Abstract
Distal biliary strictures (DBS) are common and may be caused by both malignant and benign pathologies. While endoscopic procedures play a major role in their management, a comprehensive review of the subject is still lacking. Our consensus statements were formulated by a group of expert Asian pancreatico‐biliary interventional endoscopists, following a proposal from the Digestive Endoscopy Society of Taiwan, the Thai Association for Gastrointestinal Endoscopy, and the Tokyo Conference of Asian Pancreato‐biliary Interventional Endoscopy. Based on a literature review utilizing Medline, Cochrane library, and Embase databases, a total of 19 consensus statements on DBS were made on diagnosis, endoscopic drainage, benign biliary stricture, malignant biliary stricture, and management of recurrent biliary obstruction and other complications. Our consensus statements provide comprehensive guidance for the endoscopic management of DBS.
Keywords: distal biliary stricture, endoscopic retrograde cholangiopancreatography, endosonography, stents
Introduction
Distal biliary strictures (DBS) are common and may be caused by both malignant and benign pathologies. While endoscopic procedures play a major role in their management, a comprehensive review of the subject is still lacking, which has impact on diagnosis (imaging and pathological) and treatment (indications, procedure options, and outcomes). The consensus statements presented here were formulated following a literature review and discussion among international experts. The aim is to provide comprehensive guidance for the endoscopic management of DBS.
Methods
The consensus statements were formulated by a group of expert Asian pancreatico‐biliary interventional endoscopists, some of whom perform interventional radiology procedures and operative surgical procedures in their routine clinical practice, following a proposal from the Digestive Endoscopy Society of Taiwan (DEST), the Thai Association for Gastrointestinal Endoscopy (TAGE), and the Tokyo Conference of Asian Pancreato‐biliary Interventional Endoscopy (T‐CAP).
Based on a literature review utilizing Medline, Cochrane library, and Embase databases, first draft clinical questions (CQs) were developed by a planning panel (HI, RR, HPW, IY, YN, and HK). These first draft CQs were discussed via the internet and revised by each author. A face‐to‐face meeting was conducted in June 2017 in Tokyo, Japan. After further discussion and revision, the statements were voted on by all attendees (YN, HI, HPW, RR, CK, IY, HK, JHM, JL, SL, TR, DWS, DKL, DM, FD, WL, PVD, MA, AI, AK, MK, SR, and TF). The evidence level and recommendation grade were evaluated using the evidence leveling system (Table 1).1 The discussion continued until an agreement of greater than 80% was achieved for recommendation Level A or B. Finally, after voting, a total of 19 consensus statements were agreed upon. The second draft of consensus statements was presented for open discussion at Asia Pacific Digestive Week (APDW) in September 2017 in Hong Kong. Subsequently, the revised statements were discussed among the faculty members via the internet. The resultant manuscript was evaluated by three expert endoscopists (MW, TI, and BD) and was finalized by each author.
Table 1.
Quality of evidence, classification of recommendations and voting schema of the modified Canadian Task Force on the periodic health examination
| Level/grade | Description |
| Levels of evidence | |
| A (strong) | Strongly confident in the effect of estimate. |
| B (moderate) | Moderately confident in the effect of estimate. |
| C (weak) | Confidence in the effect of estimate is limited. |
| D (very weak) | Almost no confidence in the effect of estimate. |
| Classification of the recommendation | |
| 1 | Strong recommendation |
| 2 | Weak recommendation |
| Voting on the recommendation | |
| A | Accept completely |
| B | Accept with some reservations |
| C | Accept with major reservations |
| D | Reject with reservations |
| E | Reject completely |
Definition of distal biliary stricture
Numerous studies have reported on DBS, but anatomical definitions vary. The most commonly used definitions used include stenosis of the distal third of the extrahepatic bile duct,2 the intrapancreatic portion of the common bile duct, or the distal half of the extrahepatic bile duct.3 Some papers on the subject do not provide a definition. As the focus of our consensus statements is on endoscopic management and the intra‐pancreatic portion4 of the common bile duct cannot be determined on endoscopic retrograde cholangio‐pancreatography (ERCP), we have excluded the use of the intrapancreatic bile duct as a definition. There was a debate during the face‐to‐face meeting, but there were no clear reasons to select one over the other. Hence, we define DBS as abnormal narrowing of the distal half, which includes the distal third, of the extrahepatic bile duct.
Consensus statements
Diagnosis
CQ1. What strategy is recommended for diagnosis of distal biliary strictures?
Statement 1‐1
The diagnostic strategy for evaluating a DBS is determined by the possible etiology, clinical features, abnormal liver biochemistries, and/or cross‐sectional imaging.
Evidence level: D
Recommendation: 1
Agreement: A, 0%; B, 100%; C, 0%; D, 0%
Statement 1‐2
In patients with DBS, ERCP and/or endoscopic ultrasound (EUS) are often helpful in establishing the etiology.
Evidence level: A
Recommendation: 1
Agreement: A, 96%; B, 4%; C, 0%; D, 0%
First of all, detailed history taking and physical examination should be performed. Painless jaundice with Courvoisier's sign5 and a previous history of malignancy raises the possibility of malignant biliary stricture (MBS). Meanwhile, a history of pancreatitis in heavy drinkers suggests the presence of chronic pancreatitis as a cause of biliary stricture. In addition to hepatobiliary enzymes, some serum markers may help in the diagnosis of DBS. Serum carbohydrate antigen (CA) 19–9 is a tumor marker useful for pancreato‐biliary cancer with a sensitivity of 70–80% in pancreatic cancer and 50–80% in biliary tract cancer.6, 7 However, CA19–9 increases in the presence of cholestasis and cholangitis, and false positive results are possible in cases with DBS. IgG4 related diseases including autoimmune pancreatitis can be a cause of DBS and serum IgG4 level is high in about 80% of autoimmune pancreatitis.
The diagnostic strategy for evaluating DBS should take into account the presence of jaundice or abnormal liver biochemistry, findings of cross‐sectional imaging, and probability of resectability, if a tumor is detected (Fig. 1). When a pancreatic tumor causing DBS is noted on cross‐sectional imaging, EUS‐guided fine needle aspiration (EUS‐FNA) is helpful in establishing a tissue diagnosis. Notably, EUS may detect occult periampullary tumors in patients who present with DBS but without a mass on cross‐sectional imaging.8, 9 In jaundiced patients, ERCP is an appropriate first‐line procedure as both diagnostic and therapeutic maneuvers can be performed including transpapillary brush cytology or forceps biopsy and biliary drainage. The diagnostic yield of EUS‐guided and transpapillary tissue sampling is discussed later in CQ3. In summary, the diagnostic strategy should be directed by the presence of jaundice and initial cross‐sectional imaging.
Figure 1.
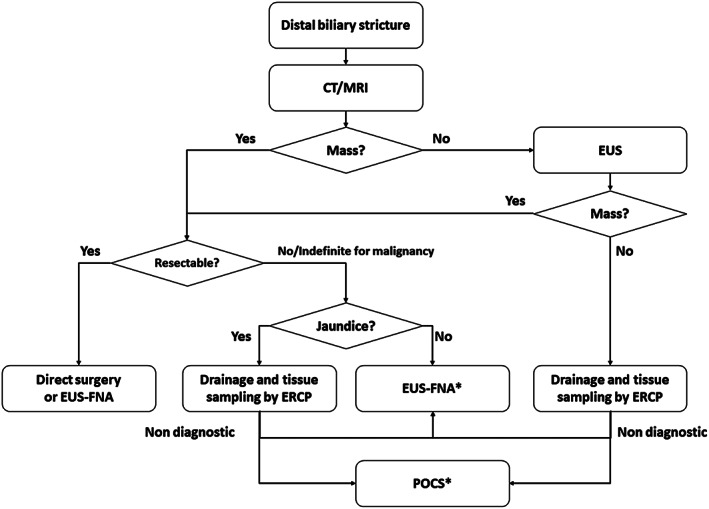
The diagnostic strategy and tissue sampling approach for a distal biliary stricture. *EUS‐FNA is recommended for an extrinsic biliary stricture and POCS for an intrinsic biliary stricture. CT, computed tomography; ERCP, endoscopic retrograde cholangio‐pancreatography; EUS, endoscopic ultrasound; EUS‐FNA, endoscopic ultrasound‐guided fine needle aspiration; MRI, magnetic resonance imaging; POCS, peroral cholangioscopy.
CQ2. What is the role of cross‐sectional imaging?
Statement 2
Contrast‐enhanced computed tomography (CT) or magnetic resonance imaging (MRI) is necessary for the detection and characterization of a mass and to determine resectability of malignant lesions.
Evidence level: A
Recommendation: 1
Agreement: A, 100%; B, 0%; C, 0%; D, 0%
Cross‐sectional imaging such as contrast‐enhanced CT or MRI is commonly utilized in clinical practice to assess patients with DBS. Both CT and MRI have high diagnostic accuracy for identification and characterization of primary lesions10, 11 and to determine resectability of malignancy.11, 12 Therefore, multiphasic contrast‐enhanced CT or MRI should be performed in the initial evaluation of patients with DBS prior to invasive procedures such as EUS or ERCP. CT and MRI confer the additional advantage of potentially detecting distant metastasis.
CQ3. What is the optimal approach for tissue acquisition?
Statement 3
ERCP‐guided tissue acquisition should be attempted when biliary drainage is required. EUS‐FNA is preferred when cross‐sectional imaging reveals a mass associated with the stricture or when ERCP‐guided tissue acquisition is unsuccessful.
Evidence level: A
Recommendation: 1
Agreement: A, 100%; B, 0%; C, 0%; D, 0%
EUS‐FNA provides both high sensitivity and specificity in diagnosing a pancreatic mass. In a meta‐analysis, the pooled sensitivity and specificity of EUS‐FNA in correctly diagnosing pancreatic masses was 86.8% (95% confidence interval, CI, [85.5, 87.9]) and 95.8% (95% CI [94.6, 96.7]).13 In cases with biliary stricture, the sensitivity and specificity of EUS‐FNA for characterizing the biliary stricture was 80% and 97%, respectively.14 The diagnostic yield is lower in the absence of a mass,15 but EUS can detect small (<2 cm) pancreatic lesions. In one retrospective study, the sensitivity of EUS‐FNA was 87.3% in pancreatic neoplasm without a definitive mass on CT.16
In cases requiring endoscopic biliary drainage, a tissue diagnosis may be made during ERCP. In a meta‐analysis,17 the pooled sensitivity and specificity of brush cytology for the diagnosis of a MBS was 45% (95% CI [40%, 50%]) and 99% (95% CI [98%, 100%]), respectively. Intraductal forceps biopsies had a pooled sensitivity and specificity of 48.1% (95% CI [42.8%, 53.4%]) and 99.2% (95% CI [97.6%, 99.8%]), respectively. In another study, the sensitivity of transpapillary tissue sampling during ERCP was 49% compared with 75% by EUS‐FNA.18 The cause of the biliary stricture also affects diagnostic yield.19 Diagnostic accuracy of ERCP‐guided tissue sampling was 81.8% and 67.8% in intrinsic and extrinsic biliary strictures (P = 0.023), respectively. As most DBS are associated with obstructive jaundice or cholangitis, transpapillary biliary drainage is usually necessary to relieve symptoms. Therefore, we recommend that transpapillary tissue sampling should be attempted at the time of biliary drainage during ERCP. If transpapillary tissue sampling is non‐diagnostic or if early biliary drainage is unnecessary, we recommend EUS‐FNA to secure the pathological diagnosis, especially when the DBS is associated with a mass. Despite concerns that the presence of a biliary stent may compromise the accuracy of EUS imaging, most studies have reported that an indwelling biliary stent does not adversely affect the diagnostic yield of EUS‐FNA.20 Alternatively, EUS and ERCP may be performed in a single session for evaluation of obstructive jaundice as a result of a pancreatic mass.21, 22 While EUS‐FNA can provide a histopathological diagnosis with high sensitivity, biliary drainage may be effected via ERCP in the same session. With this single session approach, EUS‐FNA may be performed without interference from an indwelling stent, and repeated sedation can be avoided. Our proposal for the pathological diagnostic approach is shown in Figure 1. The diagnostic approach, with either transpapillary tissue sampling during ERCP or EUS‐FNA, should also be determined according to local expertise and/or the costs associated with each procedure and the requisite devices.
CQ4. What are the roles of advanced imaging modalities for distal biliary stricture?
Statement 4
Per‐oral cholangioscopy (POCS) and intraductal ultrasonography may improve the characterization of DBS.
Evidence level: C
Recommendation: 2
Agreement: A, 50%; B, 45%; C, 5%; D, 0%
The addition of POCS and intraductal ultrasonography (IDUS) during ERCP may increase diagnostic yield. In a prospective study of 26 cases of indeterminate biliary stricture, cholangioscopy‐guided biopsy using biopsy forceps dedicated for POCS provided higher sensitivity and accuracy (76.5% and 84.6%) compared with brush cytology (5.8% and 38.5%) and conventional forceps biopsies (29.4% and 53.9%).23 Notably, in another prospective study, the accuracy of POCS‐directed biopsies was 82% in 33 patients in whom ERCP‐guided brushing or biopsy was inconclusive.24 Recently, a digital, single operator cholangioscope has been introduced,25, 26 which provides improved intraductal visualization and maneuverability. It has shown promising results, with sensitivity and specificity in excess of 80% in both visual inspection and cholangioscopy‐guided biopsy. As we discussed in CQ3, however, the indication of POCS should be considered after EUS because EUS‐FNA showed better diagnostic yield in the extrinsic stricture as a result of a pancreatic mass. The EUS first approach would reduce the need, cost, and adverse events (AEs) of POCS.27 Furthermore, maneuverability and visualization of POCS are often limited in the very DBS because of the unstable scope position. In a retrospective analysis of 397 cases with indeterminate biliary stricture, IDUS was useful in predicting malignancy with a sensitivity, specificity, and accuracy of 93%, 89%, and 91%, respectively.28 However, most studies on the utility of IDUS and POCS included both hilar and DBS and did not evaluate their specific use in DBS. Probe‐based confocal laser endomicroscopy, which can provide in vivo imaging during ERCP, is reportedly useful for diagnosing indeterminate biliary strictures, but its role in clinical practice is yet to be determined because of poor inter‐observer agreement and cost.29
Endoscopic drainageCQ5. When is endoscopic biliary drainage indicated?
Statement 5‐1
Endoscopic biliary drainage is indicated for the treatment of cholangitis and for relief of cholestasis.
Evidence level: A
Recommendation: 1
Agreement: A, 95%; B, 0%; C, 5%; D, 0%
Statement 5‐2
Endoscopic stenting is generally considered to be primary therapy for benign biliary strictures (BBS).
Evidence level: A
Recommendation: 1
Agreement: A, 90%; B, 5%; C, 5%; D, 0%
Statement 5‐3
Endoscopic stenting is the preferred therapy for palliation of cholestasis in unresectable MBS.
Evidence level: A
Recommendation: 1
Agreement: A, 68%; B, 32%; C, 0%; D, 0%
Benign and malignant pathologies may cause DBS. Whereas the etiology of DBS should be sought in every patient, not all patients with DBS require biliary drainage. Where there is no evidence of cholestasis or cholangitis, drainage may not be necessary. Biliary drainage should be performed if there is cholangitis and/or cholestasis to relieve the associated clinical symptoms. There are a number of treatment options for biliary drainage: Surgical bypass, percutaneous transhepatic biliary (or gallbladder) drainage, and endoscopic biliary drainage, either by the transpapillary route during ERCP or EUS guided. In general, endoscopic biliary drainage is considered less invasive and affords a better quality of life. When biliary drainage is indicated, endoscopic stenting is considered the initial technique of choice. The indication for endoscopic biliary drainage differs between benign and malignant etiologies. For BBS, such as post‐surgical strictures and strictures secondary to chronic pancreatitis, endoscopic biliary stenting is performed for the relief of cholestasis and to dilate the stricture. For patients with obstructive jaundice due to benign pathologies like autoimmune pancreatitis or lymphoma, in whom the stricture is expected to fully resolve with medical management, biliary drainage may not be required. In patients with unresectable malignant DBS, however, endoscopic biliary stenting is performed primarily for palliation. For resectable malignant DBS, please see CQ10.
CQ6. What type of endoscopic biliary drainage is available for distal biliary stricture?
Statement 6
ERCP with transpapillary stenting is the mainstay of biliary drainage; EUS‐guided biliary drainage (EUS‐BD) is an alternative endoscopic treatment when ERCP is not possible.
Evidence level: A
Recommendation: 1
Agreement: A, 68%; B, 32%; C, 0%; D, 0%
In patients with DBS, biliary drainage may be achieved during ERCP or percutaneous transhepatic biliary drainage (PTBD). ERCP is preferred over PTBD as no fluid or electrolyte losses occur and cutaneous puncture‐site pain is avoided. ERCP, in expert hands, is also associated with significantly higher clinical success and requires fewer re‐interventions.30, 31 However, difficult biliary cannulation at ERCP, which accounts for up to 10% of all ERCPs, is associated with a higher risk of ERCP‐related complications.32 In addition, ERCP may not be technically possible in the presence of duodenal obstruction and surgically altered anatomy. PTBD has long been utilized as an alternative non‐endoscopic drainage method in cases with failed ERCP and is still the standard alternative technique in many centers. EUS‐guided biliary drainage (EUS‐BD) has been reported as an alternative modality after failed ERCP where expertise is available33, 34; this is further discussed in CQ8.
CQ7. How should we follow patients after biliary stent placement?
Statement 7‐1
In patients with benign DBS, ERCP with planned stent exchange or removal is recommended.
Evidence level: C
Recommendation: 2
Agreement: A, 100%; B, 0%; C, 0%; D, 0%
Statement 7‐2
In patients with malignant DBS treated with a metal stent, endoscopic re‐intervention is performed on demand.
Evidence level: C
Recommendation: 2
Agreement: A, 100%; B, 0%; C, 0%; D, 0%
Statement 7‐3
In patients with MBS treated with plastic stent (PS), planned or on‐demand endoscopic re‐intervention may be done.
Evidence level: C
Recommendation: 2
Agreement: A, 61%; B, 39%; C, 0%; D, 0%
Stent dysfunction, due to either stent occlusion or migration, is a potentially serious adverse event (AE) that can result in life‐threatening cholangitis. The optimal surveillance strategy to prevent or detect early recurrent biliary obstruction (RBO) is yet to be determined due to the limited number of studies directly addressing this question.35, 36, 37 At the minimum, we recommend that all patients receive education so as to recognize the signs and symptoms of RBO and the importance of seeking early medical intervention.
Two main post‐stent placement follow‐up strategies have been described.
Planned stent exchange
Planned stent exchange is utilized for all patients with BBS and can be considered in patients with MBS treated with PS if close clinical follow up is not available and/or if life expectancy is greater than 3 months.
On‐demand endoscopic intervention
In this strategy, ERCP is performed when signs of RBO are present. Monitoring strategies to detect early RBO have not been well studied and are mostly based on empiric evidence and extrapolation from the published literature. Furthermore, the management approach is dependent on local resources (availability and cost of laboratory and imaging evaluations) and patient factors (distance a patient has to travel for follow‐up visits).38 Possible monitoring options include outpatient clinic visits with evaluation of liver chemistry, with or without supplemental periodic remote monitoring of liver chemistry. When physical examination or liver chemistry suggests possible stent dysfunction, transabdominal ultrasound and/or plain abdominal film can be performed to assess stent position and to evaluate for the presence or absence of pneumobilia. The absence of pre‐existing pneumobilia may indicate stent occlusion. On‐demand endoscopic intervention is the preferred strategy in patients with MBS treated with self‐expandable metallic stents (SEMS). On‐demand stent exchange can be also considered in patients with MBS treated with PS if close clinical follow up is available and/or if the patient has a life expectancy of less than 3 months. However, this approach is not recommended in those without full knowledge of the signs and symptoms of cholangitis because delayed re‐intervention can lead to serious consequences.
CQ8. When should endoscopic ultrasound‐guided biliary drainage be considered?
Statement 8
Where expertise is available, EUS‐BD may be an option in these situations: (i) failed ERCP, (ii) Post‐surgical anatomy, and (iii) difficult biliary cannulation.
Evidence level: B
Recommendation: 1
Agreement: A, 67%; B, 33%; C, 0%; D, 0%
Transpapillary biliary stent placement is the mainstay of endoscopic management of DBS. ERCP can be technically difficult or may fail to achieve biliary drainage due to duodenal obstruction by benign or malignant pathologies, periampullary tumor involvement, the presence of a periampullary diverticulum, or surgically altered anatomy. Although surgical or percutaneous techniques have long been utilized in these situations, the development of EUS‐BD has provided a less invasive therapeutic approach. EUS‐BD may be categorized according to the route of approach and site of biliary drainage: choledochoduodenostomy, hepaticogastrostomy (HGS), rendezvous technique and antegrade biliary stenting. Initially, PS were the only device used in EUS‐BD. Over the last decade, SEMS have become an option for EUS‐BD.33 Recently, the use of lumen apposing metal stents has been reported for choledochoduodenostomy, with the advantage of a lower theoretical risk of bile leakage.39 In addition, a specially designed SEMS and PS have been proposed for use in HGS.40, 41 The technique of EUS‐BD has not been standardized and can be complicated by severe AEs such as bile leak and stent dislocation.42 EUS‐BD should therefore be performed by experienced therapeutic endoscopists. A recent consensus guideline discusses key issues associated with EUS‐BD in detail.43 Although there are some reports on the utility of EUS‐BD for hepaticojejunostomy strictures,44 the role of EUS‐BD in the management of BBS requires further study. Given the lack of standardization of EUS‐BD and the potential risk of severe AEs, PTBD should be considered the standard of care where expertise in EUS‐BD is not available.
Benign biliary stricture
CQ9. What strategy is recommended for benign biliary stricture?
Statement 9
Multiple PS or a fully covered SEMS are recommended options for the treatment of benign biliary strictures.
Evidence level: A
Recommendation: 1
Agreement: A, 89%; B, 11%; C, 0%; D, 0%
Endoscopic management is considered as a first‐line treatment for BBS because of its safety, effectiveness, and less‐invasive nature when compared with surgery or percutaneous techniques. The recently published Asian‐Pacific consensus guidelines summarized the endoscopic management of BBS.45 Our proposal for endoscopic management of BBS is shown in Figure 2. First, a guidewire should be passed across the stricture. If the stricture is tight, pre‐stenting dilation may be necessary using a bougie or a balloon dilator. In general, balloon dilation alone or placement of a single PS is insufficient treatment for BBS,45, 46, 47 and placement of multiple large‐bore PS has been established as standard treatment.48, 49 The initial reports of uncovered SEMS for treatment of BBS showed this to be a poor option because of stent occlusion by ingrowth of reactive tissue, rendering the stent unremovable.46 Several prospective studies have demonstrated the utility of fully covered SEMS for the treatment of BBS.50, 51 The use of a fully covered SEMS resulted in a similar success rate, with fewer procedures needed and shorter procedure duration, when compared with placement of multiple PS.52, 53, 54 Local resources and expertise can guide the decision to use either multiple PS or fully covered SEMS. In cases with BBS, fully covered SEMS should be removed after completion of treatment as per protocol (4–6 months in general or per manufacturer recommendation) to prevent degradation of the covering membrane and ingrowth of hyperplastic tissue.50 Magnetic compression anastomosis is also reported as a treatment option for BBS.55
Figure 2.
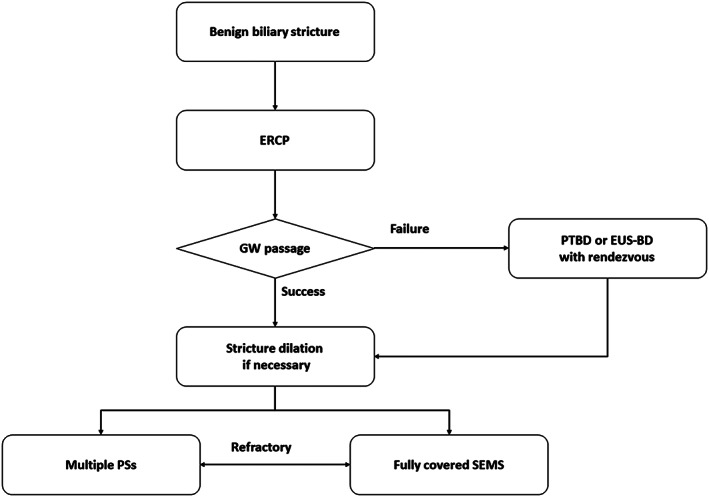
Treatment algorithm for management of benign biliary stricture. ERCP, endoscopic retrograde cholangio‐pancreatography; EUS‐BD, EUS‐guided biliary drainage; GW, guidewire; PS, plastic stent; PTBD, percutaneous transhepatic biliary drainage; SEMS, self‐expandable metallic stent.
Malignant biliary stricture
CQ10–1. Which patients require endoscopic biliary drainage before surgery?
Statement 10‐1
Pre‐operative drainage should not be routinely performed, but is indicated in patients with cholangitis, in those planned for neo‐adjuvant chemotherapy, and when a delay in surgery is anticipated.
Evidence level: A
Recommendation: 1
Agreement: A, 80%; B, 20%; C, 0%; D, 0%
CQ10–2. What type of stents should be used before surgery?
Statement 10‐2
In patients who require endoscopic drainage before surgery, a SEMS may be preferred over a PS.
Evidence level: A
Recommendation: 1
Agreement: A, 90%; B, 10%; C, 0%; D, 0%
Currently, routine preoperative biliary drainage (PBD) is not recommended. A randomized controlled trial (RCT) reported an increase in perioperative complications when PBD was performed for patients with MBS due to pancreatic cancer, in whom early surgery was planned. While a subsequent Dutch study56 showed that the use of fully covered SEMS reduced perioperative AE compared with PS (24% vs 46%), a Korean RCT57 failed to show superiority of fully covered SEMS over PS in terms of AE (16.3% vs 16.3%) and re‐interventions (14.0% vs 16.3%). This discrepancy probably arises from the different lead times to surgery: 5 weeks in the Dutch study and 2 weeks in the Korean study. In clinical practice, however, surgery is often delayed due to the limited operation room time. Surgery is also increasingly delayed when neoadjuvant chemotherapy is employed. Therefore, PBD is required to treat cholangitis when surgery is delayed due to operation room unavailability or neoadjuvant chemotherapy. PS may prematurely occlude delaying surgery or neoadjuvant chemotherapy. PBD using fully covered SEMS may assure longer stent patency and be more cost effective.58
CQ11–1. What type of stent is preferred for unresectable malignant distal biliary stricture?
Statement 11‐1
A SEMS is preferred over a PS in patients with unresectable malignant DBS.
Evidence level: A
Recommendation: 1
Agreement: A, 88%; B, 12%; C, 0%; D, 0%
CQ11–2. What type of metal stent is preferred for unresectable malignant distal biliary stricture?
Statement 11‐2
A covered SEMS may be preferred over an uncovered SEMS, especially in patients with unresectable pancreatic cancer.
Evidence level: B
Recommendation: 1
Agreement: A, 67%; B, 33%; C, 0%; D, 0%
Six RCTs59, 60, 61, 62, 63, 64 and two meta‐analyses65, 66 have compared covered and uncovered SEMS's for unresectable malignant DBS resulting from various pathologies including pancreatic cancer. The conclusions of the two meta‐analyses differed in terms of stent patency.65, 66 However, two RCTs63, 64 and subgroup analysis in a third RCT59 focusing on pancreatic cancer reported longer patency of covered SEMS. Regarding AE, covered and uncovered SEMS are comparable. The two meta‐analyses reported no difference in rates of cholecystitis and pancreatitis.65, 66 Some endoscopists avoid covering the cystic duct takeoff to prevent cholecystitis after covered SEMS placement. Three RCTs showed that covered SEMS are prone to migration but covered SEMS with anti‐migration features may reduce the risk of migration.64 Finally, there is no cost effectiveness analysis comparing covered and uncovered SEMS for unresectable MBS. As the differential cost of covered SEMS varies by the insurance system and market, the cost–benefit analysis is likely to vary based on the cost of using a covered SEMS in each country.
CQ12. What is the impact of chemotherapy on patients with a biliary stent in situ?
Statement 12
Chemotherapy does not appear to increase the risk of stent occlusion, but may increase the stent migration risk in patients with a biliary stent in situ.
Evidence level: C
Recommendation: No
Agreement: A, 80%; B, 20%; C, 0%; D, 0%
Data on the effects of chemotherapy on the outcomes of biliary stents are limited. Theoretically, neutropenia induced by chemotherapy can lead to bacterial overgrowth and subsequent cholangitis. It can also result in tumor shrinkage with partial resolution of biliary strictures leading to stent migration. It has been reported that preoperative chemoradiation therapy in locally advanced pancreatic cancer67 and chemotherapy in unresectable pancreatobiliary cancer68 were not associated with increased stent‐related complications. In patients with unresectable pancreatic cancer, gemcitabine chemotherapy did not increase stent occlusion or stent‐related complications of SEMS. However, stent migration tended to be higher in the gemcitabine group (17% vs 3%).69 In a further recent, large‐scale study of 291 biliary stents (151 SEMS and 140 PS), stent patency was comparable in cases with and without chemo (radio)therapy.70 Neoadjuvant treatment is increasingly utilized in patients with pancreatic cancer without distant metastasis. SEMS have demonstrated longer stent patency than PS in this neoadjuvant setting. Overall, regardless of resectability status, a covered SEMS is recommended in terms of stent patency and cost effectiveness.58
CQ13. What is the recommended endoscopic treatment for patients with unresectable malignant dual biliary and duodenal strictures?
Statement 13
Endoscopic biliary and duodenal metallic stenting is an option in patients with unresectable malignant dual biliary and duodenal strictures. EUS‐BD is a promising treatment option for patients with an indwelling duodenal stent.
Evidence level: C
Recommendation: 2
Agreement: A, 50%; B, 45%; C, 5%; D, 0%
The reported rate of combined MBS and gastric outlet obstruction (GOO) in advanced pancreatic cancer is approximately 30%.71, 72, 73 Historically, double bypass surgery was performed; however, the development of endoscopic interventions allows less invasive palliation. While endoscopic biliary stent placement is the standard of care for MBS, endoscopic duodenal stent placement is performed for GOO in patients with a poor prognosis and surgical gastrojejunostomy in patients with longer life expectancy. Surgical gastrojejunostomy is more invasive, but it enables a longer duration of oral intake.74, 75 In patients with combined MBS and GOO, retrospective studies report that endoscopic double stenting is associated with a high technical success rate and an acceptable AE rate.71, 76, 77, 78, 79, 80, 81 In the presence of a duodenal stent, ERCP is technically challenging,82 and the long‐term outcomes are suboptimal.83 EUS‐BD is increasingly reported as a rescue therapy for failed ERCP. GOO is one of the major causes of ERCP failure. EUS‐BD, and especially EUS‐HGS, which results in bile drainage away from the duodenal stent, may potentially provide longer biliary stent patency84, 85 and may be an alternative to transpapillary stenting in patients with combined MBS and GOO. Meanwhile, the trans‐gastric approach is reportedly associated with a higher risk of AEs.86 The procedure selected should therefore be determined by local expertise.
CQ14. What are adjunctive endoscopic treatments for malignant distal biliary stricture?
Statement 14
Endo‐biliary photodynamic therapy (PDT), radiofrequency ablation (RFA) and drug‐eluting stents (DES) are considered investigational therapies for malignant DBS.
Evidence level: B (PDT), C (RFA), D (DES)
Recommendation: 2
Agreement: A, 85%; B, 15%; C, 0%; D, 0%.
Adjunctive endoscopic therapy for unresectable malignant DBS includes intraductal PDT, RFA, and DES,87 of which the first two are more promising therapies. PDT, primarily used for cholangiocarcinoma, is a two‐step process in which a photosensitizing drug having affinity for neoplastic tissue (photosensitizer) is administered, followed by selective tumor irradiation with light of a defined wavelength (intraductal laser photo‐radiation). The interaction between light and photosensitizing agent causes tumor cell death due to the formation of oxygen free radicals. PDT has been reported to increase stent patency, quality of life, and survival.88, 89, 90, 91 The major drawback is systemic photosensitivity, which occurs in 10% of cases. Intraductal RFA is primarily used in MBS (cholangiocarcinoma or pancreatic cancer) prior to stent placement.92 It has high technical success rates and an acceptable safety profile.93 As biliary RFA locally ablates the tumor, it is expected to improve stent patency. It reportedly provides similar survival to PDT, with a good cost effectiveness profile and avoidance of photosensitivity.94 Intraductal RFA has also been reported to be useful in treating tissue ingrowth or overgrowth resulting in occlusion of biliary SEMS.95, 96, 97, 98 RCTs are required to further assess the efficacy of adjunctive endoscopic therapies.
Management of recurrent biliary obstruction and other complications
The current literature assesses numerous variants of biliary stents that have occluded or malfunctioned, making inter‐study comparisons difficult. For this consensus guideline, we chose the term RBO, which can result from either stent occlusion or migration, as described in the Tokyo Criteria 201499 and defined it as the recurrence of jaundice and/or cholangitis following stent insertion. Causes of stent occlusion may be tumor‐related (tissue ingrowth and overgrowth) or non‐tumor related (sludge, food impaction, mucosal hyperplasia, hemobilia, and kinking of the bile duct). The definition of each cause of stent occlusion is shown in Table 2.
Table 2.
Definition of causes of recurrent biliary obstruction
| Cause of recurrent biliary obstruction | Definition |
|---|---|
| Tissue ingrowth/mucosal hyperplasia | Growth of tumor or hyperplastic mucosa into the lumen of SEMS |
| Tissue overgrowth | Tumor or tissue growth beyond the ends of SEMS |
| Sludge, hemobilia, and food impaction | Occlusion of stent lumen by biliary sludge accumulation, clots, and food impaction |
| Bile duct kinking | Obstruction at a proximal or distal end of SEMS due to an angulated bile duct |
| Stent kinking | Obstruction of stent lumen due to sharp bending of a SEMS because of an angulated bile duct or tumor growth |
| Stent collapse | Collapse of stent structure due to tumor growth or other causes (compression by another stent etc.) |
SEMS, self‐expandable metallic stent.
Complications other than RBO are also clinically important as they may necessitate additional interventions or hospitalization and may significantly impair the patient's quality of life. Stent‐related complications include pancreatitis, cholecystitis, non‐occlusion cholangitis, bleeding, ulceration, penetration, perforation, and complications associated with the stent placement procedure (perforation, desaturation, aspiration pneumonia, etc.).
CQ15. What is the approach to cholangitis in patients with an indwelling biliary stent?
Statement 15
Cholangitis in patients with an indwelling biliary stent suggests RBO and may require early endoscopic re‐intervention.
Evidence level: C
Recommendation: 1
Agreement: A, 100%; B, 0%; C, 0%; D, 0%
Patients with an indwelling biliary stent may present with symptoms of cholangitis such as fever, pain, and jaundice. RBO is not uncommon in patients with an indwelling biliary stent who present with acute cholangitis. Careful physical examination as well as cross‐sectional imaging are recommended prior to endoscopic procedures. Cholangitis may be life threatening if untreated and may require early re‐intervention to resolve RBO. The therapeutic approach to cholangitis is determined by an evaluation of the etiology and severity.
CQ16. What management is recommended for abdominal pain in patients with an indwelling biliary stent?
Statement 16
In a patient with an indwelling biliary stent who presents with unexplained abdominal pain, evaluation with CT should be considered.
Evidence level: C
Recommendation: 2
Agreement: A, 80%; B, 20%; C, 0%; D, 0%
Patients may develop delayed stent‐related complications. The complication, as well as the time point at which it occurs, relates to the type of stent used (plastic vs SEMS;100 covered vs uncovered SEMS).66 Some of the complications that can manifest as abdominal pain are proximal or distal migration with or without biliary obstruction, impaction, or perforation.
A plain radiograph of the abdomen only allows assessment of stent position in a 2‐D plane. This might be useful if the stent has migrated significantly (e.g. a migrated PS to the colon101) or if a perforation has occurred as suggested by extraluminal gas. The sensitivity of plain X‐ray is low, however, and the study may not detect retroperitoneal perforation. The optimal imaging modality to investigate abdominal pain in a patient with an indwelling stent is CT. CT accurately defines biliary stent position and the presence of biliary dilatation and also detects, with high sensitivity, the presence of free gas (both intra‐ and retroperitoneal). Contrast‐enhanced CT better defines stent position in relation to the surrounding organs and vasculature and may demonstrate a cause for abdominal pain other than stent dysfunction (e.g. mesenteric ischemia from vascular occlusion).102, 103 While CT is associated with a significant cost, radiation exposure, and the potential for contrast‐associated complications, it has distinct advantages over plain radiography and endoscopic assessment (which is relatively invasive) and represents the best modality for initial evaluation.
If no cause for abdominal pain has been determined after appropriate imaging, the possibility of stent‐related mucosal ulceration should be considered and endoscopy should be performed.104 Occluded or migrated plastic biliary stents can be readily removed and can be replaced. The removal of a SEMS may be challenging.104 If a SEMS is found to have migrated distally and is causing mucosal ulceration, the intra‐luminal section may be trimmed.104, 105, 106 Further details on the management of SEMS dysfunction are discussed later.
CQ17. What strategy is recommended for the prevention and management of stent migration?
Statement 17
To reduce the risk of migration of covered metal stents, partially covered metal stents or fully covered metal stents with special anti‐migration design can be used.
Evidence level: C
Recommendation: 2
Agreement: A, 74%; B, 26%; C, 0%; D, 0%
Compared with PS, SEMS have a larger diameter and in general offer a longer duration of stent patency.100 For benign DBS, a covered SEMS is preferred to uncovered SEMS as the stent needs to be removed after stricture resolution. However, stent migration is a key disadvantage of covered SEMS. To reduce the risk of stent migration, partially or fully covered SEMS with special anti‐migration design features may be used, although there is a paucity of supporting data. Partially covered SEMS have bare ends, which embed into the biliary epithelium, thereby preventing migration.
Different designs of fully covered SEMS are currently available. Anti‐migration features include anchoring flaps, flared ends, or differential radial expansive forces.107 There are few well‐designed comparative studies, however, to determine which design works best. One randomized study108 showed that a stent with an anti‐migration flap migrated less often than a flared‐end stent. A double pigtail PS may be deployed within a fully covered SEMS, overlapping its ends, thereby serving as an anchor.108
CQ18. What complications may occur after biliary stenting, other than recurrent biliary obstruction?
Statement 18
Biliary stent‐related AEs other than RBO include pancreatitis, cholecystitis, bleeding, ulceration, non‐occlusive cholangitis, penetration, and perforation.
Evidence level: A
Recommendation: No
Agreement: A, 100%; B, 0%; C, 0%; D, 0%
Biliary stent‐related AEs other than RBO can diminish the quality of life of patients with either MBS or BBS and may necessitate the interruption of chemotherapy, potentially reducing survival of patients with MBS. Physicians should be aware of the risk factors for and the management of stent related AEs. The rates of post‐procedure pancreatitis were 0–5.1% in SEMS and 0–12.5% in PS.100 The risk factors for pancreatitis were the use of SEMS, non‐pancreatic cancer, and SEMS with high axial force.109, 110 The rates of cholecystitis was 0–12.2% in metal stents and 0–8.9% in PS.100 The risk factors for cholecystitis after MS placement were tumor involvement to the cystic duct, gallstones, and SEMS with high axial force.111, 112, 113
CQ19. When is removal of a metallic stent indicated?
Statement 19‐1
SEMS removal or replacement should be considered if stent‐related complications occur.
Evidence level: C
Recommendation: 1
Agreement: A, 100%; B, 0%; C, 0%; D, 0%
Statement 19‐2
Where an uncovered SEMS or partially covered SEMS has been erroneously placed in a patient with BBS, endoscopic removal should be considered as soon as possible.
Evidence level: C
Recommendation: 1
Agreement: A, 90%; B, 10%; C, 0%; D, 0%
In cases of RBO and stent‐related complications, stent removal and/or replacement should be considered whenever possible. Removal of SEMS, particularly uncovered SEMS, can be technically difficult.114, 115, 116 The approach to RBO in patients with distal MBS depends on the etiology. Placement of a fully covered SEMS within an uncovered SEMS, obstructed by tumor ingrowth, is recommended (stent in stent technique).117, 118, 119 An obstructed fully covered SEMS is best managed by stent removal and placement of a new fully covered SEMS rather than the stent‐in‐stent technique or attempts at clearance by balloon sweeping.120
A fully covered SEMS is a treatment option for BBS (see Statement 9). Uncovered or partially covered SEMS may occlude due to tissue hyperplasia through the stent mesh. Stent embedment occurs within 1 week making removal difficult or impossible. Therefore, uncovered or partially covered SEMS should not be used for BBS. If one of these SEMS variants has been erroneously placed, endoscopic removal should be considered as soon as possible. The stent‐in‐stent technique utilizing a fully covered SEMS may be useful.121
Nakai, Y. , Isayama, H. , Wang, H.‐P. , Rerknimitr, R. , Khor, C. , Yasuda, I. , Kogure, H. , Moon, J. H. , Lau, J. , Lakhtakia, S. , Ratanachu‐ek, T. , Seo, D. W. , Lee, D. K. , Makmun, D. , Dy, F. , Liao, W.‐C. , Draganov, P. V. , Almadi, M. , Irisawa, A. , Katanuma, A. , Kitano, M. , Ryozawa, S. , Fujisawa, T. , Wallace, M. B. , Itoi, T. , and Devereaux, B. (2020) International consensus statements for endoscopic management of distal biliary stricture. Journal of Gastroenterology and Hepatology, 35: 967–979. 10.1111/jgh.14955.
Author contributions: HI and YN was involved with study conception, study design, creation of the preliminary statements list and with the allocated statement, drafting of the manuscript, critical revision of the manuscript, obtaining funding, face‐to‐face meetings, and voting. HW, RR, IY, YN and HK were involved with creation of the preliminary statements list and the allocated statement, drafting of the manuscript, critical revision of the manuscript, face‐to‐face meetings and voting. CK was involved with creation of the allocated statement, drafting of the manuscript, critical revision and integration of the manuscript, face‐to‐face meetings and voting. JHM, JL, SL, TR, DWS, DKL, DM, FD, DL, PD, MA, AI, AK, MK, SR, TF were involved with creation of the allocated statement, drafting of the manuscript, critical revision of the manuscript, face‐to‐face meetings and voting. MW, TI, BD were involved with critical revision of the manuscript.
Financial support: An unrestricted education grant was provided by Boston Scientific Japan, Tokyo, Japan. Boston Scientific Japan did not participate in the literature search, consensus discussion, voting, lecture preparation, or manuscript preparation. Tokyo Conference of Pancreatobiliary Interventional Endoscopy (T‐CAP) also supported this activity.
Statement of previous publication: These consensus statements were presented at the Asian Pacific Digestive Week (APDW) 2017 in Hong Kong.
References
- 1. Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Developing clinical guidelines. West. J. Med. 1999; 170: 348–351. [PMC free article] [PubMed] [Google Scholar]
- 2. Domagk D, Poremba C, Dietl KH et al Endoscopic transpapillary biopsies and intraductal ultrasonography in the diagnostics of bile duct strictures: a prospective study. Gut 2002; 51: 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miyazaki M, Ohtsuka M, Miyakawa S et al Classification of biliary tract cancers established by the Japanese Society of Hepato‐Biliary‐Pancreatic Surgery: 3(rd) English edition. J. Hepatobiliary Pancreat. Sci. 2015; 22: 181–196. [DOI] [PubMed] [Google Scholar]
- 4. Mohamadnejad M, DeWitt JM, Sherman S et al Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single‐center experience. Gastrointest. Endosc. 2011; 73: 71–78. [DOI] [PubMed] [Google Scholar]
- 5. Fitzgerald JE, White MJ, Lobo DN. Courvoisier's gallbladder: Law or sign? World J. Surg. 2009; 33: 886–891. [DOI] [PubMed] [Google Scholar]
- 6. Yamaguchi K, Okusaka T, Shimizu K et al Clinical practice guidelines for pancreatic cancer 2016 from the Japan Pancreas Society: A synopsis. Pancreas 2017; 46: 595–604. [DOI] [PubMed] [Google Scholar]
- 7. Miyazaki M, Yoshitomi H, Miyakawa S et al Clinical practice guidelines for the management of biliary tract cancers 2015: The 2nd English edition. J. Hepatobiliary Pancreat. Sci. 2015; 22: 249–273. [DOI] [PubMed] [Google Scholar]
- 8. Cannon ME, Carpenter SL, Elta GH et al EUS compared with CT, magnetic resonance imaging, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest. Endosc. 1999; 50: 27–33. [DOI] [PubMed] [Google Scholar]
- 9. DeWitt J, Devereaux B, Chriswell M et al Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann. Intern. Med. 2004; 141: 753–763. [DOI] [PubMed] [Google Scholar]
- 10. Takakura K, Sumiyama K, Munakata K et al Clinical usefulness of diffusion‐weighted MR imaging for detection of pancreatic cancer: comparison with enhanced multidetector‐row CT. Abdom. Imaging 2011; 36: 457–462. [DOI] [PubMed] [Google Scholar]
- 11. Fusari M, Maurea S, Imbriaco M et al Comparison between multislice CT and MR imaging in the diagnostic evaluation of patients with pancreatic masses. Radiol. Med. 2010; 115: 453–466. [DOI] [PubMed] [Google Scholar]
- 12. Zhao WY, Luo M, Sun YW et al Computed tomography in diagnosing vascular invasion in pancreatic and periampullary cancers: a systematic review and meta‐analysis. Hepatobiliary Pancreat. Dis. Int. 2009; 8: 457–464. [PubMed] [Google Scholar]
- 13. Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound‐guided fine‐needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta‐analysis and systematic review. Pancreas 2013; 42: 20–26. [DOI] [PubMed] [Google Scholar]
- 14. Sadeghi A, Mohamadnejad M, Islami F et al Diagnostic yield of EUS‐guided FNA for malignant biliary stricture: a systematic review and meta‐analysis. Gastrointest. Endosc. 2016; 83: 290–298 e1. [DOI] [PubMed] [Google Scholar]
- 15. Moura DTH, de Moura EGH, Matuguma SE et al EUS‐FNA versus ERCP for tissue diagnosis of suspect malignant biliary strictures: a prospective comparative study. Endosc Int Open. 2018; 6: E769–E777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang W, Shpaner A, Krishna SG et al Use of EUS‐FNA in diagnosing pancreatic neoplasm without a definitive mass on CT. Gastrointest. Endosc. 2013; 78: 73–80. [DOI] [PubMed] [Google Scholar]
- 17. Navaneethan U, Njei B, Venkatesh PG, Lourdusamy V, Sanaka MR. Endoscopic ultrasound in the diagnosis of cholangiocarcinoma as the etiology of biliary strictures: a systematic review and meta‐analysis. Gastroenterology report. 2015; 3: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Moura DTH, Moura EGH, Bernardo WM et al Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: Systematic review and meta‐analysis. Endoscopic ultrasound. 2018; 7: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee YN, Moon JH, Choi HJ et al Diagnostic approach using ERCP‐guided transpapillary forceps biopsy or EUS‐guided fine‐needle aspiration biopsy according to the nature of stricture segment for patients with suspected malignant biliary stricture. Cancer Med. 2017; 6: 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ranney N, Phadnis M, Trevino J, Ramesh J, Wilcox CM, Varadarajulu S. Impact of biliary stents on EUS‐guided FNA of pancreatic mass lesions. Gastrointest. Endosc. 2012; 76: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ross WA, Wasan SM, Evans DB et al Combined EUS with FNA and ERCP for the evaluation of patients with obstructive jaundice from presumed pancreatic malignancy. Gastrointest. Endosc. 2008; 68: 461–466. [DOI] [PubMed] [Google Scholar]
- 22. Aslanian HR, Estrada JD, Rossi F, Dziura J, Jamidar PA, Siddiqui UD. Endoscopic ultrasound and endoscopic retrograde cholangiopancreatography for obstructing pancreas head masses: combined or separate procedures? J. Clin. Gastroenterol. 2011; 45: 711–713. [DOI] [PubMed] [Google Scholar]
- 23. Draganov PV, Chauhan S, Wagh MS et al Diagnostic accuracy of conventional and cholangioscopy‐guided sampling of indeterminate biliary lesions at the time of ERCP: A prospective, long‐term follow‐up study. Gastrointest. Endosc. 2012; 75: 347–353. [DOI] [PubMed] [Google Scholar]
- 24. Ramchandani M, Reddy DN, Gupta R et al Role of single‐operator peroral cholangioscopy in the diagnosis of indeterminate biliary lesions: A single‐center, prospective study. Gastrointest. Endosc. 2011; 74: 511–519. [DOI] [PubMed] [Google Scholar]
- 25. Navaneethan U, Hasan MK, Kommaraju K et al Digital, single‐operator cholangiopancreatoscopy in the diagnosis and management of pancreatobiliary disorders: A multicenter clinical experience (with video). Gastrointest. Endosc. 2016; 84: 649–655. [DOI] [PubMed] [Google Scholar]
- 26. Ogura T, Imanishi M, Kurisu Y et al Prospective evaluation of digital single‐operator cholangioscope for diagnostic and therapeutic procedures (with videos). Digestive endoscopy: official journal of the Japan Gastroenterological Endoscopy Society. 2017; 29: 782–789. [DOI] [PubMed] [Google Scholar]
- 27. Nguyen NQ, Schoeman MN, Ruszkiewicz A. Clinical utility of EUS before cholangioscopy in the evaluation of difficult biliary strictures. Gastrointest. Endosc. 2013; 78: 868–874. [DOI] [PubMed] [Google Scholar]
- 28. Meister T, Heinzow HS, Woestmeyer C et al Intraductal ultrasound substantiates diagnostics of bile duct strictures of uncertain etiology. World J. Gastroenterol. 2013; 19: 874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Talreja JP, Sethi A, Jamidar PA et al Interpretation of probe‐based confocal laser endomicroscopy of indeterminate biliary strictures: Is there any interobserver agreement? Dig. Dis. Sci. 2012; 57: 3299–3302. [DOI] [PubMed] [Google Scholar]
- 30. Moole H, Bechtold ML, Forcione D, Puli SR. A meta‐analysis and systematic review: Success of endoscopic ultrasound guided biliary stenting in patients with inoperable malignant biliary strictures and a failed ERCP. Medicine 2017; 96: e5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharaiha RZ, Khan MA, Kamal F et al Efficacy and safety of EUS‐guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: a systematic review and meta‐analysis. Gastrointest. Endosc. 2017; 85: 904–914. [DOI] [PubMed] [Google Scholar]
- 32. Halttunen J, Meisner S, Aabakken L et al Difficult cannulation as defined by a prospective study of the Scandinavian Association for Digestive Endoscopy (SADE) in 907 ERCPs. Scand. J. Gastroenterol. 2014; 49: 752–758. [DOI] [PubMed] [Google Scholar]
- 33. Iwashita T, Doi S, Yasuda I. Endoscopic ultrasound‐guided biliary drainage: A review. Clinical journal of gastroenterology. 2014; 7: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iwashita T, Yasuda I, Mukai T et al EUS‐guided rendezvous for difficult biliary cannulation using a standardized algorithm: a multicenter prospective pilot study (with videos). Gastrointest. Endosc. 2016; 83: 394–400. [DOI] [PubMed] [Google Scholar]
- 35. Prat F, Chapat O, Ducot B et al A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest. Endosc. 1998; 47: 1– 7. [DOI] [PubMed] [Google Scholar]
- 36. Byrne MF, Chan CH, Branch MS, Jowell PS, Baillie J. Repeat procedures within 30 days in patients stented for malignant distal biliary strictures: Experience of 508 patients at a tertiary referral center. Gastroenterology Res. 2012; 5: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Di Giorgio P, Manes G, Grimaldi E et al Endoscopic plastic stenting for bile duct stones: stent changing on demand or every 3 months. A prospective comparison study. Endoscopy 2013; 45: 1014–1017. [DOI] [PubMed] [Google Scholar]
- 38. Tarnasky P, Miller C, Wang H et al Should stents be changed prophylactically in malignant obstructive jaundice? Computer modeling to optimize the use of health care resources. Gastrointest. Endosc. 1995; 41: 331. [Google Scholar]
- 39. Kunda R, Perez‐Miranda M, Will U et al EUS‐guided choledochoduodenostomy for malignant distal biliary obstruction using a lumen‐apposing fully covered metal stent after failed ERCP. Surg. Endosc. 2016; 30: 5002–5008. [DOI] [PubMed] [Google Scholar]
- 40. Cho DH, Lee SS, Oh D et al Long‐term outcomes of a newly developed hybrid metal stent for EUS‐guided biliary drainage (with videos). Gastrointest. Endosc. 2017; 85: 1067–1075. [DOI] [PubMed] [Google Scholar]
- 41. Mukai S, Itoi T, Tsuchiya T, Tanaka R, Tonozuka R. EUS‐guided right hepatic bile duct drainage in complicated hilar stricture. Gastrointest. Endosc. 2017; 85: 256–257. [DOI] [PubMed] [Google Scholar]
- 42. Wang K, Zhu J, Xing L, Wang Y, Jin Z, Li Z. Assessment of efficacy and safety of EUS‐guided biliary drainage: A systematic review. Gastrointest. Endosc. 2016; 83: 1218–1227. [DOI] [PubMed] [Google Scholar]
- 43. Teoh AYB, Dhir V, Kida M et al Consensus guidelines on the optimal management in interventional EUS procedures: results from the Asian EUS group RAND/UCLA expert panel. Gut 2018; 67: 1209–1228. [DOI] [PubMed] [Google Scholar]
- 44. Umeda J, Itoi T, Tsuchiya T et al A newly designed plastic stent for EUS‐guided hepaticogastrostomy: A prospective preliminary feasibility study (with videos). Gastrointest. Endosc. 2015; 82: 390–396 e2. [DOI] [PubMed] [Google Scholar]
- 45. Hu B, Sun B, Cai Q et al Asia‐Pacific consensus guidelines for endoscopic management of benign biliary strictures. Gastrointest. Endosc. 2017; 86: 44–58. [DOI] [PubMed] [Google Scholar]
- 46. van Boeckel PG, Vleggaar FP, Siersema PD. Plastic or metal stents for benign extrahepatic biliary strictures: A systematic review. BMC Gastroenterol. 2009; 9: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huszar O, Kokas B, Matrai P et al Meta‐analysis of the long term success rate of different interventions in benign biliary strictures. PLoS ONE 2017; 12: e0169618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Draganov P, Hoffman B, Marsh W, Cotton P, Cunningham J. Long‐term outcome in patients with benign biliary strictures treated endoscopically with multiple stents. Gastrointest. Endosc. 2002; 55: 680–686. [DOI] [PubMed] [Google Scholar]
- 49. Costamagna G, Tringali A, Mutignani M et al Endotherapy of postoperative biliary strictures with multiple stents: Results after more than 10 years of follow‐up. Gastrointest. Endosc. 2010; 72: 551–557. [DOI] [PubMed] [Google Scholar]
- 50. Deviere J, Nageshwar Reddy D, Puspok A et al Successful management of benign biliary strictures with fully covered self‐expanding metal stents. Gastroenterology 2014; 147: 385–395 quiz e15. [DOI] [PubMed] [Google Scholar]
- 51. Schmidt A, Pickartz T, Lerch MM et al Effective treatment of benign biliary strictures with a removable, fully covered, self‐expandable metal stent: A prospective, multicenter European study. United European gastroenterology journal. 2017; 5: 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haapamaki C, Kylanpaa L, Udd M et al Randomized multicenter study of multiple plastic stents vs. covered self‐expandable metallic stent in the treatment of biliary stricture in chronic pancreatitis. Endoscopy 2015; 47: 605–610. [DOI] [PubMed] [Google Scholar]
- 53. Cote GA, Slivka A, Tarnasky P et al Effect of covered metallic stents compared with plastic stents on benign biliary stricture resolution: A randomized clinical trial. JAMA 2016; 315: 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Khan MA, Baron TH, Kamal F et al Efficacy of self‐expandable metal stents in management of benign biliary strictures and comparison with multiple plastic stents: A meta‐analysis. Endoscopy 2017; 49: 682–694. [DOI] [PubMed] [Google Scholar]
- 55. Jang SI, Choi J, Lee DK. Magnetic compression anastomosis for treatment of benign biliary stricture. Digestive endoscopy: official journal of the Japan Gastroenterological Endoscopy Society. 2015; 27: 239–249. [DOI] [PubMed] [Google Scholar]
- 56. Tol JA, van Hooft JE, Timmer R et al Metal or plastic stents for preoperative biliary drainage in resectable pancreatic cancer. Gut 2016; 65: 1981–1987. [DOI] [PubMed] [Google Scholar]
- 57. Song TJ, Lee JH, Lee SS et al Metal versus plastic stents for drainage of malignant biliary obstruction before primary surgical resection. Gastrointest. Endosc. 2016; 84: 814–821. [DOI] [PubMed] [Google Scholar]
- 58. Kahaleh M, Brock A, Conaway MR et al Covered self‐expandable metal stents in pancreatic malignancy regardless of resectability: A new concept validated by a decision analysis. Endoscopy 2007; 39: 319–324. [DOI] [PubMed] [Google Scholar]
- 59. Isayama H, Komatsu Y, Tsujino T et al A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut 2004; 53: 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kullman E, Frozanpor F, Soderlund C et al Covered versus uncovered self‐expandable nitinol stents in the palliative treatment of malignant distal biliary obstruction: Results from a randomized, multicenter study. Gastrointest. Endosc. 2010; 72: 915–923. [DOI] [PubMed] [Google Scholar]
- 61. Telford JJ, Carr‐Locke DL, Baron TH et al A randomized trial comparing uncovered and partially covered self‐expandable metal stents in the palliation of distal malignant biliary obstruction. Gastrointest. Endosc. 2010; 72: 907–914. [DOI] [PubMed] [Google Scholar]
- 62. Krokidis M, Fanelli F, Orgera G, Bezzi M, Passariello R, Hatzidakis A. Percutaneous treatment of malignant jaundice due to extrahepatic cholangiocarcinoma: Covered Viabil stent versus uncovered Wallstents. Cardiovasc. Intervent. Radiol. 2010; 33: 97–106. [DOI] [PubMed] [Google Scholar]
- 63. Krokidis M, Fanelli F, Orgera G et al Percutaneous palliation of pancreatic head cancer: Randomized comparison of ePTFE/FEP‐covered versus uncovered nitinol biliary stents. Cardiovasc. Intervent. Radiol. 2011; 34: 352–361. [DOI] [PubMed] [Google Scholar]
- 64. Kitano M, Yamashita Y, Tanaka K et al Covered self‐expandable metal stents with an anti‐migration system improve patency duration without increased complications compared with uncovered stents for distal biliary obstruction caused by pancreatic carcinoma: A randomized multicenter trial. Am. J. Gastroenterol. 2013; 108: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 65. Saleem A, Leggett CL, Murad MH, Baron TH. Meta‐analysis of randomized trials comparing the patency of covered and uncovered self‐expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest. Endosc. 2011; 74: 321–327 e1–3. [DOI] [PubMed] [Google Scholar]
- 66. Almadi MA, Barkun AN, Martel M. No benefit of covered vs uncovered self‐expandable metal stents in patients with malignant distal biliary obstruction: A meta‐analysis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2013; 11: 27–37 e1. [DOI] [PubMed] [Google Scholar]
- 67. Pisters PW, Hudec WA, Lee JE et al Preoperative chemoradiation for patients with pancreatic cancer: Toxicity of endobiliary stents. J. Clin. Oncol. 2000; 18: 860–867. [DOI] [PubMed] [Google Scholar]
- 68. Lofts FJ, Evans TR, Mansi JL, Glees JP, Knight MJ. Bile duct stents: Is there an increased rate of complications in patients receiving chemotherapy? Eur. J. Cancer 1997; 33: 209–213. [DOI] [PubMed] [Google Scholar]
- 69. Nakai Y, Isayama H, Kawabe T et al Efficacy and safety of metallic stents in patients with unresectable pancreatic cancer receiving gemcitabine. Pancreas 2008; 37: 405–410. [DOI] [PubMed] [Google Scholar]
- 70. Haal S, van Hooft JE, Rauws EAJ, Fockens P, Voermans RP. Stent patency in patients with distal malignant biliary obstruction receiving chemo (radio)therapy. Endoscopy international open. 2017; 5: E1035–E1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Maire F, Hammel P, Ponsot P et al Long‐term outcome of biliary and duodenal stents in palliative treatment of patients with unresectable adenocarcinoma of the head of pancreas. Am. J. Gastroenterol. 2006; 101: 735–742. [DOI] [PubMed] [Google Scholar]
- 72. Shah A, Fehmi A, Savides TJ. Increased rates of duodenal obstruction in pancreatic cancer patients receiving modern medical management. Dig. Dis. Sci. 2014; 59: 2294–2298. [DOI] [PubMed] [Google Scholar]
- 73. Nakai Y, Hamada T, Isayama H, Itoi T, Koike K. Endoscopic management of combined malignant biliary and gastric outlet obstruction. Digestive endoscopy: official journal of the Japan Gastroenterological Endoscopy Society. 2017; 29: 16–25. [DOI] [PubMed] [Google Scholar]
- 74. Jeurnink SM, van Eijck CH, Steyerberg EW, Kuipers EJ, Siersema PD. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: A systematic review. BMC Gastroenterol. 2007; 7: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jeurnink SM, Steyerberg EW, van Hooft JE et al Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): A multicenter randomized trial. Gastrointest. Endosc. 2010; 71: 490–499. [DOI] [PubMed] [Google Scholar]
- 76. Kaw M, Singh S, Gagneja H. Clinical outcome of simultaneous self‐expandable metal stents for palliation of malignant biliary and duodenal obstruction. Surg. Endosc. 2003; 17: 457–461. [DOI] [PubMed] [Google Scholar]
- 77. Vanbiervliet G, Demarquay JF, Dumas R, Caroli‐Bosc FX, Piche T, Tran A. Endoscopic insertion of biliary stents in 18 patients with metallic duodenal stents who developed secondary malignant obstructive jaundice. Gastroenterol. Clin. Biol. 2004; 28: 1209–1213. [DOI] [PubMed] [Google Scholar]
- 78. Mutignani M, Tringali A, Shah SG et al Combined endoscopic stent insertion in malignant biliary and duodenal obstruction. Endoscopy 2007; 39: 440–447. [DOI] [PubMed] [Google Scholar]
- 79. Moon JH, Choi HJ, Ko BM et al Combined endoscopic stent‐in‐stent placement for malignant biliary and duodenal obstruction by using a new duodenal metal stent (with videos). Gastrointest. Endosc. 2009; 70: 772–777. [DOI] [PubMed] [Google Scholar]
- 80. Kim KO, Kim TN, Lee HC. Effectiveness of combined biliary and duodenal stenting in patients with malignant biliary and duodenal obstruction. Scand. J. Gastroenterol. 2012; 47: 962–967. [DOI] [PubMed] [Google Scholar]
- 81. Tonozuka R, Itoi T, Sofuni A, Itokawa F, Moriyasu F. Endoscopic double stenting for the treatment of malignant biliary and duodenal obstruction due to pancreatic cancer. Digestive endoscopy: official journal of the Japan Gastroenterological Endoscopy Society. 2013; 25: 100–108. [DOI] [PubMed] [Google Scholar]
- 82. Khashab MA, Valeshabad AK, Leung W et al Multicenter experience with performance of ERCP in patients with an indwelling duodenal stent. Endoscopy 2014; 46: 252–255. [DOI] [PubMed] [Google Scholar]
- 83. Hamada T, Nakai Y, Isayama H et al Duodenal metal stent placement is a risk factor for biliary metal stent dysfunction: an analysis using a time‐dependent covariate. Surg. Endosc. 2013; 27: 1243–1248. [DOI] [PubMed] [Google Scholar]
- 84. Hamada T, Isayama H, Nakai Y et al Transmural biliary drainage can be an alternative to transpapillary drainage in patients with an indwelling duodenal stent. Dig. Dis. Sci. 2014; 59: 1931–1938. [DOI] [PubMed] [Google Scholar]
- 85. Ogura T, Chiba Y, Masuda D et al Comparison of the clinical impact of endoscopic ultrasound‐guided choledochoduodenostomy and hepaticogastrostomy for bile duct obstruction with duodenal obstruction. Endoscopy 2016; 48: 156–163. [DOI] [PubMed] [Google Scholar]
- 86. Dhir V, Artifon EL, Gupta K et al Multicenter study on endoscopic ultrasound‐guided expandable biliary metal stent placement: Choice of access route, direction of stent insertion, and drainage route. Digestive endoscopy: official journal of the Japan Gastroenterological Endoscopy Society. 2014; 26: 430–435. [DOI] [PubMed] [Google Scholar]
- 87. Song TJ, Lee SS, Yun SC et al Paclitaxel‐eluting covered metal stents versus covered metal stents for distal malignant biliary obstruction: A prospective comparative pilot study. Gastrointest. Endosc. 2011; 73: 727–733. [DOI] [PubMed] [Google Scholar]
- 88. Berr F, Wiedmann M, Tannapfel A et al Photodynamic therapy for advanced bile duct cancer: Evidence for improved palliation and extended survival. Hepatology (Baltimore, Md) 2000; 31: 291–298. [DOI] [PubMed] [Google Scholar]
- 89. Ortner ME, Caca K, Berr F et al Successful photodynamic therapy for nonresectable cholangiocarcinoma: A randomized prospective study. Gastroenterology 2003; 125: 1355–1363. [DOI] [PubMed] [Google Scholar]
- 90. Zoepf T, Jakobs R, Arnold JC, Apel D, Riemann JF. Palliation of nonresectable bile duct cancer: Improved survival after photodynamic therapy. Am. J. Gastroenterol. 2005; 100: 2426–2430. [DOI] [PubMed] [Google Scholar]
- 91. Kahaleh M, Mishra R, Shami VM et al Unresectable cholangiocarcinoma: comparison of survival in biliary stenting alone versus stenting with photodynamic therapy. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2008; 6: 290–297. [DOI] [PubMed] [Google Scholar]
- 92. Figueroa‐Barojas P, Bakhru MR, Habib NA et al Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: A novel palliation technique. J. Oncol. 2013; 2013: 910897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Steel AW, Postgate AJ, Khorsandi S et al Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest. Endosc. 2011; 73: 149–153. [DOI] [PubMed] [Google Scholar]
- 94. Strand DS, Cosgrove ND, Patrie JT et al ERCP‐directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma. Gastrointest. Endosc. 2014; 80: 794–804. [DOI] [PubMed] [Google Scholar]
- 95. Dolak W, Schreiber F, Schwaighofer H et al Endoscopic radiofrequency ablation for malignant biliary obstruction: A nationwide retrospective study of 84 consecutive applications. Surg. Endosc. 2014; 28: 854–860. [DOI] [PubMed] [Google Scholar]
- 96. Sharaiha RZ, Sethi A, Weaver KR et al Impact of radiofrequency ablation on malignant biliary strictures: Results of a collaborative registry. Dig. Dis. Sci. 2015; 60: 2164–2169. [DOI] [PubMed] [Google Scholar]
- 97. Sharaiha RZ, Natov N, Glockenberg KS, Widmer J, Gaidhane M, Kahaleh M. Comparison of metal stenting with radiofrequency ablation versus stenting alone for treating malignant biliary strictures: Is there an added benefit? Dig. Dis. Sci. 2014; 59: 3099–3102. [DOI] [PubMed] [Google Scholar]
- 98. Kallis Y, Phillips N, Steel A et al Analysis of endoscopic radiofrequency ablation of biliary malignant strictures in pancreatic cancer suggests potential survival benefit. Dig. Dis. Sci. 2015; 60: 3449–3455. [DOI] [PubMed] [Google Scholar]
- 99. Isayama H, Hamada T, Yasuda I et al TOKYO criteria 2014 for transpapillary biliary stenting. Digestive endoscopy: official journal of the Japan Gastroenterological Endoscopy Society. 2015; 27: 259–264. [DOI] [PubMed] [Google Scholar]
- 100. Almadi MA, Barkun A, Martel M. Plastic vs. self‐expandable metal stents for palliation in malignant biliary obstruction: A series of meta‐analyses. Am. J. Gastroenterol. 2017; 112: 260–273. [DOI] [PubMed] [Google Scholar]
- 101. Chittleborough TJ, Mgaieth S, Kirkby B, Zakon J. Remove the migrated stent: sigmoid colon perforation from migrated biliary stent. ANZ J. Surg. 2016; 86: 947–948. [DOI] [PubMed] [Google Scholar]
- 102. Catalano O, De Bellis M, Sandomenico F, de Lutio di Castelguidone E, Delrio P, Petrillo A. Complications of biliary and gastrointestinal stents: MDCT of the cancer patient. AJR Am. J. Roentgenol. 2012; 199: W187–W196. [DOI] [PubMed] [Google Scholar]
- 103. Chou ND, Burbridge RA, Jowell PS. Colonic perforation secondary to retained biliary stent. Am. J. Gastroenterol. 2017; 112: 13. [DOI] [PubMed] [Google Scholar]
- 104. Ishii K, Itoi T, Sofuni A et al Endoscopic removal and trimming of distal self‐expandable metallic biliary stents. World J. Gastroenterol. 2011; 17: 2652–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Dumonceau JM, Tringali A, Blero D et al Biliary stenting: Indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 2012; 44: 277–298. [DOI] [PubMed] [Google Scholar]
- 106. Ito K, Ogawa T, Horaguchi J, Koshita S, Fujita N. Reintervention for occluded biliary metal stent for patients with malignant distal biliary stricture. Digestive endoscopy: official journal of the Japan Gastroenterological Endoscopy Society. 2013; 25: 126–131. [DOI] [PubMed] [Google Scholar]
- 107. Walter D, Laleman W, Jansen JM et al A fully covered self‐expandable metal stent with antimigration features for benign biliary strictures: A prospective, multicenter cohort study. Gastrointest. Endosc. 2015; 81: 1197–1203. [DOI] [PubMed] [Google Scholar]
- 108. Park DH, Lee SS, Lee TH et al Anchoring flap versus flared end, fully covered self‐expandable metal stents to prevent migration in patients with benign biliary strictures: A multicenter, prospective, comparative pilot study (with videos). Gastrointest. Endosc. 2011; 73: 64–70. [DOI] [PubMed] [Google Scholar]
- 109. Cote GA, Kumar N, Ansstas M et al Risk of post‐ERCP pancreatitis with placement of self‐expandable metallic stents. Gastrointest. Endosc. 2010; 72: 748–754. [DOI] [PubMed] [Google Scholar]
- 110. Kawakubo K, Isayama H, Nakai Y et al Risk factors for pancreatitis following transpapillary self‐expandable metal stent placement. Surg. Endosc. 2012; 26: 771–776. [DOI] [PubMed] [Google Scholar]
- 111. Isayama H, Kawabe T, Nakai Y et al Cholecystitis after metallic stent placement in patients with malignant distal biliary obstruction. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2006; 4: 1148–1153. [DOI] [PubMed] [Google Scholar]
- 112. Suk KT, Kim HS, Kim JW et al Risk factors for cholecystitis after metal stent placement in malignant biliary obstruction. Gastrointest. Endosc. 2006; 64: 522–529. [DOI] [PubMed] [Google Scholar]
- 113. Nakai Y, Isayama H, Kawakubo K et al Metallic stent with high axial force as a risk factor for cholecystitis in distal malignant biliary obstruction. J. Gastroenterol. Hepatol. 2014; 29: 1557–1562. [DOI] [PubMed] [Google Scholar]
- 114. Familiari P, Bulajic M, Mutignani M et al Endoscopic removal of malfunctioning biliary self‐expandable metallic stents. Gastrointest. Endosc. 2005; 62: 903–910. [DOI] [PubMed] [Google Scholar]
- 115. Shin HP, Kim MH, Jung SW et al Endoscopic removal of biliary self‐expandable metallic stents: A prospective study. Endoscopy 2006; 38: 1250–1255. [DOI] [PubMed] [Google Scholar]
- 116. Kida M, Miyazawa S, Iwai T et al Endoscopic management of malignant biliary obstruction by means of covered metallic stents: primary stent placement vs. re‐intervention. Endoscopy 2011; 43: 1039–1044. [DOI] [PubMed] [Google Scholar]
- 117. Togawa O, Kawabe T, Isayama H et al Management of occluded uncovered metallic stents in patients with malignant distal biliary obstructions using covered metallic stents. J. Clin. Gastroenterol. 2008; 42: 546–549. [DOI] [PubMed] [Google Scholar]
- 118. Cho JH, Jeon TJ, Park JY et al Comparison of outcomes among secondary covered metallic, uncovered metallic, and plastic biliary stents in treating occluded primary metallic stents in malignant distal biliary obstruction. Surg. Endosc. 2011; 25: 475–482. [DOI] [PubMed] [Google Scholar]
- 119. Lee BS, Ryu JK, Jang DK et al Reintervention for occluded metal stent in malignant bile duct obstruction: A prospective randomized trial comparing covered and uncovered metal stent. J. Gastroenterol. Hepatol. 2016; 31: 1901–1907. [DOI] [PubMed] [Google Scholar]
- 120. Togawa O, Isayama H, Tsujino T et al Management of dysfunctional covered self‐expandable metallic stents in patients with malignant distal biliary obstruction. J. Gastroenterol. 2013; 48: 1300–1307. [DOI] [PubMed] [Google Scholar]
- 121. Tan DM, Lillemoe KD, Fogel EL. A new technique for endoscopic removal of uncovered biliary self‐expandable metal stents: Stent‐in‐stent technique with a fully covered biliary stent. Gastrointest. Endosc. 2012; 75: 923–925. [DOI] [PubMed] [Google Scholar]


