Abstract
Parasites may have strong eco‐evolutionary interactions with their hosts. Consequently, they may contribute to host diversification. The radiation of cichlid fish in Lake Victoria provides a good model to study the role of parasites in the early stages of speciation. We investigated patterns of macroparasite infection in a community of 17 sympatric cichlids from a recent radiation and 2 older species from 2 nonradiating lineages, to explore the opportunity for parasite‐mediated speciation. Host species had different parasite infection profiles, which were only partially explained by ecological factors (diet, water depth). This may indicate that differences in infection are not simply the result of differences in exposure, but that hosts evolved species‐specific resistance, consistent with parasite‐mediated divergent selection. Infection was similar between sampling years, indicating that the direction of parasite‐mediated selection is stable through time. We morphologically identified 6 Cichlidogyrus species, a gill parasite that is considered a good candidate for driving parasite‐mediated speciation, because it is host species‐specific and has radiated elsewhere in Africa. Species composition of Cichlidogyrus infection was similar among the most closely related host species (members of the Lake Victoria radiation), but two more distantly related species (belonging to nonradiating sister lineages) showed distinct infection profiles. This is inconsistent with a role for Cichlidogyrus in the early stages of divergence. To conclude, we find significant interspecific variation in parasite infection profiles, which is temporally consistent. We found no evidence that Cichlidogyrus‐mediated selection contributes to the early stages of speciation. Instead, our findings indicate that species differences in infection accumulate after speciation.
Keywords: adaptive radiation, cichlid fish, diversification, host–parasite interaction, Lake Victoria, parasite‐mediated selection, temporal consistency
Species composition of Cichlidogyrus infection was similar among the closely related cichlid species of the Lake Victoria radiation (in orange), but differed between this lineage and two distantly related species (belonging to nonradiating sister lineages, in blue).
1. INTRODUCTION
Ecological speciation, the evolutionary process by which ecologically based divergent selection leads to species divergence, can be driven by adaptation to both abiotic and biotic factors. Antagonistic interactions among species (i.e. prey‐predator, resource competition) are commonly considered examples of biotic factors that may drive ecological speciation (Maan & Seehausen, 2011; Rundle & Nosil, 2005; Schluter, 1996, 2000).
Parasites form another ubiquitous selective pressure (Poulin & Morand, 2000; Schmid‐Hempel, 2013) and engage with their hosts in co‐evolutionary dynamics of adaptation and counter‐adaptation (Decaestecker et al., 2007). Heterogenous parasite‐mediated selection, as different infection levels of a parasite species and/or different parasite community compositions may initiate, promote or reinforce host diversification and ecological speciation. Studies investigating the role of parasites in host diversification have begun to accumulate (Eizaguirre, Lenz, Kalbe, & Milinski, 2012; Eizaguirre et al., 2011; Feulner et al., 2015; Greischar & Koskella, 2007; Karvonen, Lucek, Marques, & Seehausen, 2015; Stutz, Lau, & Bolnick, 2014). However, parasite‐mediated selection has received relatively little attention in the context of adaptive radiation (El Nagar & MacColl, 2016; Vanhove & Huyse, 2015).
Adaptive radiations are characterized by the rapid evolution of ecologically distinct taxa in response to new ecological opportunities or challenges (Rundle & Nosil, 2005; Schluter, 2000). Parasites may contribute to this process if three prerequisites are met (Karvonen & Seehausen, 2012; Rundle & Nosil, 2005). First, parasite‐mediated selection should differ within or between host populations in terms of parasite abundance and/or community composition. Consistent with this, previous studies have reported infection differences among closely related host species across a wide range of animal taxa (mammals: Boundenga et al., 2018; reptiles: Carbayo, Martin, & Civantos, 2018; fish: Thomas, Renaud, Rousset, Cezilly, & Meeuûs, 1995, MacColl, 2009; bivalves: Coustau, Renaud, Maillard, Pasteur, & Delay, 1991; crustaceans: Galipaud, Bollache, & Lagrue, 2017). Second, parasitic infection should impose a cost on host fitness, thereby exerting selection for resistance or tolerance on the host. This prerequisite is also supported by empirical evidence from a wide range of taxa (mammals: Careau, Thomas, & Humphries, 2010; fish Milinski & Bakker, 1990; crustaceans: Stirnadel & Ebert, 1997, Tellenbach, Wolinska, & Spaak, 2007; angiosperms: Segar, Mardiastuti, Wheeler, & Cook, 2018; birds: Hamilton & Zuk, 1982). Third, the direction of parasite‐mediated selection between host populations should be stable over time. Stochastic or frequency‐dependent temporal fluctuations in parasite abundances could cause variation in the strength of parasite‐mediated selection, but the direction of divergent selection is stable if the differences between host populations in parasite exposure or impact are maintained. Temporally consistent infection differences have been observed in cichlids of Lake Tanganyika (Raeymaekers et al., 2013) and in icefish from the Antarctic Sea (Mattiucci et al., 2015). In response to parasite‐mediated divergent selection, host (sub)populations may adapt either by evolving a specialized immune response or by evolving increased tolerance (depending on their respective costs and benefits). Such adaptive responses can lead to an increasingly different parasite infection pattern between host (sub)populations. Here, we investigate two prerequisites of parasite‐mediated speciation in the same study system, by analysing infection differences—in terms of parasite communities and individual parasite taxa—between several sympatric host species within an adaptive radiation of cichlid fish, at two different time points.
Parasite transmission is associated with specific habitats and foraging strategies; therefore, host populations with different ecological specializations may encounter different parasites, even in geographical sympatry (Hablützel et al., 2017; Hayward et al., 2017). Host populations that are exposed to different parasites are expected to respond to parasite‐mediated divergent selection, potentially strengthening host species differentiation. According to the hybrid/immigrant disadvantage hypothesis (Fritz, Nichols‐Orians, & Brunsfeld, 1994), hybrids between two diverging host populations may not cope well with the infection of either parental species because of their recombinant resistance genotype. For example, hybrids may have a super‐optimal MHC diversity, causing a reduced T‐cell repertoire (through elimination of T cells that are binding self‐peptides; Janeway, Travers, Walport, & Shlomchik, 2005) and making them more susceptible to parasites (Eizaguirre et al., 2012). As a result, parasite‐mediated selection against recombinants can reduce gene flow between parental species. Alternatively, the recombinant resistance genotype of hybrids outperforms parental resistance genotypes (Baird et al., 2012). In that case, parasite‐mediated selection could promote gene flow and reduce the opportunity for speciation. Since specific MHC alleles may confer resistance to specific parasites (Bonneaud, Pérez‐Tris, Federici, Chastel, & Sorci, 2006; Eizaguirre, Yeates, Lenz, Kalbe, & Milinski, 2009a; Paterson, Wilson, & Pemberton, 1998), both scenarios may occur at the same time: for some infections, recombinants are favoured, but not for others.
Cichlid fish of the Great African Lakes (Lakes Malawi, Tanganyika and Victoria) are a well‐studied example of adaptive radiation (Kocher, 2004; Kornfield & Smith, 2000; Seehausen, 2006). At the same time, cichlids also provide many examples of no diversification, as most lineages never radiated into multiple species despite extensive ecological opportunity (Seehausen, 2015). Within radiations, the Lake Victoria rock cichlids are a classical example of species divergence in macro‐habitat, micro‐habitat and trophic specialization (Bouton, Seehausen, & van Alphen, 1997; Seehausen & Bouton, 1997, 1998). This suggests that they may be exposed to different parasite taxa (Karvonen, Wagner, Selz, & Seehausen, 2018; Maan, van Rooijen, van Alphen, & Seehausen, 2008) and thus good candidates for responding to parasite‐mediated divergent selection.
Here, we investigate the potential role of parasites in host diversification by analysing macroparasite infection in Lake Victoria cichlid fish. In addition to higher taxon‐level identification, we assess morphospecies diversity of Cichlidogyrus, a genus of flatworm gill parasites (Monogenea, Ancyrocephalidae) that primarily infects members of the Cichlidae family (but also killifishes belonging to Aphyosemion, Messu Mandeng et al., 2015, and the nandid Polycentropsis abbreviata, Pariselle & Euzet, 2009). Cichlidogyrus is the most species‐rich parasite taxon infecting old world cichlids (Scholz, Vanhove, Smit, Jayasundera, & Gelnar, 2018) and has undergone at least one radiation (in Lake Tanganyika, Vanhove et al., 2015). Host specificity of representatives of Cichlidogyrus has been observed in Lake Tanganyika, but is poorly investigated in other lakes (Pariselle, Muterezi Bukinga, Steenberge, & Vanhove, 2015). Recent studies experimentally confirmed that monogeneans cause an immune response in their host (Chen et al., 2019; Zhi et al., 2018), providing evidence for the second prerequisite for parasite‐mediated speciation. Together, the often relatively high host specificity, large species number and high morphological diversity within the genus, make Cichlidogyrus a good model to study the evolution of host–parasite interactions (Pariselle, Morand, Deveney, & Pouyaud, 2003; Vanhove et al., 2016).
In a previous study, ectoparasite infections in a cichlid fish species assemblage of a rocky island in Lake Victoria were found to differ between host species and to be correlated with host species differences in water depth occupation, diet and abundance (Karvonen et al., 2018). Here, we study the same assemblage, allowing us to test the temporal consistency in these patterns. We also expand on the earlier findings by including endoparasites and by identifying monogenean parasites to species level. We expect divergent infections between host species of the radiation, in both parasite community composition and parasite abundance, in line with the first prerequisite for parasite‐mediated speciation. Moreover, parasite‐mediated selection should generate species differences in infection that are not explained by ecological factors alone. If variation in parasite infection across host species is fully explained by variation in host capture depth and diet, it could be driven entirely by environmental variation in exposure and would not constitute evidence for divergent evolution of host‐specific defence mechanisms. Following the third prerequisite for parasite‐mediated speciation, we also expect that the direction of infection differences between host species is constant through time, thus maintaining the direction of divergent selection even in the presence of temporal fluctuations in parasite abundances.
We include two cichlid species (Astatoreochromis alluaudi and Pseudocrenilabrus multicolor) that have not been investigated previously for their Cichlidogyrus infection. They are not part of the radiation of cichlids in Lake Victoria and only distantly related to the radiation (Schedel, Musilova, & Schliewen, 2019), yet they co‐occur with the radiation cichlids. If parasite‐mediated selection contributed to the Lake Victoria cichlid radiation, we predict that radiation members have adapted to parasites by evolving specific immune responses, whereas these two older lineages that did not diversify in response to parasites (nor to other factors), evolved an unspecialized defence (i.e. generalist tolerance or resistance). This would result in different infection patterns, possibly characterized by higher within‐host–parasite diversity (more species of Cichlidogyrus) and parasite abundance (more individuals of Cichlidogyrus) in the nondiversifying lineages. Variation in infection patterns of Cichlidogyrus within and between cichlid lineages could emerge from at least two evolutionary scenarios. First, worms colonized the radiation cichlids from the ancient nonradiating cichlids, with different worm species colonizing the differentiating hosts in different numbers. This would impose different selection pressures on different host species and could initiate host‐specific evolutionary responses. This scenario would lead to a pattern in which Cichlidogyrus species are shared among the radiation cichlids and the older, nonradiating lineages. Alternatively, ancestral worms may have diverged after colonizing the radiation cichlids, co‐speciating with their hosts. This latter pattern, with Cichlidogyrus species not shared between radiation members and the older nonradiating lineages, would support a contribution of Cichlidogyrus‐mediated selection to the Lake Victoria cichlid radiation.
2. MATERIALS AND METHODS
2.1. Fish collection
Cichlid fish were collected in May–August 2010 at Makobe Island and in June–October 2014 at three locations in southern Lake Victoria, Tanzania (Makobe Island, Sweya swamp and Kissenda Island, Figure 1). At Makobe, we collected 18 sympatric cichlid species representing different ecological specializations (diet and water depth, Bouton et al., 1997; Seehausen, 1996; Seehausen & Bouton, 1998; Witte & Oijen, 1990; Table 1), and also different levels of genetic differentiation (Karvonen et al., 2018; Wagner, McCune, & Lovette, 2012). Of those, 17 species belong to the Lake Victoria radiation and one species (Astatoreochromis alluaudi) represents an old lineage that has not radiated. Since Makobe is inhabited by only one of the two nonradiating haplochromine species that occur in Lake Victoria, it was necessary to sample a second location, Sweya, to obtain the other one (Pseudocrenilabrus multicolor). The divergence between the two nonradiating species, and between them and the ancestors of the radiations in Lake Victoria, Lake Malawi and other lakes, dates back to ~15 million years ago (Schedel et al., 2019). Including Sweya introduced geographical variation as an additional variable. To assess the effects of geographical distance on parasite infection patterns, we therefore also collected additional specimens of A. alluaudi from this second location (Sweya). For the same reason, we also added a third location, the rocky island Kissenda, where we sampled two species of the radiation (P. sp. ‘pundamilia‐like’ and P. sp. ‘nyererei‐like’), that are closely related and ecologically similar to two Makobe species (P. pundamilia and P. nyererei, respectively). Finally, to increase the number of molluscivore species, we also sampled Ptyochromis xenognathus (belonging to the radiation) at Kissenda.
FIGURE 1.
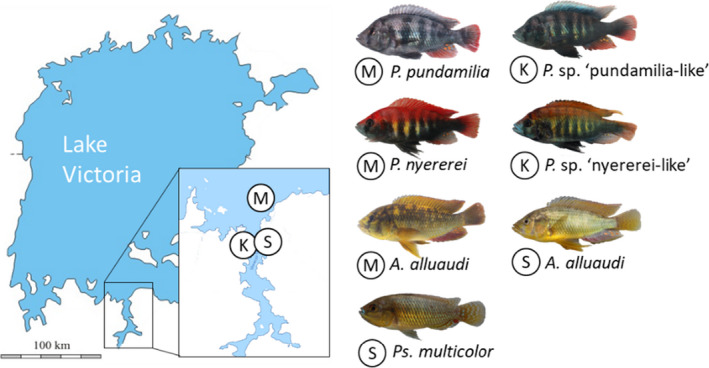
Geographical location of the three sampling sites in southern Lake Victoria, Tanzania: rocky islands Makobe (M) and Kissenda (K) and the Sweya swampy inlet stream (S). Depicted are the two nonradiating lineages, represented by A. alluaudi (collected from both Makobe and Sweya) and Ps. multicolor (collected from Sweya), as well as representatives of the radiation: two closely related species pairs collected from Makobe (P. pundamilia, P. nyererei) and at Kissenda (P. sp. ‘pundamilia‐like’, P. sp. ‘nyererei‐like’)
TABLE 1.
Characteristics of host species sampled in 2014 at Makobe, Sweya and Kissenda islands: diet, number of fish individuals, water depth, SL standard length, weight, CF condition factor
| Host species | Diet | nr fish | nr identified Cichlidogyrus | Depth (m) | SL (mm) | Weight (g) | CF | nr fish | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | Mean | (Min–Max) | Mean | (Min–Max) | Mean | (Min–Max) | mean | (Min–Max) | 2010 | |||||
| Makobe | ||||||||||||||
| ■ | ● | Astatoreochromis alluaudi | Mollusc | 17 | 38 | 9.59 | (0.75–18.5) | 111.28 | (70.9–130.8) | 46.59 | (10.8–71.5) | 3.09 | (2.72–3.46) | 10 |
| Harpagochromis vonlinnei | Fish | 2 | 15 | (11–19) | 133.29 | (125.3–141.3) | 68.54 | (68.5–68.5) | 2.32 | (2.2–2.43) | 0 | |||
| ■ | ● | Labrochromis sp. ‘stone’ | Mollusc | 1 | 3 | 19 | (19–19) | 130.75 | (130.8–130.8) | 65.45 | (65.5–65.5) | 2.84 | (2.84–2.84) | 14 |
| ● | Lipochromis melanopterus | Fry | 2 | 8.75 | (5.5–12) | 91.96 | (80.8–103.1) | 24.76 | (16.5–33) | 2.94 | (2.9–2.99) | 8 | ||
| Lithochromis sp. ‘yellow chin pseudonigricans’ | Insect | 10 | 10.95 | (9–19) | 92.05 | (79.7–113) | 34.57 | (21.3–47.9) | 2.52 | (2.23–3.26) | 0 | |||
| ■ | ● | Mbipia lutea | Algae | 7 | 14 | 1 | (1–1) | 139.68 | (136–142) | 76.87 | (67.1–83.4) | 2.81 | (2.56–3.08) | 13 |
| ■ | ● | Mbipia mbipi | Algae | 16 | 22 | 1.88 | (1–2.5) | 97.33 | (84.7–113.2) | 30.31 | (20.3–40.5) | 2.87 | (2.54–3.72) | 16 |
| ■ | ● | Neochromis gigas | Algae | 8 | 15 | 1.22 | (1–2.75) | 114.99 | (86.2–127.3) | 43.11 | (17.9–52.4) | 2.75 | (2.52–2.94) | 13 |
| ■ | ● | Neochromis omnicaeruleus | Algae | 26 | 25 | 4.84 | (2.5–9.5) | 91.86 | (74–110.5) | 23.78 | (11.3–41.6) | 2.82 | (2.28–3.54) | 9 |
| ■ | ● | Neochromis rufocaudalis | Algae | 16 | 13 | 2.61 | (0.75–3.5) | 89.21 | (61.4–100) | 20.28 | (6.4–26.3) | 2.7 | (2.41–3.08) | 9 |
| ■ | ● | Neochromis sp. ‘unicuspid scraper’ | Algae | 32 | 23 | 13.18 | (1.25–19) | 96.73 | (76.6–114.4) | 26.16 | (10.9–49.4) | 2.69 | (2.19–3.21) | 8 |
| ■ | ● | Pundamilia nyererei | Plankton | 71 | 34 | 10.61 | (2.5–18.5) | 81.28 | (63–106.7) | 17.69 | (7–41.9) | 2.74 | (2.06–3.41) | 10 |
| ■ | ● | Pundamilia sp. ‘pink anal’ | Plankton | 18 | 15 | 9.92 | (5.5–19) | 91.79 | (77.9–120.8) | 24.78 | (12.2–59.1) | 2.8 | (2.37–3.43) | 10 |
| ■ | ● | Pundamilia pundamilia | Insect | 56 | 21 | 1.69 | (0.5–16) | 95.32 | (52.1–128.8) | 33.54 | (3.7–71.3) | 3.15 | (2.5–3.76) | 9 |
| ■ | ● | Paralabidochromis chilotes | Insect | 9 | 5 | 12.28 | (1.5–19) | 106.35 | (81.1–120.8) | 47.13 | (34.1–53.7) | 2.46 | (2.09–2.95) | 11 |
| ■ | ● | “Haplochromis” cyaneus | Insect | 14 | 16 | 2.71 | (1–6.5) | 100.16 | (81.4–107.9) | 24.43 | (12.3–33.7) | 2.32 | (2.08–2.63) | 9 |
| ● | Paralabidochromis sauvagei | Insect | 11 | 7.5 | (3.5–14) | 103.18 | (93.7–115.4) | 30.74 | (11.3–44.8) | 2.76 | (1.06–3.42) | 11 | ||
| ● | Paralabidochromis sp. ‘short snout scraper’ | Algae | 11 | 4.59 | (3–6) | 105.31 | (93.5–115.5) | 37.32 | (22.8–44.8) | 3.04 | (2.7–3.29) | 9 | ||
| Sweya | ||||||||||||||
| ■ | Astatoreochromis alluaudi | Mollusc | 6 | 19 | 0.5 | (0.5–0.5) | 63.63 | (48.2–80.3) | 8.85 | (2.9–15.6) | 2.89 | (2.5–3.26) | 0 | |
| ■ | Pseudocrenilabrus multicolor | Insect | 20 | 12 | 0.5 | (0.5–0.5) | 39.6 | (32.8–46.8) | 1.94 | (1.1–2.7) | 3.01 | (2.19–3.86) | 0 | |
| Kissenda | ||||||||||||||
| ■ | Pundamilia sp. ‘nyererei‐like’ | Insect | 32 | 6 | 4.16 | (0.75–7.5) | 73.42 | (60.1–88.9) | 11.56 | (4.8–26.7) | 2.68 | (1.92–3.68) | 0 | |
| ■ | Pundamilia sp. ‘pundamilia‐like’ | Insect | 31 | 13 | 3.04 | (0.75–7.5) | 76.21 | (49.3–108.1) | 13.96 | (2.8–38.5) | 2.58 | (1.58–3.46) | 0 | |
| ■ | Ptyochromis xenognathus | Mollusc | 0 | 18 | 3.03 | (1.5–7) | 107.76 | (97.4–115.4) | 37.39 | (29.8–44.9) | 2.93 | (2.63–3.16) | 10 | |
Species labelled with a circle (●) were also sampled in 2010 (only sample sizes reported, other data available in Karvonen et al., 2018), and those with a square (■) were used to assess Cichlidogyrus diversity (number of identified worm specimens reported).
Collection was done by angling and with gillnets of variable mesh sizes, set at different water depths (0–19 m). Males and females may differ in infection pattern (Maan, van der Spoel, Jimenez, van Alphen, & Seehausen, 2006). However, females are difficult to identify reliably in the field, due to their generally cryptic coloration. We therefore included only males. Fish were euthanized with an overdose of 2‐phenoxyethanol immediately after capture. Their body cavity was slit open ventrally to allow preservation of organs and internal parasites. Some fish were preserved in 4% formalin and subsequently transferred on 70% ethanol, and other fish were directly preserved in 100% ethanol for future genetic analysis. Each individual fish was subsequently measured (SL standard length, BD body depth, to the nearest 0.1 mm) and weighed (to the nearest 0.1 g).
2.2. Parasite screening
We examined gill arches (right side of the fish only), abdominal cavity, gonads, liver and gastrointestinal tract under a dissecting stereoscope. All macroparasites were identified following Paperna (1996) and monogenean literature (Muterezi Bukinga, Vanhove, Steenberge, & Pariselle, 2012; Vanhove, Snoeks, Volckaert, & Huyse, 2011; Zahradníčková, Barson, Luus‐Powell, & Přikrylová, 2016) and counted. Five ectoparasite taxa and two endoparasite taxa were found. Encysted skin trematodes of the ‘neascus’ type (Paperna, 1996) were not included because consistency of detection was low due to their cryptic appearance. All monogenean worms infecting gills were individually preserved in 100% ethanol. With the exception of one individual of Gyrodactylus sp., these all belonged to Cichlidogyrus. For morphological identification, we selected a subset of Cichlidogyrus specimens (n = 640) from 17 host species (the two species from the two nonradiating lineages, 15 species from the radiation). We aimed to identify 15 Cichlidogyrus specimens per host population, by sampling all worms infesting each fish individual from a randomly selected pool of each host population. If the total number of worms available per host population was less than 15, then all worms of that host population were identified (see Table 1 for sample sizes).
2.3. Cichlidogyrus morphospecies identification
For morphological analysis, specimens of Cichlidogyrus were mounted on slides in Hoyer's medium, after prior treatment with 20% sodium dodecyl sulphate to soften tissues. Specimens of Cichlidogyrus were examined with a microscope (Olympus BX41TF) under 1,000x magnification using differential interference phase contrast. None of the morphospecies of Cichlidogyrus that we found have been formally described; species were discriminated based on shape and size of sclerotized parts of the attachment organ (haptor) and, in particular, on those of the male copulatory organ (MCO) (e.g. Grégoir et al., 2015).
2.4. Data analysis
2.4.1. Divergent parasite infection
To compare parasite communities between host species inhabiting Makobe Island, we performed one‐way analysis of similarities, based on the zero‐adjusted Bray–Curtis distances of parasite abundance data (i.e. the number of parasites in infected and uninfected host individuals) and on the Jaccard index of presence/absence of parasite species (ANOSIM, 9,999 permutations, PAST 3.18, Hammer, Harper, & Ryan, 2001). Pairwise comparisons were made using the false discovery rate correction for P values (Benjamini & Hochberg, 1995). Such analyses were performed on fish individuals for which we established both endo‐ and ectoparasite infection (2014 only; fish were not screened for endoparasites in 2010) and on fish individuals for which we established ectoparasite infection in both years (2014 and 2010). To evaluate the extent to which these differences could be explained by differences in diet or depth habitat, we performed PERMANOVA (PAST). Since PERMANOVA considers categorical variables, individual capture depths were categorized into depth ranges of different resolution (1 m, 2 m, 3 m, 5 m, 10 m). To investigate the contribution of each parasite taxon to parasite community differences, similarity percentages analysis (SIMPER, PAST) was performed (reported in Appendix S1).
Ectoparasite (pooling all species of Cichlidogyrus) and endoparasite taxa infecting the Makobe cichlid community in 2014 were analysed separately for prevalence (percentage of infected individuals of total host population) and infection intensity (number of parasites per infected individual), using generalized linear models in R (3.4.1. R Core Team, 2018) with binomial distribution for prevalence and Poisson distribution for intensity. Fixed effects included host species, individual capture water depth and diet. Fish standard length was not included because its correlation with infection was inconsistent across species (Figure S1). However, to account for the effect of fish length in species variation in parasite infection, we performed an additional analysis that included fish standard length as a fixed effect. We determined the significance of fixed effects by likelihood ratio tests (LRT) to select the minimum adequate model (MAM). The MAM was confirmed by bootstrapping (bootStepAIC package). We then used model comparison to test the MAM against models including the removed terms (LRT bootstrap and Akaike information criterion) to obtain parameter estimates for all terms.
2.4.2. Temporal consistency of infection
To investigate temporal consistency in infection, we compared ectoparasite infection profiles (endoparasites were not assessed in 2010) for 16 of the 18 host species from Makobe between samples collected in 2014 and samples collected in 2010 at the same location (from Karvonen et al., 2018), using ANOSIM as described above. For each ectoparasite taxon, we performed generalized linear models on parasite prevalence and intensity (both years) to assess temporal consistency. Fixed effects included host species, diet, individual capture water depth, sampling year and the interaction between sampling year and host species. Fish standard length was not included in the model, because species differences in fish length were consistent between the two years (Figure S2) and because its correlation with infection was inconsistent across species (Figure S1).
We also assessed temporal consistency of parasite‐mediated divergent selection within pairs of closely related species (following Brawand et al., 2014; Keller et al., 2013; Magalhaes, Lundsgaard‐Hansen, Mwaiko, & Seehausen, 2012; Seehausen, 1996; Wagner et al., 2013). We plotted the mean infection intensity and prevalence in 2014 against that in 2010 (Figures S3 and S4); then, we established the slope of the line connecting the two species (for species pairs) and the slope of the correlation for all species (for the community‐level analysis). A positive correlation slope would indicate temporal consistency in infection differences.
2.4.3. Divergent parasite infection at morphospecies level for Cichlidogyrus
Differences between host species of the radiation in the community composition of Cichlidogyrus morphospecies were analysed using ANOSIM as described above. Pairwise comparisons were made using the false discovery rate correction for P values (Benjamini & Hochberg, 1995). The same analysis was performed to compare communities of Cichlidogyrus between the three haplochromine lineages (radiation members, A. alluaudi, Ps. multicolor). To investigate the contribution of each morphospecies of Cichlidogyrus to parasite community differences, similarity percentages analysis (SIMPER, PAST) was performed (reported in Appendix S1).
3. RESULTS
We observed five ectoparasite taxa and two endoparasite taxa (Table 2; not considering species diversity of Cichlidogyrus). The ectoparasites were as follows: Cichlidogyrus spp. (Monogenea: Dactylogyridea), Gyrodactylus sturmbaueri (Monogenea: Gyrodactylidea), Lamproglena monodi (Copepoda: Cyclopoida), Ergasilus lamellifer (Copepoda: Poecilostomatoida) and glochidia mussel larvae (Bivalvia: Unionoidea). Among endoparasites, we found nematodes and trematodes.
TABLE 2.
Parasite infection (% prevalence, mean intensity, mean abundance, abundance range) of cichlid fish at Makobe, Kissenda and Sweya locations in 2014
| Host species | Cichlidogyrus spp. | Lamproglena monodi | Ergasilus lamellifer | Glochidia | Nematode | Trematode | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | Intensity | Abundance | % | Intensity | Abundance | % | Intensity | Abundance | % | Intensity | Abundance | % | Intensity | Abundance | % | Intensity | Abundance | |||||||
| Makobe | ||||||||||||||||||||||||
| A. alluaudi | 100.0 | 20.3 | 20.3 | (2–59) | 18.5 | 1.8 | 0.3 | (0–3) | 7.4 | 1.0 | 0.1 | (0–1) | 25.9 | 2.3 | 0.6 | (0–5) | 60.0 | 4.2 | 2.5 | (0–15) | 0.0 | 0.0 | 0.0 | (0–0) |
| Ha. serranus | 0.0 | 0.0 | 0.0 | (0–0) | 0.0 | 0.0 | 0.0 | (0–0) | 0.0 | 0.0 | 0.0 | (0–0) | 0.0 | 0.0 | 0.0 | (0–0) | 0.0 | 0.0 | 0.0 | (0–0) | 0.0 | 0.0 | 0.0 | (0–0) |
| La. sp. 'stone' | 53.3 | 1.3 | 0.7 | (0–2) | 53.3 | 2.3 | 1.2 | (0–7) | 0.0 | 0.0 | 0.0 | (0–0) | 20.0 | 1.3 | 0.3 | (0–2) | 0.0 | 0.0 | 0.0 | (0–0) | 0.0 | 0.0 | 0.0 | (0–0) |
| Li. melanopterus | 70.0 | 1.6 | 1.1 | (0–3) | 40.0 | 3.8 | 1.5 | (0–5) | 0.0 | 0.0 | 0.0 | (0–0) | 0.0 | 0.0 | 0.0 | (0–0) | 0.0 | 0.0 | 14.0 | (0–28) | 0.0 | 0.0 | 0.0 | (0–0) |
| Li. sp. ‘yellow chin pseudonigricans’ | 30.0 | 3.0 | 0.9 | (0–3) | 80.0 | 2.8 | 2.2 | (0–6) | 10.0 | 1.0 | 0.1 | (0–1) | 20.0 | 1.5 | 0.3 | (0–2) | 30.0 | 19.0 | 8.9 | (0–38) | 0.0 | 0.0 | 0.0 | (0–0) |
| M. lutea | 80.0 | 6.0 | 5.1 | (0–18) | 85.0 | 4.8 | 4.3 | (0–21) | 5.0 | 1.0 | 0.1 | (0–1) | 10.0 | 1.5 | 0.2 | (0–2) | 100.0 | 17.7 | 17.7 | (1–34) | 11.1 | 1.0 | 0.1 | (0–1) |
| M. mbipi | 90.6 | 6.0 | 5.8 | (0–16) | 50.0 | 1.8 | 0.9 | (0–3) | 6.3 | 1.0 | 0.1 | (0–1) | 28.1 | 1.8 | 0.5 | (0–4) | 62.5 | 3.4 | 2.3 | (0–9) | 0.0 | 0.0 | 0.0 | (0–0) |
| N. gigas | 90.5 | 6.9 | 6.2 | (0–17) | 90.5 | 2.1 | 1.9 | (0–5) | 0.0 | 0.0 | 0.0 | (0–0) | 19.1 | 1.3 | 0.2 | (0–2) | 37.5 | 4.7 | 1.8 | (0–6) | 0.0 | 0.0 | 0.0 | (0–0) |
| N. omnicaeruleus | 88.6 | 6.0 | 5.3 | (0–18) | 54.3 | 1.7 | 0.9 | (0–4) | 8.6 | 1.0 | 0.1 | (0–1) | 5.7 | 2.0 | 0.1 | (0–3) | 27.3 | 3.0 | 1.1 | (0–10) | 0.0 | 0.0 | 0.0 | (0–0) |
| N. rufocaudalis | 96.0 | 4.4 | 4.2 | (0–17) | 20.0 | 2.0 | 0.4 | (0–3) | 8.0 | 1.0 | 0.1 | (0–1) | 8.0 | 1.0 | 0.1 | (0–1) | 33.3 | 3.2 | 1.1 | (0–12) | 6.7 | 1.0 | 0.1 | (0–1) |
| N. sp. 'unicuspid scraper' | 67.5 | 2.6 | 1.7 | (0–7) | 82.5 | 3.3 | 2.7 | (0–14) | 10.0 | 1.0 | 0.1 | (0–1) | 10.0 | 1.5 | 0.2 | (0–2) | 40.0 | 2.8 | 1.1 | (0–4) | 10.0 | 1.0 | 0.1 | (0–1) |
| P. nyererei | 49.4 | 2.1 | 1.1 | (0–9) | 76.5 | 3.0 | 2.3 | (0–13) | 11.1 | 1.1 | 0.1 | (0–2) | 22.2 | 2.0 | 0.5 | (0–8) | 63.6 | 1.7 | 1.4 | (0–3) | 0.0 | 0.0 | 0.0 | (0–0) |
| P. sp. 'pink anal' | 57.1 | 2.6 | 1.5 | (0–6) | 60.7 | 1.6 | 1.0 | (0–5) | 3.6 | 1.0 | 0.0 | (0–1) | 10.7 | 1.0 | 0.1 | (0–1) | 16.7 | 3.0 | 0.6 | (0–5) | 0.0 | 0.0 | 0.0 | (0–0) |
| P. pundamilia | 44.6 | 2.5 | 1.1 | (0–6) | 52.3 | 1.9 | 1.0 | (0–7) | 1.5 | 1.0 | 0.0 | (0–1) | 20.0 | 4.2 | 0.9 | (0–26) | 80.0 | 58.6 | 52.3 | (3–152) | 0.0 | 0.0 | 0.0 | (0–0) |
| Pa. chilotes | 60.0 | 3.4 | 2.1 | (0–24) | 45.0 | 2.3 | 1.1 | (0–6) | 30.0 | 1.3 | 0.4 | (0–2) | 10.0 | 2.5 | 0.3 | (0–3) | 11.1 | 3.0 | 17.1 | (0–151) | 0.0 | 0.0 | 0.0 | (0–0) |
| Ha. cyaneus | 95.7 | 7.6 | 7.3 | (0–20) | 87.0 | 2.6 | 2.3 | (0–7) | 8.7 | 1.0 | 0.1 | (0–1) | 4.4 | 1.0 | 0.0 | (0–1) | 42.9 | 2.7 | 1.1 | (0–6) | 0.0 | 0.0 | 0.0 | (0–0) |
| Pa. sauvagei | 13.6 | 1.7 | 0.2 | (0–3) | 68.2 | 2.9 | 2.0 | (0–9) | 9.1 | 1.0 | 0.1 | (0–1) | 0.0 | 0.0 | 0.0 | (0–0) | 72.7 | 1.6 | 1.3 | (0–4) | 0.0 | 0.0 | 0.0 | (0–0) |
| Pa. sp. 'short snout scraper' | 0.0 | 0.0 | 0.0 | (0–0) | 60.0 | 6.4 | 3.9 | (0–16) | 15.0 | 2.3 | 0.4 | (0–4) | 0.0 | 0.0 | 0.0 | (0–0) | 18.2 | 1.0 | 0.2 | (0–1) | 0.0 | 0.0 | 0.0 | (0–0) |
| Sweya | ||||||||||||||||||||||||
| A. alluaudi | 66.7 | 9.0 | 6.0 | (0–33) | 0.0 | 0.0 | 0.0 | (0–0) | 0.0 | 0.0 | 0.0 | (0–0) | 66.7 | 17.0 | 11.3 | (0–37) | ||||||||
| Ps. multicolor | 25.0 | 2.4 | 0.6 | (0–5) | 0.0 | 0.0 | 0.0 | (0–0) | 5.0 | 1.0 | 0.1 | (0–1) | 10.0 | 7.0 | 0.7 | (0–13) | 27.3 | 4.7 | 1.3 | (0–10) | 0.0 | 0.0 | 0.0 | (0–0) |
| Kissenda | ||||||||||||||||||||||||
| P. sp. 'nyererei‐like' | 81.0 | 4.3 | 3.5 | (0–25) | 42.9 | 1.9 | 0.8 | (0–5) | 52.4 | 1.8 | 0.9 | (0–4) | 50.0 | 7.0 | 3.5 | (0–20) | 20.0 | 1.0 | 0.2 | (0–1) | 0.0 | 0.0 | 0.0 | (0–0) |
| P. sp. 'pundamilia‐like' | 80.5 | 5.3 | 4.3 | (0–17) | 43.9 | 1.7 | 0.8 | (0–4) | 39.0 | 1.7 | 0.7 | (0–4) | 46.3 | 11.3 | 5.2 | (0–44) | 44.4 | 1.0 | 0.6 | (0–1) | 11.1 | 1.0 | 0.1 | (0–1) |
| Pt. xenognathus | 60.0 | 3.5 | 2.1 | (0–9) | 50.0 | 1.6 | 0.8 | (0–4) | 70.0 | 3.0 | 2.1 | (0–7) | 90.0 | 16.0 | 14.4 | (0–83) | ||||||||
Trematodes, E. lamellifer and glochidia were rarely observed. Only three individuals (from three different species) were infected by trematodes; therefore, we did not perform statistical analyses on these. Representatives of Cichlidogyrus and L. monodi were common, with prevalence generally higher than 50%. Gyrodactylus sturmbaueri was encountered only once (in Pt. xenognathus from Kissenda Island). The latter parasite was originally described from Simochromis diagramma, a tropheine cichlid from Lake Tanganyika (Vanhove et al., 2011) and was also observed in the haplochromine Pseudocrenilabrus philander in Zimbabwe and South Africa (Zahradníčková et al., 2016). The current study is hence the first report of this monogenean species in Lake Victoria.
At Makobe, within radiation members, ectoparasites were more prevalent than endoparasites (84.45% of fish infected with ectoparasites and 48.85% with endoparasites, LR1 = 41.56, p < .0001). Individuals infected by endoparasites tended to have those in larger numbers than ectoparasites that were usually present in low numbers (mean intensity 11.77 ± 2.73 endoparasites and 7.03 ± 0.72 ectoparasites, LR1 = 83.34, p < .0001). Individuals infected by endoparasites carried more ectoparasites than individuals without endoparasites (7.03 ± 0.72 versus. 4.25 ± 0.51, LR1 = 9.17, p = .002). Also, when considering both lineages, radiation members and A. alluaudi, prevalence and intensity of endoparasites were higher than those of ectoparasites (prevalence: 85.3% ectoparasites, 49.2% endoparasites, LR1 = 46.27, p < .0001; mean intensity 11.30 ± 2.56 endoparasites and 8.89 ± 1.12 ectoparasites, LR1 = 21.26, p < .0001; Figure 2).
FIGURE 2.
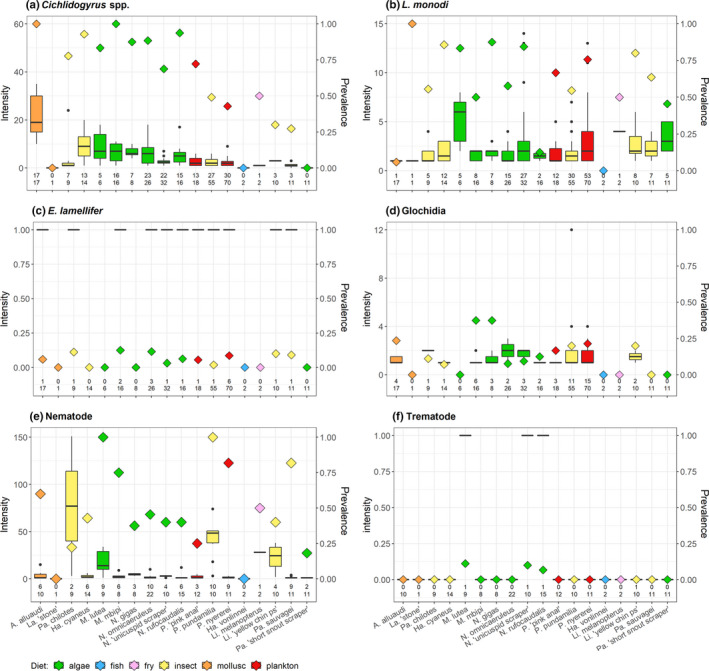
Parasite intensity (boxes) and prevalence (diamonds) of cichlid species at Makobe Island in 2014. Colours represent host diet. (a) Cichlidogyrus spp., (b) L. monodi, (c) E. lamellifer, (d) glochidia, (e) nematodes, (f) trematodes. Numbers indicate the number of infected fish individuals per species (upper line) and total sample size per species (lower line)
3.1. Divergent parasite infection across host species
Within the radiation, host species were infected by different parasite communities (ANOSIM on zero‐adjusted Bray–Curtis distances R = 0.3675, p < .0001): each species differed in its infection profile from at least five other species and on average from 11 other species (of 16; Table 3). Including A. alluaudi did not change this pattern, but the parasite community composition of this nonradiating lineage differed from every radiation member (Table 3). The differences in parasite infection profiles were largely driven by the numbers of parasites of each taxon, rather than by the presence or absence of parasite taxa. Indeed, the same five parasite taxa were shared by all host species, as illustrated by the few differences in Jaccard indices within the radiation (Table S1a). To exclude possible effects of uneven sample sizes between host species, we repeated community analysis on host species represented by at least 10 individuals and we performed ectoparasite community analysis on host species from both years. These analyses confirmed the aforementioned patterns (Tables S1b,c and S4).
TABLE 3.
Differences in parasite community (not considering Cichlidogyrus morphospecies diversity) between cichlid host species at Makobe Island in 2014
| A. alluaudi | Pa. chilotes | Ha. cyaneus | M. lutea | M. mbipi | N. gigas | N. omnicaeruleus | N. sp.'unicuspid scraper' | N. rufocaudalis | P. sp.' pink anal' | P. pundamilia | P. nyererei | Ha. vonlinnei | Li. melanopterus | Li. sp. 'yellow chin pseudonigricans' | Pa. sauvagei | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonradiating | Radiation | |||||||||||||||
| Pa. chilotes | 0.782*** | |||||||||||||||
| Ha. cyaneus | 0.290** | 0.490** | ||||||||||||||
| M. lutea | 0.861*** | 0.604* | 0.757*** | |||||||||||||
| M. mbipi | 0.476** | 0.305* | 0.044 | 0.793** | ||||||||||||
| N. gigas | 0.617*** | 0.405** | −0.025 | 0.81** | 0.009 | |||||||||||
| N. omnicaeruleus | 0.294** | 0.330* | −0.024 | 0.663*** | −0.077 | −0.059 | ||||||||||
| N. sp. 'unicuspid scraper' | 0.981*** | 0.060 | 0.419** | 0.92*** | 0.403** | 0.392** | 0.333** | |||||||||
| N. rufocaudalis | 0.592*** | 0.414* | 0.179* | 0.851*** | 0.086 | 0.153 | 0.072 | 0.467*** | ||||||||
| P. sp.' pink anal' | 0.894*** | −0.01 | 0.378** | 0.905*** | 0.324* | 0.325** | 0.31** | −0.084 | 0.364** | |||||||
| P. pundamilia | 0.915*** | 0.661** | 0.917*** | 0.248* | 0.822*** | 0.846*** | 0.868*** | 0.871*** | 0.904*** | 0.901*** | ||||||
| P. nyererei | 0.970*** | 0.217. | 0.444*** | 0.921*** | 0.402** | 0.454** | 0.372** | −0.019 | 0.496*** | 0.056 | 0.862*** | |||||
| Ha. vonlinnei | 1.000* | −0.052 | 0.867* | 1.000. | 0.806* | 0.987* | 0.790* | 0.472* | 0.669* | 0.107 | 0.944* | 0.755* | ||||
| Li. melanopterus | 0.937* | 0.094 | 0.763* | 0.365 | 0.849* | 0.735. | 0.741* | 0.523. | 0.896* | 0.496. | 0.422 | 0.604* | 0.000 | |||
| Li. sp. 'yellow chin pseudonigricans' | 0.742*** | −0.019 | 0.438*** | 0.215. | 0.268* | 0.201* | 0.343** | 0.060 | 0.494*** | 0.092 | 0.75** | 0.175* | −0.007 | −0.029 | ||
| Pa. sauvagei | 0.989*** | 0.264* | 0.596*** | 0.928*** | 0.565** | 0.19*** | 0.537*** | 0.059 | 0.602*** | 0.066 | 0.865*** | −0.041 | 0.346. | 0.586* | 0.158* | |
| Pa. sp. 'short snout scraper' | 1.000*** | 0.272* | 0.73*** | 0.941** | 0.804*** | 0.785*** | 0.724*** | 0.239* | 0.74*** | 0.118. | 0.938*** | 0.35** | −0.177 | 0.523. | 0.152* | 0.168. |
Parasite community composition of A. alluaudi (nonradiating lineage) differed from all radiation members. Within the radiation (separate analysis), each host species differed from at least five other species in parasite community. Differences are expressed as R values, derived from ANOSIM pairwise comparisons (Benjamini‐Hochberg correction) based on zero‐adjusted Bray–Curtis distances of parasite abundance, 9,999 permutations.
Considering each parasite taxon separately, we found that host species had significantly heterogeneous prevalence and intensity of Cichlidogyrus, L. monodi and nematodes (Table 4). The prevalence of glochidia tended to differ among host species as well. We found the same pattern of infection differences among host species when including A. alluaudi (Table S2a) and also when accounting for fish standard length (Table S3). Infected A. alluaudi had a significantly higher intensity of Cichlidogyrus than all other infected host species (mean 23.23 ± 2.86 versus. 0.45 ± 0.28–8.43 ± 1.53, all p < .001). As above, we repeated this analysis on the subset of host species represented by at least 10 individuals. These confirmed the aforementioned patterns, with the exception of L. monodi intensity that no longer differed between host species (Table S2b,c).
TABLE 4.
Variation in prevalence and intensity of parasites (not considering Cichlidogyrus morphospecies diversity) among host species of the radiation at Makobe Island, in 2014
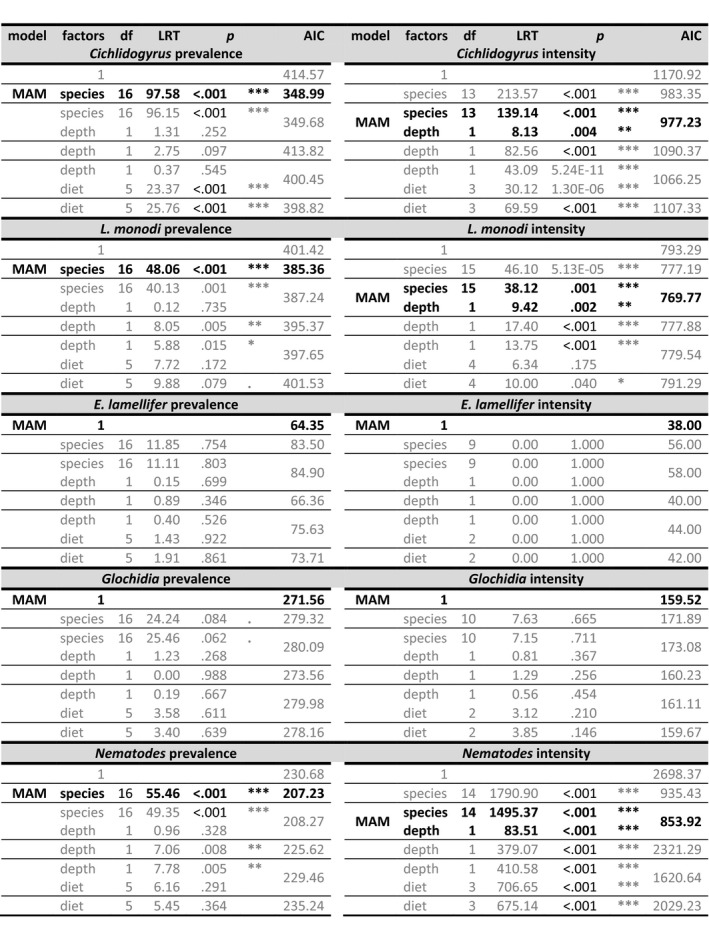
The minimum adequate model (MAM) was established by stepwise removal of nonsignificant variables (shown in grey), and the contribution of each fixed effect was assessed through LRT. Model fits were also compared through AIC.
3.2. Water depth and diet do not fully explain infection variation
Since haplochromine species occupy different water depth ranges, we investigated if parasite infection covaried with the typical water depth range of each species. Variation in parasite community among radiation members inhabiting Makobe was best explained by host species (15.39%, PERMANOVA p = .0001, F 16 = 0.269), rather than diet (2.84%) or water depth (5.30% for 3 m ranges). The contribution of water depth increased with higher‐resolution depth categorization (10 m 1.22%, 5 m 3.68%, 3 m 5.30%, 2 m 7.79%, 1 m 9.49%). However, the species contribution was dominant regardless of the depth bin chosen. Including A. alluaudi gave similar results (species 18.08%, diet 3.84%, 3‐m depth range 4.80%).
A similar pattern was observed for individual parasite taxa: variation in prevalence of Cichlidogyrus, L. monodi and nematodes was best explained by host species, rather than individual capture depth and/or diet (Table 4). Intensities of Cichlidogyrus, L. monodi and nematodes were explained by both host species and water depth. Fish individuals from deeper waters had more L. monodi and fewer Cichlidogyrus and nematodes (Table 4). However, the effect of depth on the intensities of Cichlidogyrus and nematodes differed among host species (follow‐up analysis revealed significant species by depth interactions; Cichlidogyrus: LRT10 = 53.99, p < .0001; nematodes: LRT7 = 122.57, p < .0001). Variation in E. lamellifer and glochidia (both in terms of prevalence and intensity) was not significantly associated with host species identity, nor with ecological factors (water depth, diet)—at species nor at individual level. Including A. alluaudi gave similar results (Table S2a), as well as including host standard length in the analyses (Table S3).
3.3. Temporal consistency in infection
Ectoparasite community composition did not differ between the two sampling years (R = 0.001, p = .423; note that endoparasites were not screened in 2010). Temporal fluctuations in the abundance of parasites were observed for some parasite taxa but not others (Table S5). Overall, prevalence was similar in both sampling years for Cichlidogyrus (LRT1 = 0.03, p = .861), L. monodi (LRT1 = 0.43, p = .551) and glochidia (LRT1 = 1.28, p = .256). Prevalence of E. lamellifer was higher in 2010 (LRT1 = 7.86, p = .005). Infection intensity was lower in 2014 for L. monodi (LRT1 = 11.56, df = 1, p = .001) and glochidia (LRT1 = 14.51, p < .0001), but similar for Cichlidogyrus (LRT1 = 1.45, df = 1, p = .227) and E. lamellifer (LRT1 = 0.37, df = 1, p = .541).
Despite temporal fluctuations in some parasite taxa, differences in infection profile between host species were consistent over time (Table S5). Most importantly, variation among radiation members in both prevalence and intensity of the two most common parasites, Cichlidogyrus and L. monodi, were positively correlated between 2010 and 2014 (Figure 3, Figure S5). Interspecific variation in Cichlidogyrus prevalence and in glochidia intensity differed between years. Including A. alluaudi gave a similar pattern (Table S5b).
FIGURE 3.
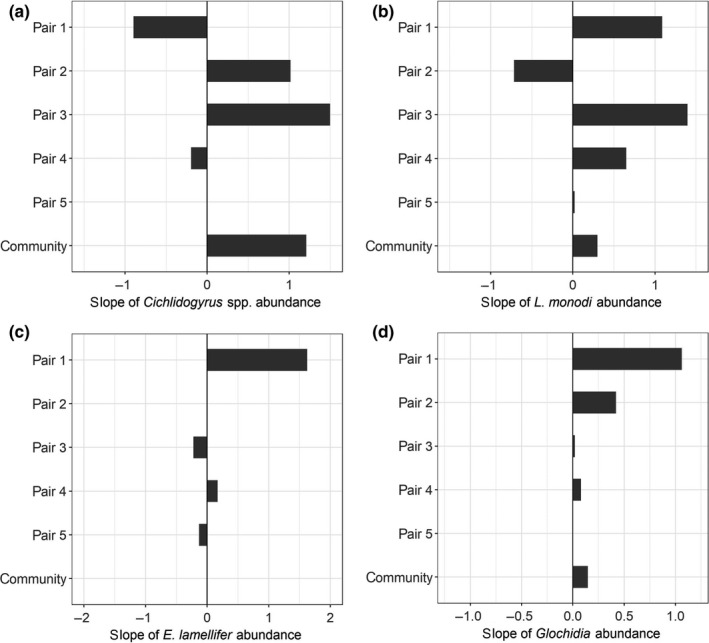
Temporal consistency in infection intensity. Correlations between species differences in infection intensity of (a) Cichlidogyrus spp., (b) L. monodi, (c) E. lamellifer, (d) glochidia between sampling years, for members of the radiation at community wide level and for sister species pairs. After plotting the mean intensity in 2014 against that in 2010 (Figure S3), we established the slope of the line connecting the two species within a pair and the slope of the correlation line for all species (for the community‐level analysis). A positive correlation slope indicates temporal consistency in infection differences. Sister species pairs are as follows: (1) M. mbipi – M. lutea, (2) M. mbipi – P. sp. ‘pink anal’, (3) N. omnicaeruleus – N. sp. ‘unicuspid scraper’, (4) P. pundamilia – P. nyererei, (5) Pa. sauvagei – Pa. sp. ‘short snout scraper’. Intensity of Cichlidogyrus, L. monodi and glochidia was consistent for most sister pairs
We focused on several pairs of closely related host species (following Brawand et al., 2014; Keller et al., 2013; Magalhaes et al., 2012; Seehausen, 1996; Wagner et al., 2013) to assess temporal consistency of parasite‐mediated divergent selection within those pairs. If parasite‐mediated divergent selection contributes to speciation, its signature should be especially visible in species pairs that are in the process of evolving reproductive isolation. The direction of the infection difference between sister species depended on the ectoparasite taxon and the host pair considered, but in general the direction was maintained over time (visual inspection of Figure 3, Figure S5; endoparasites were not assessed in 2010). We excluded cases in which prevalence or mean intensity was identical for the two species within a pair in one or both years (respectively, 3 and 4 of 20 comparisons). Prevalence of glochidia was temporally consistent among all sister pairs; prevalence of Cichlidogyrus and L. monodi was consistent among most pairs (3 of 4, 3 of 5, respectively). Sister species differences in prevalence of E. lamellifer were maintained in both years only in the P. pundamilia – P. nyererei pair. Intensity of Cichlidogyrus, L. monodi and glochidia (but not of E. lamellifer) was consistent for most sister pairs (3 of 4; 4 of 5; 3 of 4, respectively).
3.4. Species differences in infection at Cichlidogyrus morphospecies level
Morphological assessment of Cichlidogyrus revealed the presence of six morphospecies among the cichlids of the Makobe Island assemblage. Since all observed species of Cichlidogyrus appear to be undescribed (formal taxonomic description in prep.), they are provisionally named with roman numbers.
Within the radiation, host species at Makobe harboured similar assemblages of Cichlidogyrus, consisting of six morphospecies (Figure 4). Only two host species (P. pundamilia, P. nyererei) differed from another radiation member, N. gigas (both p = .036; Table S6a). This difference was not significant when considering only morphospecies presence/absence (Jaccard indices, Table S6b). When excluding host species represented by less than 5 individuals, we observed the same pattern (Table S6c, d).
FIGURE 4.
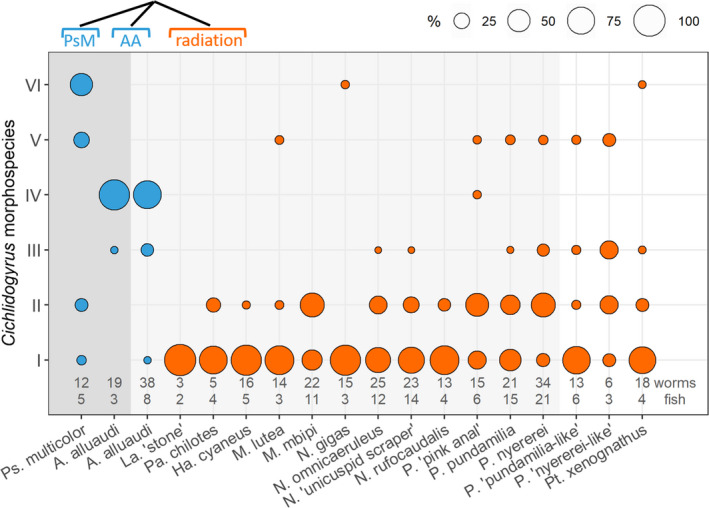
Morphospecies of Cichlidogyrus infecting cichlid species at Sweya (dark grey background), Makobe Island (light grey background) and Kissenda Island (white background). Infection profiles did not differ among species of the radiation (orange), except for seven (of 105) comparisons. Infection profiles differed among host lineages, as highlighted by the simplified host phylogeny on top right (PsM Ps. multicolor, AA A. alluaudi)
To explore differences between species of the radiation and the two species from nonradiating lineages, we examined populations of A. alluaudi from Makobe and Sweya, and Ps. multicolor from Sweya. Compared to the radiation members, the two populations of A. alluaudi had a very different morphospecies assemblage of Cichlidogyrus, dominated by one morphospecies in both populations (no. VI) that was extremely rare in radiation members (seen only twice, in only one species). At Makobe, A. alluaudi differed significantly from almost all radiation members, both considering zero‐adjusted Bray–Curtis distances and Jaccard indices (except La. sp. ‘stone’ and M. lutea, both p = .064, probably not reaching statistical significance because of the low sample sizes for these two species; Table 5 and Table S7b). The characteristic morphospecies community of Cichlidogyrus of A. alluaudi at Makobe was also found in the Sweya population of this species. Analysis revealed a significant difference in monogenean community composition between allopatric A. alluaudi, but this is probably due to their very different sample size (both in terms of fish—8 Makobe versus. 3 Sweya—and parasite numbers—38 Makobe versus. 19 Sweya). The difference disappeared when simulating a larger sample size for Sweya. Ps. multicolor had yet another infection profile, significantly different from the sympatric A. alluaudi (zero‐adjusted Bray–Curtis p = .047, Jaccard p = .035), from A. alluaudi inhabiting Makobe (p = .008, p = .007) and from several radiation members at Makobe (5 of 12 species). Both diversity indices (zero‐adjusted Bray–Curtis and Jaccard) revealed the same pattern, indicating that differences observed in Cichlidogyrus communities are due to both numbers and presence/absence of morphospecies of Cichlidogyrus. When excluding host species represented by less than 5 individuals, we observed the same patterns (Table S7c, d).
TABLE 5.
Differences in Cichlidogyrus community between cichlid host species of the radiating and nonradiating lineages at Makobe, Sweya and Kissenda locations
| Ps. multicolor | A. alluaudi | A. alluaudi | La. sp. 'stone' | Pa. chilotes | Ha. cyaneus | M. lutea | M. mbipi | N. gigas | N. omnicaeruleus | N. sp. 'unicuspid scraper' | N. rufocaudalis | P. sp. 'pink anal' | P. pundamilia | P. nyererei | P. sp. 'pundamilia‐like' | P. sp. 'nyererei‐like' | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sweya | Makobe | Kissenda | |||||||||||||||||
| Nonradiating lineages | Radiating lineage | ||||||||||||||||||
| Nonradiating | Sweya | A. alluaudi | 0.893* | ||||||||||||||||
| Radiation | Makobe | A. alluaudi | 0.480** | 0.393 | |||||||||||||||
| La. sp. 'stone' | 0.344 | 0.834 | 0.357 | ||||||||||||||||
| Pa. chilotes | 0.367 | 0.845. | 0.625* | 0.125 | |||||||||||||||
| Ha. cyaneus | 0.688* | 0.924* | 0.775* | −0.073 | 0.344 | ||||||||||||||
| M. lutea | 0.604 | 0.972 | 0.750 | 1.000 | 1.000 | −0.100 | |||||||||||||
| M. mbipi | 0.427* | 0.872** | 0.711** | 0.176 | 0.025 | 0.355. | 0.516. | ||||||||||||
| N. gigas | 0.787. | 0.964. | 0.759* | 1.000 | 1.000. | −0.036 | 0.000 | 0.620* | |||||||||||
| N. omnicaeruleus | 0.362* | 0.763* | 0.528** | −0.235 | −0.235 | −0.010 | 0.143 | 0.013 | 0.257 | ||||||||||
| N. sp. 'unicuspid scraper' | 0.543** | 0.875** | 0.795** | −0.133 | −0.199 | 0.166 | 0.535* | 0.082 | 0.553* | −0.016 | |||||||||
| N. rufocaudalis | 0.601. | 0.919. | 0.719* | 0.125 | 0.427 | −0.153 | −0.071 | 0.250. | 0.222 | −0.077 | 0.111 | ||||||||
| P. sp. 'pink anal' | 0.171 | 0.700 | 0.315** | −0.195 | −0.152 | 0.132 | 0.234 | 0.066 | 0.375 | −0.030 | 0.126 | 0.026 | |||||||
| P. pundamilia | 0.560* | 0.853** | 0.770** | −0.297 | −0.046 | 0.073 | 0.578. | 0.276 | 0.639** | −0.102 | −0.003 | 0.119 | 0.009 | ||||||
| P. nyererei | 0.292. | 0.795* | 0.523** | −0.112 | −0.228 | 0.154* | 0.346. | 0.006 | 0.493** | −0.025 | 0.001. | 0.046 | −0.025 | −0.015 | |||||
| Kissenda | P. sp. 'pundamilia‐like' | 0.120 | 0.811 | 0.333* | 0.125 | 0.398 | 0.615. | 0.333 | 0.211 | 0.796 | 0.097 | 0.330 | 0.491. | −0.083 | 0.491 | 0.045 | |||
| P. sp. 'nyererei‐like' | 0.281* | 0.792* | 0.546** | 0.256 | 0.056 | 0.440 | 0.548 | 0.022. | 0.781. | 0.129 | 0.149 | 0.272 | 0.098 | 0.281 | 0.038. | −0.003. | |||
| Pt. xenognathus | 0.620. | 0.876. | 0.667* | −0.125 | 0.352 | −0.103 | −0.417 | 0.346 | 0.037 | 0.025 | 0.277 | −0.111 | 0.046 | 0.167 | 0.222. | 0.315 | 0.485 | ||
Cichlidogyrus community composition of A. alluaudi (nonradiating lineage) was similar at Makobe and Sweya but differed from most radiation species. Within the radiation, most species at Makobe had similar Cichlidogyrus communities, also similar to radiation members at Kissenda. Differences are expressed as R values, derived from ANOSIM based on zero‐adjusted Bray–Curtis distances of morphospecies abundances, Benjamini–Hochberg correction, 9,999 permutations.
The highly similar infection profiles of Cichlidogyrus morphospecies in A. alluaudi from different habitats and locations (Sweya and Makobe) suggest that host species identity determines infection much more than geographical location. To verify this, we also analysed three additional species of the radiation from a third location, Kissenda. At Kissenda, P. sp. ‘pundamilia‐like’ and P. sp. ‘nyererei‐like’ had infection profiles that were highly similar to that of their counterparts at Makobe, P. pundamilia and P. nyererei (p = .614, p = .547, respectively) despite their substantial geographical distance (23.1 km).
The Makobe sample included only two molluscivore species (La. sp. ‘stone’ and A. alluaudi). To assess whether the distinct infection profile of A. alluaudi could be explained by its molluscivore diet, we therefore also sampled Pt. xenognathus at Kissenda, which is a radiation member (but does not occur at Makobe). The two radiation molluscivores (Pt. xenognathus at Kissenda and La. sp. ‘stone’ at Makobe) had similar Cichlidogyrus assemblages (p = .758) that differed from that of A. alluaudi at Makobe (p = .034, Table 5, Figure 4). Thus, molluscivory does not explain the characteristic Cichlidogyrus infection profile of A. alluaudi. Within the radiation, Cichlidogyrus community composition did not significantly differ among the three Kissenda species (all p > .093) and among them and other radiation members at Makobe (all p > .051), confirming the modest influence of geographical distance.
4. DISCUSSION
We investigated patterns of ecto‐ and endoparasite infection in Lake Victoria cichlid fish, to explore potential occurrence of parasite‐mediated selection. Consistent with parasite‐mediated speciation, we found significant differences between members of the haplochromine radiation in parasite infection levels and parasite communities. These infection differences could not be attributed to host ecology (depth and diet) and were largely consistent over two sampling years. These findings are in line with two prerequisites of parasite‐mediated speciation: infection differences between closely related host species that are temporally consistent. However, at the morphospecies level for Cichlidogyrus, a common and species‐rich genus of monogeneans, we found homogeneous infection profiles within the Lake Victoria radiation, inconsistent with a role of Cichlidogyrus species in host speciation. We observed divergent Cichlidogyrus infections, that were not due to host ecology nor to geography, only between the radiation cichlids and two distantly related, nonradiating haplochromine lineages. These results suggest that parasite resistance may differ between radiating and nonradiating lineages, but do not support a role of Cichlidogyrus in driving divergence within the Lake Victoria haplochromine radiation.
4.1. Parasite infection differences among species and the role of ecology
Host species had different parasite infection profiles, as also found by previous studies on the same host assemblage (Karvonen et al., 2018; Maan et al., 2008) and as predicted by the first prerequisite of parasite‐mediated speciation (Karvonen & Seehausen, 2012). Significant differences between host species were observed both at the parasite community level and for three of five individual parasite taxa. Cichlid species in Lake Victoria display different ecological specializations, inhabiting different water depth ranges and specializing on different dietary resources (Bouton et al., 1997; Seehausen, 1996; Seehausen & Bouton, 1997). This likely translates into differences in parasite exposure. Intensity of some parasites (Cichlidogyrus spp., L. monodi and nematodes) was indeed associated with water depth, but water depth and diet did not fully explain the variation in infection profile between host species.
Hosts from deeper waters had more L. monodi and fewer Cichlidogyrus and nematodes, consistent with differences in parasite ecology and thereby exposure to those parasites. L. monodi is a fully limnetic copepod with a direct life cycle and its infective stage can survive a few days without a host (Paperna, 1996). These characteristics may lead to high dispersal and allow L. monodi to infect deep‐water dwelling fish. Representatives of Cichlidogyrus have a direct life cycle: eggs are released by adults from the fish host and the infective free‐swimming larvae have only a few hours to find a suitable host (Paperna, 1996). Higher host densities in shallow waters may provide favourable conditions for Cichlidogyrus transmission. Nematodes were found in the abdominal cavity only, indicating that cichlids are intermediate hosts (Yanong, 2002). Most nematodes have an indirect life cycle with birds as intermediate hosts that release eggs through faeces. Thus, nematode transmission is highest close to the shoreline, where birds live, and in shallow waters, as discussed below. Some parasites (E. lamellifer and glochidia) were not linked to host species, diet or water depth, suggesting that other factors may determine their infection prevalence and intensity, or that E. lamellifer and glochidia are generalist parasites that equally infect all sampled radiation members. Many ergasilids are known to specialize on specific infection sites on fish gills, rather than specific host species (Fryer, 1968; Scholz et al., 2018). Although glochidia are the parasitic larval forms of several bivalve species, they were not more common in molluscivore hosts than in other trophic groups, suggesting that glochidia are not directly ingested trophically.
Endoparasites (dominated by nematodes) showed different prevalences among host species, and variation in intensity across species and water depth ranges, suggesting that they could contribute to divergent selection. In particular, all individuals of two host species (P. pundamilia and M. lutea) were infected by high numbers of nematodes. Both species live cryptically in very shallow water (1 m) and close to the rocky shore (Seehausen, 1996), which likely exposes them to nematode eggs released through faeces of piscivorous birds. Similar patterns were observed in 2003 by Maan et al. (2008), who found that all P. pundamilia were infected by nematodes, and with higher intensity than its deeper‐ and more offshore‐dwelling sister species P. nyererei.
Overall, our results are in line with a previous study on the same host species assemblage (Karvonen et al., 2018). In both that study and ours (sampling years 2010 and 2014), some parasite taxa were related to host depth and diet, but host species identity was always the strongest predictor of infection. The observation that infection divergence between host species could not be explained by ecological factors alone suggests the presence of host species‐specific resistance or tolerance, against the parasites that are most important for that particular host species. However, disentangling the contributions of exposure, resistance, tolerance and susceptibility to variation in infection requires experimental manipulation.
4.2. Variation in parasite infections between years
For the two most prevalent ectoparasite taxa, Cichlidogyrus and L. monodi, differences between host species in infection parameters were similar between sampling years. This was true within the radiation but also within sister species pairs: most pairs maintained the direction of the infection difference between them for these two taxa (as well as for glochidia). In an earlier study in one of those species pairs (Pundamilia), sampled in 2003, Maan et al. (2008) reported the same direction of infection difference. In the context of rapid evolution, as for the Lake Victoria radiation, even short‐term fluctuations in divergent selection may be important for the evolution of reproductive isolation (Siepielski, DiBattista, & Carlson, 2009). Therefore, the maintenance of species differences in infection, even over the relatively short time frames studied here (a period of 4 years for most species; a 16‐year period for Pundamilia sp. when including the 2003 investigation by Maan et al. (2008)), is noteworthy and suggests that an important prerequisite for divergent selection may be met. However, the potentially rapid turnover of MHC alleles and stochasticity in the direction of parasite‐mediated selection (Eizaguirre et al., 2009b; Lenz, Eizaguirre, Scharsack, Kalbe, & Milinski, 2009) shows that longer‐term studies are still needed. Also, we did not find consistency for all parasites. For example, E. lamellifer did not show temporal constancy for most of the host species pairs. Because of the low prevalence of this parasite, this finding is difficult to interpret.
The observed consistency in the direction of parasite‐mediated selection occurred despite variation in its strength, that is despite fluctuations between years in overall ectoparasite intensity. Both copepods and glochidia showed lower infection intensity in 2014 than in 2010. This is in line with Maan et al. (2006), who found that the abundance of parasites varies between years. This variation could result from temporal variation in various ecological factors (e.g. host abundance, water chemistry, climate) and/or from interspecific competition between parasites. For example, Maan et al. (2006) observed that an increase in the abundance of L. monodi coincided with a decrease in Cichlidogyrus. We found a similar pattern: the abundance of Cichlidogyrus tended to increase from 2010 to 2014, while the abundance of L. monodi decreased. Observations in other fish species have also suggested antagonistic interactions between gill‐infecting copepods and monogeneans (Baker, Pante, & de Buron, 2005).
4.3. Host phylogenetic signature of Cichlidogyrus infection
Within the radiation, host species differed in the prevalence and intensity of Cichlidogyrus infection. However, the species community of Cichlidogyrus was similar within the radiation, contrary to our prediction of parasite‐driven diversification. This homogeneity in infection among recently arisen host species indicates that Cichlidogyrus morphospecies‐mediated selection does not contribute to the early stages of speciation.
In contrast to the pattern within the radiation, prevalence and intensity of Cichlidogyrus differed significantly between all radiation members and A. alluaudi (Figure 2). This host species showed a 100% prevalence and harboured high numbers of Cichlidogyrus (2.5 times higher than the most heavily infected radiation species, Ha. cyaneus). Species identification of Cichlidogyrus revealed that this high intensity in A. alluaudi was not due to the accumulation of many worm morphospecies, but resulted from a high number of individuals from a limited number of morphospecies. These findings are partially in contrast to our hypothesis of parasite‐mediated selection. Cichlidogyrus‐mediated divergent selection should result in lower infection intensities in radiation members, which we observed, but also in fewer morphospecies per host, and more differentiated morphospecies communities among hosts—which we did not observe. Moreover, the other representative from a nonradiating lineage, Ps. multicolor, did not exhibit higher Cichlidogyrus infection than radiation members (Table 2). Thus, our findings suggest that while radiating and nonradiating lineages may differ in Cichlidogyrus resistance, variation in infection profiles within the radiation do not result from species‐specific resistance.
The high intensity of Cichlidogyrus in A. alluaudi cannot be explained by its molluscivore diet, as two molluscivore radiation members (La. sp. ‘stone’ and Pt. xenognathus) had much lower infections. Likewise, the community composition of morphospecies of Cichlidogyrus was significantly different between A. alluaudi and the two molluscivore radiation members (Figure 4). The other old and nonradiating lineage, represented by Ps. multicolor, harboured a community of Cichlidogyrus that differed from radiation members as well as from A. alluaudi. The pattern that emerges is that, with a few exceptions, morphospecies of Cichlidogyrus that infect members of the radiation do not infect old lineages and vice versa. This lineage specificity occurs even in the presence of many sympatric host species, providing ample opportunity for host switching. Possibly, cross‐infection between lineages is hampered by specific co‐evolutionary adaptations in the old lineages of A. alluaudi and Ps. multicolor, which prevents these morphospecies from infecting the radiating lineage. Colonization of other host species, phylogenetically related or co‐occurring with the original host, has been observed previously in monogeneans infecting gobies (Huyse & Volckaert, 2005) and cichlids (Mendlová, Desdevises, Civáňová, Pariselle, & Šimková, 2012), indicating that parasites can colonize host species that represent a similar resource without any prior novelty evolution (Agosta & Klemens, 2008). The Lake Victoria radiation may be too recent to represent multiple different resources for parasites and thus to allow for co‐evolutionary differentiation. A similar pattern was observed in closely related cichlids of West African rivers and lakes, which were infected by similar monogenean assemblages (Pariselle et al., 2003). In contrast, the representatives of Cichlidogyrus infecting the much older Lake Tanganyika cichlid tribes generally exhibit higher host specificity (Pariselle et al., 2015).
Morphospecies of Cichlidogyrus that dominate in radiation members were rare in the two nonradiating lineages and vice versa. This suggests that Cichlidogyrus species did not simply sort among cichlid species during the radiation. Instead, ancestral Cichlidogyrus may have adapted to the new niche provided by the radiation, and subsequently diversified into the currently observed morphospecies—thus specializing on the radiating lineage as a whole, without within‐radiation differentiation. Genetic analysis is required to resolve this (as in Vanhove et al., 2015). Such analysis may also reveal genetic variation within morphospecies, potentially uncovering more differentiated infections within the radiation. Indeed, molecular investigations have already revealed the presence of several cryptic Cichlidogyrus species that are more host‐specific than the currently recognized morphospecies (e.g. monogeneans Pouyaud, Desmarais, Deveney, & Pariselle, 2006; trematodes Donald, Kennedy, Poulin, & Spencer, 2004, Jousson, Bartoli, & Pawlowski, 2000).
In addition to differences at the level of host lineages, assemblages of Cichlidogyrus morphospecies may align with host genus. For example, Pundamilia spp. (including five species, from two locations) had infection patterns that were more similar to each other than to other radiation members. The same was observed for three species of Neochromis (not for N. gigas, but sample size was low for this species). These patterns corroborate the phylogenetic signature of Cichlidogyrus infections, but require more systematic analysis.
Monogenean intensity differed between sampling sites. At Makobe, A. alluaudi harboured a high Cichlidogyrus intensity, whereas its allopatric conspecifics at Sweya, as well as the representative of the other old lineage sampled there (Ps. multicolor), had low numbers of Cichlidogyrus. Abundances of all ectoparasites at Sweya were very low in both host species sampled there, compared to those observed in the radiation members at Makobe. Differences in parasite abundances among sampling sites were also found in Lake Tanganyika haplochromine cichlids (Hablützel et al., 2017; Raeymaekers et al., 2013). The overall lower ectoparasite abundance at Sweya may be explained by habitat conditions. Sweya is a vegetated swampy stream inlet, inhabited by only five fish species, at low abundances. Makobe is a rocky offshore reef inhabited by a large cichlid community with several highly abundant species and several noncichlids (Seehausen, 1996). Low abundance and low diversity of hosts may therefore explain the low numbers of parasites at Sweya. This is in line with Karvonen et al. (2018), who found that within the Makobe community, host‐specific parasite abundance was positively correlated with host‐specific population abundance.
Despite differences in overall Cichlidogyrus abundance, the community composition of Cichlidogyrus in different host lineages was consistent across sampling sites. Allopatric A. alluaudi at Makobe and Sweya were infected by identical assemblages (Figure 4). The same pattern was observed in four host species from the radiating lineage, sampled at Makobe and Kissenda: two closely related species pairs (P. nyererei and P. sp. ‘nyererei‐like’, P. pundamilia and P. sp. ‘pundamilia‐like’) and allopatric species from the same guild (molluscivores La. sp. ‘stone’ and Pt. xenognathus) had the same community of morphospecies of Cichlidogyrus at the two locations. The maintenance of parasite community composition despite geographical separation is consistent with observations in Lake Tanganyika, where allopatric populations of tropheine cichlids harboured the same Cichlidogyrus species, while sympatric host species had different infection profiles (Grégoir et al., 2015; Vanhove et al., 2015).
5. CONCLUSION
At parasite community level, we found significant differences in infection profiles between host species that were consistent over time. These findings support parasite‐mediated selection in Lake Victoria cichlids. However, the association between host species divergence and parasite infection depended on the parasite taxon considered. At the level of morphospecies community of Cichlidogyrus, infection profiles were similar within the radiation but different between host lineages. This is not consistent with parasite‐mediated diversification within the Lake Victoria radiation. Future genetic analysis of Cichlidogyrus morphospecies may reveal cryptic parasite diversity between host species within the radiation that could be congruent with parasite‐mediated diversification.
AUTHOR CONTRIBUTIONS
OS, MEM and TGG conceived the study. TPG collected the data. TPG, MPMV and AP identified parasite species. TPG analysed data, with contribution from MEM and OS. TPG, MEM, MPMV and OS wrote the manuscript with input from all authors. All authors approved the final version of the manuscript.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
This research was funded by the University of Bern and the University of Groningen (Ubbo Emmius Programme). Infrastructure was provided by the Natural History Museum in Lugano and Hasselt University (EMBRC Belgium ‐ FWO project GOH3817N). We acknowledge Iva Přikrylová for confirmation of Gyrodactylus identification and Anssi Karvonen for sharing data. We also thank Michiel Jorissen and Chahrazed Rahmouni for the hospitality at Hasselt University to TPG and for sharing their insights on Cichlidogyrus morphology, Oliver Selz for help with cichlid species identification and Ariane LeGrand for help with endoparasite screening.
Gobbin TP, Vanhove MPM, Pariselle A, Groothuis TGG, Maan ME, Seehausen O. Temporally consistent species differences in parasite infection but no evidence for rapid parasite‐mediated speciation in Lake Victoria cichlid fish. J Evol Biol. 2020;33:556–575. 10.1111/jeb.13615
Maan and Seehausen contributed equally.
Data deposited at Dryad: https://doi.org/10.5061/dryad.44j0zpc9s
REFERENCES
- Agosta, S. J. , & Klemens, J. A. (2008). Ecological fitting by phenotypically flexible genotypes: Implications for species associations, community assembly and evolution. Ecology Letters, 11, 1123–1134. [DOI] [PubMed] [Google Scholar]
- Baird, S. J. E. , Ribas, A. , Macholán, M. , Albrecht, T. , Piálek, J. , & Goüy de Bellocq, J. (2012). Where are the wormy mice? A reexamination of hybrid parasitism in the European house mouse hybrid zone. Evolution, 66, 2757–2772. [DOI] [PubMed] [Google Scholar]
- Baker, T. G. , Pante, E. , & de Buron, I. (2005). Co‐occurrence of Naobranchia lizae (Copepoda) and Metamicrocotyla macracantha (Monogenea), gill parasites of the striped mullet Mugil cephalus . Parasitology Research, 97, 515–520. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Bonneaud, C. , Pérez‐Tris, J. , Federici, P. , Chastel, O. , & Sorci, G. (2006). Major histocompatibility alleles associated with local resistance to malaria in a passerine. Evolution, 60, 383–389. [PubMed] [Google Scholar]
- Boundenga, L. , Moussadji, C. , Mombo, I. M. , Ngoubangoye, B. , Lekana‐Douki, J. B. , & Hugot, J.‐P. (2018). Diversity and prevalence of gastrointestinal parasites in two wild Galago species in Gabon. Infection, Genetics and Evolution, 63, 249–256. [DOI] [PubMed] [Google Scholar]
- Bouton, N. , Seehausen, O. , & van Alphen, J. J. M. (1997). Resource partitioning among rock‐dwelling haplochromines (Pisces: Cichlidae) from Lake Victoria. Ecology of Freshwater Fish, 6, 225–240. [Google Scholar]
- Brawand, D. , Wagner, C. , Li, Y. I. , Malinsky, M. , Keller, I. , Fan, S. , … Di Palma, F. (2014). The genomic substrate for adaptive radiation in African cichlid fish. Nature, 513, 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbayo, J. , Martin, J. , & Civantos, E. (2018). Habitat type influences parasite load in Algerian Psammodromus lizards (Psammodromus algirus). Canadian Journal of Zoology, 97, 172–180. [Google Scholar]
- Careau, V. , Thomas, D. W. , & Humphries, M. M. (2010). Energetic cost of bot fly parasitism in free‐ranging eastern chipmunks. Oecologia, 162, 303–312. [DOI] [PubMed] [Google Scholar]
- Chen, J. , Zhi, T. , Xu, X. , Zhang, S. , Zheng, Y. , & Yang, T. (2019). Molecular characterization and dynamic expressions of three Nile tilapia (Oreochromis niloticus) complement genes after Gyrodactylus cichlidarum (Monogenea) infection. Aquaculture, 502, 176–188. [Google Scholar]
- Coustau, C. , Renaud, F. , Maillard, C. , Pasteur, N. , & Delay, B. (1991). Differential susceptibility to a trematode parasite among genotypes of the Mytilus edulis/galloprovincialis complex. Genetical Research, 57, 207–212. [DOI] [PubMed] [Google Scholar]
- Decaestecker, E. , Gaba, S. , Raeymaekers, J. A. M. , Stoks, R. , Van Kerckhoven, L. , Ebert, D. , & De Meester, L. (2007). Host–parasite ‘Red Queen’ dynamics archived in pond sediment. Nature, 450, 870–873. [DOI] [PubMed] [Google Scholar]
- Donald, K. M. , Kennedy, M. , Poulin, R. , & Spencer, H. G. (2004). Host specificity and molecular phylogeny of larval Digenea isolated from New Zealand and Australian topshells (Gastropoda: Trochidae). International Journal for Parasitology, 34, 557–568. [DOI] [PubMed] [Google Scholar]
- Eizaguirre, C. , Lenz, T. L. , Kalbe, M. , & Milinski, M. (2012). Divergent selection on locally adapted major histocompatibility complex immune genes experimentally proven in the field. Ecology Letters, 15, 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizaguirre, C. , Lenz, T. L. , Sommerfeld, R. D. , Harrod, C. , Kalbe, M. , & Milinski, M. (2011). Parasite diversity, patterns of MHC II variation and olfactory based mate choice in diverging three‐spined stickleback ecotypes. Evolutionary Ecology, 25(3), 605–622. 10.1007/s10682-010-9424-z [DOI] [Google Scholar]
- Eizaguirre, C. , Yeates, S. E. , Lenz, T. L. , Kalbe, M. , & Milinski, M. (2009a). MHC‐based mate choice combines good genes and maintenance of MHC polymorphism. Molecular Ecology, 18, 3316–3329. [DOI] [PubMed] [Google Scholar]
- El Nagar, A. , & MacColl, A. D. C. (2016). Parasites contribute to ecologically dependent postmating isolation in the adaptive radiation of three‐spined stickleback. Proceedings of the Royal Society B: Biological Sciences, 283(1836), 20160691– 10.1098/rspb.2016.0691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feulner, P. G. D. , Chain, F. J. J. , Panchal, M. , Huang, Y. , Eizaguirre, C. , Kalbe, M. , … Milinski, M. (2015). Genomics of Divergence along a Continuum of Parapatric Population Differentiation. PLOS Genetics, 11, e1004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz, R. S. , Nichols‐Orians, C. M. , & Brunsfeld, S. J. (1994). Interspecific hybridization of plants and resistance to herbivores: Hypotheses, genetics, and variable responses in a diverse herbivore community. Oecologia, 97, 106–117. [DOI] [PubMed] [Google Scholar]
- Fryer, G. (1968). The parasitic Crustacea of African freshwater fishes; their biology and distribution. Journal of Zoology, 156, 45–95. [Google Scholar]
- Galipaud, M. , Bollache, L. , & Lagrue, C. (2017). Variations in infection levels and parasite‐induced mortality among sympatric cryptic lineages of native amphipods and a congeneric invasive species: Are native hosts always losing? International Journal for Parasitology: Parasites and Wildlife, 6, 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grégoir, A. F. , Hablützel, P. I. , Vanhove, M. P. M. , Pariselle, A. , Bamps, J. , Volckaert, F. A. M. , & Raeymaekers, J. A. M. (2015). A link between host dispersal and parasite diversity in two sympatric cichlids of Lake Tanganyika. Freshwater Biology, 60, 323–335. [Google Scholar]
- Greischar, M. A. , & Koskella, B. (2007). A synthesis of experimental work on parasite local adaptation. Ecology Letters, 10, 418–434. [DOI] [PubMed] [Google Scholar]
- Hablützel, P. I. , Vanhove, M. P. M. , Deschepper, P. , Grégoir, A. F. , Roose, A. K. , Volckaert, F. , & Raeymaekers, J. a. M. (2017). Parasite escape through trophic specialization in a species flock. Journal of Evolutionary Biology, 30, 1437–1445. [DOI] [PubMed] [Google Scholar]
- Hamilton, W. , & Zuk, M. (1982). Heritable true fitness and bright birds: A role for parasites? Science, 218, 384–387. [DOI] [PubMed] [Google Scholar]
- Hammer, O. , Harper, D. A. T. , & Ryan, P. D. (2001). PAST: Paleontological Statistics Software Package for Education and Data Analysis.
- Hayward, A. , Tsuboi, M. , Owusu, C. , Kotrschal, A. , Buechel, S. D. , Zidar, J. , … Kolm, N. (2017). Evolutionary associations between host traits and parasite load: Insights from Lake Tanganyika cichlids. Journal of Evolutionary Biology, 30, 1056–1067. [DOI] [PubMed] [Google Scholar]
- Huyse, T. , & Volckaert, F. A. M. (2005). Comparing Host and Parasite Phylogenies: Gyrodactylus Flatworms Jumping from Goby to Goby. Systematic Biology, 54, 710–718. [DOI] [PubMed] [Google Scholar]
- Janeway, C. A. , Travers, P. , Walport, M. , & Shlomchik, M. J. (2005). Immunobiology: The immune system in health and disease (6th edn.). London: Garland Publishing. [Google Scholar]
- Jousson, O. , Bartoli, P. , & Pawlowski, J. (2000). Cryptic speciation among intestinal parasites (Trematoda: Digenea) infecting sympatric host fishes (Sparidae). Journal of Evolutionary Biology, 13, 778–785. [Google Scholar]
- Karvonen, A. , Lucek, K. J. O. , Marques, D. A. , & Seehausen, O. (2015). Divergent Macroparasite Infections in Parapatric Swiss Lake‐Stream Pairs of Threespine Stickleback (Gasterosteus aculeatus). PLoS ONE, 10, e0130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvonen, A. , & Seehausen, O. (2012). The Role of parasitism in adaptive radiations—when might parasites promote and when might they constrain ecological speciation? International Journal of Ecology, 2012, 1–20. [Google Scholar]
- Karvonen, A. , Wagner, C. E. , Selz, O. M. , & Seehausen, O. (2018). Divergent parasite infections in sympatric cichlid species in Lake Victoria. Journal of Evolutionary Biology, 31, 1313–1329. [DOI] [PubMed] [Google Scholar]
- Keller, I. , Wagner, C. E. , Greuter, L. , Mwaiko, S. , Selz, O. M. , Sivasundar, A. , … Seehausen, O. (2013). Population genomic signatures of divergent adaptation, gene flow and hybrid speciation in the rapid radiation of Lake Victoria cichlid fishes. Molecular Ecology, 22, 2848–2863. [DOI] [PubMed] [Google Scholar]
- Kocher, T. D. (2004). Adaptive evolution and explosive speciation: The cichlid fish model. Nature Reviews Genetics, 5, 288–298. [DOI] [PubMed] [Google Scholar]
- Kornfield, I. , & Smith, P. F. (2000). African cichlid fishes: Model systems for evolutionary biology. Annual Review of Ecology and Systematics, 31, 163–196. [Google Scholar]
- Lenz, T. L. , Eizaguirre, C. , Scharsack, J. P. , Kalbe, M. , & Milinski, M. (2009). Disentangling the role of MHC‐dependent ‘good genes' and ‘compatible genes' in mate‐choice decisions of three‐spined sticklebacks Gasterosteus aculeatus under semi‐natural conditions. Journal of Fish Biology, 75, 2122–2142. [DOI] [PubMed] [Google Scholar]
- Maan, M. , van Rooijen, A. , van Alphen, J. , & Seehausen, O. (2008). Parasite‐mediated sexual selection and species divergence in Lake Victoria cichlid fish. Biological Journal of the Linnean Society, 94, 53–60. [Google Scholar]
- Maan, M. E. , & Seehausen, O. (2011). Ecology, sexual selection and speciation. Ecology Letters, 14, 591–602. [DOI] [PubMed] [Google Scholar]
- Maan, M. E. , van der Spoel, M. , Jimenez, P. Q. , van Alphen, J. J. M. , & Seehausen, O. (2006). Fitness correlates of male coloration in a Lake Victoria cichlid fish. Behavioral Ecology, 17, 691–699. [Google Scholar]
- MacColl, A. D. C. (2009). Parasite burdens differ between sympatric three‐spined stickleback species. Ecography, 32, 153–160. [Google Scholar]
- Magalhaes, I. S. , Lundsgaard‐Hansen, B. , Mwaiko, S. , & Seehausen, O. (2012). Evolutionary divergence in replicate pairs of ecotypes of Lake Victoria cichlid fish. Evolutionary Ecology, 14, 381–401. [Google Scholar]
- Mattiucci, S. , Cipriani, P. , Paoletti, M. , Nardi, V. , Santoro, M. , Bellisario, B. , & Nascetti, G. (2015). Temporal stability of parasite distribution and genetic variability values of Contracaecum osculatum sp. D and C. osculatum sp. E (Nematoda: Anisakidae) from fish of the Ross Sea (Antarctica). International Journal for Parasitology: Parasites and Wildlife, 4, 356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendlová, M. , Desdevises, Y. , Civáňová, K. , Pariselle, A. , & Šimková, A. (2012). Monogeneans of West African Cichlid Fish: Evolution and Cophylogenetic Interactions. PLoS ONE, 7, e37268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messu Mandeng, F. D. , Bilong Bilong, C. F. , Pariselle, A. , Vanhove, M. P. M. , Bitja Nyom, A. R. , & Agnèse, J.‐F. (2015). A phylogeny of Cichlidogyrus spp. (Monogenea, Dactylogyridea) clarifies a host‐switch between fish families and reveals an adaptive component to attachment organ morphology of this parasite genus. Parasites & Vectors, 8, 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milinski, M. , & Bakker, T. C. M. (1990). Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature, 344(6264), 330–333. 10.1038/344330a0. [DOI] [Google Scholar]
- Muterezi Bukinga, F. , Vanhove, M. P. M. , Steenberge, M. V. , & Pariselle, A. (2012). Ancyrocephalidae (Monogenea) of Lake Tanganyika: III: Cichlidogyrus infecting the world’s biggest cichlid and the non‐endemic tribes Haplochromini, Oreochromini and Tylochromini (Teleostei, Cichlidae). Parasitology Research, 111, 2049–2061. [DOI] [PubMed] [Google Scholar]
- Paperna, I. (1996). Parasites, infections and diseases of fishes in Africa, an update. Parasites, infections and diseases of fishes in Africa, an update. CIFA Technical Paper. [Google Scholar]
- Pariselle, A. , & Euzet, L. (2009). Systematic revision of dactylogyridean parasites (Monogenea) from cichlid fishes in Africa, the Levant and Madagascar. Zoosystema, 31, 849–898. [Google Scholar]
- Pariselle, A. , Morand, S. , Deveney, M. R. , & Pouyaud, L. (2003). Parasite species richness of closely related hosts: historical scenario and "genetic" hypothesis In Combes C., Jourdane J., Pages J. R., & Modat A. (Eds.), Taxonomie, écologie et évolution des métazoaires parasites (livre hommage à Louis Euzet (pp. 147–163). Perpignan: Etudes. Presses Universitaires de Perpignan. [Google Scholar]
- Pariselle, A. , Muterezi Bukinga, F. , Van Steenberge, M. , & Vanhove, M. P. M. (2015). Ancyrocephalidae (Monogenea) of Lake Tanganyika: IV: Cichlidogyrus parasitizing species of Bathybatini (Teleostei, Cichlidae): Reduced host‐specificity in the deepwater realm? Hydrobiologia, 748, 99–119. [Google Scholar]
- Paterson, S. , Wilson, K. , & Pemberton, J. M. (1998). Major histocompatibility complex variation associated with juvenile survival and parasite resistance in a large unmanaged ungulate population. Proceedings of the National Academy of Sciences of the United States of America, 95, 3714–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin, R. , & Morand, S. (2000). The diversity of parasites. The Quarterly Review of Biology, 75, 277–293. [DOI] [PubMed] [Google Scholar]
- Pouyaud, L. , Desmarais, E. , Deveney, M. , & Pariselle, A. (2006). Phylogenetic relationships among monogenean gill parasites (Dactylogyridea, Ancyrocephalidae) infesting tilapiine hosts (Cichlidae): Systematic and evolutionary implications. Molecular Phylogenetics and Evolution, 38, 241–249. [DOI] [PubMed] [Google Scholar]
- R Core Team . (2019). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Raeymaekers, J. A. M. , Hablützel, P. I. , Grégoir, A. F. , Bamps, J. , Roose, A. K. , Vanhove, M. P. M. , … Volckaert, F. A. M. (2013). Contrasting parasite communities among allopatric colour morphs of the Lake Tanganyika cichlid Tropheus. BMC Evolutionary Biology, 13, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle, H. D. , & Nosil, P. (2005). Ecological speciation. Ecology Letters, 8, 336–352. [Google Scholar]
- Schedel, F. D. B. , Musilova, Z. , & Schliewen, U. K. (2019). East African cichlid lineages (Teleostei: Cichlidae) might be older than their ancient host lakes: New divergence estimates for the east African cichlid radiation. BMC Evolutionary Biology, 19, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter, D. (1996). Ecological causes of adaptive radiation. The American Naturalist, 148, S40–S64. 10.1086/285901. [DOI] [Google Scholar]
- Schluter, D. (2000). The ecology of adaptive radiation. Oxford, UK: Oxford University Press. [Google Scholar]
- Schmid‐Hempel, P. (2013). Evolutionary Parasitology: The integrated study of infections, immunology, ecology, and genetics. Oxford: Oxford University Press. [Google Scholar]
- Scholz, T. , Vanhove, M. P. M. , Smit, N. , Jayasundera, Z. , & Gelnar, M. (2018). A guide to the parasites of African freshwater fishes. Brussels, Belgium: CEBioS , Royal Belgian Institute of Natural Sciences. [Google Scholar]
- Seehausen, O. (1996). Lake Victoria rock cichlids: Taxonomy, ecology, and distribution (1st edn.). Zevenhuizen, the Netherlands: Verduyn Cichlids. [Google Scholar]
- Seehausen, O. (2006). African cichlid fish: a model system in adaptive radiation research. Proceedings of the Royal Society B: Biological Sciences, 273(1597), 1987–1998. 10.1098/rspb.2006.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen, O. (2015). Process and pattern in cichlid radiations ‐ inferences for understanding unusually high rates of evolutionary diversification. New Phytologist, 207, 304–312. [DOI] [PubMed] [Google Scholar]
- Seehausen, O. , & Bouton, N. (1997). Microdistribution and fluctuations in niche overlap in a rocky shore cichlid community in Lake Victoria. Ecology of Freshwater Fish, 6, 161–173. [Google Scholar]
- Seehausen, O. , & Bouton, N. (1998). The community of rock dwelling cichlids in Lake Victoria. Bonner Zoologische Beiträge, 47, 301–311. [Google Scholar]
- Segar, S. T. , Mardiastuti, A. , Wheeler, P. M. , & Cook, J. M. (2018). Detecting the elusive cost of parasites on fig seed production. Acta Oecologica, 90, 69–74. [Google Scholar]
- Siepielski, A. M. , DiBattista, J. D. , & Carlson, S. M. (2009). It’s about time: The temporal dynamics of phenotypic selection in the wild. Ecology Letters, 12, 1261–1276. [DOI] [PubMed] [Google Scholar]
- Stirnadel, H. A. , & Ebert, D. (1997). Prevalence, host specificity and impact on host fecundity of microparasites and epibionts in three sympatric daphnia species. Journal of Animal Ecology, 66, 212–222. [Google Scholar]
- Stutz, W. E. , Lau, O. L. , & Bolnick, D. I. (2014). Contrasting patterns of phenotype‐dependent parasitism within and among populations of threespine stickleback. The American Naturalist, 183, 810–825. [DOI] [PubMed] [Google Scholar]
- Tellenbach, C. , Wolinska, J. , & Spaak, P. (2007). Epidemiology of a Daphnia brood parasite and its implications on host life‐history traits. Oecologia, 154, 369–375. [DOI] [PubMed] [Google Scholar]
- Thomas, F. , Renaud, F. , Rousset, F. , Cezilly, F. , & Meeuûs, T. D. (1995). Differential mortality of two closely related host species induced by one parasite. Proceedings of the Royal Society of London, Series B: Biological Sciences, 260, 349–352. [Google Scholar]
- Vanhove, M. P. M. , Hablützel, P. I. , Pariselle, A. , Šimková, A. , Huyse, T. , & Raeymaekers, J. A. M. (2016). Cichlids: A host of opportunities for evolutionary parasitology. Trends in Parasitology, 32, 820–832. [DOI] [PubMed] [Google Scholar]
- Vanhove, M. P. M. , & Huyse, T. (2015). Host‐specificity and species‐jumps in fish‐parasite systems In Morand S., Krasnov B., & Littlewood D. T. J. (Eds.), Parasite diversity and diversification: evolutionary ecology meets phylogenetics (pp. 401–419). Cambridge: Cambridge University Press. [Google Scholar]
- Vanhove, M. P. M. , Pariselle, A. , Van Steenberge, M. , Raeymaekers, J. A. , Hablützel, P. I. , Gillardin, C. , … Huyse, T. (2015). Hidden biodiversity in an ancient lake: phylogenetic congruence between Lake Tanganyika tropheine cichlids and their monogenean flatworm parasites. Scientific Reports, 5, 13669 10.1038/srep13669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhove, M. P. M. , Snoeks, J. , Volckaert, F. A. M. , & Huyse, T. (2011). First description of monogenean parasites in Lake Tanganyika: The cichlid Simochromis diagramma (Teleostei, Cichlidae) harbours a high diversity of Gyrodactylus species (Platyhelminthes, Monogenea). Parasitology, 138, 364–380. [DOI] [PubMed] [Google Scholar]
- Wagner, C. E. , Keller, I. , Wittwer, S. , Selz, O. M. , Mwaiko, S. , Greuter, L. , … Seehausen, O. (2013). Genome‐wide RAD sequence data provide unprecedented resolution of species boundaries and relationships in the Lake Victoria cichlid adaptive radiation. Molecular Ecology, 22, 787–798. [DOI] [PubMed] [Google Scholar]
- Wagner, C. E. , McCune, A. R. , & Lovette, I. J. (2012). Recent speciation between sympatric Tanganyikan cichlid colour morphs. Molecular Ecology, 21, 3283–3292. [DOI] [PubMed] [Google Scholar]
- Witte, F. , & Oijen, V. M. J. P. (1990). Taxonomy, ecology and fishery of Lake Victoria haplochromine trophic groups. Zoologische Verhandelingen, 262, 1–47. [Google Scholar]
- Yanong, R. P. E. (2002). Nematode (roundworm) infections in fish. Fisheries and Aquatic Sciences Department, Institute of Food and Agricultural Sciences. University of Florida. http://edis.ifas.ufl.edu/fa091
- Zahradníčková, P. , Barson, M. , Luus‐Powell, W. J. , & Přikrylová, I. (2016). Species of Gyrodactylus von Nordmann, 1832 (Platyhelminthes: Monogenea) from cichlids from Zambezi and Limpopo river basins in Zimbabwe and South Africa: Evidence for unexplored species richness. Systematic Parasitology, 93, 679–700. [DOI] [PubMed] [Google Scholar]
- Zhi, T. , Xu, X. , Chen, J. , Zheng, Y. , Zhang, S. , Peng, J. , … Yang, T. (2018). Expression of immune‐related genes of Nile tilapia Oreochromis niloticus after Gyrodactylus cichlidarum and Cichlidogyrus sclerosus infections demonstrating immunosuppression in coinfection. Fish & Shellfish Immunology, 80, 397–404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


