Abstract
Objective
The aim of this study was to evaluate the diagnostic and prognostic role of multiparameter flow cytometry (FC) in patients with idiopathic cytopenia of undetermined significance (ICUS) and clonal cytopenia of undetermined significance (CCUS).
Methods
We performed FC using a standardized panel and two different diagnostic algorithms (Ogata, Wells) in a well‐characterized cohort of 79 patients with ICUS/CCUS and compared it with a retrospective blinded morphological evaluation and data from targeted next‐generation DNA sequencing of 20 myelodysplastic syndrome (MDS)‐related genes.
Results
Our data show that FC has low sensitivity in distinguishing CCUS from ICUS patients (40.5% for Ogata score and 59.5% for Wells score). The Wells score was suggestive of dysplasia in ICUS/CCUS patients with concurrent morphological signs of dysplasia in the bone marrow (following re‐evaluation by two hematopathologists) and in CCUS patients with a higher mutational burden. Eight patients with ICUS/CCUS from our cohort progressed to another myeloid malignancy (MDS, acute myeloid leukemia, or chronic myelomonocytic leukemia), all showing flow cytometric signs of dysplasia.
Conclusion
FC performs poorly in diagnosing CCUS versus ICUS. However, it can potentially provide prognostic information in cytopenic patients by identifying a subgroup of patients with a higher grade of dysplasia, higher mutational burden, and higher risk of progression and, together with mutational screening, also identify a group of patients who might require morphological reassessment of dysplastic changes in their bone marrow.
1. INTRODUCTION
The term idiopathic cytopenia of undetermined significance (ICUS) is used to describe cases with persistent cytopenia (more than 6 months) without evidence of dysplasia in the bone marrow and normal cytogenetics (Valent et al., 2007), that is, they do not fulfill the diagnostic criteria for myelodysplastic syndrome (MDS). However, a subgroup of ICUS patients has been shown to carry one or more somatic mutations in genes that are frequently mutated in MDS, a condition characterized as clonal cytopenia of undetermined significance (CCUS), associated with increased risk of developing MDS (Cargo et al., 2015; Hansen et al., 2016; Kwok et al., 2015; Steensma et al., 2015). Even though ICUS and CCUS have by definition no traits of significant dysplasia (>10% on bone marrow morphological assessment) (Valent & Valent, 2019), we previously reported that patients with CCUS and a high mutational burden (more than two somatic mutations) can also exhibit variable grades of bone marrow dysplasia upon morphological re‐evaluation (Hansen et al., 2016). However, little is known about the flow cytometric changes in patients with ICUS and CCUS.
Multiparameter flow cytometry (FC) is a very commonly used, robust diagnostic tool for hematological malignancies, allowing a reliable and quantitative immunophenotyping of hematopoietic cells. Several studies have shown that FC can also detect changes in hematopoiesis related to MDS, and both the European Leukemia Net (ELN) and the International Working Group for FC in MDS (IMDS‐Flow) recommend its use for the diagnostic evaluation of MDS (Porwit et al., 2014; Westers et al., 2012). However, the role of FC in patients with ICUS and CCUS has not yet been evaluated, and it remains unknown whether FC may confer additional diagnostic and prognostic value together with targeted sequencing and morphological evaluation of the bone marrow. Therefore, we conducted this single‐center study, analyzing a cohort of 79 patients with cytopenia who did not fulfill the criteria for MDS (>10% dysplasia) upon routine morphological assessment (i.e., ICUS and CCUS patients) with targeted DNA sequencing of 20 of the most commonly mutated genes in MDS, reassessment of bone marrow histomorphology, and a standardized panel of multi‐parameter FC to evaluate the importance of FC in these patients. We aimed to evaluate, first, the diagnostic value of FC in distinguishing CCUS from ICUS patients, second, whether FC could offer any prognostic information in ICUS/CCUS patients, and, third, how well flow cytometric changes correlate with the mutational burden and the grade of dysplasia following the morphological re‐assessment.
2. MATERIALS AND METHODS
2.1. Patient samples
A total of 79 patients with unexplained cytopenia were included in this study. The patients were referred to the Department of Hematology, Rigshospitalet, Copenhagen, between 2013 and 2017. Patients were only included in the study if their cytopenias were persistent for more than 6 months and other common causes of cytopenia, such as vitamin deficiencies, HIV infection, and other malignant disorders in the bone marrow, were ruled out. Importantly, routine bone marrow morphological examination and cytogenetic analysis were not diagnostic for MDS. Next, morphological reassessment and targeted DNA sequencing and FC were performed on diagnostic samples for all patients, as described in further detail below. This study was approved by the Ethical Committee of Capital Region of Denmark (HD‐2009‐003) and all participants signed an informed consent upon written and oral information about the study.
2.2. Mutational analysis
DNA was extracted either from peripheral blood neutrophils or from mononuclear cells from the bone marrow and was stored at −20 or −80°C. Samples were analyzed using a custom‐designed multiplex Ion Ampliseq panel (Ampliseq designer, Thermo Fischer Scientific, Waltham, MA) including the following 20 genes: IDH1, IDH2, TET2, DNMT3A, ASXL1, TP53, NRAS, KRAS, CBL, JAK2, GATA2, CEBPA, RUNX1, SF3B1, U2AF1, SRSF2, ZRSR2, EZH2, SETBP1, and ETV6. A more detailed description of the targeted next‐generation sequencing protocol and subsequent data analysis performed in this study can be found in (Hansen et al., 2016).
2.3. Flow cytometry
FC was performed on bone marrow samples from all patients at diagnosis, using a slightly modified acute myeloid leukemia (AML) panel, as described by the Euroflow Consortium (van Dongen et al., 2012). Briefly, samples were analyzed using a total of four tubes (Euroflow AML panel tubes 1–4) with eight antibodies in each tube. The antibodies used in this study were the following: HLA‐DR‐Pacific Blue, CD45‐Pacific Orange, CD34‐PerCPCy5.5, CD117‐PECy7, CD16‐FITC, CD13‐PE, CD11b‐APC, CD10‐APCH7, CD35‐FITC, CD64‐PE, IREM2‐APC, CD14‐APCH7, CD36‐FITC, CD105‐PE, CD33‐APC, CD71‐APCH7, NuTdT‐FITC, CD56‐PE, CD7‐APC, CD19‐APCH7.
Acquisition of flow data was performed on an FACS Canto (Becton Dickinson Immunocytometry Systems). Data analysis was performed using the Infinicyt software (Cytognos, Salamanca, Spain). The FC evaluation was done in a randomized and blinded manner (with no knowledge of the mutational status and/or pathological evaluation of the samples). All samples were analyzed using two different algorithms: the Ogata algorithm (Porta et al., 2012) and the Wells algorithm (Wells et al., 2003). The Ogata algorithm is based on four parameters (CD45 ratio between lymphocytes and myeloblasts, SSC ratio between lymphocytes and granulocytes, the proportion of CD34+ blasts out of all leukocytes, and the proportion of lymphoid progenitors out of all CD34+ blasts); thus, the Ogata score for the samples could range from 0 to 4. The Wells algorithm has a more complex scoring system based on specific aberrations of myeloblasts, granulocytes, and monocytes and the score for the samples ranged from 0 to 6. Briefly, 0 points were given when there were no observed abnormalities on granulocytes, monocytes, or myeloblasts; 1 point was given if granulocytes or monocytes had a single abnormality; 2 points were given if granulocytes or monocytes had 2–3 abnormalities, or if both populations had one abnormality each, or if any population was CD34+: 3 points were given if one population (granulocytes or monocytes) had 4 abnormalities or if one population had 1–3 abnormalities and was also CD34+; and 4 points were given if both populations had 2–3 abnormalities each. Additional points could be given if myeloblasts were clearly aberrant, or if the lymphoid‐to‐myeloid ratio was 1.0 or above (severe granulopenia). For the evaluation of aberrant expression of markers such as CD34 and CD56 on granulocytes and monocytes, we superimposed the gated populations on positive control populations (CD34+ myeloblasts, CD56+ lymphocytes), allowing for controlling intersample variations in fluorescence intensity. We applied a cutoff of 20% or more for calling the population positive for an aberrant marker. Samples with a flow cytometric score (FC‐score) equal to or higher than 2 with either algorithm were classified as suggestive of dysplasia by FC.
2.4. Morphological examination of bone marrow samples
All patients had an initial routine morphology assessment of an experienced hematopathologist, and for 57 cases, which by routine evaluation did not fulfill the MDS diagnostic criteria (>10% dysplasia), bone marrow smears from aspirate, peripheral blood smears, and bone marrow trephine biopsies were available for a blinded morphological assessment. Importantly, the morphological review was performed retrospectively by two hematopathologists and results were blinded and reported independently. Each reviewing pathologist scored the samples from a scale between 0 and 2 (0: “no signs of dysplasia,” 1: “dysplasia of unknown significance,” and 2: “significant dysplasia, highly suspicious for MDS”). In the current analysis, the two different scores were added together, and cases were further categorized into three groups: 0: “no morphological signs of dysplasia,” 1–2: “mild dysplasia,” and 3–4: “severe dysplasia.” A detailed overview of the morphological analysis of the samples can be found in (Hansen et al., 2016).
2.5. Statistics
Statistical analyses were performed using the statistical program R (version 3.4.0). For comparisons of numerical variables between patients with ICUS and CCUS, we used Mann–Whitney U test, and we used Pearson's chi‐squared test or Fisher's exact test for comparisons of categorical variables. The cutoff for statistical significance was set at 0.05 (p‐value < .05).
3. RESULTS
3.1. Patient characteristics
We included a total of 79 patients with a median age of 70 years (range: 39–88). By performing targeted NGS, we identified at least one mutation in 37 of the cases (46.8%), which were thus classified as CCUS. Thus, our cohort included 42 patients with ICUS and 37 patients with CCUS. The frequency of the mutations discovered is shown in Supporting Information Figure S1. The demographics and baseline clinical characteristics of the entire cohort are shown in Table 1. There was a slightly bigger proportion of males having CCUS compared with ICUS, but we observed no significant differences in any of the other parameters.
Table 1.
Baseline clinical features of the patients included in our study
| Characteristics (median, range) | Total (n = 79) | ICUS (n = 42) | CCUS (n = 37) | p‐value |
|---|---|---|---|---|
| Age | 70 (39–88) | 57 (44–84) | 69 (39–88) | .06 |
| Male | 52 | 25 | 19 | .59 |
| Female | 39 | 17 | 18 | |
| Hemoglobin (g/L) | 11.0 (7.3–16.8) | 11.6 (7.3–16.8) | 6.8 (7.3–16.4) | .003 |
| Leucocytes (109/L) | 4.3 (1.6–22.4) | 4.8 (1.6–10.1) | 4.0 (2.1–22.4) | .76 |
| Neutrophils (109/L) | 2.3 (0.1–18.1) | 2.8 (0.1–7.8) | 2.0 (0.5–18.1) | .40 |
| Thrombocytes (109/L) | 110 (8–427) | 110 (22–353) | 106 (18–427) | .82 |
| MCV (fL) | 92 (68–119) | 91 (68–111) | 91 (79–114) | .56 |
| Ferritin (μg/L) | 220 (5–2980) | 175 (5–2980) | 240 (19–2590) | .89 |
| LDH (U/L) | 201 (60–621) | 198 (60–391) | 208 (121–621) | .62 |
Note: There was no significant age difference between patients with MDS and ICUS/CCUS, but patients with low‐risk MDS had lower hemoglobin levels.
Abbreviations: CCUS, clonal cytopenia of undetermined significance; ICUS, idiopathic cytopenia of undetermined significance.
3.2. Diagnostic performance of flow cytometry in ICUS and CCUS
Initially, we compared the diagnostic efficacy of the two algorithms in identifying CCUS patients among patients, which by routine assessment had unexplained cytopenia. An FC‐score for all 79 samples was obtained using the Ogata and Wells algorithm separately. The Ogata score is based on four different parameters, the value of which was provided directly from the analysis software. The Wells algorithm is more complex and requires careful evaluation of different parameters and the maturation pattern of granulocytes and monocytes. Some of the most commonly observed flow cytometric abnormalities in our cohort are shown in Figure 1.
Figure 1.
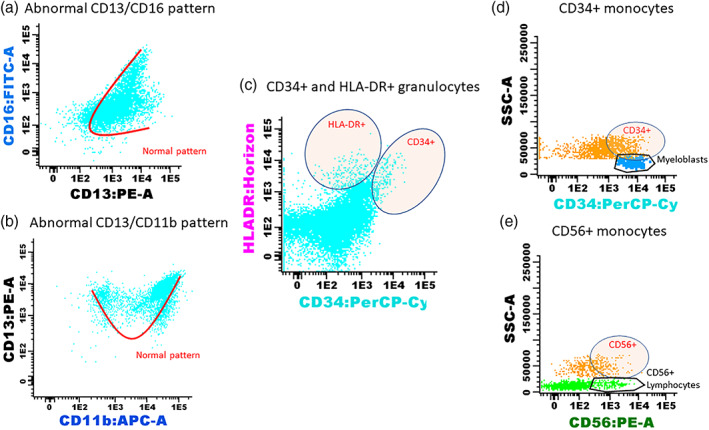
Some of the most commonly observed flow cytometric abnormalities in the granulocyte and monocyte populations in patients with ICUS/CCUS. (a) Abnormal maturation pattern of granulocytes on a CD13/CD16 plot, seen in a patient with CCUS with mutations in ASXL1, CEBPα, TET2, EZH2, and CBL. (b) Abnormal maturation pattern on a CD13/CD11b plot. The patient had CCUS with mutations in GATA2 and U2AF1. (c) Aberrant expression of HLA‐DR and CD34 on granulocytes in a patient with CCUS with mutations in TET2 and ASXL1. Expression of aberrant antigens on monocytes is a common feature in myelodysplasia. We evaluated the presence of D) CD34 (with CD34+ myeloblasts as control population) and E) CD56 (with CD56‐positive lymphocytes—natural‐killer cells as control population) on monocytes and, in this example, on the sample of a patient with ICUS. Aberrant expression of CD34 and CD56 on monocytes was compared with positive control populations and deemed positive when more than 20% of monocytes were CD34+ or CD56+. CCUS, clonal cytopenia of undetermined significance; ICUS, idiopathic cytopenia of undetermined significance [Color figure can be viewed at wileyonlinelibrary.com]
The Ogata score was suggestive of dysplasia in 18 (22.8%) samples with unexplained cytopenia, while the Wells score was suggesting dysplasia in a total of 35 (44.3%) of the samples. The sensitivity, specificity, positive predictive value, and negative predictive value of both algorithms in distinguishing CCUS from ICUS are shown in Figure 2a. Notably, all the samples that were positive using the Ogata score were also positive with the Wells score. We then performed a receiving operating characteristic (ROC) curve analysis to evaluate the predictive value of FC in distinguishing CCUS from ICUS. The Ogata score had an area under the curve of 65.4%, compared with 66.8% for Wells score (Figure 2b). Overall, our data suggest that Ogata score alone has a much higher specificity (92.9% vs. 71.4%) while Wells score has a higher sensitivity (59.5% vs. 40.5%) when used as a diagnostic tool for CCUS, but overall, both algorithms performed rather poor in the classification of ICUS/CCUS.
Figure 2.
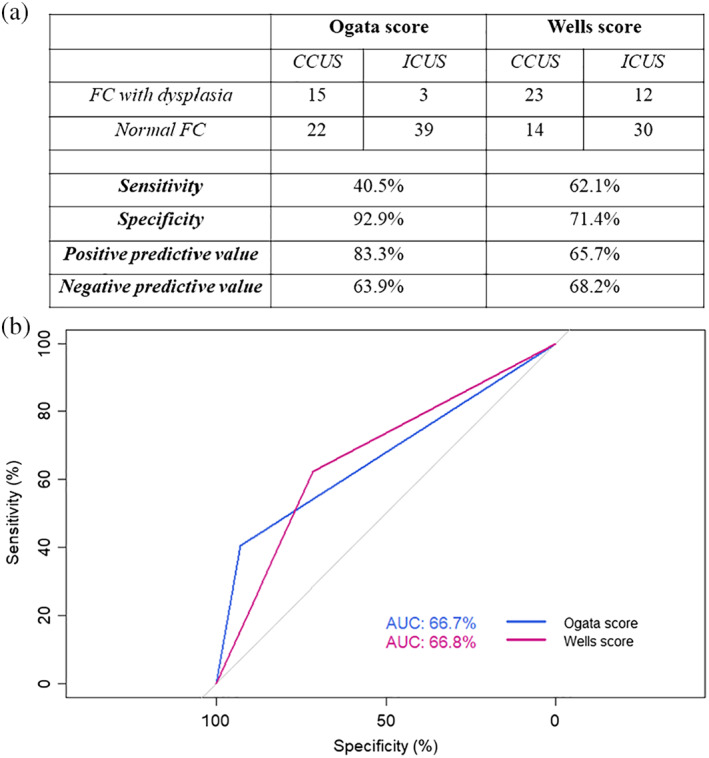
(a) Diagnostic efficacy of FC in 79 ICUS/CCUS patients using the Ogata and the Wells score. Samples were suggestive of dysplasia for either of the algorithms if the score was 2 or more. (b) ROC curve with calculated area under the curve for Ogata score and Wells score. CCUS, clonal cytopenia of undetermined significance; ICUS, idiopathic cytopenia of undetermined significance [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Flow cytometric changes are associated with morphological signs of dysplasia and higher mutational burden
The blinded morphological evaluation revealed that 33 of 57 samples (57.9%) showed no signs of dysplasia, while 14 (24.6%) samples had mild dysplasia and 10 (17.5%) samples severe dysplasia.
Positive scores from both algorithms were significantly associated with dysplasia (Ogata: p < .001, Wells: p = .005, Figure 3a). Notably, the Ogata score had a low false‐positive rate in cases with no morphological signs of dysplasia, with only 3/33 patients scoring positive, yielding a specificity of 90.9%. The Wells score was suggestive of dysplasia in 9/10 (90%) patients with severe dysplasia, but at the cost of a higher false‐positive rate for the lower grades of dysplasia. There were 12 samples, where at least one reviewer found more than 10% of dysplastic changes; 10 of these 12 patients had mutations in at least one gene (Hansen et al., 2016). The Ogata score was positive in 9 (75%) of those samples and the Wells score in 11 (91.7%) of the samples. Interestingly, when FC and DNA sequencing were combined, they could identify all 12 misclassified MDS patients, possibly suggesting that FC and mutational analysis might be slightly more robust in detecting early dysplastic changes in the bone marrow that may not be picked up by routine morphological assessment.
Figure 3.
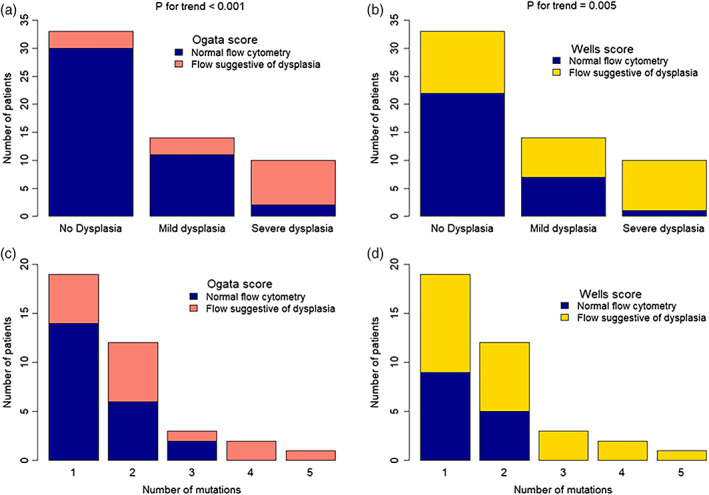
Barplots showing the performance of Ogata (a) and Wells (b) FC‐scores in identifying patients with ICUS/CCUS with morphological signs of dysplasia. Barplots showing the number of mutated genes in CCUS patients and the performance of the Ogata (c) and Wells (d) score. CCUS, clonal cytopenia of undetermined significance; ICUS, idiopathic cytopenia of undetermined significance [Color figure can be viewed at wileyonlinelibrary.com]
By performing a subanalysis on our 37 CCUS patients, we found that the Wells algorithm could identify all the patients with more than two mutations (Figure 3b). By contrast, the Ogata algorithm performed slightly poorer in identifying patients with a higher mutational burden. Thus, our data show that FC using the Wells score might be able to detect CCUS patients with a higher mutational burden.
3.4. Flow cytometric dysplasia as a prognostic marker in ICUS and CCUS
We also evaluated the presence of “high‐risk” mutations and their association with FC changes. The genes, whose mutations were considered to confer a more adverse prognosis were ASXL1, SRSF2, GATA2, RUNX1, EZH2, TP53, ETV6, IDH2, and U2AF1, based on previous studies (Bejar, 2017; Bejar et al., 2011). We identified a total of 21 patients with a mutation in at least one of these “high‐risk” genes. The Wells algorithm was suggestive of dysplasia in 18 (85.7%) of the 21 cases, while the Ogata score was positive for 12 (57.1%) of the patients (Table 2). Morphological examination was available for 17 of the samples, and 10 of the 17 samples (59%) showed signs of mild or severe dysplasia.
Table 2.
FC score and morphological evaluation of the 21 patients with high‐risk mutations (shown in bold)
| “High‐risk” mutations (bold) | Ogata score | Wells score | Grade of dysplasia |
|---|---|---|---|
| CBL, ASXL1, ZRSR2, SETBP1 | 2 | 3 | Severe dysplasia |
| ASXL1 | 3 | 4 | No dysplasia |
| TET2, EZH2, ASXL1, ZRSR2 | 2 | 2 | Mild dysplasia |
| TET2, RUNX1, SRSF2 | 1 | 2 | No dysplasia |
| TET2, SRSF2 | 2 | 4 | Mild dysplasia |
| TET2, ASXL1 | 1 | 3 | Mild dysplasia |
| ASXL1 | 0 | 4 | Severe dysplasia |
| ASXL1 | 0 | 2 | No dysplasia |
| TET2, SRSF2 | 3 | 3 | Severe dysplasia |
| TET2, RUNX1, SRSF2 | 3 | 4 | Severe dysplasia |
| ASXL1, CEBPa, TET2, EZH2, CBL | 2 | 4 | Severe dysplasia |
| TET2, SRSF2 | 2 | 3 | Severe dysplasia |
| U2AF1, TP53 | 3 | 3 | Severe dysplasia |
| TP53 | 0 | 3 | No dysplasia |
| SRSF2 | 1 | 1 | No dysplasia |
| SRSF2, TET2 | 0 | 0 | No dysplasia |
| TET2, ASXL1 | 3 | 4 | No dysplasia |
| SRSF2, TET2, IDH2 | 1 | 3 | NA |
| ETV6 | 1 | 1 | NA |
| ASXL1 | 2 | 2 | NA |
| U2AF1, GATA2 | 3 | 2 | NA |
Abbreviation: FC, flow cytometry.
After a median follow‐up of 28.6 months (range: 2.5–56.3), we observed progression to MDS, AML, or chronic myelomonocytic leukemia (CMML) in eight patients in our cohort. Using the Wells algorithm, all the patients had a high FC score, even though three of these patients had either none (one patient) or only one mutation (two patients), while another patient had no visible changes in morphological evaluation (Table 3). Thus, our data suggest that the Wells score might be able to distinguish patients with cytopenia, who have a higher mutational burden and are at a higher risk of progressing to overt myeloid cancer.
Table 3.
A summary of the eight patients with ICUS/CCUS that have so far progressed to MDS, AML, or CMML
| Mutations | Ogata score | Wells score | Grade of dysplasia |
|---|---|---|---|
| TET2, EZH2, ASXL1, ZRSR2 | 2 | 2 | Mild dysplasia |
| TET2, RUNX1, SRSF2 | 1 | 2 | No dysplasia |
| TET2, SRSF2 | 2 | 4 | Mild dysplasia |
| ASXL1 | 0 | 4 | Severe dysplasia |
| None | 1 | 2 | Mild dysplasia |
| U2AF1, TP53 | 3 | 3 | Severe dysplasia |
| DNMT3A | 2 | 7 | Mild dysplasia |
| U2AF1, GATA2 | 3 | 2 | NA |
Note: Flow cytometry (Wells score) was suggestive of dysplasia in all these patients, while morphological evaluation only showed severe dysplasia in two of the patients.
AML, acute myeloid leukemia; CCUS, clonal cytopenia of undetermined significance; CMML, chronic myelomonocytic leukemia; ICUS, idiopathic cytopenia of undetermined significance; MDS, myelodysplastic syndrome.
4. DISCUSSION
Multiparameter FC is a major diagnostic tool in laboratory hematology and is also being used to evaluate the presence of myelodysplasia in cases presenting with peripheral blood cytopenias (Duetz, Westers, & Van De Loosdrecht, 2018). It is important not only to distinguish MDS from ICUS and CCUS, but also to identify patients with unexplained cytopenia which are at high risk of progression.
In this study, we used two well‐known FC scores for the assessment of myelodysplasia. The Ogata score consists of only four different parameters: percentage of myeloblasts in all nucleated cells, percentage of B‐cell progenitors in CD34+ cells, lymphocyte‐to‐myeloblast CD45 ratio and granulocyte to lymphocyte side scatter ratio (Porta et al., 2012). The Wells score on the other hand is calculated by multiple parameters, including the maturation pattern of granulocytes (Wells et al., 2003) and many of these parameters have also been included in the FC guidelines for MDS from the European Leukemia Net (Porwit et al., 2014). In our cohort of 79 patients with unexplained cytopenia (ICUS/CCUS) according to routine assessment, the Ogata score had a higher specificity, while the Wells score had a higher sensitivity in distinguishing CCUS from ICUS patients. All patients with ICUS or CCUS and a positive Ogata score also had a positive Wells score. Using the Wells score, we found that 23/37 (85.1%) of patients with CCUS also had flow cytometric changes that suggested dysplasia. In ICUS patients, FC using the Wells score was suggestive of dysplasia in 12/42 of the cases (28.6%). These findings can somehow be equated to the genetic landscape of patients with MDS, where it has been shown that around 20% of patients do not have any detectable genetic mutations (Haferlach et al., 2014). However, our data suggest that, overall, both scores performed poorly in differentiating between ICUS and CCUS, and FC can therefore not substitute DNA sequencing of relevant genes.
Our analysis included two of the most widely used diagnostic algorithms for flow cytometric evaluation of bone marrow dysplasia. The European LeukemiaNet Working Group's recommendations suggest a thorough evaluation of granulocytes, monocytes, and myeloblasts, similar to the one employed by Wells et al. (Wells et al., 2003; Westers et al., 2012). However, there is still a lack of standardized gating strategies and cutoffs that can minimize observer bias in such an analysis. We used a cutoff of 20% when examining the aberrant expression of CD34 or CD56 on monocytes; however, this was an arbitrary choice, since precise gating strategies and guidelines for the evaluation of all the different population parameters are still missing. There is no doubt that standardization of FC in evaluating bone marrow dysplasia is a necessity to increase its accuracy and diagnostic value.
It must also be noted that our FC evaluation was based on scores that exclusively consider aberrations involving leukocytes and their progenitors, thus not including the erythroid compartment. However, anemia is a very common symptom in both MDS and ICUS/CCUS and dyserythropoiesis is a hallmark of MDS (Greenberg et al., 2012). Importantly, FC changes in the erythroid progenitors have been broadly examined during the last decade and have been shown to improve the performance of existing algorithms. The RED‐score, which is solely based on the coefficient of variation of the mean expression of only two markers, CD71 and CD36, was introduced in 2013 by Mathis et al. (Mathis et al., 2013). Its sensitivity in diagnosing MDS was estimated at 80% but was increased to 88% when combined with the Ogata algorithm (Mathis et al., 2013). Another recent study by the HOVON group proposed an improved version of the RED‐score, including three markers: CD71, CD36, and CD117 (Cremers et al., 2017). The addition of these three erythroid markers to the Ogata and Wells algorithms increased their sensitivity from 74% to 86% and from 69% to 80%, respectively, without significant changes in specificity (Cremers et al., 2017). A large, multicenter study from the IMDSFlow working group evaluated dyserythropoiesis by FC using four markers: CD36, CD71, CD117, and CD105 (Westers et al., 2017). A weighted score was then calculated based on these four parameters and tested in two different cohorts, yielding a sensitivity of 24–33% and a specificity of 90–92% (Westers et al., 2017). It might be possible that the evaluation of the erythroid compartment can provide additional information in patients with ICUS/CCUS and should be addressed in future studies.
An interesting finding in our study is that FC in ICUS/CCUS patients was in concordance with morphological signs of dysplasia—especially severe dysplasia. It must be emphasized that the initial morphological evaluation in all the patients in our cohort had reported less than 10% of dysplasia and, hence, not a diagnosis of MDS. After a blinded reevaluation of 57 of the samples by two hematopathologists, the cases were divided according to their degree of dysplasia. For a total of 12 samples, at least one of the reviewers found more than 10% of dysplasia in the bone marrow, which would have led to a diagnosis of MDS. FC was suggestive of dysplasia in 11 of these 12 (91.6%) of these samples, while 10 of the samples presented with at least one mutation at diagnosis. If these patients’ BMs had been re‐evaluated morphologically following a positive FC score and/or a positive mutational screening, they might potentially have been reclassified as having MDS instead of ICUS/CCUS and subsequently selected for a different follow‐up and treatment. Thus, FC combined with NGS for MDS‐related genes might have an important role in the evaluation of patients with unexplained cytopenia, since it might be more sensitive in identifying early dysplastic changes, subsequently guiding to a potential morphological reassessment to confirm a diagnosis of MDS according to current criteria.
While our data suggest that FC might not be useful in distinguishing CCUS patients from ICUS patients, we show that FC might offer some additional prognostic information. In our cohort, CCUS patients with a high mutational burden were also more likely to have dysplastic changes by FC evaluation. Moreover, FC could identify 85.7% of patients with high‐risk mutations and, importantly, all the patients that progressed to a myeloid malignancy. It is impossible to estimate whether we, by using a combination of NGS, FC, and morphological reevaluation, may have identified a subgroup of ICUS/CCUS patients with a more adverse prognosis, mainly due to the relatively short follow‐up of our cohort. Eight patients in our cohort have so far progressed to either MDS, AML, or CMML; seven had mutations in one or more genes, only two of them had signs of severe dysplasia by morphology, while all eight patients had FC changes suggestive of dysplasia when using the Wells score. Thus, a combined approach with both FC and genomic analyses might provide more precise prognostic information in patients with ICUS/CCUS. Additional studies with bigger cohorts are needed to define the precise diagnostic and prognostic role of FC in patients with ICUS and/or CCUS.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
KD and OKH designed this study, collected and analyzed the data, and wrote the manuscript. LDS and LS contributed with morphological analysis of the samples. JWH performed the NGS analysis and gathered all the relevant clinical data for this study. KG and JWH were supervisors of this study and contributed to the design of this study, data interpretation, and preparation of the manuscript.
Supporting information
Supplementary Fig. 1 Frequency of single gene mutations across our cohort of 79 patients with ICUS. The most common mutated gene is TET2, followed by ASXL1.
Supplementary Table 1: Mutation frequencies in our population of patients initially diagnosed with ICUS. The most commonly mutated gene is TET2. We detected no mutations in KRAS, NRAS or JAK2. The FC‐score applied to evaluate dysplasia (top row: purple, yellow) was based on the Wells algorithm.
ACKNOWLEDGMENTS
The authors would like to thank Steen Mortensen for his technical assistance. This study was supported by the University of Copenhagen (KD), Rigshospitalet (KD). The K.G. lab is also funded by center grants from the Danish Cancer Society (Danish Research Centre for Precision Medicine in Blood Cancer; grant 223‐A13071‐18‐S68), the Novo Nordisk Foundation (Novo Nordisk Foundation Centre for Stem Cell Biology, DanStem; grant NNF17CC0027852) and the Greater Copenhagen Health Science Partners (Clinical Academic Group in Translational Hematology).
Dimopoulos K, Hansen OK, Sjö LD, et al. The diagnostic and prognostic role of flow cytometry in idiopathic and clonal cytopenia of undetermined significance (ICUS/CCUS): A single‐center analysis of 79 patients. Cytometry. 2020;98:250–258. 10.1002/cyto.b.21842
Funding information Novo Nordisk Foundation, Grant/Award Number: NNF17CC0027852; Danish Cancer Society; University of Copenhagen
Contributor Information
Konstantinos Dimopoulos, Email: konstantinos.dimopoulos@regionh.dk.
Kirsten Grønbæk, Email: kirsten.groenbaek@regionh.dk.
REFERENCES
- Bejar, R. (2017). Implications of molecular genetic diversity in MDS. Current Opinion in Hematology, 24(2), 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejar, R. , Stevenson, K. , Abdel‐Wahab, O. , Galili, N. , Nilsson, B. , Garcia‐Manero, G. , … Ebert, B. L. (2011). Clinical effect of point mutations in myelodysplastic syndromes. The New England Journal of Medicine [Internet, 364(26), 2496–2506. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/21714648%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3159042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargo, C. A. , Rowbotham, N. , Evans, P. A. , Barrans, S. L. , Bowen, D. T. , Crouch, S. , & Jack, A. S. (2015). Targeted sequencing identifies patients with preclinical MDS at high risk of disease progression. Blood, 126(21), 2362–2365. [DOI] [PubMed] [Google Scholar]
- Cremers, E. M. P. , Westers, T. M. , Alhan, C. , Cali, C. , Visser‐Wisselaar, H. A. , Chitu, D. A. , … A study on behalf of the HOVON89 study group . (2017). Implementation of erythroid lineage analysis by flow cytometry in diagnostic models for myelodysplastic syndromes. Haematologica, 102(2), 320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duetz, C. , Westers, T. M. , & Van De Loosdrecht, A. A. (2018). Clinical implication of multi‐parameter flow cytometry in myelodysplastic syndromes. Pathobiology [Internet], 85, 274–283. Retrieved from. http://www.karger.com/Services/OpenAccessLicense [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg, P. L. , Tuechler, H. , Schanz, J. , Sanz, G. , Garcia‐Manero, G. , Solé, F. , … Haase, D. (2012. September 20). Revised international prognostic scoring system for myelodysplastic syndromes. Blood [Internet, 120(12), 2454–2465. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/22740453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haferlach, T. , Nagata, Y. , Grossmann, V. , Okuno, Y. , Bacher, U. , Nagae, G. , … Ogawa, S. (2014). Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia [Internet, 28(2), 241–247. Retrieved from. 10.1038/leu.2013.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, J. W. , Westman, M. K. , Sjö, L. D. , Saft, L. , Kristensen, L. S. , Ørskov, A. D. , … Grønbæk, K. (2016). Mutations in idiopathic cytopenia of undetermined significance assist diagnostics and correlate to dysplastic changes. American Journal of Hematology, 91(12), 1234–1238. [DOI] [PubMed] [Google Scholar]
- Kwok, B. , Hall, J. M. , Witte, J. S. , Xu, Y. , Reddy, P. , Lin, K. , … Bejar, R. (2015. Nov 19). MDS‐associated somatic mutations and clonal hematopoiesis are common in idiopathic cytopenias of undetermined significance. Blood [Internet, 126(21), 2355–2361. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/25931582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis, S. , Chapuis, N. , Debord, C. , Rouquette, A. , Radford‐Weiss, I. , Park, S. , … Bardet, V. (2013). Flow cytometric detection of dyserythropoiesis: A sensitive and powerful diagnostic tool for myelodysplastic syndromes. Leukemia, 27(10), 1981–1987. [DOI] [PubMed] [Google Scholar]
- Porta, M. G. D. , Picone, C. , Pascutto, C. , Malcovati, L. , Tamura, H. , Handa, H. , … Ogata, K. (2012). Multicenter validation of a reproducible flow cytometric score for the diagnosis of low‐grade myelodysplastic syndromes: Results of a European LeukemiaNET study. Haematologica, 97(8), 1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porwit, A. , Van De Loosdrecht, A. A. , Bettelheim, P. , Eidenschink Brodersen, L. , Burbury, K. , Cremers, E. , … Béné, M. C. (2014). Revisiting guidelines for integration of flow cytometry results in the WHO classification of myelodysplastic syndromes ‐ proposal from the international/European LeukemiaNet working Group for Flow Cytometry in MDS. Leukemia, 28(9), 1793–1798. [DOI] [PubMed] [Google Scholar]
- Steensma, D. P. , Bejar, R. , Jaiswal, S. , Lindsley, R. C. , Sekeres, M. A. , Hasserjian, R. P. , & Ebert, B. L. (2015. Jul 2). Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood [Internet, 126(1), 9–16. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/25931582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valent, P. , Horny, H.‐P. , Bennett, J. M. , Fonatsch, C. , Germing, U. , Greenberg, P. , … Wells, D. A. (2007. Jun). Definitions and standards in the diagnosis and treatment of the myelodysplastic syndromes: Consensus statements and report from a working conference. Leukemia Research [Internet, 31(6), 727–736. Available from. http://www.ncbi.nlm.nih.gov/pubmed/17257673 [DOI] [PubMed] [Google Scholar]
- Valent, P. , & Valent, P. (2019). ICUS, IDUS, CHIP and CCUS: Diagnostic criteria, separation from MDS and clinical implications. Pathobiology, 86(1), 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen, J. J. M. , Lhermitte, L. , Böttcher, S. , Almeida, J. , van der Velden, V. H. J. , Flores‐Montero, J. , … Orfao, A. (2012. Sep [cited 2014 Nov 28]). EuroFlow antibody panels for standardized n‐dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia [Internet, 26(9), 1908–1975. Retrieved from. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3437410&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, D. A. , Benesch, M. , Loken, M. R. , Vallejo, C. , Myerson, D. , Leisenring, W. M. , & Deeg, H. J. (2003). Myeloid and monocytic dyspoiesis as determined by flow cytometric scoring in myelodysplastic syndrome correlates with the IPSS and with outcome after hematopoietic stem cell transplantation. Blood, 102(1), 394–403. [DOI] [PubMed] [Google Scholar]
- Westers, T. M. , Cremers, E. M. P. , Oelschlaegel, U. , Johansson, U. , Bettelheim, P. , Matarraz, S. , … IMDSFlow Working Group . (2017). Immunophenotypic analysis of erythroid dysplasia in myelodysplastic syndromes. A report from the IMDSFlow working group. Haematologica, 102(2), 308–319.27758818 [Google Scholar]
- Westers, T. M. , Ireland, R. , Kern, W. , Alhan, C. , Balleisen, J. S. , Bettelheim, P. , … van de Loosdrecht, A. A. (2012. July). Standardization of flow cytometry in myelodysplastic syndromes: A report from an international consortium and the European LeukemiaNet working group. Leukemia [Internet, 26(7), 1730–1741. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/22307178 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 Frequency of single gene mutations across our cohort of 79 patients with ICUS. The most common mutated gene is TET2, followed by ASXL1.
Supplementary Table 1: Mutation frequencies in our population of patients initially diagnosed with ICUS. The most commonly mutated gene is TET2. We detected no mutations in KRAS, NRAS or JAK2. The FC‐score applied to evaluate dysplasia (top row: purple, yellow) was based on the Wells algorithm.


