Abstract
Aim
To assess the effects of dapagliflozin plus saxagliptin plus metformin versus glimepiride plus metformin on liver fat (proton density fat fraction) and visceral and subcutaneous adipose tissue volumes over 52 weeks of treatment.
Materials and methods
This was a magnetic resonance imaging substudy of a 52‐week, multicentre, randomized, double‐blind, parallel‐group trial that evaluated the efficacy and safety of dapagliflozin 10 mg/day plus saxagliptin 5 mg/day versus titrated glimepiride 1–6 mg (1, 2, 3, 4 or 6 mg) in 82 patients with type 2 diabetes (HbA1c 7.5%–10.5%) on metformin ≥1500 mg/day background. Analyses were exploratory and not controlled for multiplicity; P‐values are nominal.
Results
Magnetic resonance imaging was performed on 59 patients; liver fat and adipose tissue volumes were analysed for 59 and 57 patients, respectively. There was a significant >30% reduction from baseline in liver fat (P = 0.007) and >10% reduction in adipose tissue volumes (P < 0.01) with dapagliflozin plus saxagliptin plus metformin at week 52 versus glimepiride plus metformin. In the full‐study population, dapagliflozin plus saxagliptin plus metformin decreased body weight and serum alanine aminotransferase and aspartate aminotransferase levels over 52 weeks.
Conclusions
Dapagliflozin plus saxagliptin significantly decreased liver fat and adipose tissue volume versus glimepiride, and reduced serum liver enzyme levels, indicating a favourable metabolic profile of dapagliflozin plus saxagliptin in patients with type 2 diabetes on metformin therapy.
Keywords: dapagliflozin, ectopic fat, fixed‐dose combination, glimepiride, liver fat, magnetic resonance imaging‐estimated proton density fat fraction, metformin, nonalcoholic fatty liver disease, saxagliptin, sodium‐glucose co‐transporter‐2 inhibitors
1. INTRODUCTION
In patients with type 2 diabetes (T2D), weight loss is associated with improvement in glycaemic control and reduction in cardiovascular (CV) risk factors.1 T2D is a risk factor for nonalcoholic fatty liver disease (NAFLD), which can progress to nonalcoholic steatohepatitis (NASH) and ultimately cirrhosis and/or liver cancer.2 NAFLD has been defined by ≥5% macrovesicular hepatocyte steatosis in the absence of significant alcohol use, and is the most common cause of liver enzyme increases.3 Almost three quarters of patients with T2D have NAFLD.4, 5 Body weight reduction is one of the few measures that may prevent liver dysfunction.6, 7 Given the well‐known contribution of some commonly used T2D drugs, such as sulfonylureas, to weight gain,8 alternative drug therapies are desirable in patients with T2D with or at risk of concomitant liver disease.
Ectopic fat accumulation, including that of the liver, is related to increased insulin resistance.9 Weight loss results in a reduction in liver fat content.7 Reducing liver fat content in addition to weight loss may therefore help prevent liver disease progression in patients with T2D. Dapagliflozin is a sodium‐glucose co‐transporter‐2 (SGLT2) inhibitor that reduces hyperglycaemia independent of insulin and also results in weight loss and moderate blood pressure reduction in patients with T2D.10, 11 Most of the weight loss associated with dapagliflozin has been shown to be because of reductions in total body fat mass, abdominal visceral adipose tissue (VAT) volume and subcutaneous adipose tissue (SAT) volume.12
Saxagliptin, a selective dipeptidyl peptidase‐4 (DPP‐4) inhibitor, also reduces HbA1c regardless of T2D stage, although with no clinically significant effects on body weight.13 The fixed‐dose combination of dapagliflozin and saxagliptin was approved by the United States Food and Drug Administration on February 28, 2017, for improving glycaemic control in adults with T2D as an adjunct to diet and exercise for those who have inadequate control with dapagliflozin or who are already being treated with dapagliflozin and saxagliptin.14 Evidence from phase 3 studies of dapagliflozin plus saxagliptin indicates that weight loss observed with concomitant administration of dapagliflozin and saxagliptin is similar to that of dapagliflozin alone.15, 16 A global phase 3b study (NCT02419612) found better efficacy (as shown by a significantly decreased HbA1c) for dapagliflozin plus saxagliptin versus the sulfonylurea glimepiride, both groups on metformin background, in patients with T2D who had inadequate glycaemic control on metformin alone.17 Safety profiles were similar between the two groups, although the incidence of hypoglycaemia was lower in the dapagliflozin plus saxagliptin group.17 Here, we report a substudy of this trial that used magnetic resonance imaging (MRI) to determine liver fat deposition via assessment of proton density fat fraction (PDFF), VAT volume and SAT volume over 52 weeks of treatment.
2. MATERIALS AND METHODS
2.1. Study design and patients
This was a 52‐week, international, multicentre, randomized, double‐blind, active‐controlled, parallel‐group, phase 3b study, with a patient‐ and site‐blinded, 104‐week, long‐term extension period (Figure 1). At week 52, the least‐squares mean change (95% confidence interval [CI]) in HbA1c was −1.35% (−1.49, −1.22) for the dapagliflozin plus saxagliptin group, and −0.98% (−1.12, −0.84) for the glimepiride group, with similar rates of adverse events between groups.17
Figure 1.
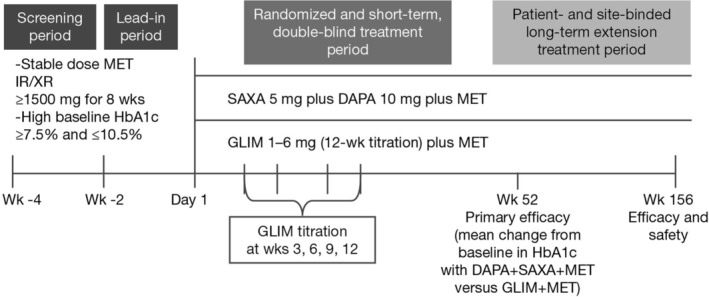
Study design (NCT02419612). DAPA, dapagliflozin; GLIM, glimepiride; IR, instant release; MET, metformin; SAXA, saxagliptin; XR, extended release
The study was conducted at 87 centres in 10 countries beginning on August 14, 2015 and comprised a 2‐week screening period and a 2‐week lead‐in period. The study enrolled male or female patients (aged ≥18 years) with T2D, with HbA1c from ≥7.5% (58.5 mmol/mol) to ≤10.5% (91.3 mmol/mol), with a body mass index (BMI) of 20.0–45.0 kg/m2 (inclusive) at enrolment, and on the same daily dose of metformin ≥1500 mg for at least 8 weeks before enrolment. Key exclusion criteria comprised clinical diagnosis of type 1 diabetes, malignancy within 5 years of the screening visit (with the exception of treated basal cell or treated squamous cell carcinoma), pregnancy, history of diabetic ketoacidosis and history of CV/vascular disease within 3 months of enrolment, including transient ischaemic attack or significant cerebrovascular disease, and unstable or previously undiagnosed arrhythmia. Patients with impaired renal function (creatinine clearance <60 mL/min/1.73m2 [estimated by Cockcroft–Gault] or serum creatinine ≥1.5 mg/dL [132.6 μmol/L] in males or ≥1.4 mg/dL [123.8 μmol/L] in females), severe hepatic insufficiency and/or significant abnormal liver function (aspartate aminotransferase [AST] >3 × upper limit of normal [ULN] and/or alanine aminotransferase [ALT] >3 × ULN), positive serologic evidence of current infectious liver disease, including patients who were positive for hepatitis B viral antibody immunoglobulin M, hepatitis B surface antigen and hepatitis C antibody, and/or history of current, acute or chronic pancreatitis were also excluded. Further details are in the primary study article.17
2.2. Interventions
Patients on metformin were randomly assigned, based on a randomization schedule stratified by site and using an interactive voice response system, to the following double‐blinded treatment groups in a 1:1 ratio: dapagliflozin 10 mg plus saxagliptin 5 mg plus placebo, each administered orally once daily (QD), or glimepiride 1–6 mg (specifically 1, 2, 3, 4 or 6 mg) plus placebo, each administered orally QD. A fasting plasma glucose level of ≤270 mg/dL (15.0 mmol/L) was required at randomization. The starting dose for glimepiride was 1 mg QD, and was titrated stepwise using doses of 2, 3, 4 and 6 mg QD at 3‐week intervals to a maximum of 6 mg QD. In patients for whom titration was not medically indicated at week 3, reassessment for titration occurred at weeks 6, 9 and 12. Glimepiride could be downtitrated in the event of hypoglycaemia.
2.3. MRI substudy
The MRI substudy included patients from the full study who provided informed consent, had no contraindications to MRI scanning, and had a BMI of 20.0–40.0 kg/m2 (inclusive) at enrolment and a maximum weight of 140 kg to accommodate MRI scanning. No other selection criteria were applied for participation in the substudy. The MRI substudy was conducted at nine MRI centres in Hungary, the Czech Republic, Poland, Russia and Sweden using GE HealthCare, Siemens or Philips 1.5 or 3 T MRI scanners. MRI centres were selected based on proximity to study sites willing to participate in the MRI substudy and ability to perform PDFF measurements. To qualify, at each site a healthy volunteer was scanned and PDFF values in adipose tissue and muscle were checked to test that the PDFF measurements gave reasonable results. No phantom was used because MRI‐PDFF has been shown to have excellent linearity, bias and precision across different field strengths, imager manufacturers and reconstruction methods.18, 19, 20 The MRI substudy endpoint was 52 weeks. SAT and VAT volumes were assessed using abdominal MRIs of patients. Liver fat content was assessed using MRI‐PDFF. This well‐validated, noninvasive imaging technique exploits the difference in MRI chemical shift of triglyceride‐based mobile protons from that of the water to enable accurate quantitative liver fat assessment over the entire liver.21 In adults with NAFLD, MRI‐PDFF correlates highly with steatosis grade22 and is a useful method in NASH clinical trials.21 MRI is a comprehensive tool to quantify fat, able to differentiate between VAT and SAT with multidimensional images and to quantify ectopic fat safely and with minimal examination time.23
2.4. Assessments
The mean change from baseline in liver fat percentage by MRI‐PDFF and VAT and SAT volumes was assessed by MRI in both treatment groups at week 52 in the subpopulation of patients in the MRI substudy.
2.5. Statistical analyses
Efficacy analyses were performed using the randomized patient dataset (all randomized patients who received at least one dose of study medication) during the double‐blind treatment period. Analysis for change in serum liver enzyme levels was conducted on the treated patient dataset (all patients who received at least one dose of study medication during the study treatment period) during the short‐term double‐blind treatment period. Analyses of change from baseline in liver fat percentage and VAT and SAT volumes in the MRI substudy were performed using the analysis of covariance (ANCOVA) model based on last observation carried forward (LOCF) data. MRI analyses were not controlled for multiplicity and nominal P‐values are presented. Analysis of mean change from baseline in total body weight was performed using a longitudinal repeated measures model. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
3. RESULTS
3.1. Patient disposition
Overall, 823 patients were enrolled. Of these, 444 entered the double‐blind treatment period; 227 were randomized to dapagliflozin plus saxagliptin plus metformin and 217 were randomized to glimepiride plus metformin (Figure S1). A total of 82 patients provided informed consent to participate in the MRI substudy, of whom 46 were in the dapagliflozin plus saxagliptin plus metformin treatment group and 36 were in the glimepiride plus metformin treatment group.
3.2. Baseline demographics
Key baseline characteristics of the MRI substudy patients were well balanced between the groups (Table 1).
Table 1.
Demographics and baseline disease characteristics (randomized patient dataset) for the MRI substudy
| Characteristic | DAPA+SAXA+MET (n = 46) | GLIM+MET (n = 36) | Total (N = 82) |
|---|---|---|---|
| Age, years | 58.9 (8.2) | 56.9 (10.0) | 58.0 (9.0) |
| Female, n (%) | 22 (47.8) | 19 (52.8) | 41 (50.0) |
| Weight, kg | 94.4 (16.5) | 90.9 (17.3) | 92.9 (16.8) |
| BMI, kg/m2 | 32.9 (4.7) | 32.3 (5.1) | 32.6 (4.9) |
| BMI group, kg/m2, n (%) | |||
| <25 | 1 (2.2) | 3 (8.3) | 4 (4.9) |
| ≥25– <27 | 4 (8.7) | 1 (2.8) | 5 (6.1) |
| ≥27– <30 | 11 (23.9) | 8 (22.2) | 19 (23.2) |
| ≥30 | 30 (65.2) | 24 (66.7) | 54 (65.9) |
| Race, n (%) | |||
| White | 46 (100.0) | 36 (100.0) | 82 (100.0) |
| Region, n (%) | |||
| Europe | 46 (100.0) | 36 (100.0) | 82 (100.0) |
| Duration of T2D, years | 6.4 (5.8) | 6.5 (6.4) | 6.4 (6.0) |
| HbA1c, % | 8.4 (0.8) | 8.6 (0.9) | 8.5 (0.9) |
| FPG, mg/dL | 10.2 (3.1) | 10.5 (2.6) | 10.4 (2.8) |
Abbreviations: BMI, body mass index; DAPA, dapagliflozin; FPG, fasting plasma glucose; GLIM, glimepiride; MET, metformin; MRI, magnetic resonance imaging; SAXA, saxagliptin; SD, standard deviation; T2D, type 2 diabetes.
All data are reported as mean (SD) unless specified otherwise. Includes all patients who consented to be in the MRI substudy.
3.3. MRI substudy
MRI was performed on 59 patients and liver fat and adipose tissue volume were analysed for 59 and 57 patients, respectively. There was a significant >30% reduction from baseline in liver fat (P = 0.007) and a >10% reduction in VAT volume (P < 0.001) and SAT volume (P = 0.006) with dapagliflozin plus saxagliptin plus metformin at week 52 versus glimepiride plus metformin (Table 2 and Figure 2).
Table 2.
Liver fat and adipose tissue volumes over the 52‐week, double‐blind treatment period for the magnetic resonance imaging (MRI) substudy
| Variable | DAPA+SAXA+MET | GLIM+MET | P ‐value† |
|---|---|---|---|
| N | 35 | 24 | |
| Liver fat, PDFF% | |||
| Baseline | 14.3 (6.4) | 13.7 (8.3) | |
| Week 52 | 9.9 (7.1) | 12.9 (8.6) | 0.007 |
| N | 34 | 23 | |
| VAT, L | |||
| Baseline | 3.6 (1.1) | 2.9 (1.1) | |
| Week 52 | 3.2 (1.1) | 3.0 (1.1) | < 0.001 |
| SAT, L | |||
| Baseline | 4.7 (2.2) | 4.1 (1.8) | |
| Week 52 | 4.2 (2.0) | 4.1 (1.7) | 0.006 |
Abbreviations: ANCOVA, analysis of covariance; DAPA, dapagliflozin; GLIM, glimepiride; LOCF, last observation carried forward; LS, least squares; MET, metformin; PDFF, proton density fat fraction; SAT, subcutaneous adipose tissue; SAXA, saxagliptin; SD, standard deviation; VAT, visceral adipose tissue.
All data are reported as mean (SD) unless specified otherwise.
Nominal P‐value for LS mean difference in change from baseline to week 52 between groups. Analysis of mean change from baseline in total body weight was performed using a longitudinal repeated measures model. Analyses of change from baseline in liver fat percentage and VAT and SAT volumes in the MRI substudy were performed using the ANCOVA model based on LOCF data.
Figure 2.
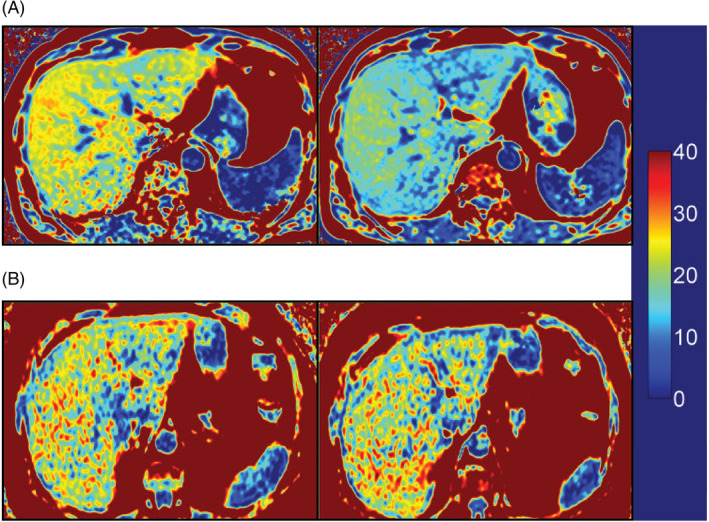
Representation of liver magnetic resonance imaging‐estimated proton density fat fraction (MRI‐PDFF) pre‐ and post‐treatment. (A) Pre‐ and post‐treatment MRI‐PDFF for dapagliflozin plus saxagliptin plus metformin and (B) pre‐ and post‐treatment MRI‐PDFF for glimepiride plus metformin
3.4. Full study
For patients in the dapagliflozin plus saxagliptin plus metformin treatment group, body weight (mean ± standard deviation [SD]) reduced from 90.8 ± 19.7 kg at baseline to 88.4 ± 18.1 kg at week 52. Patients in the glimepiride plus metformin treatment group experienced weight gain (mean ± SD) from 88.4 ± 17.0 kg at baseline to 90.6 ± 17.4 kg at week 52. The decrease in mean total body weight from baseline to week 52 in the dapagliflozin plus saxagliptin plus metformin treatment group was significantly different from that in the glimepiride plus metformin treatment group (difference [95% CI], −4.1 kg [−4.8, −3.3]; P < 0.001). The difference in body weight between treatment groups was evident at week 3 (difference [95% CI], −1.1 kg [−1.4, −0.9]; P < 0.001; Figure 3).
Figure 3.
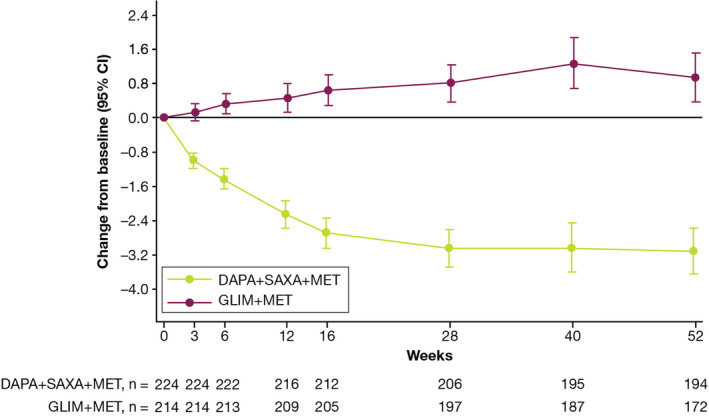
Adjusted mean change from baseline in total body weight during the 52‐week, double‐blind treatment period†.
Body weight assessments collected after initiation of rescue treatment or collected more than 8 days after the last dose in the short‐term, double‐blind treatment period were excluded from the analysis.
†Patients from the randomized patient dataset with nonmissing baseline assessment at week 52 were included in this analysis. Baseline is defined as patients in the randomized patient dataset with nonmissing baseline assessment and at least one postbaseline assessment. DAPA, dapagliflozin; GLIM, glimepiride; MET, metformin; SAXA, saxagliptin
At week 52, there was a decrease from baseline in the serum levels of both ALT and AST in the dapagliflozin plus saxagliptin plus metformin treatment group. Although ALT and AST levels decreased they remained within the normal range. By contrast, enzyme levels increased in the glimepiride plus metformin treatment group (Figure S2).
4. DISCUSSION
In this 52‐week MRI substudy of a phase 3b, randomized, active‐controlled study, the SGLT2 inhibitor dapagliflozin, co‐administered with the DPP‐4 inhibitor saxagliptin, significantly decreased liver fat content, adipose tissue volume and body weight versus the sulfonylurea glimepiride, with both groups on metformin background. Serum liver enzyme levels in the dapagliflozin group were lower than in the saxagliptin group, and although still in the normal range, did show a decreasing trend. Elevated ectopic fat is a significant contributor to the pathogenesis of T2D.24 Because liver fat correlates with increased mortality from liver disease,25 the significant reduction in liver fat content observed in this substudy indicates that the combination of dapagliflozin plus saxagliptin added to metformin could help prevent the progression of liver disease in patients with T2D.
Although the mechanisms by which liver fat reduction occurs with SGLT2 inhibition are not fully established, there are several possibilities. These include reduced hepatic lipogenesis, increased hepatic insulin extraction as seen with dapagliflozin,26 improvements in insulin concentrations and/or resistance, and/or, as seen in some animal models, suppression of inflammatory cytokines and/or mitigation of oxidative stress.27, 28, 29 Additionally, dapagliflozin's possible role as an alpha cell secretagogue that has been shown to promote glucagon secretion30 is consistent with its ability to reduce intrahepatic lipid content.
There may also be several mechanisms by which reductions in liver fat content could lead to therapeutic benefit in patients with T2D. The strong association between VAT and liver fat content with hepatic insulin resistance in patients with T2D31 suggests one possibility. The liver fat reduction results we found are consistent with those of other studies of dapagliflozin. In a 12‐week, randomized controlled study, dapagliflozin significantly reduced liver fat in patients with T2D and NAFLD.32 In a 6‐month Japanese study, dapagliflozin significantly reduced liver size and hepatic fat accumulation in patients with inadequately controlled T2D. However, the study's open‐label and nonrandomized design precludes firm conclusions.26 Liver enzymes were decreased with empagliflozin treatment in the EMPA‐REG OUTCOME trial.33
Although the direct contribution of each drug in the dapagliflozin plus saxagliptin combination to liver improvement could not be determined, a previous study with a DPP‐4 inhibitor did not find a reduction in hepatic fibrosis or steatosis in patients with T2D,34 suggesting that the beneficial liver effects observed in the current study were a result of dapagliflozin. However, significant reductions in body weight, VAT volume and liver fat content assessed by MRI‐PDFF were found with 26‐week DPP‐4 inhibitor treatment in patients with T2D and NAFLD.35 Baseline DPP‐4 activity has been reported to be higher in insulin‐resistant patients with NAFLD than in healthy controls,36 and a small uncontrolled study reported a significant decrease in hepatic lipid content after 6 months of vildagliptin or sitagliptin treatment, although only in females.37
Elevated ALT levels sometimes indicate the presence of NAFLD, and in NAFLD cases with normal liver enzyme levels, assessment of liver fibrosis markers is recommended.2, 38 In the current study, ALT levels decreased in the dapagliflozin plus saxagliptin versus glimepiride treatment group. SGLT2 inhibitors have been shown to result in significantly greater reductions in ALT levels than incretin‐based drugs in patients with T2D,39 lending support to the notion that the ALT decreases observed in the current study were primarily attributable to dapagliflozin.
The weight loss observed with dapagliflozin plus saxagliptin was consistent with that observed in previous studies.16 The difference in weight loss between the treatment groups was evident by week 3, suggesting that the trend towards weight maintenance or decrease among patients receiving dapagliflozin plus saxagliptin versus those receiving glimepiride is rapid and sustainable. However, weight loss alone may not be sufficient to entail a reduction in liver fat content. This is supported by the fact that glucagon‐like peptide‐1 receptor agonism40 and thiazolidinedione treatment41, 42, 43 in patients with T2D have been shown to result in liver fat reductions independent of effects on body weight. In the current study, at least some of the reduction in liver fat content with dapagliflozin plus saxagliptin compared with glimepiride could have been because of a reduction in body weight; however, because glimepiride resulted in weight gain, it is not possible to compare the differential effects of the two treatment groups with respect to liver fat reduction. Although the reduction in liver fat in the current study was accompanied by overall weight loss, there is some evidence to suggest that reductions in liver fat with SGLT2 inhibition may be independent of SGLT2 inhibitors’ effects on weight loss.44 We did not, however, investigate if this was true for dapagliflozin.
In conclusion, dapagliflozin co‐administered with saxagliptin significantly decreased liver fat and adipose tissue volume versus glimepiride. The decrease in liver fat and adipose tissue volume was accompanied by a significant reduction in body weight and a reduction in the levels of serum liver enzymes for patients in the full study in the dapagliflozin plus saxagliptin group. These results indicate a favourable metabolic profile of dapagliflozin plus saxagliptin versus glimepiride in patients with T2D.
CONFLICT OF INTEREST
ND, JM and EJ are employees of AstraZeneca. RG‐S was an employee of AstraZeneca at the time the work was conducted. PDH and LJ are employees of Antaros Medical AB, which received payment from AstraZeneca for conducting the study. LJ is a shareholder in Antaros Medical AB. JPHW, outside the submitted work, has grants, personal fees and consultancy fees (paid to his institution) from AstraZeneca, Novo Nordisk and Takeda; personal fees and consultancy fees (paid to his institution) from Boehringer Ingelheim, Janssen, Lilly, Mundipharma, Napp and Sanofi; and consultancy fees (paid to his institution) from Wilmington Healthcare.
AUTHOR CONTRIBUTIONS
JPHW contributed to data interpretation. PDH contributed to study conduct, data collection and data analysis. EJ and LJ contributed to study design, study conduct, data collection and data analysis. All authors contributed to critical review of the manuscript.
ETHICAL APPROVAL
All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was conducted in accordance with the International Council for Harmonisation Good Clinical Practice Guidelines, and followed applicable regulatory requirements, including AstraZeneca's policy on bioethics. The local Institutional Review Boards or Independent Ethics Committees approved the final protocol and amendment. All patients provided written informed consent. Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data‐sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
FUNDING INFORMATION
This study was funded by AstraZeneca.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
Medical writing assistance, funded by AstraZeneca, was provided by Steven Tresker of Cactus Life Sciences (part of Cactus Communications).
Johansson L, Hockings PD, Johnsson E, et al. Dapagliflozin plus saxagliptin add‐on to metformin reduces liver fat and adipose tissue volume in patients with type 2 diabetes. Diabetes Obes Metab. 2020;22:1094–1101. 10.1111/dom.14004
Funding information AstraZeneca
REFERENCES
- 1. Horton ES, Silberman C, Davis KL, Berria R. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. 2010;33:1759‐1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- 3. Ahmed A, Wong RJ, Harrison SA. Nonalcoholic fatty liver disease review: diagnosis, treatment, and outcomes. Clin Gastroenterol Hepatol. 2015;13:2062‐2070. [DOI] [PubMed] [Google Scholar]
- 4. Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non‐alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359‐1368. [DOI] [PubMed] [Google Scholar]
- 5. Leite NC, Salles GF, Araujo AL, Villela‐Nogueira CA, Cardoso CR. Prevalence and associated factors of non‐alcoholic fatty liver disease in patients with type‐2 diabetes mellitus. Liver Int. 2009;29:113‐119. [DOI] [PubMed] [Google Scholar]
- 6. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005‐2023. [DOI] [PubMed] [Google Scholar]
- 7. Ratziu V, Bellentani S, Cortez‐Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372‐384. [DOI] [PubMed] [Google Scholar]
- 8. Thule PM, Umpierrez G. Sulfonylureas: a new look at old therapy. Curr Diab Rep. 2014;14:473. [DOI] [PubMed] [Google Scholar]
- 9. Trouwborst I, Bowser SM, Goossens GH, Blaak EE. Ectopic fat accumulation in distinct insulin resistant phenotypes; targets for personalized nutritional interventions. Front Nutr. 2018;5:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med. 2015;66:255‐270. [DOI] [PubMed] [Google Scholar]
- 11. Yu H, Woo VC. Emerging use of combination therapies for the management of type 2 diabetes ‐ focus on saxagliptin and dapagliflozin. Diabetes Metab Syndr Obes. 2017;10:317‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bolinder J, Ljunggren Ö, Johansson L, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014;16:159‐169. [DOI] [PubMed] [Google Scholar]
- 13. Jain R. Utility of saxagliptin in the treatment of type 2 diabetes: review of efficacy and safety. Adv Ther. 2015;32:1065‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.AstraZeneca. https://www.astrazeneca.com/media-centre/press-releases/2017/fda-approves-once-daily-qtern-dapagliflozin-and-saxagliptin-tablets-for-adults-with-type-2-diabetes-240217.html. Accessed October 23, 2018.
- 15. Matthaei S, Catrinoiu D, Celinski A, et al. Randomized, double‐blind trial of triple therapy with saxagliptin add‐on to dapagliflozin plus metformin in patients with type 2 diabetes. Diabetes Care. 2015;38:2018‐2024. [DOI] [PubMed] [Google Scholar]
- 16. Rosenstock J, Hansen L, Zee P, et al. Dual add‐on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double‐blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38:376‐383. [DOI] [PubMed] [Google Scholar]
- 17. Frias JP, Gonzalez‐Galvez G, Johnsson E, et al. Efficacy and safety of dual add‐on therapy with dapagliflozin plus saxagliptin versus glimepiride in patients with poorly controlled type 2 diabetes on a stable dose of metformin: results from a 52‐week, randomized, active‐controlled trial. Diabetes Obes Metab. 2020. 10.1111/dom.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayashi T, Fukuzawa K, Yamazaki H, et al. Multicenter, multivendor phantom study to validate proton density fat fraction and T2* values calculated using vendor‐provided 6‐point DIXON methods. Clin Imaging. 2018;51:38‐42. [DOI] [PubMed] [Google Scholar]
- 19. Hernando D, Sharma SD, Aliyari Ghasabeh M, et al. Multisite, multivendor validation of the accuracy and reproducibility of proton‐density fat‐fraction quantification at 1.5T and 3T using a fat‐water phantom. Magn Reson Med. 2017;77:1516‐1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yokoo T, Serai SD, Pirasteh A, et al. Linearity, bias, and precision of hepatic proton density fat fraction measurements by using MR imaging: a meta‐analysis. Radiology. 2018;286:486‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, quantitative assessment of liver fat by MRI‐PDFF as an endpoint in NASH trials. Hepatology. 2018;68:763‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Permutt Z, Le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non‐alcoholic fatty liver disease ‐ MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu HH, Nayak KS, Goran MI. Assessment of abdominal adipose tissue and organ fat content by magnetic resonance imaging. Obes Rev. 2011;12:e504‐e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sattar N, Gill JM. Type 2 diabetes as a disease of ectopic fat? BMC Med. 2014;12:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Unalp‐Arida A, Ruhl CE. Liver fat scores predict liver disease mortality in the United States population. Aliment Pharmacol Ther. 2018;48:1003‐1016. [DOI] [PubMed] [Google Scholar]
- 26. Kurinami N, Sugiyama S, Yoshida A, et al. Dapagliflozin significantly reduced liver fat accumulation associated with a decrease in abdominal subcutaneous fat in patients with inadequately controlled type 2 diabetes mellitus. Diabetes Res Clin Pract. 2018;142:254‐263. [DOI] [PubMed] [Google Scholar]
- 27. Jojima T, Tomotsune T, Iijima T, Akimoto K, Suzuki K, Aso Y. Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP‐4 inhibitor), prevents steatohepatitis in a novel mouse model of non‐alcoholic steatohepatitis and diabetes. Diabetol Metab Syndr. 2016;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Komiya C, Tsuchiya K, Shiba K, et al. Ipragliflozin improves hepatic steatosis in obese mice and liver dysfunction in type 2 diabetic patients irrespective of body weight reduction. PLoS One. 2016;11:e0151511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakano S, Katsuno K, Isaji M, et al. Remogliflozin etabonate improves fatty liver disease in diet‐induced obese male mice. J Clin Exp Hepatol. 2015;5:190‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bonner C, Kerr‐Conte J, Gmyr V, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21:512‐517. [DOI] [PubMed] [Google Scholar]
- 31. Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496‐506. [DOI] [PubMed] [Google Scholar]
- 32. Eriksson JW, Lundkvist P, Jansson PA, et al. Effects of dapagliflozin and n‐3 carboxylic acids on non‐alcoholic fatty liver disease in people with type 2 diabetes: a double‐blind randomised placebo‐controlled study. Diabetologia. 2018;61:1923‐1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sattar N, Fitchett D, Hantel S, George JT, Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA‐REG OUTCOME® trial. Diabetologia. 2018;61:2155‐2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smits MM, Tonneijck L, Muskiet MH, et al. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo‐controlled trial. Diabetologia. 2016;59:2588‐2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yan J, Yao B, Kuang H, et al. Liraglutide, sitagliptin and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and NAFLD. Hepatology. 2019;69:2414‐2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Firneisz G, Varga T, Lengyel G, et al. Serum dipeptidyl peptidase‐4 activity in insulin resistant patients with non‐alcoholic fatty liver disease: a novel liver disease biomarker. PLoS One. 2010;5:e12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kosi‐Trebotic L, Thomas A, Harreiter J, Chmelik M, Trattnig S, Kautzky‐Willer A. Gliptin therapy reduces hepatic and myocardial fat in type 2 diabetic patients. Eur J Clin Invest. 2017;47:829‐838. [DOI] [PubMed] [Google Scholar]
- 38. European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64:1388‐1402. [DOI] [PubMed] [Google Scholar]
- 39. Bajaj HS, Brown RE, Bhullar L, Sohi N, Kalra S, Aronson R. SGLT2 inhibitors and incretin agents: associations with alanine aminotransferase activity in type 2 diabetes. Diabetes Metab. 2018;44:493‐499. [DOI] [PubMed] [Google Scholar]
- 40. Cuthbertson DJ, Irwin A, Gardner CJ, et al. Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon‐like peptide‐1 (GLP‐1) receptor agonists. PLoS One. 2012;7:e50117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Belfort R, Harrison SA, Brown K, et al. A placebo‐controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297‐2307. [DOI] [PubMed] [Google Scholar]
- 42. Gupta AK, Bray GA, Greenway FL, Martin CK, Johnson WD, Smith SR. Pioglitazone, but not metformin, reduces liver fat in type‐2 diabetes mellitus independent of weight changes. J Diabetes Complications. 2010;24:289‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tiikkainen M, Hakkinen AM, Korsheninnikova E, Nyman T, Makimattila S, Yki‐Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53:2169‐2176. [DOI] [PubMed] [Google Scholar]
- 44. Shibuya T, Fushimi N, Kawai M, et al. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non‐alcoholic fatty liver disease: a prospective randomized controlled pilot study. Diabetes Obes Metab. 2018;20:438‐442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


