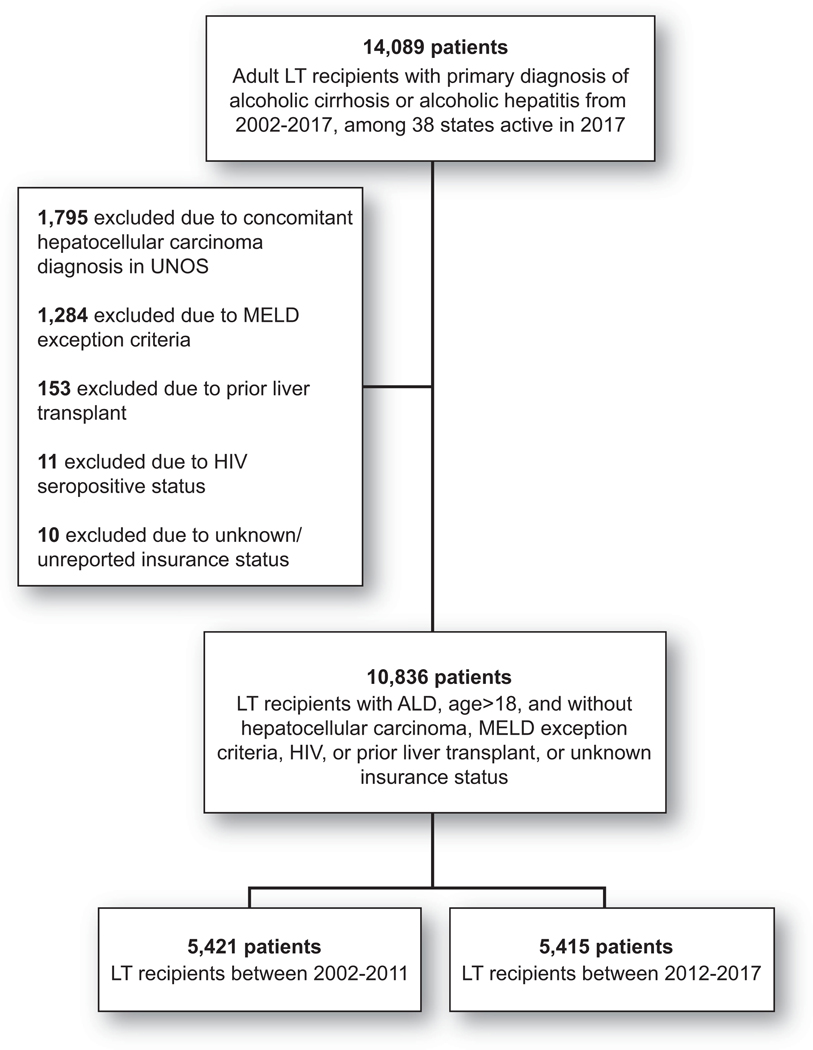
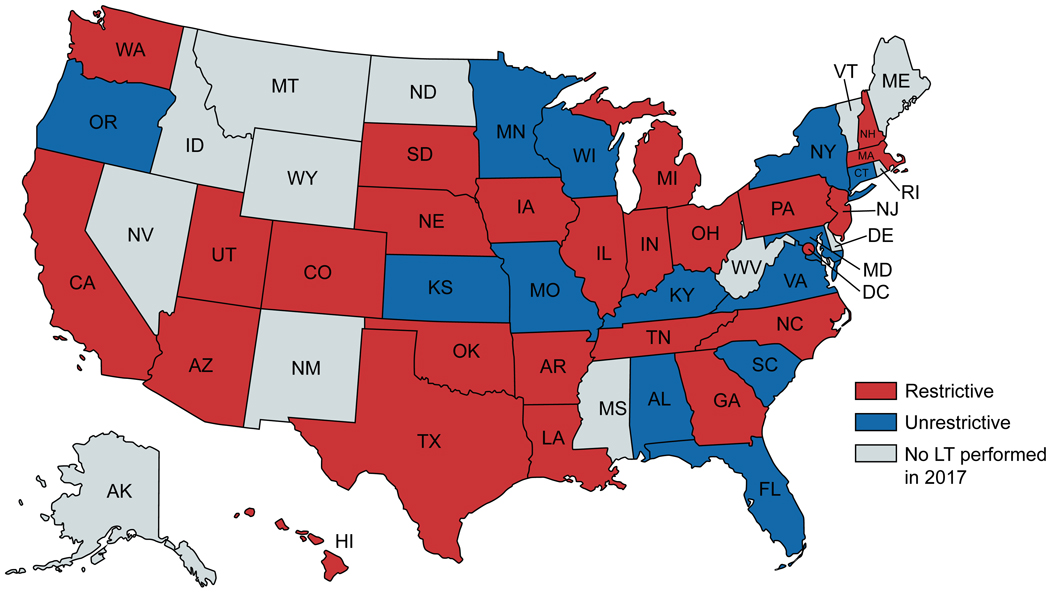
A total of 10,836 LT recipients for ALD from 2002–2017 from 38 states (for this study, District of Columbia and Puerto Rico were considered states) were included; derivation of the cohort for our primary analysis is outlined in Fig. 1. The restrictive policy group included 24 states, 78 centers, and 7,091 patients. The unrestrictive group included 14 states, 36 centers, and 3,745 patients. Categorization of states by restrictive versus unrestrictive policy is summarized in Supporting Table S1 and Fig. 2. Patients in the restrictive group (versus unrestrictive) were more frequently with private insurance (4,273/7,091 [60%] versus 2,116/3,745 [56%], P < 0.001) and from a high–wait time region (1,831/7,091 [26%] versus 617/3,745 [16%], P < 0.001). They were less likely to be white (5,430/7,091 [77%] versus 3,191/3,745 [85%], P < 0.001) and to have a college education (3,064/7,091 [43%] versus 1,796/3,745 [48%], P < 0.001). They had higher MELD (26 versus 24, P < 0.001) and more often required renal replacement therapy at LT (1,689/7,091 [24%] versus 643/3,745 [15%], P < 0.001). Baseline characteristics are summarized in Table 1.
FIG. 1.
Study cohort for primary analysis. This study used prospectively collected UNOS registry data from LT recipients between 2002 and 2017 reflecting the period of MELD-based allocation and available UNOS data. We only included LT recipients with ALD as primary listing diagnosis, defined as alcoholic cirrhosis or alcoholic hepatitis. Any patients with age < 18, human immunodeficiency virus, acute hepatic failure, MELD exception, prior LT, and unknown or unreported primary insurance status were excluded. Abbreviation: HIV, human immunodeficiency virus.
FIG. 2.
Classification of state policies. This US map represents states with restrictive (red) versus unrestrictive (blue) Medicaid policies for ALD. Puerto Rico has an unrestrictive policy and is not represented in the figure. The following states were not included in this study as no adult liver transplants meeting study inclusion criteria were performed in 2017: Alaska, Idaho, Maine, Mississippi, Montana, Nevada, New Hampshire, New Mexico, North Dakota, Rhode Island, Vermont, West Virginia, Wyoming.
Table 1.
Characteristics of Patients Receiving a Liver Transplant for Alcohol-Associated Liver Disease*
| Recipient Characteristic | Transplant in a State with a Restrictive Policy (N=7,091) | Transplant in a State with an Unrestrictive Policy (N=3,745) | P |
|---|---|---|---|
| Age – yr – median (IQR) | 53 (47–59) | 54 (47–60) | 0.01 |
| Male, n (%) | 5,379 (76) | 2,871 (77) | 0.35 |
| Medicaid Insurance, n (%) | 1,295 (18) | 718 (19) | 0.25 |
| Private Insurance, n (%) | 4,273 (60) | 2,116 (56) | <0.001 |
| Race / Ethnicity, n (%) Caucasian African American Hispanic Asian Other |
5,430 (77) 303 (4) 1164 (16) 98 (1) 96 (1) |
3,191 (85) 131(4) 335 (9) 46 (1) 42 (1) |
<0.001 |
| Highest Education Level, n (%) High School or Below College or Above Unknown |
3,161 (45) 3,064 (43) 866 (12) |
1,430 (38) 1,796 (48) 519 (14) |
<0.001 |
| Body Mass Index, median (IQR) | 27.8 (24.5–31.9) | 27.9 (24.5–32.1) | 0.24 |
| Diabetes**, n (%) | 1,298 (18) | 673 (18) | 0.72 |
| Renal Replacement Therapy, n (%) | 1,689 (24) | 642 (17) | <0.001 |
| Portal Vein Thrombosis at LT***, n (%) | 577 (8) | 294 (8) | 0.60 |
| MELD Score at LT, median (IQR) | 26 (20–34) | 24 (18–33) | <0.001 |
| SLK Recipient, n (%) | 821 (12) | 358 (10) | <0.001 |
| Region Wait Time, n (%) Low Medium High |
2,349 (33) 2,929 (41) 1,813 (26) |
1,659 (44) 1,469 (39) 617 (16) |
<0.001 |
| Days on Waitlist, median (IQR) | 30 (8–122) | 28 (8–104) | 0.007 |
At time of liver transplant
For diabetes status, 80 (1%) missing values in restrictive group, 48 (1%) missing values in unrestrictive group
For portal vein thrombosis at LT, 107 (2%) missing values in restrictive group, 67 (2%) missing values in unrestrictive group
Baseline characteristics stratified by era (2002–2011 versus 2012–2017) are summarized in Supporting Table S2.
Among medium-volume and high-volume states (performing at least 100 LTs between 2002 and 2017), the proportion of LT for ALD paid by Medicaid (versus non-Medicaid) ranged from 6.1% (North Carolina; 12 of 196 LTs) to 29.3% (California; 327 of 1,117 LTs). Among low-volume states, the proportion ranged from 13.5% (Oklahoma; 7 of 52 LTs) to 61.3% (New Jersey; 19 of 31 LTs). Values for all states can be found in Supporting Table S1.
PROPORTION OF PAYMENT BY MEDICAID FOR LT AMONG PATIENTS WITH ALD
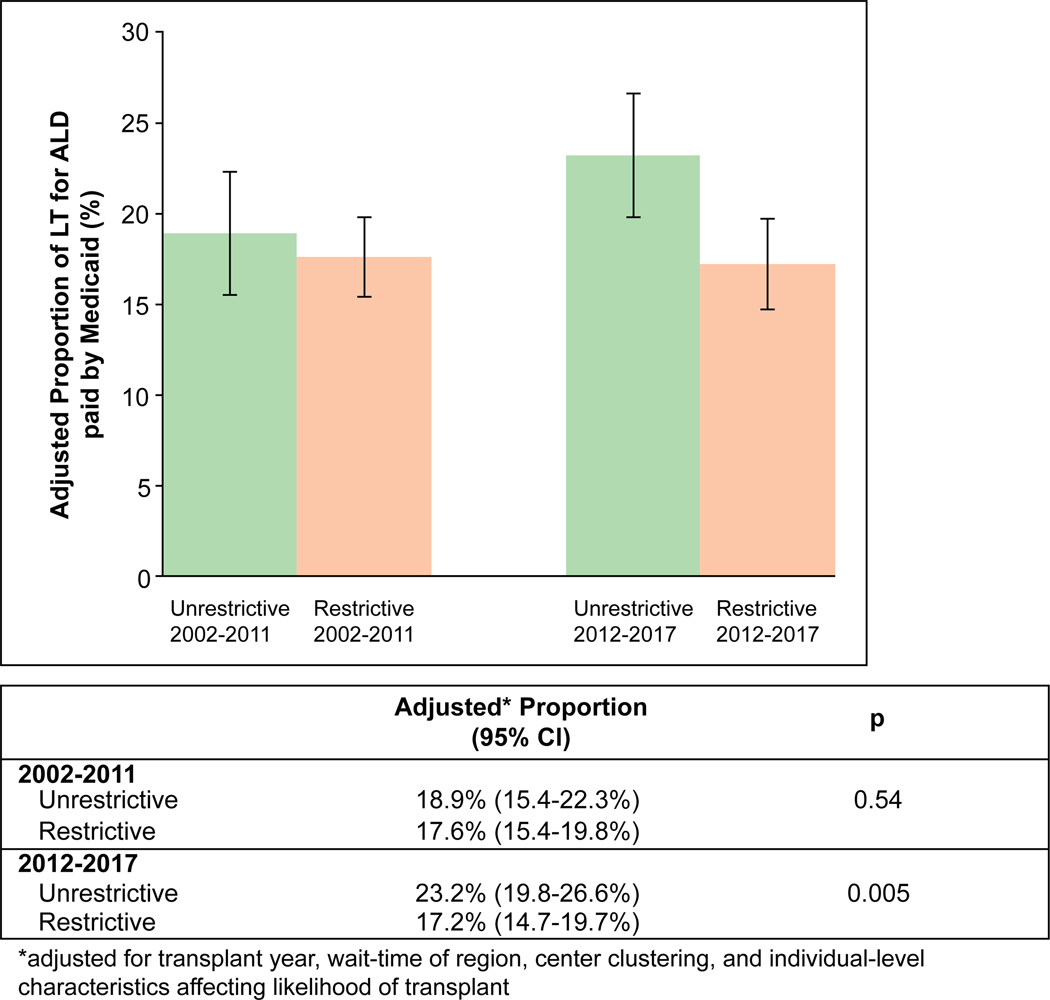
In an analysis adjusting for individual-level characteristics affecting likelihood of LT, region wait time, differences by era in unrestrictive states, and differences between restrictive and unrestrictive states in 2002–2011, a restrictive policy was associated with lower odds of LT for ALD paid by Medicaid in 2012–2017 (adjusted odds ratio [aOR], 0.74; 95% confidence interval [CI], 0.57–0.96; P = 0.02). Between 2002 and 2011, the adjusted proportion of LT paid by Medicaid was 17.6% (95% CI 15.4%−19.8%) versus 18.9% (95% CI, 15.4%−22.3%) among restrictive versus unrestrictive states (P = 0.54). Between 2012 and 2017, the adjusted proportion of LT paid by Medicaid was 17.2% (95% CI, 14.7%−19.7%) versus 23.2% (95% CI, 19.8%−26.6%) among restrictive versus unrestrictive states (P = 0.005). In difference-of-differences analysis, restrictive (versus unrestrictive) policies were associated with a 4.7% (95% CI, 0.8%−8.6%; P = 0.02) absolute lower adjusted proportion of LT paid by Medicaid after 2011. Adjusted probabilities by era are summarized in Fig. 3. Results for patients with HCC are included in the Supporting Information.
FIG. 3.
Adjusted proportion of LT for ALD paid by Medicaid among restrictive versus unrestrictive states. This figure shows the proportion of LT for ALD paid by Medicaid among restrictive versus unrestrictive states, adjusted for transplant year, wait time of region, center clustering, and individual-level characteristics affecting likelihood of transplant between 2002 and 2011 and between 2012 and 2017. Error bars indicate 95% CIs.
In adjusted analysis to ensure that our findings were not due to different prevalence of ALD, we conducted a sensitivity analysis among all LT recipients (n = 46,113) during the study period. We confirmed that a restrictive (versus unrestrictive) policy was not associated with a significant difference in the odds of ALD among all LT recipients between 2012 and 2017 versus between 2002 and 2011 (aOR, 1.14; 95% CI, 0.97–1.35; P = 0.12).
PROPORTION OF PAYMENT BY PRIVATE INSURANCE FOR LT IN ALD
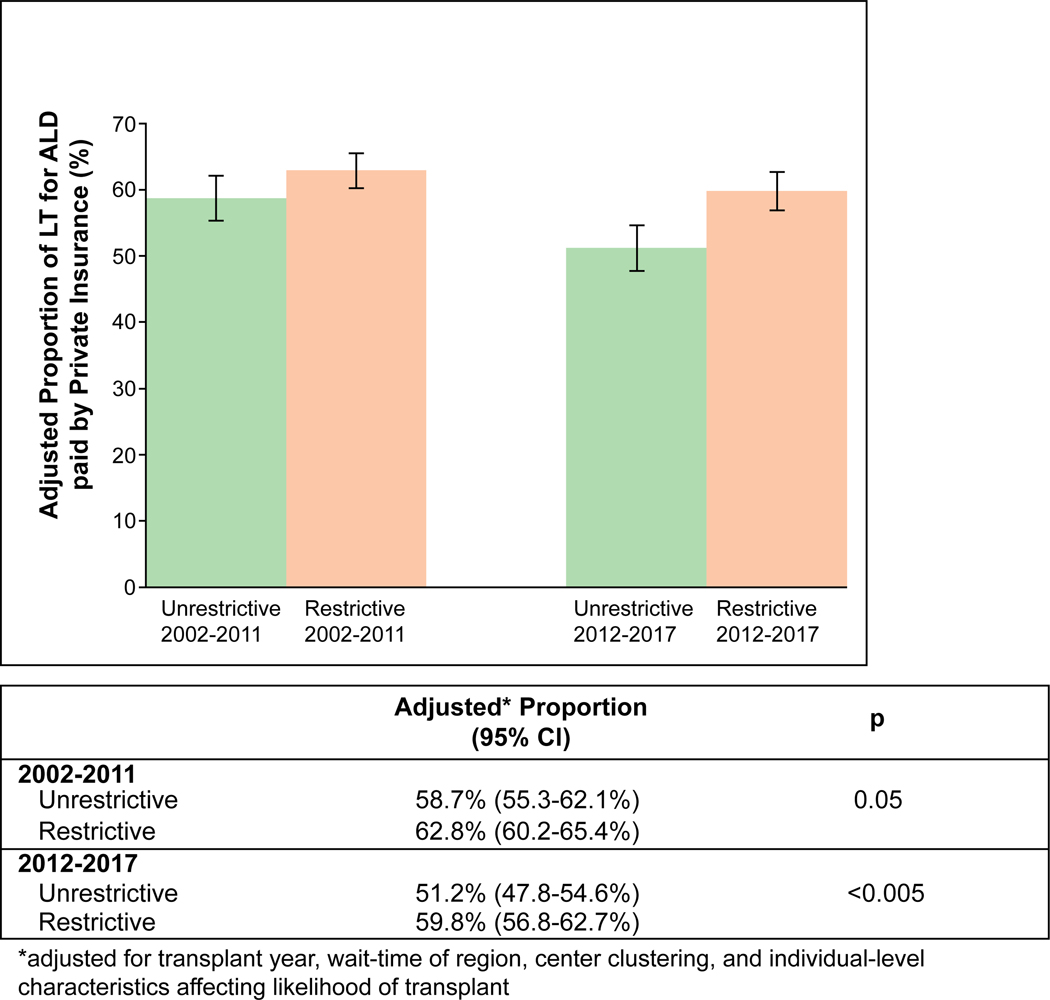
To assess the proportion of private insurance payment among LT for ALD recipients, in an analysis adjusting for individual-level characteristics affecting likelihood of LT, region wait-time, differences by era in unrestrictive states, and differences between restrictive and unrestrictive states in 2002–2011, a restrictive policy was associated with similar odds of LT in ALD paid by private insurance in 2012–2017 (aOR, 1.20; 95% CI, 0.97–1.50; P = 0.10). Between 2002 and 2011, the adjusted proportion of LT paid by private insurance was 62.8% (95% CI, 60.2%−65.4%) versus 58.7% (95% CI, 55.3%−62.1%) among restrictive versus unrestrictive states (P = 0.05). Between 2012 and 2017, the adjusted proportion of LT paid by private insurance was 59.8% (95% CI, 56.8–62.7%) versus 51.2% (95% CI, 47.8–54.6%) among restrictive versus unrestrictive states (P < 0.0005). In difference-of-differences analysis, restrictive (versus unrestrictive) policies were associated with an absolute increase of 4.4% (95% CI, –0.6 to +8.6%; P = 0.08) in adjusted proportion of LT paid by private insurance after 2011. Adjusted proportions by era are summarized in Fig. 4. Results for patients with HCC are included in the Supporting Information.
FIG. 4.
Adjusted proportion of LT for ALD paid by private insurance among restrictive versus unrestrictive states. This figure shows the proportion of LT for ALD paid by private insurance among restrictive versus unrestrictive states, adjusted for transplant year, wait time of region, center clustering, and individual-level characteristics affecting likelihood of transplant, between 2002 and 2011 and between 2012 and 2017. Error bars indicate 95% CIs.
SENSITIVITY ANALYSES
We performed a sensitivity analysis that divided the study period into three eras instead of two: 2002–2006, 2007–2011, and 2012–2017. In difference-of-differences analysis with 2002–2006 as the reference group, restrictive (versus unrestrictive) policies were associated with no significant difference in adjusted proportion of LT paid by Medicaid between 2007 and 2011: −2.3% (95% CI, −6.6% to +2.1%; P = 0.31). In difference-of-differences analysis with 2002–2006 as the reference group, restrictive (versus unrestrictive) policies were associated with an absolute decrease of 5.4% (95% CI, −9.9% to −0.9%; P = 0.02) in adjusted proportion of LT paid by Medicaid between 2012 and 2017.
In difference-of-differences analysis, restrictive (versus unrestrictive) policies were associated with no significant difference in adjusted proportion of LT paid by Medicare insurance after 2011: −0.5% (95% CI, −4.5% to +3.5%; P = 0.80).
In difference-of-differences analysis, among LT recipients with hepatitis C virus, restrictive (versus unrestrictive) policies were associated with no significant difference in adjusted proportion of LT paid by Medicaid insurance after 2011: −2.2% (95% CI, −6.6% to +2.2%; P = 0.33).
In difference-of-differences analysis, among LT recipients with nonalcoholic steatohepatitis, restrictive (versus unrestrictive) policies were associated with no significant difference in adjusted proportion of LT paid by Medicaid insurance after 2011: −0.1% (95% CI, −3.7% to +3.6%; P = 0.97).
Discussion
Shifting attitudes toward the requirement of specific durations of abstinence or rehabilitation for LT eligibility have likely contributed to the ascent of ALD as the most common indication for LT in the United States.(2) We show that Medicaid policy may pose a barrier for patients with ALD who rely on Medicaid for medical coverage. This study found that the majority of active LT centers are subject to Medicaid restrictions for financial coverage of LT for ALD. Specifically, restrictive policies encompass 63% of states and 68% of LT centers actively performing LT, and restrictive policies may be associated with a decreased proportion of Medicaid payment for LT in ALD since 2011. This study shows that while the increase in access to LT for ALD has likely increased nationally, this increase is not distributed equally across states. Similarly, in other countries, differences in local and regional policies to ALD may also exist and contribute to disparities in access to lifesaving care.
LT for ALD has increased over time in the United States, particularly since 2011, reflecting shifting attitudes toward early LT for ALD following the landmark trial in early LT for AH published that year,(4) plus other temporal trends in the United States, including declining need for LT for hepatitis C virus due to antiviral therapy,(10,11) increasing rates of harmful drinking,(12) and Medicaid expansion with passage of the Affordable Care Act.(2,4) Additionally, there has been increasing scrutiny regarding the use of a “6-month” abstinence rule for LT for ALD, with studies showing that duration of pre-LT abstinence was an unreliable predictor of alcohol relapse post-LT.(13,14) As more providers acknowledge these data and use other criteria to determine LT candidacy, the number of eligible patients with ALD for LT would be expected to increase. Further, while center policies have adapted to allow early LT with mandated periods of sobriety or rehabilitation, state Medicaid policies have been static. We found that the absolute difference in proportion of LT for ALD paid by Medicaid was a net 4.7% higher among unrestrictive states compared to restrictive states in the early LT era. Conversely, incremental changes in the post-2011 early LT era for proportion and odds of LT for ALD paid by private insurance were higher among restrictive states compared to unrestrictive states, although not statistically significant. This analysis suggests that access to early LT may be distributed unevenly, in part due to restrictive Medicaid policies. Given recent data suggesting that changing attitudes toward the requirement of 6 months of abstinence from drinking may be contributing to geographic disparities in access to LT,(2) reevaluation of Medicaid policy to be more aligned with center-level practices may help to address these disparities in the care of Medicaid versus non- Medicaid patients with ALD.
We found that the proportion of LT recipients with Medicaid was highly variable among states, with a 5-fold difference between states even within policy category—this finding was present even when excluding low-volume states and is unlikely to be explained fully by differences in population demographics. First, there was some variability in the degree of Medicaid policy restriction; for example, while most restrictive policies adhered to the “6-month rule,” some states, such as North Carolina, were even more restrictive, with patients with less than 12 months of abstinence needing to complete at least 6 months of counseling and patients with less than 2 years of abstinence still requiring consultation with counseling for financial eligibility of LT. Indeed, North Carolina (lowest) and California (highest) were the extremes for proportion of Medicaid among LT for ALD among medium- volume to large-volume states—the proportion of Medicaid among LT for ALD was underrepresented in North Carolina (6%) compared to Medicaid coverage of the state population (ranging 9%−12%(15) between 2012 and 2017 for adults aged 19–64), whereas the proportion was 29% in California and thus overrepresented (Medicaid coverage of state population ranging 12%−22% between 2012 and 2017(15)). Second, studies have found that patients in lower socioeconomic groups, with ALD as the primary diagnosis, without a college education, and with nonprivate medical insurance are less likely to be listed for LT.(16,17) While this may be evidence for implicit bias by transplant providers charged with selection of LT candidates and is likely variable across centers, an alternative reason may be that ALD is often accompanied by psychosocial comorbidities that may preclude LT beyond policies focused on alcohol. Nevertheless, our findings highlight the importance of identifying disparities and increasing the awareness surrounding them.
There were limitations to our study. First, this study relies on registry data, and our findings are by association and not necessarily causal. Second, our study does not account for possible misclassification of restrictive policy, changes in policies over time, or varying degrees of restriction. However, recent studies suggest significant liberalization in attitudes toward early LT policies over time(2,7); thus, if such confounders were present, our results would likely be biased toward the null hypothesis. Further, policies specific to alcohol use are rarely publicly available, but we were able to obtain direct responses from authoritative sources (Medicaid representatives and practicing transplant physicians) in 100% of states to summarize all policies. Third, payment for LT is captured in UNOS as the insurance used for “primary source of payment”—we could not assess the possibility of a secondary insurance covering LT costs rather than the primary insurance. Finally, our study includes only LT recipients, and factors influencing referral patterns or eligibility could not be assessed. However, our models adjust for a number of variables associated with the likelihood of receiving LT, encompassing socioeconomic demographics, geography, and clinical factors, though residual confounding cannot be excluded.
In conclusion, restrictive Medicaid policies are present in the majority of states with active LT centers in the United States and are associated with reduced proportion of payment by Medicaid for LT in ALD after 2011 compared to states without restrictive Medicaid policies. Reevaluation of Medicaid policy to align more closely with contemporary center-level practices may be warranted in the United States. Other countries may be experiencing such geography-based disparities that should be investigated, and a broader discussion regarding the effect of regional policy on access to transplant may be warranted.
Supplementary Material
Acknowledgment:
This work was supported in part by the UCSF Liver Center (P30 DK026743).
Supported by the National Institute of Diabetes and Digestive and Kidney Diseases (UCSF T32 DK060414, to B.P.L.).
Abbreviations:
- AH
alcohol-associated hepatitis
- ALD
alcohol-associated liver disease
- aOR
adjusted odds ratio
- CI
confidence interval
- HCC
hepatocellular carcinoma
- LT
liver transplant
- MELD
Model for End-Stage Liver Disease
- UNOS
United Network of Organ Sharing
Footnotes
Supporting Information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.31027/suppinfo.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1).Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol 2018;113:175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Lee BP, Vittinghoff E, Dodge JL, Cullaro G, Terrault NA. National trends and long-term outcomes of liver transplant for alcohol-associated liver disease in the United States. JAMA Intern Med 2019;179:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Lee BP, Mehta N, Platt L, Gurakar A, Rice JP, Lucey MR, et al. Outcomes of early liver transplantation for patients with severe alcoholic hepatitis. Gastroenterology 2018;155:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011;365:1790–1800. [DOI] [PubMed] [Google Scholar]
- 5).Mitchell MC, Maddrey WC. Changing times in liver transplantation for alcohol-associated liver disease. JAMA Intern Med 2019;179:348–350. [DOI] [PubMed] [Google Scholar]
- 6).National Institutes of Health. Liver transplantation. National Institutes of Health Consensus Development Conference statement June 20–23, 1983. Available at: https://consensus.nih.gov/1983/19831livertransplantation036html.htm. Accessed August 1, 2019.
- 7).Zhu J, Chen PY, Frankel M, Selby RR, Fong TL. Contemporary policies regarding alcohol and marijuana use among liver transplant programs in the United States. Transplantation 2018;102:433–439. [DOI] [PubMed] [Google Scholar]
- 8).Lee BP, Chen P-H, Haugen C, Hernaez R, Gurakar A, Philosophe B, et al. Three-year results of a pilot program in early liver transplantation for severe alcoholic hepatitis. Ann Surg 2017;265:20–29. [DOI] [PubMed] [Google Scholar]
- 9).Im GY, Kim-Schluger L, Shenoy A, Schubert E, Goel A, Friedman SL, et al. Early liver transplantation for severe alcoholic hepatitis in the United States—a single-center experience. Am J Transplant 2016;16:841–849. [DOI] [PubMed] [Google Scholar]
- 10).Kim D, Li AA, Gadiparthi C, Khan MA, Cholankeril G, Glenn JS, et al. Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology 2018;155:1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Cholankeril G, Ahmed A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin Gastroenterol Hepatol 2018;16:1356–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 2017;74:911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).De Gottardi A A simple score for predicting alcohol relapse after liver transplantation. Arch Intern Med 2007;167:1183–1188. [DOI] [PubMed] [Google Scholar]
- 14).Dimartini A, Dew MA, Day N, Fitzgerald MG, Jones BL, DeVera ME, et al. Trajectories of alcohol consumption following liver transplantation. Am J Transplant 2010;10:2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Kaiser Family Foundation. Health Insurance Coverage of Adults 2017:19–64. https://www.kff.org/other/state-indicator/adults-19-64/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed August 1, 2019. [Google Scholar]
- 16).Wilder JM, Oloruntoba OO, Muir AJ, Moylan CA. Role of patient factors, preferences, and distrust in health care and access to liver transplantation and organ donation. Liver Transpl 2016;22:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Julapalli VR, Kramer JR, El-Serag HB; American Association for the Study of Liver Diseases. Evaluation for liver transplantation: adherence to AASLD referral guidelines in a large Veterans Affairs center. Liver Transpl 2005;11:1370–1378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.