Abstract
Synaptotagmin‐4 (SYT4) is a membrane protein that regulates membrane traffic in neurons in a calcium‐dependent or calcium‐independent manner. In melanocytes, the intracellular free calcium ion (Ca2+) may be important for dendrite growth and melanogenesis. Mammalian melanocytes originating from neural crest cells produce melanins. Therefore, we predicted that SYT4 might play a role in melanogenesis and the dendrite morphology of melanocytes. To investigate whether SYT4 is involved in melanocyte physiology, SYT4 was overexpressed in alpaca melanocytes and B16‐F10 cells. The results showed that SYT4 overexpression resulted in a phenotype consistent with melanogenesis and dendrite extension. At the molecular level, SYT4 interacted with extracellular regulated MAP kinase (ERK) to decrease p‐ERK activity, which negatively regulated CREB expression. Furthermore, cyclic AMP‐responsive element‐binding protein (CREB) was upregulated and caused the downregulation of the expression of melanogenic regulatory proteins, including microphthalmia‐associated transcription factor (MITF), tyrosinase (TYR), tyrosinase‐related protein‐1 (TYRP1), dopachrome tautomerase (DCT), and transient receptor potential melastatin 1 (TRPM1). Intracellular free Ca2+ promoted the upregulation of calcium/calmodulin dependent protein kinase IV (CAMK4) expression, which phosphorylated CREB (p‐CREB). In turn, p‐CREB participated in the transcription of MITF. These results demonstrated that SYT4 promoted melanogenesis through dendrite extension and tyrosinase activity, during which the regulation of Ca2+ influx via the TRPM1 channel was a key factor.
Significance of the study
Intracellular Ca2+ is important for the function and survival of melanocytes and melanoma cells. SYT4 stimulated melanogenesis through calcium. These results provide evidence that SYT4 regulates Ca2+ influx through TRPM1 to cause melanogenesis and axonal elongation in alpaca melanocytes and further suggesting that the growth and metastasis of melanoma is controlled by the inhibited expression of SYT4 in melanoma cells.
Keywords: Ca2+, melanocyte, melanogenesis, SYT4, TRPM1
1. INTRODUCTION
Synaptotagmins (SYTs) constitute a large family of membrane proteins that regulate membrane traffic in neurons and other types of cells1, which are characterized by their distinct distributions patterns and different biochemical features.2 The members of this protein family are likely to function as calcium sensors in calcium‐regulated events in neurons.2 One isoform, SYT4, serves as a postsynaptic Ca2+ sensor that releases retrograde signals to stimulate enhanced presynaptic function through the activation of the cyclic adenosine monophosphate (cAMP)‐dependent protein kinase pathway.3 SYT4 inhibited SNAP [soluble N‐ethylmaleimide–sensitive factor attachment protein] receptor (SNARE)‐catalysed membrane fusion in both the absence and presence of Ca2+. Rat SYT4 could stimulate fusion in response to Ca2+ when the conserved Asp‐to‐Ser Ca2+ ligand substitution to the C2A domain was changed.4 SYT4 contains two internal repeats that have homology with the C2 domain of protein kinase C (PKC).5 This domain is known to regulate the calcium‐dependent translocation of PKC to membranes and is present in many proteins with different functions.5, 6 In SYTs, the two C2 domains are responsible for different calcium‐dependent and calcium‐independent reactions. SYT4 was shown to be expressed in muscle cells at the Drosophila neuromuscular junction.3 SYT4 is localized on insulin granules and transcriptional regulation of SYT4 affects insulin secretion.7 SYT4 is an integral vesicle protein present on brain‐derived neurotrophic factor (BDNF)‐harbouring dense core vesicles (DCVs) in neurons.8 SYT4 regulates the capture and spatial distribution the DCVs in hippocampal neurons.9 In a previous study, we found that SYT4 is expressed in mouse melanocytes and B16 melanoma cells.10
In the electron transfer system, calcium is important for both melanocyte function and survival.11 An increase in intracellular free Ca2+ may be important in stimulating melanogenesis.12 Transient receptor potential melastatin (TRPM) is a subfamily of ion channels that was originally identified as melanoma metastasis suppressor based on its expression in normal pigment cells in the skin and eye.13 TRPM1 (also known as melastatin) expression and intracellular Ca2+ levels are significantly lower in rapidly proliferating melanocytes compared with their levels in slow growing, differentiated melanocytes.14 Knocking down TRPM1 resulted in decreases in tyrosinase activity and intracellular melanin pigment levels.15 Calcium‐ and CAMK4, a cytoplasm and nucleus protein, is activated by Ca2+/calmodulin and is a multifunctional serine/threonine protein kinase,14 and nuclear effector of the Ca2+/calmodulin kinase (CAMK) pathway, where it coordinates transcriptional responses.15 CAMK4 phosphorylates CREB by acting upstream of CREB activation in β cells16 and in neurons.17 CREB activates transcription of MITF, which consequently triggers the transcription of downstream melanogenic genes such as TYR, TYRP1 and DCT.18 Mammalian melanocytes originate from neural crest cells.19 Therefore, we predicted that SYT4 leads to pigmentation in a process that is not completely understood.
2. MATERIALS AND METHODS
2.1. Construction of DNA recombination
The complete coding sequences (CDSs) of wild‐type alpaca and mouse SYT4 were amplified by reverse transcription polymerase chain reaction (PCR) using a kit and gene‐specific primers (Table 1) and subcloned into a pMD‐19T vector to generate pMD‐19T‐SYT4 plasmids. The SYT4 inserts were liberated from pMD‐19T‐SYT4 using endonucleases NotI and XhoI and ligated into a pVAX1 expression vector to construct pVAX1‐SYT4. The sequence of the construction was verified by restriction digestion and DNA sequencing. In addition, based on the mouse SYT4 CDS sequence, three SYT4 short hairpin RNAs (shRNAs) and negative controls (NCs) designed to target the mouse SYT4 mRNA were obtained from Sangon Biotech (Shanghai, China).
Table 1.
Primers used in this study
| Primer Name | Primer Sequence 5′‐3′ | Annealing Temperature, °C |
|---|---|---|
| SYT4‐F | ATAGCGGCCGCATGGCTCCTATCACCACCAGCCGC | 64 |
| SYT4‐R | ATCCTCGAGTATGGCTCCTATCACCACCAG | |
| SYT4‐F‐RT | CCTCGTCTTCACTGTCTCTCTCTTT | 58 |
| SYT4‐R‐RT | TTCACTTCACTCTTGTCATCTCCTC | |
| MITF‐F (alpaca) | TCCCAAGTCAAATGATCCAG | 52 |
| MITF‐R (alpaca) | GAGCCTGCATTTCAAGTTCC | |
| DCT‐F (alpaca) | CCAGGGCGCCCATACAAGGC | 60 |
| DCT‐R (alpaca) | GCTGGTCTGTGCACACN(1/2G,1/2A)TCA | |
| 18S‐F (alpaca) | GAAGGGCACCACCAGGAGT | 50‐64 |
| 18S‐R (alpaca) | CAGACAAATCACTCCACCAA | |
| MITF‐F (mus) | GAGCAGAGCAGGGCAGAGAGTGAGT | 60 |
| MITF‐R (mus) | TGGGAAGGTTGGCTGGACAGGAGTT | |
| TYR‐F (mus) | TGAAAATCCTAACTTACTCAGCCCA | 60 |
| TYR‐R (mus) | TCAAACTCAGACAAAATTCCACATC | |
| TYRP1‐F (mus) | CCATTGCTGTAGTGGCTGCGTTGTT | 61 |
| TYRP1‐R (mus) | GGAGAGGCTGGTTGGCTTCATTCTT | |
| DCT‐F (mus) | TTGCTCTTGGGGTTGCTGGCTTTTC | 61 |
| DCT‐R (mus) | TCCTCCGTGTATCTCTTGCTGCTGA | |
| β‐actin (mus) | TTGCTGACAGGATGCAGAAG | 50‐64 |
| β‐actin (mus) | ACATCTGCTGGAAGGTGGAC |
2.2. Cell culture and transfection
Melanocytes used in this study were established by our lab and were maintained as previously described. B16‐F10 cells are commercially got from Cell Research (Shanghai,China) Melanocytes and B16‐F10 cells were transfected with pVAX1‐SYT4, Sh‐SYT4 and the negative control plasmid using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Three days after transfection, the cells were collected. Cell lysates and total RNA were prepared and subjected to Western blot and real‐time quantitative PCR analyses, respectively.
2.3. Total RNA isolation and quantitative real‐time PCR analysis
Total RNA from transfected cells was extracted with TRIzol reagent (Invitrogen) according to the manufacturer's instructions and treated with DNase I (MilliporeSigma, Burlington, MA, USA). For mRNA quantification, 1 μg of total RNA was converted by a cDNA synthesis kit (TaKaRa, Dalian, China) according to the manufacturer's instructions, and qPCR was performed using SYBR Green PCR master mix (TaKaRa). For analyses, qPCR was performed with SYBR Green PCR Master Mix (TaKaRa) on a 7500 Fast Real‐Time PCR system (Thermo Fisher Scientific). The primer sequences are listed in Table 1. Melting curves for each sample were analysed to validate the amplification specificity. All mRNAs were normalized to the amount of 18S mRNA. Quantification of mRNA transcript abundance was performed by using the comparative threshold cycle method. The significance the differences in the levels of mRNA was determined by ANOVA (SPSS 11.5 Software; IBM, Armonk, NY, USA).
2.4. Western blotting
Protein samples from transfected cells were got by and were separated on 10% or 8% SDS‐PAGE gels and transferred to nitrocellulose membranes. The membranes were blocked with 5% skim milk for 1 hour and incubated overnight at 4°C with the following diluted primary antibodies against SYT4 (1:1000, Abcam, Cambridge, UK), CAMK4 (1:1000, OmnimAbs), TRM1 (1:1000, Abcam, Cambridge, UK), ERK (1:1000, Cell Signaling Technology, Beverly, MA), MITF (1:1000, Abcam, Cambridge, UK), TYR (1:1000, Abcam, Cambridge, UK), TYRP1 (1:1000, Abcam, Cambridge, UK), DCT (1:1000, Abcam, Cambridge, UK), and β‐actin (1:1000, CWBIO, Beijing, China). After the membranes were washed three times with TBST for 10 minutes and washed, they were incubated at 37°C for 1 hour with horseradish peroxidase‐conjugated secondary antibodies against rabbit or mouse IgG (1:10 000, Invitrogen). After the membranes were washed again with TBST six times for 5 minutes per wash, the bound antibodies were visualized using enhanced chemiluminescence. Immunoblots were scanned on a ChemiDoc XRS+ Imager (Bio‐Rad, Hercules, CA, USA), and protein levels were quantified with Image‐Pro Plus Software (Olympus, Tokyo, Japan).
2.5. Ca2+ imaging
Ca2+ imaging was performed according to the manufacturer's instructions. Melanocytes were harvested and rinsed with phosphate‐buffered saline (PBS). Then, the melanocytes were labelled with Rhod‐3 (Invitrogen) for 1 hour at room temperature, washed with PBS three times for 5 minutes per wash, and incubated for 1 hour to de‐esterify the dye.20 Labelled cells are subjected to live cell imaging with laser scanning confocal microscope (FV1000, Olympus, Japan).
2.6. Immunocytochemistry
Melanocytes were washed three times for 3 minutes per wash with PBS, fixed in 4% paraformaldehyde for 20 minutes, and incubated at room temperature in 3% hydrogen peroxide for 15 minutes to block the action of the endogenous peroxidases. After three 5‐minute washes with PBS, the cells were immersed in bovine serum albumin (BSA) at 37°C for 25 minutes and incubated at 4°C overnight in rabbit anti‐SYT4 antibody solution. After three 5‐minute washes with PBS, the cells were incubated with horseradish peroxidase‐conjugated anti‐rabbit IgG for 30 minutes at 37°C and observed under a microscope (Leica Microsystems, Buffalo Grove, IL, USA). For the negative control (NC), IgG was substituted for the primary antibody.
2.7. Tyrosinase activity measurement
Melanocytes were washed three times with PBS for 3 minutes per wash. Then, the cells were seeded into microtiter plates containing 100 μL of 1% Triton X‐100 in each well, and the plate was vortexed for 5 minutes. After a 1‐hour incubation at room temperature, 50 μL of 1% Levodopa was added (LJ350101; Cool Chemistry) and the plate was further incubated at 37°C for 2 hours. Tyrosinase activity was determined spectrophotometrically by measuring the absorbance at 460 nm.
2.8. Melanin measurement
Melanocytes transfected with SYT4‐CDS and Sh‐SYT4 were harvested and rinsed with PBS. For the assay of the total alkali‐soluble melanin (ASM), 1 mL of 0.2M NaOH was added, and the absorbance of the solution was measured spectrophotometrically at 475 nm. For the assay of eumelanin (EM), the cells were hydrolysed in hot 30% hypophosphoric acid and hydriodic acid. After cooling, 50% ethanol was added, and the cell samples were centrifuged at 2, 234 g for 10 minutes. Insoluble eumelanic pigments were selectively solubilized in hot sodium hydroxide and hydrogen peroxide, and the solution was cleared by centrifuging it at 10 , 700 g for 1 minute. The absorbance of the supernatant was measured at 350 nm. For pheomelanin (PM) assay, the cells were solubilized in phosphate buffer (pH 10.5) and cleared by centrifugation at 10, 700 g for 10 minutes before the determination of the absorbance at 400 nm. The melanin levels were normalized to the total number of cells. All experiments were performed in triplicate.
2.9. Statistical analysis
Data related to the levels of mRNA, protein, tyrosinase activity, and melanin levels in the control and experimental groups were analysed using the Statistical Analysis System (GraphPad Prism 5). All experiments were repeated three times, and the data are expressed as the mean ± standard error (SE). Statistical comparisons between different treatments were performed using one‐way analysis of variance followed by a T test.
3. RESULTS
3.1. The effect of SYT4 on the phenotypes of the melanocytes with the overexpression of SYT4
To investigate the effect of SYT4 on cell morphology, an immunocytochemistry method was used. The results showed that SYT4 immunoreactivity was observed in the cytoplasm of melanocytes, and it was stronger in melanocytes overexpressing SYT4 than in NC cells (Figure 1A). At the same time, the melanocytes transfected with the NC plasmid showed a normal fusiform or polygonal shape, while the dendrites of melanocytes overexpressing SYT4 were obviously slender (Figure 1B).
Figure 1.
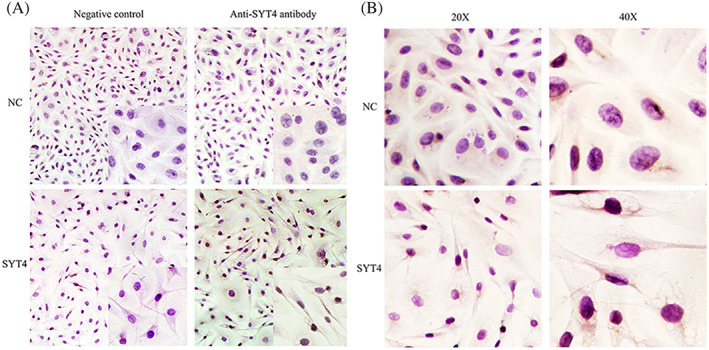
The effect of SYT4 on cell morphology. A,Analysis of SYT4 protein expression in melanocytes transfected with SYT4 overexpressing plasmids using immunocytochemistry. B, Overexpression of SYT4 in alpaca melanocytes makes cell synapse slender
3.2. The effect of SYT4 on free intracellular Ca2+ and the protein expression of the related genes
By calcium imaging, the fluorescence signal of Ca2+ in melanocytes overexpressing SYT4 was significantly stronger than that in the negative control (Figure 2A), which indicated that SYT4 overexpression could upregulate free intracellular Ca2+. Furthermore, the protein expression of the genes related to Ca2+, CAMK4, and TRPM1 was unregulated, while that of p‐ERK was downregulated in the melanocytes overexpressing SYT4, compared with the expression of these proteins in the NC (Figure 2B).
Figure 2.
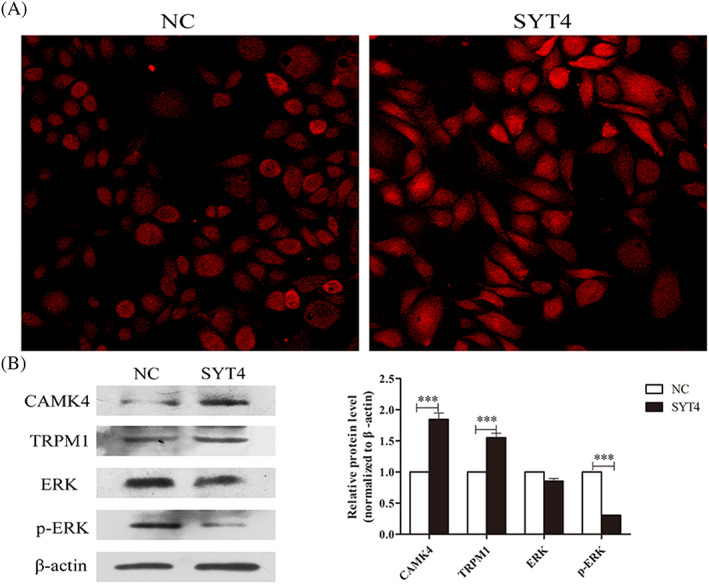
Effect of the overexpression of SYT4 on calcium influx and Ca2+‐related protein expression levels in alpaca melanocytes. A, Overexpression of SYT4 in alpaca melanocytes promotes calcium influx. B , The results from the Western blot and abundance analyses of the protein expression of CAMK4, TRPM1, ERK in SYT4‐overexpressing melanocytes compared with that in the NC group. Data represent the mean ± standard error (n = 3). ** P < .05. *** P < .001
3.3. The effect of SYT4 on the tyrosinase activity and melanins production
To determine whether SYT4 affected tyrosinase activity, tyrosinase activity was measured after SYT4 was overexpressed or knocked down in alpaca melanocytes. The results showed that the tyrosinase activity was increased by >100% in alpaca melanocytes overexpressing SYT4 (Figure 3A). To determine whether SYT4 affected melanins production, the production ofASM, EM, and PM was measured in alpaca melanocytes after SYT4 was overexpressed or knocked down. The results showed that the production of ASM, EM, and PM was significantly higher in melanocytes overexpressing SYT4 than that in NC cells overexpressing SYT4 (Figure 3B). In contrast, the tyrosinase activity and the production of ASM, EM, and PM was significantly lower in melanocytes with SYT4 knocked down than that in the NC cells (Figure 3C,D).
Figure 3.
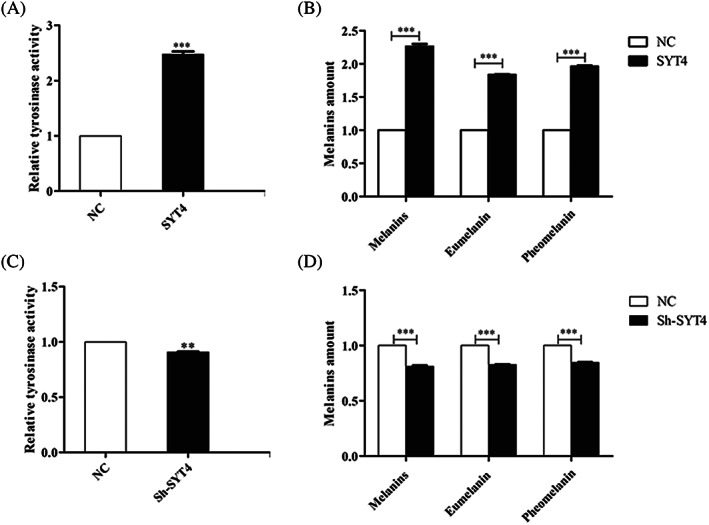
Effects of SYT4 on tyrosinase activity and melanins production. A, Effect of SYT4 overexpression on tyrosinase activity in alpaca melanocytes. B, Effect of SYT4 overexpression on the production of ASM, EM, and PM in alpaca melanocytes. C, Effect of SYT4 knock down on tyrosinase activity in alpaca melanocytes. D, Effect of SYT4 knock down on the production of ASM, EM, and PM in alpaca melanocytes. Data represent the mean ± standard error (n = 3). ** P < .05. *** P < .001
3.4. SYT4 regulated the expression of melanogenesis related genes in alpaca melanocytes
To explore the effects of SYT4 on melanogenesis in alpaca melanocytes, we investigated the expression levels of related genes. Overexpressed and knocked down SYT4 in melanocytes resulted in the increase and decrease of quantitative levels of mRNAs encoding MITF, and DCT, respectively (Figure 4A). The results of the Western blot analysis showed that the expression of the MITF, TYR, TYRP1, and DCT proteins was significantly increased and decreased in melanocytes with SYT4 overexpressed or knocked down compared with that of the NC cell groups, respectively (Figure 4B).
Figure 4.
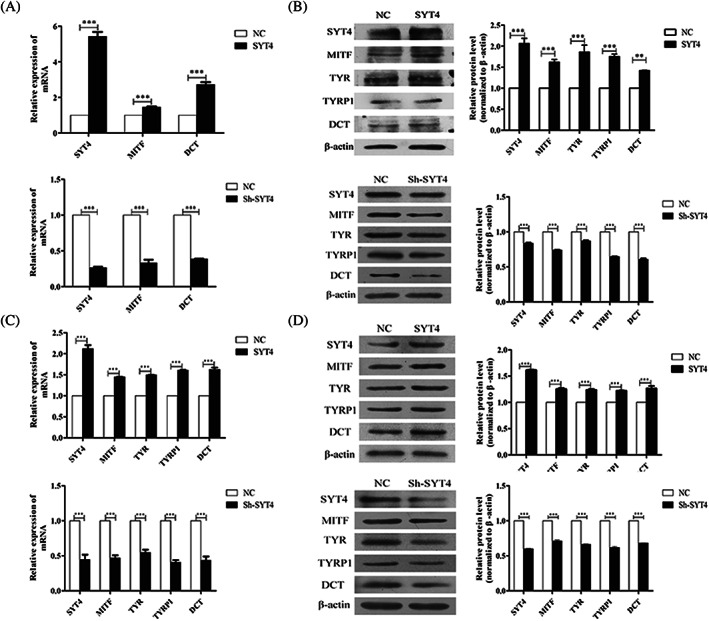
Effects of SYT4 overexpression on the protein and mRNA expression levels of melanogenesis‐related genes in alpaca melanocytes. A, Results from the qPCR analyses of SYT4, MITF, and DCT mRNA expression in melanocytes with SYT4 overexpressed or knocked down compared with the expression in the cells of the NC groups. B, Results from the Western blot and abundance analyses of SYT4, MITF, TYR, TYRP1, and DCT protein expression in melanocytes with SYT4 overexpressed or knocked down compared with the expression in the cells of the NC group. C, Results from the qPCR analyses of SYT4, MITF, TYR, TYRP1 and DCT mRNA expression in melanoma B16‐F10 cells with SYT4 overexpressed and knocked down compared with the expression in the cells of the NC groups. D, Western blot and abundance analyses of SYT4, MITF, TYR, TYRP1 and DCT protein expression in B16‐F10 melanoma cells with SYT4 overexpressed or knocked down compared with expression in the cells of the NC groups. Data represent mean ± standard error (n = 3). ** P < .05. *** P < .001
3.5. Verification of SYT4 in another melanoma cell line: B16‐F10
To verify the effects of SYT4 on melanogenesis, SYT4 was overexpressed or knocked down in B16‐F10 cells. The expression of mRNAs of MITF, TYR, TYRP1, and DCT was detected. The results showed that the expression levels of these genes were significantly increased and decreased in B16‐F10 cells with overexpressed and knocked down SYT4 compared with that in the NC groups, respectively (Figure 4C). Similarly, results from the Western blot analysis showed that the expression of MITF, TYR, TYRP1, and DCT proteins was also significantly increased and decreased in B16‐F10 cells with overexpressed and knocked down SYT4, respectively (Figure 4D).
4. DISCUSSION
SYT family members with C2 domains were found to be related to dendrite lengthening.21 It has been reported that SYT14L containing the C2 domain induces significant elongation in dendrite length that was accompanied by the induction of melanocyte differentiation‐related markers, including those for melanin synthesis, tyrosinase catalytic activity, and the expression of TYR, TYRP1, and DCT.21 Here, we investigated whether SYT4 overexpression induced the morphological phenotype of dendritic extension and the action of the melanocytes.
In mammalian skin, melanins are transferred within melanosomes through melanocytic dendrites to epidermal keratinocytes, which gives rise to the observed pigmentation, and it has been reported that dendrite formation is promoted by increases in intracellular Ca2+ in human melanocytes.22 Pigmentation can be regulated by Ca2+ influx via a transient receptor potential cation channel: the TRPM1 channel,23 the downregulation of which has been correlated with a higher metastatic risk of skin melanoma.24 Based on the results of the Rhod‐3 calcium imaging kit, the Ca2+ influx was observed to be greater in melanocytes overexpressing SYT4. The results suggested that the opening of TRPM1 was promoted by SYT4 to increase free intracellular Ca2+. Therefore, SYT4 induced Ca2+ to play important roles in the extended dendrites of melanocytes, which are responsible for melanogenesis. In turn, we found that an increase in the amount of Ca2+ in the melanocytes, as induced by SYT4, led to upregulated CAMK4. The CAMK4/CREB cascade forms a signalling pathway in some cells, such as β cells16 and neurons,17 according to the extent by which CAMK4 phosphorylates CREB. In an interesting finding, SYT4 interacted with ERK and positively regulated p‐ERK expression. The ERK signalling pathway may negatively affect the melanogenesis23, 25 induced by the p53‐POMC‐MC1R signalling cascade to enhance the phosphorylation levels of ERK1/2 and CREB.26 CREB activates the transcription of MITF, which consequently triggers the transcription of the downstream melanogenic genes TYR, TYRP1, and DCT,18 causing increases in the melanin content. However, TRPM1 expression was regulated by MITF in melanocytes.27 Thus, SYT4 regulated melanogenesis by a crosstalk.
5. CONCLUSION
Taken together, the findings indicate that SYT4 overexpression causes a positive loop depended on Ca2+ influx, a key factor that is regulated by TRPM1. Such functional morphological changes in the melanocytes of skin melanoma and the colour formation in hair and skin share the similar molecular mechanisms (Figure 5).
Figure 5.
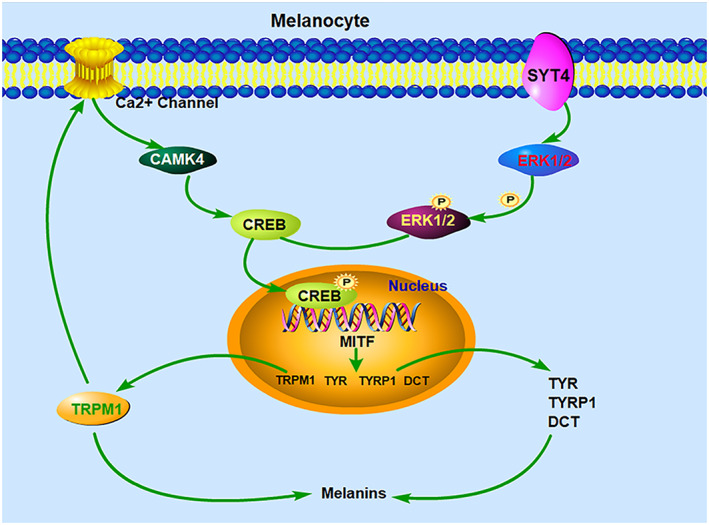
Graphic showing the signalling pathway by which SYT4 regulates melanogenesis via Ca2+ influx
DATA AVAILABILITY STATEMENT
The data generated in this study are original and available, have not been published before, and are not currently under review elsewhere.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare
ACKNOWLEDGEMENTS
The authors thank their colleagues in the laboratory for their help with this study. This work was from a research project supported by the Shanxi Scholarship Council of China (2017‐072), a Special Fund for the Young Sanjin Scholars Distinguished Professor (to Ruiwen Fan) and the Aid Program for the Innovation Team in Shanxi Agricultural University (grant number CXTD201201).
Jia Q, Hu S, Jiao D, Li X, Qi S, Fan R. Synaptotagmin‐4 promotes dendrite extension and melanogenesis in alpaca melanocytes by regulating Ca2+ influx via TRPM1 channels. Cell Biochem Funct. 2020;38:275–282. 10.1002/cbf.3465
REFERENCES
- 1. Hilbush BS, Morgan JI. A third synaptotagmin gene, Syt3, in the mouse. P Natl Acad Sci. 1994;91(17):8195‐8199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Osborne S, Herreros J, Bastiaens P, Schiavo G. Calcium‐dependent oligomerization of synaptotagmins I and II—synaptotagmins I and II are localized on the same synaptic vesicle and heterodimerize in the presence of calcium. J Biol Chem. 1999;274(1):59. [DOI] [PubMed] [Google Scholar]
- 3. Yoshihara M, Adolfsen B, Galle KT, Littleton JT. Retrograde signaling by Syt 4 induces presynaptic release and synapse‐specific growth. Science. 2005;310(5749):858‐863. [DOI] [PubMed] [Google Scholar]
- 4. Wang Z, Wang Z, Chapman ER. Rat and drosophila synaptotagmin 4 have opposite effects during SNARE‐catalyzed membrane fusion. J Biol Chem. 2010;285(40):30759‐30766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pointing CP, Parker PJ. Extending the C2 domain family: C2s in PKCs δ, ɛ, η, θ, phospholipases, GAPs, and perforin. Protein Sci. 2010;5(1):162‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schiavo G, Osborne SL, Sgouros JG. Synaptotagmins: more isoforms than functions? Biochem Bioph Res Co. 1998;248(1):0‐8. [DOI] [PubMed] [Google Scholar]
- 7. Huang C, Walker EM, Dadi PK, et al. Synaptotagmin 4 regulates pancreatic β cell maturation by modulating the Ca2+, sensitivity of insulin secretion vesicles. Dev Cell. 2018;45:S1534580718302338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dean C, Liu H, Mark Dunning F, et al. Synaptotagmin‐IV modulates synaptic function and long‐term potentiation by regulating BDNF release. Nat Neurosci. 2009;12(6):767‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bharat V, Siebrecht M, Burk K, et al. Capture of dense core vesicles at synapses by JNK‐dependent phosphorylation of synaptotagmin‐4. Cell Rep. 2017;21(8):2118‐2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ji K, Fan R, Zhang J, et al. Differential expression of mRNA between murine melanocytes and B16 melanoma cells (in Chinese). Chinese J Biochem Molec Biol. 2019;35(1). [Google Scholar]
- 11. Schallreuter‐Wood KU, Pittelkow MR, Swanson NN. Defective calcium transport in vitiliginous melanocytes. Arch Dermatol Res. 1996;288(1):11. [DOI] [PubMed] [Google Scholar]
- 12. Carsberg CJ, Jones KT, Sharpe GR, et al. Intracellular calcium modulates the responses of human melanocytes to melanogenic stimuli. J Dermatol Sci. 1995;9(3):157‐164. [DOI] [PubMed] [Google Scholar]
- 13. Devi S, Kedlaya R, Maddodi N, et al. Calcium homeostasis in human melanocytes: role of transient receptor potential melastatin 1 (TRPM1) and its regulation by ultraviolet light. Am J Physiol‐Cell Ph. 2009;297(3):C679‐C687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khattri A, Reddy VP, Pandey RK, et al. Novel mutations in calcium/calmodulin‐dependent protein kinase IV (CAMK4) gene in infertile men. Int J Androl. 2012;35(6):810‐818. [DOI] [PubMed] [Google Scholar]
- 15. Editor PE. IB4‐binding sensory neurons in the adult rat express a novel 3′ UTR‐extended isoform of CAMK4 that is associated with its localization to axons. J Comp Neurol. 2013;522(2):308‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bo L, Helena BS, Jones PM, et al. The CAMK4/CREB/IRS‐2 cascade stimulates proliferation and inhibits apoptosis of β‐cells. PLoS ONE. 2012;7(9):e45711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bilbao A, Parkitna JR, Engblom D, et al. Loss of the Ca2+/calmodulin‐dependent protein kinase type IV in dopaminoceptive neurons enhances behavioral effects of cocaine. P Natl Acad Sci. 2008;105(45):17549‐17554. 10.1073/pnas.0803959105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vachtenheim J, Borovansky J. “Transcription physiology” of pigment formation in melanocytes: central role of MITF. Exp Dermatol. 2010;19(7):617‐627. [DOI] [PubMed] [Google Scholar]
- 19. Kos L, Aronzon A, Takayama H, et al. Hepatocyte growth factor/scatter factor‐MET signaling in neural crest‐derived melanocyte development. Pigment Cell Res. 1999;12(1):13‐21. [DOI] [PubMed] [Google Scholar]
- 20. Ieda M, Fu JD, Delgado‐Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Nihon Rinsho Japanese J Clinic Med. 2011;142(3):375‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoo JC, Lim TY, Park JS, et al. SYT14L, especially its C2 domain, is involved in regulating melanocyte differentiation. J Dermatol Sci. 2013;72(3):246‐251. [DOI] [PubMed] [Google Scholar]
- 22. Abdel‐Naser MB, Seltmann H, Zouboulis CC. SZ95 sebocytes induce epidermal melanocyte dendricity and proliferation in vitro. Exp Dermatol. 2012;21(5):393‐395. [DOI] [PubMed] [Google Scholar]
- 23. Hwang E, Lee TH, Lee WJ, et al. A novel synthetic Piper amide derivative NED‐180 inhibits hyperpigmentation by activating the PI3K and ERK pathways and by regulating Ca2+ influx via TRPM1 channels. Pigm Cell Melanoma R. 2016;29(1):81‐91. [DOI] [PubMed] [Google Scholar]
- 24. Moulin AP, Zografos L. TRPM1 and MITF expression in conjunctival melanocytic proliferations. Acta Ophthalmol. 2011;89(s246):0‐0. [Google Scholar]
- 25. Kim DS, Kim SY, Chung JH, Kim KH, Eun HC, Park KC. Delayed ERK activation by ceramide reduces melanin synthesis in human melanocytes. Cell Signal. 2002;14(9):779‐785. [DOI] [PubMed] [Google Scholar]
- 26. Zhou D, Kuang Z, Zeng X, et al. p53 regulates ERK1/2/CREB cascade via a novel SASH1/MAP 2K2 crosstalk to induce hyperpigmentation. J Cell Mol Med. 2017;21(10):2465‐2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller AJ. Transcriptional regulation of the melanoma prognostic marker melastatin (TRPM1) by MITF in melanocytes and melanoma. Cancer Res. 2004;64(2):509‐516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in this study are original and available, have not been published before, and are not currently under review elsewhere.


