Abstract
Objective
To evaluate the efficacy of a novel and selective β3‐adrenoreceptor agonist vibegron on urgency urinary incontinence (UUI) in patients with overactive bladder (OAB).
Patients and Methods
A post hoc analysis was performed in patients with UUI (>0 episodes/day) who were assigned to receive vibegron or placebo in a vibegron phase 3 study. Patients were subclassified into mild/moderate (>0 to <3 UUI episodes/day) or severe UUI (≥3 UUI episodes/day) subgroup. Changes from baseline in number of UUI episodes/day, in number of urgency episodes/day, and in voided volume/micturition were compared between the groups. The percentage of patients who became UUI‐free ('diary‐dry' rate) and the response rate (percentage of patients with scores 1 [feeling much better] or 2 [feeling better] assessed by the Patient Global Impression scale [PGI]) were evaluated.
Results
Changes in numbers of UUI episodes at week 12 in the vibegron 50 mg, vibegron 100 mg and placebo groups, respectively, were −1.35, −1.47 and −1.08 in all patients, −1.04, −1.13 and −0.89 in the mild/moderate UUI subgroup, and −2.95, −3.28 and −2.10 in the severe UUI subgroup. The changes were significant in the vibegron 50 and 100 mg groups vs placebo regardless of symptom severity. Change in number of urgency episodes/day was significant in the vibegron 100 mg group vs placebo in all patients and in both severity subgroups. In the vibegron 50 mg group, a significant change vs placebo was observed in all patients and in the mild/moderate UUI subgroup. Change in voided volume/micturition was significantly greater in the vibegron 50 and 100 mg groups vs placebo in all patients, as well as in the both severity subgroups. Diary‐dry rates in the vibegron 50 and 100 mg groups were significantly greater vs placebo in all patients and in the mild/moderate UUI subgroup. In the severe UUI subgroup, however, a significant difference was observed only in the vibegron 50 mg group. Response rates assessed by the PGI were significantly higher in the vibegron groups vs placebo in all patients and in the both severity subgroups. Vibegron administration, OAB duration ≤37 months, mean number of micturitions/day at baseline <12.0 and mean number of UUI episodes/day at baseline <3.0 were identified as factors significantly associated with normalization of UUI.
Conclusions
Vibegron, a novel β3‐adrenoreceptor agonist, significantly reduced the number of UUI episodes/day and significantly increased the voided volume/micturition in patients with OAB including those with severe UUI, with the response rate exceeding 50%. These results suggest that vibegron can be an effective therapeutic option for OAB patients with UUI.
Keywords: urgency urinary incontinence, overactive bladder, β3‐adrenoreceptor agonist, vibegron, post hoc analysis
Abbreviations
- OAB
overactive bladder
- PGI
Patient Global Impression scale
- UUI
urgency urinary incontinence
Introduction
Approximately 4.3 billion individuals across the world have been estimated to experience various urinary symptoms, including overactive bladder (OAB) 1. The prevalence of urgency urinary incontinence (UUI) is estimated to be 8.2% (~350 million individuals) 1. UUI is an extremely common complaint in every part of the world. It causes a great deal of distress and embarrassment, as well as significant costs, to both individuals and societies 2. In addition, more severe urinary frequency and incontinence were reported to be associated with higher bother ratings 3; many patients therefore desire successful treatment of the symptoms. One study, however, showed that, even with pharmacotherapy, the UUI cure rate 1 year after treatment with anticholinergics was 49% 4, indicating that there is a pressing need for a more effective drug.
Vibegron, a novel β3‐adrenoreceptor agonist, is unlikely to be metabolized by CYP3A4 or CYP2D6 in the liver 5, thus is expected to exert its efficacy and safety with fewer inter‐individual differences compared with drugs that are metabolized by these enzymes. Vibegron was evaluated in a phase 3 study (trial registration no.: JapicCTI‐152936) in Japanese patients with OAB, and the study demonstrated excellent efficacy and tolerability 6.
The present study, a post hoc analysis of the vibegron phase 3 study, was performed to evaluate the efficacy of vibegron on UUI in patients with OAB.
Patients and Methods
Patients
The study design and results of the vibegron phase 3 study have been reported elsewhere 6. Briefly, a total of 1232 patients with OAB (defined as ≥ 8 micturitions/day and either ≥ 1 urgency episode/day or ≥ 1 urgency incontinence episode/day) were randomly assigned to one of four 12‐week treatment groups: vibegron (50 mg or 100 mg once daily), placebo, or imidafenacin (0.1 mg twice daily). Exclusion criteria included UTI, bladder cancer, bladder calculus, interstitial cystitis, enlarged prostate, and residual urinary volume> 100 mL. Among these patients, those who were assigned to receive vibegron or placebo and had UUI episodes (>0 per day) were enrolled in this post hoc analysis.
Methods
Eligible patient data were classified into a mild/moderate UUI subgroup (>0 to <3 UUI episodes/day) or a severe UUI subgroup (≥3 UUI episodes/day). The threshold for mild/moderate and severe UUI was set based on the classification of UUI severity reported in a previous study 7. Changes from baseline in number of UUI episodes/day, number of urgency episodes/day, and voided volume/micturition, calculated based on a 3‐day micturition diary before each scheduled visit, were compared between the groups. In addition, the percentage of patients who became UUI‐free (zero UUI episodes/day) at week 12 was calculated as the 'diary‐dry' rate. The percentage of patients with score 1 (feeling much better) or 2 (feeling better) assessed by the Patient Global Impression scale (PGI) was also calculated to identify the response rate. To identify factors associated with normalization of UUI, factorial analysis of the data using the multivariate logistic regression analysis was performed.
Statistical Analysis
For changes in individual micturition variables from baseline to week 12, a constrained longitudinal data analysis model 8 was used to calculate least squares means and 95% CIs, and between‐intervention comparison was made. For the assessment of differences in the diary‐dry rate and response rate, a chi‐squared test was used. Multivariate logistic regression analysis was performed with presence/absence of UUI at week 12 as an objective variable and the following items as explanatory variables: treatment (vibegron vs placebo), gender (male vs female), OAB duration (≤37 months [median baseline value of all patients in the present analysis] vs ≥ 38 months), treatment history (absent vs present), mean number of micturitions/day at baseline (<12.0 vs ≥ 12.0), and mean number of UUI episodes/day at baseline (<3.0 vs ≥ 3.0).
A P value <0.05 was considered to indicate significance. All analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
A total of 243 patients were excluded from the 1232 patients randomized in the phase 3 study, and 989 patients were included in the post hoc analysis (Fig. 1). Among the 989 patients, numbers of patients were 329, 327 and 333 in the vibegron 50 mg, vibegron 100 mg, and placebo group, respectively (Table 1). Background characteristics of the three patient groups were almost identical, except that the vibegron 100 mg group included slightly more patients with hypertension or longer OAB duration.
Figure 1.
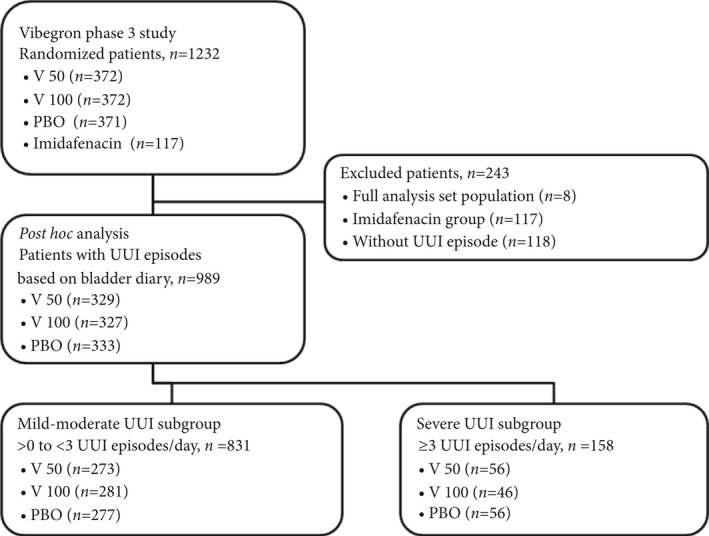
Study population. OAB, overactive bladder; PBO, placebo group; UUI, urgency urinary incontinence; V 100, vibegron 100 mg/day group; V 50, vibegron 50 mg/day group.
Table 1.
Patient characteristics.
| All patients | Mild‐moderate UUI subgroup | Severe UUI subgroup | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Vibegron 50 mg (N = 329) | Vibegron 100 mg (N = 327) | Placebo (N = 333) | Vibegron 50 mg (N = 273) | Vibegron 100 mg (N = 281) | Placebo (N = 277) | Vibegron 50 mg (N = 56) | Vibegron 100 mg (N = 46) | Placebo (N = 56) | |
| Age | 58.1 (11.4) | 58.8 (11.2) | 59.1 (11.6) | 58.0 (11.2) | 58.5 (11.2) | 59.4 (11.9) | 58.7 (12.0) | 60.7 (11.0) | 58.1 (10.0) |
| Female, n (%) | 307 (93.3) | 308 (94.2) | 315 (94.6) | 256 (93.8) | 262 (93.2) | 262 (94.6) | 51 (91.1) | 46 (100) | 53 (94.6) |
| Body weight, kg | 56.7 (11.0) | 56.8 (11.2) | 56.3 (10.6) | 56.7 (11.2) | 56.8 (11.6) | 55.6 (9.9) | 56.6 (10.0) | 56.4 (8.6) | 60.1 (12.9) |
| BMI, kg/m2 | 23.1 (4.1) | 23.1 (4.1) | 23.1 (4.0) | 23.1 (4.1) | 23.0 (4.2) | 22.8 (3.8) | 23.3 (4.4) | 23.6 (3.6) | 24.6 (4.8) |
| Hypertension, n (%) | 91 (27.7) | 99 (30.3) | 88 (26.4) | 75 (27.5) | 81 (28.8) | 70 (25.3) | 16 (28.6) | 18 (39.1) | 18 (32.1) |
| OAB duration, months | 57.6 (64.7) | 69.6 (76.0) | 59.3 (60.7) | 59.5 (67.6) | 69.2 (78.4) | 57.3 (61.6) | 48.6 (47.1) | 72.5 (60.0) | 69.4 (55.8) |
| OAB duration ≤37 months*, n (%) | 177 (53.8) | 153 (46.8) | 159 (47.7) | 143 (52.4) | 133 (47.3) | 138 (49.8) | 34 (60.7) | 20 (43.5) | 21 (37.5) |
| Anticholinergic treatment history for OAB, n (%) | 39 (11.9) | 32 (9.8) | 42 (12.6) | 27 (9.9) | 23 (8.2) | 32 (11.6) | 12 (21.4) | 9 (19.6) | 10 (17.9) |
| Number of micturitions/day | 11.1 (2.4) | 11.2 (2.3) | 11.2 (2.4) | 11.0 (2.4) | 11.0 (2.3) | 11.0 (2.1) | 11.6 (2.2) | 11.9 (2.3) | 12.4 (3.2) |
| Number of urgency episodes/day | 3.9 (2.1) | 3.9 (2.2) | 3.9 (2.2) | 3.4 (1.6) | 3.6 (2.1) | 3.4 (1.8) | 6.4 (2.2) | 5.7 (2.2) | 6.4 (2.6) |
| Number of UUI/day | 2.0 (1.5) | 1.9 (1.3) | 1.9 (1.3) | 1.4 (0.6) | 1.5 (0.6) | 1.4 (0.6) | 4.6 (1.6) | 4.3 (1.7) | 4.2 (1.4) |
| Voided volume/micturition, mL | 154.5 (44.5) | 155.7 (44.5) | 156.9 (44.7) | 157.5 (44.4) | 157.3 (44.6) | 157.7 (44.8) | 139.8 (42.4) | 146.0 (43.2) | 152.9 (44.6) |
BMI, body mass index; OAB, overactive bladder; UUI, urgency urinary incontinence.
Data are mean (sd), unless otherwise stated.
Median baseline value of all patients included in the present analysis.
The numbers of patients with mild/moderate UUI were 273, 281 and 277 in the vibegron 50 mg, vibegron 100 mg and placebo groups. The corresponding numbers of those with severe UUI were 56, 46, and 56. Patients were aged 58.0–60.7 years, 91.1–100% were women, and 8.2–21.4% had a history of anticholinergic treatment for OAB.
In the severe UUI subgroup, the vibegron 100 mg group included more older patients (60.7 years vs 58.7 and 58.1 years) and more women (100% vs 91.1% and 94.6%) compared with the vibegron 50 mg and placebo groups. With regard to baseline OAB symptoms, notable differences were not observed among the groups, with the exception of voided volume/micturition (139.8, 146.0 and 152.9 mL in the vibegron 50 mg, vibegron 100 mg and placebo groups, respectively) in the severe UUI subgroup.
In the mild/moderate UUI subgroup, the vibegron 100 mg group included patients with longer OAB duration compared with the vibegron 50 mg and placebo groups.
Changes in numbers of UUI episodes at week 12 were significant in both vibegron 50 and 100 mg groups vs placebo in all patients. In the subgroups by severity, a significant difference was also seen between both the vibegron 50 mg and 100 mg groups vs the placebo group (Fig. 2A). Change in number of urgency episodes/day was significant in the vibegron 100 mg group vs placebo in all patients (−2.54 vs −1.84; P < 0.001), in the mild/moderate UUI subgroup (−2.25 vs −1.68; P < 0.001) and in the severe UUI subgroup (−4.04 vs −2.70; P = 0.006) at week 12. In the vibegron 50 mg group, a significant change vs placebo was observed in all patients (−2.34 vs −1.84; P < 0.001) and in the mild/moderate UUI subgroup (−2.11 vs −1.68; P = 0.002), but not in the severe UUI subgroup (−3.55 vs −2.70; P = 0.064 [Fig. 2B]).
Figure 2.
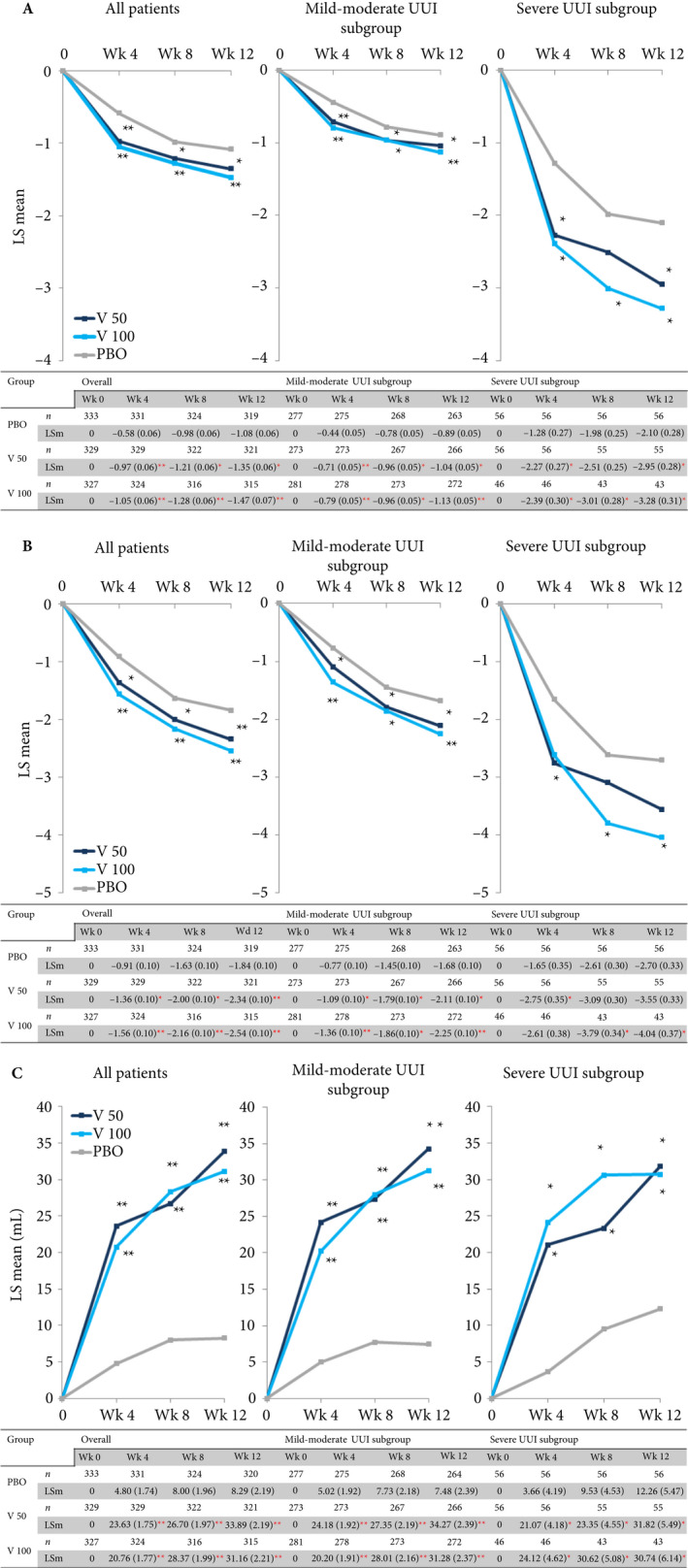
(a) Change in number of urgency urinary incontinence (UUI) episodes/day; (b) Change in number of urgency episodes/day; (c) Change in voided volume/micturition. Data are presented as least squares (LS) mean (SE). Constrained longitudinal data analysis model, *P < 0.05, **P < 0.001 vs placebo. LSm, least squares mean; PBO, placebo group; V 100, vibegron 100 mg group; V 50, vibegron 50 mg group; Wk, week(s).
Change in voided volume/micturition was significantly greater in the vibegron 50 mg and 100 mg groups vs placebo in all patients (33.89 and 31.16 vs 8.29 mL; P < 0.001 for both active doses), in the mild/moderate UUI subgroup (34.27 and 31.28 vs 7.48 mL; P < 0.001 for both active doses), and in the severe UUI subgroup (31.82 and 30.74 vs 12.26 mL; P = 0.013 for the vibegron 50 mg group, P = 0.026 for the vibegron 100 mg group vs placebo) at week 12 (Fig. 2C).
Diary‐dry rates in the vibegron 50 mg, vibegron 100 mg and placebo groups were 55.6%, 59.6% and 45.0%, respectively, in all patients (P = 0.006 for the vibegron 50 mg group; P < 0.001 for the vibegron 100 mg group vs placebo), 59.3%, 64.1% and 50.5%, respectively, in the mild/moderate UUI subgroup (P = 0.038 for the vibegron 50 mg group; P = 0.001 for the vibegron 100 mg group vs placebo), and 37.5%, 32.6% and 17.9%, respectively, in the severe UUI subgroup (P = 0.020 for the vibegron 50 mg group; P = 0.085 for the vibegron 100 mg group vs placebo) at week 12 (Fig. 3).
Figure 3.
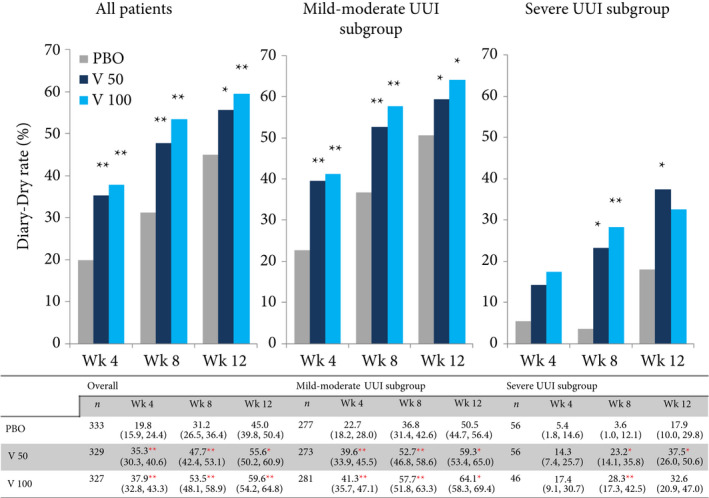
Diary‐dry rate. UUI, urgency urinary incontinence. Data are presented as mean (95% CI). Chi‐squared test, *P < 0.05, **P < 0.001 vs placebo. PBO, placebo group; V 100, vibegron 100 mg group; V 50, vibegron 50 mg group; Wk, week(s).
Response rates assessed by PGI in the vibegron 50 mg, vibegron 100 mg and placebo groups were 60.2%, 61.8% and 37.8%, respectively, in all patients, 61.2%, 63.3% and 39.7%, respectively, in the mild/moderate UUI subgroup, and 55.4%, 52.2% and 28.6%, respectively, in the severe UUI subgroup. The response rates in the vibegron groups were significantly higher vs placebo in all patients and in both the subgroups (P < 0.001 for all patients and the mild/moderate UUI subgroup; P < 0.05 for the severe UUI subgroup [Fig. 4]).
Figure 4.
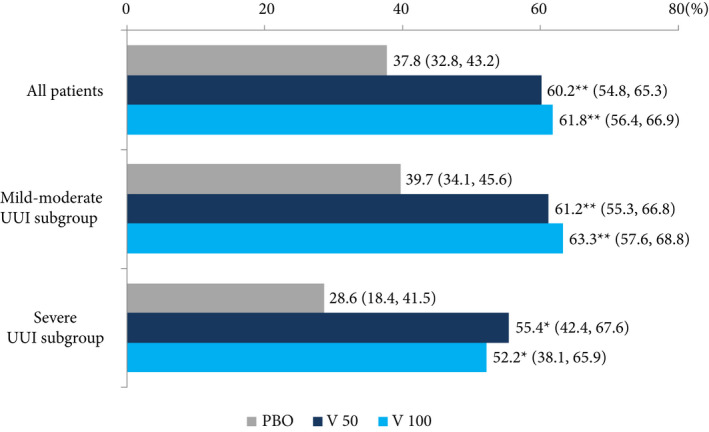
Patient global impression. Data are presented as mean (95% CI). Chi‐square test, *P < 0.05, **P < 0.001 vs placebo. PBO, placebo group; V 50, vibegron 50 mg group; V 100, vibegron 100 mg group.
Multivariate logistic regression analysis showed that vibegron administration, OAB duration ≤37 months, mean number of micturitions/day at baseline <12.0, and mean number of UUI episodes/day at baseline <3.0 were factors significantly associated with normalization of UUI (Fig. 5).
Figure 5.
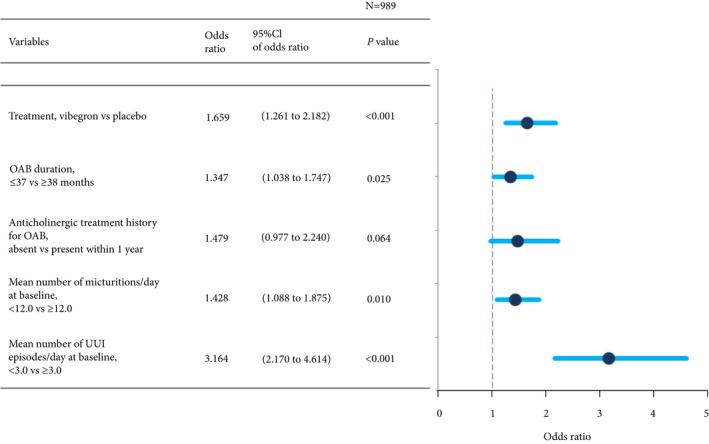
Factors associated with normalization of urgency urinary incontinence (UUI): multivariate logistic regression analysis. OAB, overactive bladder.
Treatment was discontinued in eight, 14 and 12 patients in the vibegron 50 mg, vibegron 100 mg and placebo groups, respectively. Of the 22 discontinuations in the vibegron groups, 14 were the result of patient request and six were for safety considerations by the attending physicians. Adverse events for which causal relationship with the study drug cannot be excluded were observed in 23 patients in the vibegron 50 mg group, 18 in the vibegron 100 mg group, and 15 in the placebo group. The most frequent adverse events were constipation in five patients and dry mouth in four patients, both in the vibegron 50 mg group. The remaining adverse events were noted in two patients or fewer in each treatment group. None of the adverse events were serious.
Discussion
Vibegron treatment for 12 weeks resulted in significant improvement in the number of UUI episodes/day, the number of urgency episodes/day, and voided volume/micturition, when compared with placebo. Analysis according to severity showed that vibegron 50 mg and 100 mg led to a significant improvement in the number of UUI episodes/day, in the number of urgency episodes/day and in voided volume/micturition, with the exception of number of urgency episodes/day in the severe UUI subgroup treated with vibegron 50 mg. The diary‐dry rate among vibegron‐treated patients ranged from 55.6% (vibegron 50 mg group) to 59.6% (vibegron 100 mg group) and there were significant differences from the placebo group for both vibegron groups except for the severe subgroup treated with vibegron 100 mg. The mean numbers of UUI episodes/day in the severe UUI subgroup at week 12 were reduced to 1.51 and 1.04 in the vibegron 50 and 100 mg groups, respectively, achieving the level of mild/moderate UUI (<3 per day). The response rate assessed by PGI after vibegron treatment exceeded 50% in both mild/moderate and severe subgroups at the end of the 12‐week treatment period. In the placebo group, by contrast, the response rate at week 12 was 37.8% in all patients. The results suggest that vibegron may be useful in the treatment of patients with UUI including those with severe UUI.
In the present study, the diary‐dry rate was> 50% in the vibegron‐treated patients. This high rate was considered attributable to the potent action of vibegron. The excellent potency exhibited by vibegron, a compound in the pyrrolidine series, may arise in part from a reduction of the rotational degrees of freedom imparted by the pyrrolidine ring to fix the orientation of the two substituent ‘arms’ into the appropriate binding/activation conformation of the hβ3‐adrenoreceptor. Furthermore, with its structure‐based efficacy, vibegron has been reported to demonstrate excellent selectivity for activation of β3‐adrenoreceptor over binding to β1/2‐adrenoreceptors, and there were no additional off‐target activities in a large panel of> 100 off‐target binding assays 5. Comparison of the efficacy and safety of vibegron observed in the present study vs those reported in the EIGHT trial of the anticholinergic agent fesoterodine 9 shows that vibegron did not lead to more treatment‐emergent adverse events and reduced the number of UUI episodes/day to a similar extent to that noted with fesoterodine treatment. The severity of UUI in the patients included in the two trials was equivalent. After 12‐week treatment with another β3‐adrenoreceptor agonist, mirabegron, in Japanese patients with OAB, change from baseline in number of UUI episodes/day was reported to be −1.01 10, while it was −1.35 with vibegron 50 mg and −1.47 with vibegron 100 mg. The numbers of UUI episodes/day at baseline were 1.78 for mirabegron‐treated patients and 1.9–2.0 for vibegron patients. In comparison with mirabegron, vibegron may have identical or better efficacy. Head‐to‐head studies of the two agents are warranted, however, to obtain conclusive results.
It has been reported that among women with UUI, the most frequent reason for seeing a doctor because of UUI was its adverse impact on activities of daily living 11. Furthermore, the severity rather than the presence of UUI affected their mental and physical health and was associated with increased rates of depression, anxiety and perceived stress 12. Another survey indicated that OAB symptoms reduce work productivity at levels comparable to other serious chronic conditions, including rheumatoid arthritis and asthma. The survey also showed that individuals with OAB are 1.5 times more likely to be unemployed compared to those without OAB 13. It is suggested that improving the severity of UUI is important to allow affected individuals to feel that they are in good mental and physical health, and to help them to maintain their social activities at satisfactory levels 14.
The present study demonstrated that, 12 weeks after the start of vibegron treatment, more than half of the patients achieved the diary‐dry rate, the number of UUI episodes/day in the severe UUI patients improved to that of patients with mild/moderate UUI (<3 UUI episodes/day), and 52.2–63.3% of the vibegron‐treated patients reported ‘feeling much better’ or ‘feeling better’ on the patient‐reported outcome questionnaire, the PGI. Improvement of OAB symptoms on treatment with vibegron would be expected to contribute to maintaining a perception of good mental and physical health, and to provide a positive impact on daily life and work; however, the impact of vibegron on mental/physical health was not the endpoint of the present study. Further study is required to obtain conclusive findings in this area.
In the evaluation of factors predicting UUI normalization, multivariate logistic regression analysis identified vibegron administration, shorter OAB duration, fewer micturitions/day, and fewer UUI episodes/day as factors significantly associated with normalization of UUI. Herschorn et al. 15 reported that younger age, lack of previous antimuscarinic treatment, shorter duration since OAB diagnosis, and female sex were predictors of larger changes in outcomes from baseline to week 12 in patients with OAB treated with an antimuscarinic agent, supporting that shorter OAB duration may be a predictive factor for improved outcome. By contrast, in the present analysis, history of antimuscarinic treatment was not found to be a significant factor. These differences can be explained by the difference in the adopted endpoints in the two studies. Because the endpoint in the present study was normalization of UUI, patients with milder UUI at baseline may have achieved a higher normalization rate. In the study by Herschorn et al. 15, the endpoint was changes in outcomes from baseline, therefore, greater differences after treatment may be achieved in patients with more severe UUI at baseline.
This study was performed as a post hoc analysis of the vibegron phase 3 study. A further prospective, randomized study in a larger sample is warranted to obtain data that conclusively demonstrates the efficacy and safety of vibegron for treatment of severe UUI as well as factors associated with normalization of UUI.
In conclusion, vibegron, a novel β3‐adrenoreceptor agonist, significantly reduced the number of UUI episodes and significantly increased the volume voided/micturition in patients with OAB, including those with severe UUI. Furthermore, the response rate exceeded 50%. These results suggest that vibegron could be an effective therapeutic option for OAB patients with UUI.
Conflict of Interest
Masaki Yoshida has received consultancy fees from Kyorin, grants from Astellas, and speaker fees from Kyorin, Kissei, Astellas, Daiichi‐Sankyo, Ono and Pfizer. Masayuki Takeda has received consultancy fees from Kyorin, grants from Astellas, Asahi‐Kasei Pharma, GSK, Nippon Shinyaku, Takeda and Pfizer, and speaker fees from Kyorin, Kissei, Astellas, Daiichi‐Sankyo, Nippon Shinyaku, Ono and Pfizer. Momokazu Gotoh has received consultancy fees from Kyorin, Medtronics Japan and Taiho, grants from Kyorin, Kissei, Asahi‐Kasei Pharma, Astellas, Chugai, Daiichi‐Sankyo, Nippon Shinyaku, Novartis, Ono, Pfizer, Sanofi‐Aventis, Taiho and Takeda, and speaker fees from Kyorin, Kissei, Asahi‐Kasei Pharma, Astellas, AstraZeneca, Daiichi‐Sankyo, Hisamitsu, Nippon Shinyaku, Ono, Pfizer, Sanofi‐aventis and Takeda. Osamu Yokoyama has received consultancy fees from Kyorin, Astellas, GSK, Pfizer and Taiho, grants from Kissei, Astellas, Nippon Shinyaku, Pfizer and Taiho, and speaker fees from Kissei, Astellas, Nippon Shinyaku and Pfizer. Hidehiro Kakizaki has received consultancy fees from Kyorin, Astellas and Taiho, grants from Kissei, Astellas, Daiichi‐Sankyo, Nippon Shinyaku, Taiho and Takeda, and speaker fees from Kyorin, Kissei, Astellas, Nippon Shinyaku and Pfizer. Satoru Takahashi has received consultancy fees from Kyorin, grants from Astellas, Nippon Shinyaku, and speaker fees from Kyorin, Kissei, Astellas, Nippon Shinyaku and Pfizer. Naoya Masumori has received research grants from MSD, Takeda, Bayer and Ono, and received lecture fees from Kissei, Janssen, Bayer and MSD. Shinji Nagai and Kazuyoshi Minemura are employees of Kyorin.
Acknowledgements
This research was funded by Kyorin Pharmaceutical Co., Ltd and Kissei Pharmaceutical Co., Ltd. Statistical analyses were performed by Kyorin Pharmaceutical Co., Ltd. Medical writing was assisted by Will Medical Communications Co., Ltd, for preparation of the initial and final drafts of the manuscript, which was funded by Kyorin Pharmaceutical Co., Ltd and Kissei Pharmaceutical Co., Ltd.
References
- 1. Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int 2011; 108: 1132–8 [DOI] [PubMed] [Google Scholar]
- 2. Burkhard FC, Bosch JLHR, Cruz F et al. EAU guidelines on urinary incontinence in adults. Available at: https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Urinary-Incontinence-2018-large-text.pdf. Accessed December 2019.
- 3. Liu AB, Liu Q, Yang CC et al. Patient characteristics associated with more bother from lower urinary tract symptoms. J Urol 2019; 202: 585–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riemsma R, Hagen S, Kirschner‐Hermanns R et al. Can incontinence be cured? A systematic review of cure rates. BMC Med 2017; 15: 63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edmondson SD, Zhu C, Kar NF et al. Discovery of vibegron: a potent and selective β3 adrenergic receptor agonist for the treatment of overactive bladder. J Med Chem 2016; 59: 609–23 [DOI] [PubMed] [Google Scholar]
- 6. Yoshida M, Takeda M, Gotoh M, Nagai S, Kurose T. Vibegron, a novel potent and selective β3‐adrenoreceptor agonist, for the treatment of patients with overactive bladder: a randomized, double‐blind, placebo‐controlled phase 3 study. Eur Urol 2018; 73: 783–90 [DOI] [PubMed] [Google Scholar]
- 7. Sand PK, MacDiarmid SA, Thomas H, Caramelli KE, Hoel G. Effect of baseline symptom severity on continence improvement mediated by oxybutynin chloride topical gel. J Urol 2011; 3: 145–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang KY, Zeger SL. Longitudinal data analysis of continuous and discrete response for pre‐post designs. Sankhya: Indian J Stat Ser B 2000; 62: 134–48 [Google Scholar]
- 9. Chapple C, Schneider T, Haab F et al. Superiority of fesoterodine 8 mg vs 4 mg in reducing urgency urinary incontinence episodes in patients with overactive bladder: results of the randomised, double‐blind, placebo‐controlled EIGHT trial. BJU Int 2014; 114: 418–26 [DOI] [PubMed] [Google Scholar]
- 10. Yamaguchi O, Marui E, Kakizaki H et al. Phase III, randomized, double‐blind, placebo‐controlled study of the β3‐adrenoceptor agonist mirabegron, 50 mg once daily, in Japanese patients with overactive bladder. BJU Int 2014; 113: 951–60 [DOI] [PubMed] [Google Scholar]
- 11. Biyik I, Kucuk B, Arpaci HF, Demirci H. Factors affecting doctor visits of postmenopausal women with urinary incontinence. Low Urin Tract Symptoms 2019; 11: 200–5 [DOI] [PubMed] [Google Scholar]
- 12. Siddiqui NY, Wiseman JB, Cella D et al. Mental health, sleep and physical function in treatment seeking women with urinary incontinence. J Urol 2018; 200: 848–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coyne KS, Sexton CC, Thompson CL et al. Impact of overactive bladder on work productivity. Urology 2012; 80: 97–103 [DOI] [PubMed] [Google Scholar]
- 14. Rogers RG, Bachman G, Scarpero H et al. Effects of tolterodine ER on patient‐reported outcomes in sexually active women with overactive bladder and urgency urinary incontinence. Curr Med Res Opin 2009; 25: 2159–65 [DOI] [PubMed] [Google Scholar]
- 15. Herschorn S, Kaplan SA, Sun F, Ntanios F. Do patient characteristics predict responsiveness to treatment of overactive bladder with antimuscarinic agents? Urology 2014; 83: 1023–9 [DOI] [PubMed] [Google Scholar]


