Abstract
Aim
To investigate the association of the Thrombolysis In Myocardial Infarction (TIMI) Risk Score for Heart Failure in Diabetes (TRS‐HFDM) with mortality using data from the EMPA‐REG OUTCOME trial.
Materials and Methods
In EMPA‐REG OUTCOME, patients with type 2 diabetes and atherosclerotic cardiovascular (CV) disease (N = 7020) received the sodium‐glucose co‐transporter‐2 inhibitor, empagliflozin, 10 or 25 mg or placebo. Post hoc, patients were stratified into risk categories (low‐intermediate, high, very‐high risk scores) using baseline TRS‐HFDM. Cox regression analyses evaluated the association of TRS‐HFDM categories with all‐cause mortality (ACM), CV death, hospitalization for heart failure (HHF) and CV death (excluding fatal stroke) or HHF, and whether empagliflozin reduced the risk of CV outcomes across these risk categories.
Results
In placebo patients, increasing risk category was associated with a higher risk of ACM, CV death, and HHF. Empagliflozin reduced the risk of ACM (low‐intermediate HR 0.68 [95% CI 0.48, 0.97] and very‐high 0.69 [0.52, 0.91]), CV death (0.75 [0.48, 1.18] and 0.56 [0.41, 0.78]), HHF (0.53 [0.28, 1.01] and 0.67 [0.48, 0.96]), and CV death or HHF (0.69 [0.46, 1.03]) and (0.64 [0.49, 0.82]) across all risk categories versus placebo. Higher absolute risk reductions (ARRs) were observed for CV death in the very‐high versus low‐intermediate category (P = 0.01).
Conclusions
Applied to EMPA‐REG OUTCOME, higher TRS‐HFDM was associated with increased HHF and mortality risk. Empagliflozin reduced CV outcomes across TRS‐HFDM categories. Higher ARRs were associated with higher risk scores.
Keywords: cardiovascular disease, clinical trial, heart failure, randomized trial, sodium‐glucose co‐transporter‐2 inhibitor, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes (T2D) is still on the rise worldwide with projected estimates of 700 million people with this diagnosis in 2045.1 T2D is associated with decreased life expectancy,2 as well as an increased risk of developing heart failure (HF),3, 4 with poor prognosis when both conditions are present.5 Diabetes is also one of the most common co‐morbidities among patients with established HF.6 Some glucose‐lowering drugs, such as saxagliptin and the thiazolidinediones, appear to increase HF risk.7, 8 However, sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors have been shown to reduce the risk of hospitalization for HF (HHF) in patients with T2D.9 Furthermore, in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD), the EMPA‐REG OUTCOME trial showed that, when added to standard of care, empagliflozin (compared with placebo) significantly reduced the risk of cardiovascular (CV) death by 38%, all‐cause mortality by 32%, and HHF by 35%.10, 11, 12 In addition, given the high risk of premature mortality and HF in patients with T2D, the choice of glucose‐lowering therapies in some patients will be made to reduce the risk of these outcomes.
The Thrombolysis In Myocardial Infarction (TIMI) Risk Score for Heart Failure in Diabetes (TRS‐HFDM) was developed as a practical clinical risk score for identifying patients with T2D at risk of HHF.8 The scoring system attributes numeric values to five risk indicators: prior HF, history of atrial fibrillation (AF), coronary artery disease (CAD), estimated glomerular filtration rate (eGFR), and urine albumin‐to‐creatine ratio (UACR). The risk indicators were found to be independently associated with HHF within the placebo arm of the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53 (SAVOR–TIMI 53) trial, and the scoring system was externally validated in the Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE–TIMI 58 trial).8 The investigators found that TRS‐HFDM identified patients with T2D with the greatest potential absolute risk reduction (ARR) for HHF with an SGLT2 inhibitor.8 The TRS‐HFDM score categorized patients into low‐intermediate, high or very‐high risk of HHF. The TRS‐HFDM has thus been shown to be associated with HHF risk; nonetheless, the treatment effect of dapagliflozin on HHF was consistent throughout all risk categories.8 However, the association of TRS‐HFDM with mortality is unknown. The aim of the current analyses were to determine whether a higher TRS‐HFDM score is associated with an increased risk of CV death, all‐cause mortality and HHF in the EMPA‐REG OUTCOME trial cohort. Furthermore, we evaluated whether the mortality and HHF benefit of empagliflozin was consistent across TRS‐HFDM score categories.
2. MATERIALS AND METHODS
2.1. Study design
This was a post hoc analysis of the EMPA‐REG OUTCOME randomized, double‐blind, placebo‐controlled trial. The trial methods have been previously described but, in brief, patients with T2D with HbA1c of 7.0%‐9.0% for drug‐naïve patients and 7.0%‐10.0% for those on stable glucose‐lowering therapy, established ASCVD, and eGFR ≥30 mL/min/1.73m2, were randomly assigned to empagliflozin 10 or 25 mg or placebo once‐daily in addition to standard of care. A total of 7020 patients were treated, and the trial continued until ≥691 patients experienced the primary endpoint of three‐point major adverse cardiovascular events (3P‐MACE).13 A total of 706 patients (10.1%) had investigator‐reported HF at study entry. For the current analysis, the empagliflozin arms were pooled.
2.1.1. Outcomes
We explored CV death, all‐cause mortality, HHF, and the composite of CV death (excluding fatal stroke) or HHF. In addition, we also investigated 3P‐MACE, which was the primary endpoint of the EMPA‐REG OUTCOME trial, as well as incident or worsening nephropathy defined as progression to macroalbuminuria (UACR > 300 mg/g), doubling of serum creatinine accompanied by eGFR of ≤45 mL/min/1.73m2, initiation of renal replacement therapy or death from renal disease. All CV, HF and mortality outcomes were adjudicated by blinded expert committees.
2.2. Statistical analysis
The TRS‐HFDM attributes one point to AF, CAD, eGFR < 60 mL/min/1.73m2 and UACR 30‐300 mg/g, and two points to prior HF and UACR > 300 mg/g, thus a maximum of seven points is possible.8 We defined three categories of low‐intermediate, high and very‐high risk as risk scores of 0‐1, 2 and ≥3 points, respectively, and patients were stratified based on their derived baseline TRS‐HFDM score.
Baseline demographics were calculated according to risk categories, continuous variables given as mean ± standard deviation (SD), and categorical variables as numbers and percentages.
Cox regression analysis including the variables age, sex, body mass index, HbA1c, geographical region, treatment, TRS‐HFDM score, and interaction of treatment*TRS‐HFDM score was used to investigate if the TRS‐HFDM categories were associated with the risk of all‐cause mortality, CV death, HHF, and a composite of CV death or HHF in the pooled empagliflozin and placebo groups. For the same outcomes, we calculated incidence rates (patients with events per 1000 years at risk), ARRs defined as incidence rate differences and number needed to treat (NNT). NNTs were derived as the reciprocal of the difference between the control and treatment groups in the proportion of patients who experienced a CV event within 3 years of treatment with empagliflozin, assuming exponential distribution of time to events. Poisson regression models were used to calculate the ARR, including treatment with a log‐link applied by each subgroup. In the model log (days at risk) for the time to first event, censoring was used as offset. Interaction P‐values were calculated by t‐tests, using the estimated interaction effect and variance of the interaction, as determined from the delta method following Poisson regression. NNT to prevent one event per 3 years at risk was calculated using exponential distribution.
The treatment effect of empagliflozin versus placebo for CV death, HHF, CV death or HHF, 3P‐MACE, all‐cause mortality, and incident or worsening nephropathy, across the TRS‐HFDM risk categories was assessed using the Cox models as described above. Interaction P‐values were calculated to assess whether the treatment effect was consistent across the risk categories.
All analyses were repeated in patients without HF at baseline, using the TRS‐HFDM with a maximum of five points.
3. RESULTS
3.1. Baseline characteristics
Among 7020 participants, variables to calculate TRS‐HFDM at baseline were available for 6952 patients (99%; empagliflozin, n = 4635; placebo, n = 2317). Based on TRS‐HFDM at baseline, 49.3% (n = 3429), 26.0% (n = 1807) and 24.7% (n = 1716) patients were assigned to the low‐intermediate, high and very‐high risk categories, respectively. Baseline characteristics and concomitant medications were similar between the placebo and empagliflozin groups across the risk categories (Table 1). Patients in the very‐high risk category were older (mean age 65.5 vs. 61.4 years in the low‐intermediate risk category) and more often male (74.1% vs. 69.1%). Patients in the very‐high risk category had more co‐morbidities and a worse cardiometabolic profile than those in the lower risk categories (Table 1). As TRS‐HFDM accounts for the presence of AF, CAD, eGFR and UACR, there was greater heterogeneity in these variables between the risk categories.
Table 1.
Baseline characteristics (overall population)
| TRS‐HFDM category | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Very‐high risk (≥3 points) | High risk (2 points) | Low‐intermediate risk (0/1 point) | |||||||
| Placebo (n = 600) | Pooled empagliflozin (n = 1116) | Total (N = 1716) | Placebo (n = 575) | Pooled empagliflozin (n = 1232) | Total (N = 1807) | Placebo (n = 1142) | Pooled empagliflozin (n = 2287) | Total (N = 3429) | |
| Age, years | 65.2 ± 8.5 | 65.6 ± 8.3 | 65.5 ± 8.4 | 64.6 ± 8.8 | 64.2 ± 8.5 | 64.3 ± 8.6 | 61.6 ± 8.6 | 61.3 ± 8.4 | 61.4 ± 8.5 |
| Male | 451 (75.2) | 821 (73.6) | 1272 (74.1) | 421 (73.2) | 901 (73.1) | 1322 (73.2) | 795 (69.6) | 1574 (68.8) | 2369 (69.1) |
| White race | 436 (72.7) | 824 (73.8) | 1260 (73.4) | 416 (72.3) | 876 (71.1) | 1292 (71.5) | 813 (71.2) | 1660 (72.6) | 2473 (72.1) |
| Body mass index, kg/m2 | 31.2 ± 5.3 | 31.1 ± 5.4 | 31.1 ± 5.4 | 31.0 ± 5.3 | 30.6 ± 5.2 | 30.7 ± 5.3 | 30.2 ± 5.1 | 30.3 ± 5.2 | 30.3 ± 5.2 |
| >10 years since diagnosis of diabetes | 392 (65.3) | 711 (63.7) | 1103 (64.3) | 358 (62.3) | 766 (62.2) | 1124 (62.2) | 581 (50.9) | 1166 (51.0) | 1747 (50.9) |
| HbA1c, % | 8.1 ± 0.9 | 8.1 ± 0.9 | 8.1 ± 0.9 | 8.1 ± 0.8 | 8.2 ± 0.9 | 8.1 ± 0.9 | 8.1 ± 0.8 | 8.0 ± 0.8 | 8.0 ± 0.8 |
| Baseline insulin use | 374 (62.3) | 640 (57.3) | 1014 (59.1) | 286 (49.7) | 642 (52.1) | 928 (51.4) | 466 (40.8) | 943 (41.2) | 1409 (41.1) |
| Baseline sulphonylurea use | 221 (36.8) | 445 (39.9) | 666 (38.8) | 248 (43.1) | 525 (42.6) | 773 (42.8) | 516 (45.2) | 1023 (44.7) | 1539 (44.9) |
| Diabetic retinopathy | 191 (31.8) | 312 (28.0) | 503 (29.3) | 125 (21.7) | 292 (23.7) | 417 (23.1) | 203 (17.8) | 402 (17.6) | 605 (17.6) |
| eGFR, mL/min/1.73m2 | 61.3 ± 19.1 | 60.8 ± 19.7 | 61.0 ± 19.5 | 69.5 ± 21.3 | 70.2 ± 21.3 | 70.0 ± 21.3 | 82.5 ± 17.6 | 82.8 ± 18.4 | 82.7 ± 18.2 |
| Baseline eGFR < 60 mL/min/1.73m2 | 349 (58.2) | 658 (59.0) | 1007 (58.7) | 209 (36.3) | 449 (36.4) | 658 (36.4) | 45 (3.9) | 93 (4.1) | 138 (4.0) |
| UACR | |||||||||
| Normal | 139 (23.2) | 269 (24.1) | 408 (23.8) | 213 (37.0) | 430 (34.9) | 643 (35.6) | 1030 (90.2) | 2090 (91.4) | 3120 (91.0) |
| Microalbuminuria | 240 (40.0) | 419 (37.5) | 659 (38.4) | 323 (56.2) | 721 (58.5) | 1044 (57.8) | 112 (9.8) | 197 (8.6) | 309 (9.0) |
| Macroalbuminuria | 221 (36.8) | 428 (38.4) | 649 (37.8) | 39 (6.8) | 81 (6.6) | 120 (6.6) | 0 | 0 | 0 |
| CV disease | |||||||||
| CAD† | 542 (90.3) | 999 (89.5) | 1541 (89.8) | 486 (84.5) | 1027 (83.4) | 1513 (83.7) | 723 (63.3) | 1478 (64.6) | 2201 (64.2) |
| Myocardial infarction | 355 (59.2) | 653 (58.5) | 1008 (58.7) | 266 (46.3) | 601 (48.8) | 867 (48.0) | 458 (40.1) | 911 (39.8) | 1369 (39.9) |
| Peripheral artery disease | 128 (21.3) | 254 (22.8) | 382 (22.3) | 101 (17.6) | 243 (19.7) | 344 (19.0) | 247 (21.6) | 475 (20.8) | 722 (21.1) |
| Stroke | 122 (20.3) | 207 (18.5) | 329 (19.2) | 104 (18.1) | 246 (20.0) | 350 (19.4) | 325 (28.5) | 617 (27.0) | 942 (27.5) |
| Atrial fibrillation | 101 (16.8) | 173 (15.5) | 274 (16.0) | 26 (4.5) | 51 (4.1) | 77 (4.3) | 15 (1.3) | 18 (0.8) | 33 (1.0) |
| Multivessel CAD | 341 (56.8) | 635 (56.9) | 976 (56.9) | 321 (55.8) | 646 (52.4) | 967 (53.5) | 428 (37.5) | 871 (38.1) | 1299 (37.9) |
| Hypertension | 573 (95.5) | 1070 (95.9) | 1643 (95.7) | 539 (93.7) | 1144 (92.9) | 1683 (93.1) | 1027 (89.9) | 2006 (87.7) | 3033 (88.5) |
| Current smoker | 76 (12.7) | 120 (10.8) | 196 (11.4) | 77 (13.4) | 139 (11.3) | 216 (12.0) | 147 (12.9) | 359 (15.7) | 506 (14.8) |
| Systolic blood pressure, mmHg | 137.6 ± 19.0 | 138.3 ± 18.0 | 138.1 ± 18.4 | 137.9 ± 17.4 | 136.7 ± 17.3 | 137.1 ± 17.3 | 133.9 ± 15.9 | 132.9 ± 15.8 | 133.2 ± 15.8 |
| Diastolic blood pressure, mmHg | 76.4 ± 10.5 | 76.3 ± 10.2 | 76.3 ± 10.3 | 76.9 ± 10.9 | 76.6 ± 9.9 | 76.7 ± 10.2 | 77.1 ± 9.6 | 76.8 ± 9.4 | 76.9 ± 9.5 |
Abbreviations: CAD, coronary artery disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; TRS‐HFDM, Thrombolysis In Myocardial Infarction Risk Score for Heart Failure in Diabetes; UACR, urine albumin‐to‐creatinine ratio.
CAD was defined as any of the components of a history of myocardial infarction, coronary artery bypass graft, single vessel CAD or multivessel CAD.
Note: Data are n (%) or mean ± SD.
3.2. TRS‐HFDM category association with mortality and HHF
In both the placebo and empagliflozin groups, increasing TRS‐HFDM category was associated with greater incidence of CV death and all‐cause mortality, as well as HHF. For CV death, the placebo incidence rates (N per 1000 patient years) were 9.1 (95% CI 6.2, 12.5), 22.7 (16.1, 30.5) and 41.2 (32.0, 51.5), in the low‐intermediate, high and very‐high risk categories, respectively. For all‐cause mortality, the placebo incidence rates were 16.1 (95% CI 12.1, 20.6), 32.3 (24.2, 41.4) and 51.4 (41.1, 62.9), respectively. Similarly, an association between risk categories and HHF, and a composite of CV death and HHF, was observed. Placebo incidence rates were 5.4 (95% CI 3.2, 8.1), 13.5 (8.5, 19.7) and 35.8 (26.9, 45.8) for HHF and 12.2 (8.8, 16.2), 32.5 (24.3, 41.8) and 67.6 (55.2, 81.2) for CV death or HHF, in the low‐intermediate, high and very‐high risk categories, respectively. Similar associations were observed in the empagliflozin group (Figure 1).
Figure 1.
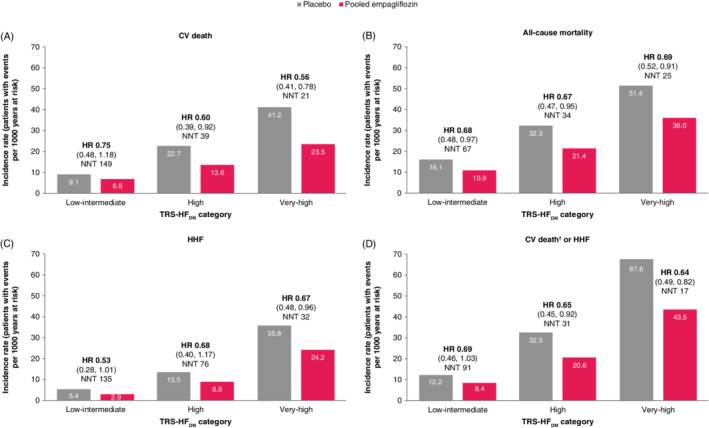
Incidence rates, number needed to treat (NNT) and treatment effect of empagliflozin versus placebo by TRS‐HFDM category for (A) cardiovascular (CV) death, (B) all‐cause mortality, (C) hospitalization for heart failure (HHF), and (D) CV death† or HHF in the overall population. CV, cardiovascular; HF, heart failure; HHF, hospitalization for heart failure; NNT, number needed to treat; TRS‐HFDM, Thrombolysis In Myocardial Infarction Risk Score for Heart Failure in Diabetes. †Excluding fatal stroke. NNTs are given as number needed to prevent one event over 3 years of treatment with empagliflozin versus placebo. 95% CIs shown in brackets: HR (95% CI)
3.3. Absolute and relative treatment effect of empagliflozin on risk of mortality and HF outcomes by TRS‐HFDM category
Table 2 depicts the ARRs and the NNT to prevent one event (CV death, all‐cause mortality, HHF, and CV death or HHF) over 3 years of treatment with empagliflozin versus placebo, as well as P‐values for the interaction between low‐intermediate and very‐high risk categories. In analyses of subgroup (risk category) interactions with treatment effect for the ARRs, we found significantly higher ARRs for CV death, and a composite of CV death or HHF in the very‐high compared with the low‐intermediate risk category with interaction P‐values of 0.0105 and 0.0107, respectively. However, no significant differences were observed between the very‐high and high risk categories, or between the low‐intermediate and high risk categories. For all‐cause mortality and HHF, similar absolute treatment effects were observed across all three risk categories with all interaction P‐values of >0.1. These ARRs are reflected in the NNT for CV death: 149, 39 and 21 in the low‐intermediate, high and very‐high risk categories, respectively; all‐cause mortality: 67, 34 and 25; CV death or HHF: 91, 31 and 17; and for HHF: 135, 76 and 32 (Figure 1 and Table 2).
Table 2.
Absolute treatment effect of empagliflozin versus placebo across TRS‐HFDM categories (very‐high, high and low‐intermediate risk), by ARR and NNT, for the overall population and in patients without HF at baseline
| TRS‐HFDM category | ||||||||
|---|---|---|---|---|---|---|---|---|
| Very‐high (≥3 points) | High (2 points) | Low‐intermediate (0/1 point) | ||||||
| NNT | ARR (95% CI) | NNT | ARR (95% CI) | NNT | ARR (95% CI) | Interaction P‐value (low‐intermediate vs. very‐high risk) | ||
| Overall population | CV death | 21 | −17.6 (−28.8, −6.5) | 39 | −9.1 (−17.3, −1.0) | 149 | −2.3 (−6.0, 1.4) | 0.0105 |
| All‐cause mortality | 25 | −15.4 (−28.2, −2.6) | 34 | −10.8 (−20.6, −1.0) | 67 | −5.2 (−10.1, −0.3) | 0.1442 | |
| HHF | 32 | −11.5 (−22.5, −0.6) | 76 | −4.6 (−11.0, 1.9) | 135 | −2.5 (−5.3, 0.3) | 0.1171 | |
| CV death† or HHF | 17 | −24.1 (−39.1, −9.1) | 31 | −11.9 (−21.8, −1.9) | 91 | −3.8 (−8.2, 0.5) | 0.0107 | |
| Patients without HF at baseline | CV death | 19 | −20.1 (−33.4, −6.7) | 37 | −9.6 (−17.9, −1.3) | 149 | −2.3 (−6.0, 1.4) | 0.0120 |
| All‐cause mortality | 22 | −17.7 (−32.9, −2.5) | 32 | −11.3 (−21.3, −1.3) | 67 | −5.2 (−10.1, −0.3) | 0.1249 | |
| HHF | 31 | −11.7 (−23.1, −0.4) | 75 | −4.6 (−11.0, 1.8) | 135 | −2.5 (−5.3, 0.3) | 0.1219 | |
| CV death† or HHF | 16 | −24.9 (−41.7, −8.2) | 30 | −12.3 (−22.3, −2.3) | 91 | −3.8 (−8.2, 0.5) | 0.0164 | |
Abbreviations: ARR, absolute risk reduction; CI, confidence interval; CV, cardiovascular; HF, heart failure; HHF, hospitalization for heart failure; NNT, number needed to treat; TRS‐HFDM, Thrombolysis In Myocardial Infarction Risk Score for Heart Failure in Diabetes.
Excluding fatal stroke. 95% CIs shown in brackets: ARR (95% CI).
The relative treatment effect of empagliflozin versus placebo was consistent across the TRS‐HFDM categories with reduction in risk of CV death, HHF, CV death or HHF, and all‐cause mortality, as well as 3P‐MACE and incident or worsening nephropathy. All P‐values for the interaction between treatment and subgroups were non‐significant: 0.6037 for CV death, 0.9489 for CV death or HHF, 0.7971 for HHF, 0.5428 for 3P‐MACE, 0.9943 for all‐cause mortality, and 0.7207 for incident or worsening nephropathy (Figure 2).
Figure 2.
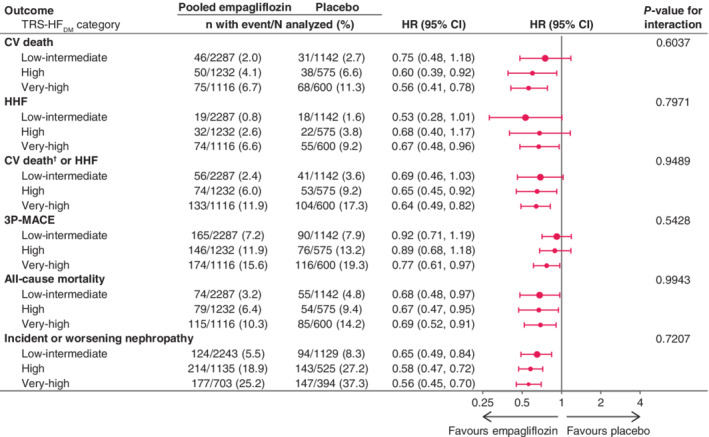
Effect of empagliflozin versus placebo on major outcomes by TRS‐HFDM category. 3P‐MACE, three‐point major adverse cardiovascular events; CV, cardiovascular; HHF, hospitalization for heart failure; TRS‐HFDM, Thrombolysis in Myocardial Infarction Risk Score for Heart Failure in Diabetes. †Excluding fatal stroke. Cox regression analysis for time to first event in patients treated with ≥1 dose of study drug
3.4. Patients without HF at baseline
When considering only those without HF at baseline, the association between TRS‐HFDM category and mortality and HHF was similar to that of the overall population (Figure 3).
Figure 3.
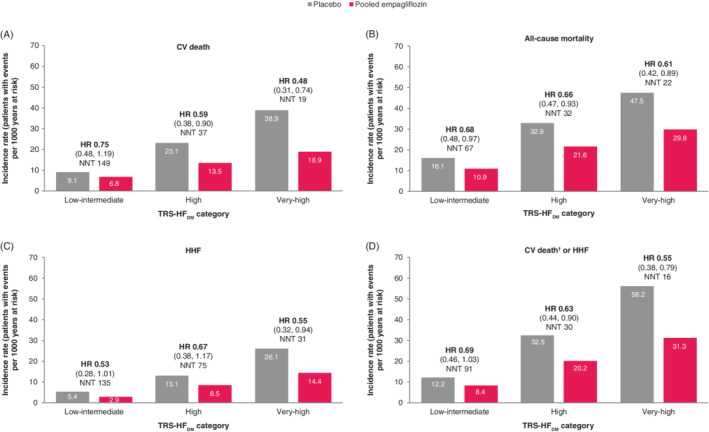
Incidence rates, NNT and treatment effect by TRS‐HFDM category for (A) CV death, (B) all‐cause mortality, (C) HHF and (D) CV death† or HHF for empagliflozin versus placebo in patients without HF at baseline. CI, confidence interval; CV, cardiovascular; HF, heart failure; HHF, hospitalization for heart failure; HR, hazard ratio; NNT, number needed to treat; TRS‐HFDM, Thrombolysis in Myocardial Infarction Risk Score for Heart Failure in Diabetes. †Excluding fatal stroke. 95% CI shown in brackets: HR (95% CI)
Similarly, ARRs for patients without HF at baseline were in line with those in the overall population: a significantly higher ARR with empagliflozin versus placebo was observed for CV death, and a composite of CV death or HHF, for the very‐high risk category versus the low‐intermediate risk category with P‐values of 0.0120 and 0.0164, respectively. ARRs with empagliflozin versus placebo of CV death were −2.3 (95% CI −6.0, 1.4) per 1000 patient years in the low‐intermediate risk category, and −20.1 (−33.4, −6.7) in the very‐high risk category, and for CV death of HHF −3.8 (−8.2, 0.5) (Table 2). The corresponding ARRs for all‐cause mortality were −5.2 (−10.1, −0.3) and −17.7 (−32.9, −2.5), and for HHF −2.5 (−5.3, 0.3) and −11.7 (−23.1, −0.4) (Table 2), with no significant differences of ARR between any of the risk categories (all interaction P‐values of >0.1).
In patients without HF at baseline, the relative treatment effect of empagliflozin versus placebo was consistent across the TRS‐HFDM categories, with non‐significant P‐values for the interaction between treatment and subgroups: 0.3652 for CV death, 0.8338 for HHF, 0.7123 for CV death or HHF, 0.5033 for 3P‐MACE, 0.9138 for all‐cause mortality and 0.8444 for incident or worsening nephropathy (Figure 4). These risk reductions are reflected in the NNT for CV death of 149, 37 and 19 in the low‐intermediate, high and very‐high risk categories, respectively; all‐cause mortality: 67, 32 and 22; HHF: 135, 75 and 31; and CV death or HHF: 91, 30 and 16 (Figure 3 and Table 2).
Figure 4.
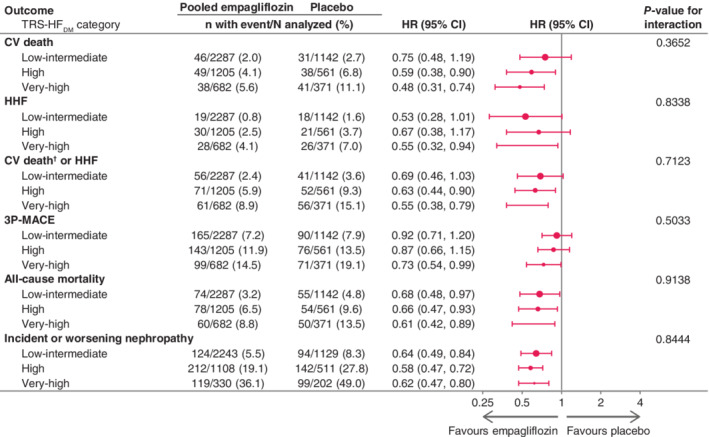
Effect of empagliflozin versus placebo on major outcomes by TRS‐HFDM category, in patients without HF at baseline. 3P‐MACE, three‐point major adverse cardiovascular events; CI, confidence interval; CV, cardiovascular; HHF, hospitalization for heart failure; TRS‐HFDM, Thrombolysis in Myocardial Infarction Risk Score for Heart Failure in Diabetes. †Excluding fatal stroke. Cox regression analysis for time to first event in patients treated with ≥1 dose of study drug
3.5. Adverse events by TRS‐HFDM category
Figure 5 depicts the incidence rate ratios of pooled empagliflozin versus placebo across TRS‐HFDM risk categories. Adverse event rates consistent with urinary tract infection, volume depletion and acute renal failure were similar between the empagliflozin and placebo groups across the risk categories. There seemed to be a slight difference between treatment arms for confirmed hypoglycaemia events in the low‐intermediate and very‐high risk categories, but the incidence rate ratios for hypoglycaemia requiring assistance were similar between treatment arms. The rates of genital infection were greater with empagliflozin than placebo in all categories (Figure 5).
Figure 5.
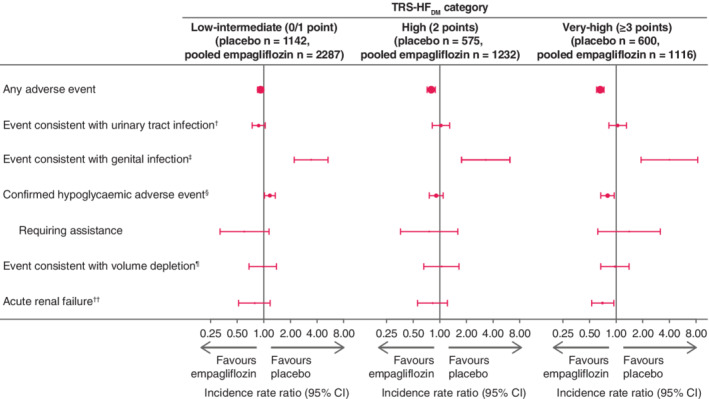
Adverse event incidence rate ratios, empagliflozin versus placebo, by TRS‐HFDM category. Incidence rates are given per 100 patient years. †Based on 79 MedDRA preferred terms. ‡Based on 88 MedDRA preferred terms. §Plasma glucose ≤70 mg/dL and/or requiring assistance. ¶Based on 8 MedDRA preferred terms. ††Based on the narrow standardized MedDRA query “acute renal failure”. CI, confidence interval; TRS‐HFDM, Thrombolysis in Myocardial Infarction Risk Score for Heart Failure in Diabetes
4. DISCUSSION
Here, we report that TRS‐HFDM is associated with all‐cause mortality, CV death, as well as HHF, in patients with T2D and established ASCVD in the EMPA‐REG OUTCOME trial cohort. Furthermore, we show that although the absolute treatment effect of empagliflozin on CV death is greater in the very‐high risk category, which was expected because of the higher underlying absolute risk, the relative treatment benefit of empagliflozin versus placebo on mortality and HF outcomes is similar across the risk categories.
The risk of developing HF is greatly increased in patients with T2D.3, 4 Furthermore, HF death contributes to a large burden of overall cause of death among patients with T2D with established ASCVD.14 Moreover, the risk of HF seems to persist, although slightly decreased, despite control of multiple CV risk factors, such as blood pressure, low‐density lipoprotein cholesterol and smoking status.15 Thus, in addition to early HF risk assessment to institute preventive measures, such as CV risk factor control, it is important to assess the residual risk of HF in patients with T2D. SGLT2 inhibitors reduce the risk of HF outcomes by one or more mechanism.16, 17, 18, 19, 20, 21 The effects of empagliflozin on HF and mortality outcomes have proven to be consistent regardless of baseline, and control of, risk factors such as HbA1c,22 blood pressure and lipids, and regardless of the number of traditional CV risk factors that are controlled.12, 23 A mediation analysis exploring the potential underlying mechanisms of empagliflozin's reduction in CV death found that changes in haematocrit and haemoglobin (suggesting haemoconcentration from reduction in plasma volume) appeared to be the most important mediators.24 Recent analyses from the EMPA‐HEART CardioLink‐6 trial also point towards an early and sustained effect of empagliflozin on erythropoietin production, which may additionally drive the rise in these haematological variables, and also potentially contribute to the reduction in adverse CV outcomes observed.18, 21
Risk scores assessing the risk of CV events or mortality in people with T2D or ASCVD have been developed,22, 25 but few assess the risk of HF. Previously, our group has shown that the Health, Ageing and Body Composition (ABC) HF risk score is a good discriminator of risk of HHF in patients without known HF at baseline in the EMPA‐REG OUTCOME trial cohort, and that the treatment effect of empagliflozin was consistent across risk categories.22 The Health ABC HF risk score was, however, developed using an elderly population, of which only approximately 7% had diabetes. By contrast, the TRS‐HFDM was developed and validated in patients with T2D and ASCVD or multiple CV risk factors (in the SAVOR‐TIMI 53 and DECLARE‐TIMI 58 trials) to identify patients at a higher risk of adverse HF outcomes, and was found to further distinguish the treatment effect on this outcome by SGLT2 inhibitors. We add to this by showing that the TRS‐HFDM is also associated with CV death. TRS‐HFDM is therefore an emerging valuable tool for physicians assessing HF or CV mortality risk in their patients with T2D.
In the DECLARE‐TIMI 58 trial, the TRS‐HFDM was shown to be strongly associated with the risk of HHF and a somewhat weaker association with MACE and non‐CV mortality was observed.8 The risk score was also shown to identify patients who had greater absolute benefit of dapagliflozin versus placebo on HHF. In the DECLARE‐TIMI 58 trial, there was no overall mortality benefit observed with dapagliflozin and the effect on mortality outcomes across the TRS‐HFDM categories was not explored. Accordingly, our results expand the evidence for the use of the TRS‐HFDM. That is, despite the presence of a greater absolute treatment benefit of empagliflozin versus placebo on CV death in the very‐high versus the low‐intermediate risk category, the relative treatment effect appears similar across all of the risk categories. For all‐cause mortality and HHF, both the absolute and the relative treatment effect of empagliflozin were consistent across the risk categories (Figure 1), as were the relative treatment effects on 3P‐MACE, and incident or worsening nephropathy (Figure 2). Thus, the findings of this study indicate that TRS‐HFDM is a prognostic marker (ie, a variable which is associated with a specified outcome) for HHF, CV mortality and all‐cause mortality, but it is not a predictive marker for the relative treatment effect of empagliflozin (ie, a variable which influences the relative effect of a treatment on a specified outcome). Of course, the numbers needed to treat to prevent one event will be lower in patients with higher absolute risks, so this risk score might inform which patients will derive the largest absolute benefit.
Previously, analysis of EMPA‐REG OUTCOME data stratified by 10‐point TIMI Risk Score for Secondary Prevention (TRS‐2P) showed that treatment with empagliflozin was associated with a consistent reduction in CV outcomes (CV death, 3P‐MACE and HHF) and all‐cause mortality, across a spectrum of CV risk.22 Despite using different variables, the incidence rates for all‐cause mortality, CV death and HHF were markedly similar between groups stratified by TRS‐2P and TRS‐HFDM, showing that empagliflozin's benefits are consistent across a broad range of baseline risk factors.
The absolute CV mortality benefit of empagliflozin was large in those at very‐high and high risk of HF, with NNT to prevent one CV death over 3.1 years of 21 and 39, respectively. Importantly, our analysis of those without known HF at baseline showed similar results to the overall population, with increasing TRS‐HFDM being associated with an increased risk of CV death, all‐cause mortality and HHF. Moreover, also in this subpopulation, the relative treatment effect of empagliflozin versus placebo was consistent across the risk categories for all outcomes explored (Figure 4). The absolute treatment effect as shown by NNT (Figure 3 and Table 2), was also similar to that in the overall population. These results suggest that the TRS‐HFDM may also be a useful tool for the assessment of mortality and HHF benefit with empagliflozin in patients with T2D without known HF.
Our study has some limitations. This is a post hoc analysis, and the EMPA‐REG OUTCOME trial was not designed to evaluate treatment effects across the TRS‐HFDM risk categories. Also, because the population included in EMPA‐REG OUTCOME all had T2D and established ASCVD, we do not know if our findings hold true in the general T2D population, where many patients have not yet developed CV disease.
The TRS‐HFDM is associated with CV death and all‐cause mortality, as well as HHF, in patients with T2D and ASCVD in the EMPA‐REG OUTCOME trial cohort. Although those with very‐high risk had a greater ARR in CV mortality with empagliflozin, empagliflozin reduced the relative risk of mortality and HF outcomes, including CV death, versus placebo consistently across the TRS‐HFDM risk categories. The TRS‐HFDM may thus be a useful tool for HF and mortality risk assessment in patients with T2D and ASCVD, and to identify those with the anticipated largest absolute benefit from empagliflozin. It does, however, require further validation in broader populations with T2D.
CONFLICT OF INTEREST
S.V. holds a Tier 1 Canada Research Chair in Cardiovascular Surgery; has also received grants and personal fees for speaker honoraria and advisory board participation from AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, Amgen, HLS, Merck, EOCI Pharmacomm Ltd, Novartis, Sun Pharmaceuticals, and Toronto Knowledge Translation Working Group; he also serves as President of the Canadian Medical and Surgical Knowledge Translation Research Group. A.S. has received funding from the FRSQ‐Junior 1 Scholars Program, Bayer‐Canadian Cardiovascular Society, Alberta Innovates Health Solution, Roche Diagnostics, Novartis, Takeda, Boehringer‐Ingelheim and Akcea. B.Z. has received research grants awarded to his institution from Boehringer Ingelheim, AstraZeneca and Novo Nordisk, and honoraria from Janssen, Sanofi, Eli Lilly and Company, Boehringer Ingelheim, Novo Nordisk, and Merck. A.P.O., M.B., I.Z. and J.T.G. are employees of Boehringer Ingelheim. D.F. has received honoraria from Sanofi, Merck & Co., Amgen, AstraZeneca, Eli Lilly and Company, and Boehringer Ingelheim, and has served on the data and safety monitoring board for Novo Nordisk. C.W. reports honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, MSD and Sanofi. S.E.I. has received honoraria for lectures, advisory work and/or clinical trial leadership from AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Sanofi/Lexicon, VTV Therapeutics, Merck, and Abbott/Alere. J.B. has received research support from the National Institutes of Health, Patient Centered Outcomes Research, and the European Union, and has served as a consultant for Abbott, Adrenomed, Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CVRx, G3 Pharmaceutical, Innolife, Janssen, LinaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, V‐Wave, and Vifor. C.D.M. has received consulting fees from Amgen, Boehringer Ingelheim and Octapharma.
AUTHOR CONTRIBUTIONS
S.V. conceived the idea, and S.V. and A.P.O. contributed to the interpretation of data and drafted the manuscript. A.S., B.Z., D.F., M.B., C.W., J.T.G., S.E.I., J.B. and C.D.M. contributed to the interpretation of data and the development of the manuscript. I.Z. contributed to the analyses and interpretation of data and the development of the manuscript, and provided statistical expertise. I.Z. and S.V. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses.
DATA STATEMENT
The sponsor of the EMPA‐REG OUTCOME Trial (Boehringer Ingelheim) is committed to responsible sharing of clinical study reports, related clinical documents and patient‐level clinical study data. Researchers are invited to submit inquiries via the Clinical Study Data Request website (https://vivli.org).
ACKNOWLEDGMENTS
The EMPA‐REG OUTCOME trial was sponsored by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance. The authors thank the patients who participated in this trial. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Sally Neath and Charlie Bellinger of Elevate Scientific Solutions, Horsham, UK, during the preparation of this article. The authors were fully responsible for all content and editorial decisions and were involved at all stages of manuscript development and have approved the final version.
Verma S, Sharma A, Zinman B, et al. Empagliflozin reduces the risk of mortality and hospitalization for heart failure across Thrombolysis In Myocardial Infarction Risk Score for Heart Failure in Diabetes categories: Post hoc analysis of the EMPA‐REG OUTCOME trial. Diabetes Obes Metab. 2020;22:1141–1150. 10.1111/dom.14015
REFERENCES
- 1. IDF Diabetes Atlas , 9th edn. Brussels, Belgium: International Diabetes Federation, 2019. http://www.diabetesatlas.org. Accessed December 3, 2019. [Google Scholar]
- 2. Di Angelantonio E, Kaptoge S, Wormser D, et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314:52‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699‐703. [DOI] [PubMed] [Google Scholar]
- 4. Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cubbon RM, Adams B, Rajwani A, et al. Diabetes mellitus is associated with adverse prognosis in chronic heart failure of ischaemic and non‐ischaemic aetiology. Diabetes Vasc Dis Res. 2013;10:330‐336. [DOI] [PubMed] [Google Scholar]
- 6. Sharma A, Zhao X, Hammill BG, et al. Trends in noncardiovascular comorbidities among patients hospitalized for heart failure: insights from the get with the guidelines‐heart failure registry. Circ Heart Fail. 2018;11:e004646. [DOI] [PubMed] [Google Scholar]
- 7. Home PD, Pocock SJ, Beck‐Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open‐label trial. Lancet. 2009;373:2125‐2135. [DOI] [PubMed] [Google Scholar]
- 8. Berg DD, Wiviott SD, Scirica BM, et al. Heart failure risk stratification and efficacy of sodium‐glucose cotransporter‐2 inhibitors in patients with type 2 diabetes mellitus. Circulation. 2019;140:1569‐1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393:31‐39. [DOI] [PubMed] [Google Scholar]
- 10. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 11. Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA‐REG OUTCOME(R) trial. Eur Heart J. 2016;37:1526‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Savarese G, Sattar N, Januzzi J, et al. Empagliflozin is associated with a lower risk of post‐acute heart failure rehospitalization and mortality. Circulation. 2019;139:1458‐1460. [DOI] [PubMed] [Google Scholar]
- 13. Zinman B, Inzucchi SE, Lachin JM, et al. Rationale, design, and baseline characteristics of a randomized, placebo‐controlled cardiovascular outcome trial of empagliflozin (EMPA‐REG OUTCOME). Cardiovasc Diabetol. 2014;13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharma A, Green JB, Dunning A, et al. Causes of death in a contemporary cohort of patients with type 2 diabetes and atherosclerotic cardiovascular disease: insights from the TECOS trial. Diabetes Care. 2017;40:1763‐1770. [DOI] [PubMed] [Google Scholar]
- 15. Rawshani A, Rawshani A, Franzen S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379:633‐644. [DOI] [PubMed] [Google Scholar]
- 16. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state‐of‐the‐art review. Diabetologia. 2018;61:2108‐2117. [DOI] [PubMed] [Google Scholar]
- 17. Lam CSP, Chandramouli C, Ahooja V, Verma S. SGLT‐2 inhibitors in heart failure: current management, unmet needs, and therapeutic prospects. J Am Heart Assoc. 2019;8:e013389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mazer CD, Hare GMT, Connelly PW, et al. Effect of empagliflozin on erythropoietin levels, iron stores and red blood cell morphology in patients with type 2 diabetes and coronary artery disease. Circulation. 2019;141:704‐707. [DOI] [PubMed] [Google Scholar]
- 19. Verma S, Rawat S, Ho KL, et al. Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. JACC Basic Transl Sci. 2018;3:575‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hess DA, Terenzi DC, Trac JZ, et al. SGLT2 inhibition with empagliflozin increases circulating provascular progenitor cells in people with type 2 diabetes mellitus. Cell Metab. 2019;30:609‐613. [DOI] [PubMed] [Google Scholar]
- 21. Verma S, Mazer CD, Yan AT, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA‐HEART CardioLink‐6 randomized clinical trial. Circulation. 2019;140:1693‐1702. [DOI] [PubMed] [Google Scholar]
- 22. Fitchett D, Inzucchi SE, Cannon CP, et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA‐REG OUTCOME trial. Circulation. 2019;139:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inzucchi SE, Khunti K, Fitchett DH, et al. 19‐LB: consistent cardiovascular (CV) benefits from empagliflozin across the spectrum of CV risk factor control: post hoc analysis from EMPA‐REG OUTCOME. Diabetes. 2019;68(1). [Google Scholar]
- 24. Inzucchi SE, Zinman B, Fitchett D, et al. How does Empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG OUTCOME trial. Diabetes Care. 2018;41:356‐363. [DOI] [PubMed] [Google Scholar]
- 25. Stevens RJ, Kothari V, Adler AI, Stratton IM. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci. 2001;101:671‐679. [PubMed] [Google Scholar]


