ABSTRACT
Objectives
To identify antenatal ultrasound markers that can differentiate between simple and complex gastroschisis and assess their predictive value.
Methods
This was a prospective nationwide study of pregnancies with isolated fetal gastroschisis that underwent serial longitudinal ultrasound examination at regular specified intervals between 20 and 37 weeks' gestation. The primary outcome was simple or complex (i.e. involving bowel atresia, volvulus, perforation or necrosis) gastroschisis at birth. Fetal biometry (abdominal circumference and estimated fetal weight), the occurrence of polyhydramnios, intra‐ and extra‐abdominal bowel diameters and the pulsatility index (PI) of the superior mesenteric artery (SMA) were assessed. Linear mixed modeling was used to compare the individual trajectories of cases with simple and those with complex gastroschisis, and logistic regression analysis was used to estimate the strength of association between the ultrasound parameters and outcome.
Results
Of 104 pregnancies with isolated fetal gastroschisis included, four ended in intrauterine death. Eighty‐one (81%) liveborn infants with simple and 19 (19%) with complex gastroschisis were included in the analysis. We found no relationship between fetal biometric variables and complex gastroschisis. The SMA‐PI was significantly lower in fetuses with gastroschisis than in healthy controls, but did not differentiate between simple and complex gastroschisis. Both intra‐ and extra‐abdominal bowel diameters were larger in cases with complex, compared to those with simple, gastroschisis (P < 0.001 and P < 0.005, respectively). The presence of intra‐abdominal bowel diameter ≥ 97.7th percentile on at least three occasions, not necessarily on successive examinations, was associated with an increased risk of the fetus having complex gastroschisis (relative risk, 1.56 (95% CI, 1.02–2.10); P = 0.006; positive predictive value, 50.0%; negative predictive value, 81.4%).
Conclusions
This large prospective longitudinal study found that intra‐abdominal bowel dilatation when present repeatedly during fetal development can differentiate between simple and complex gastroschisis; however, the positive predictive value is low, and therefore the clinical usefulness of this marker is limited. © 2019 Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: bowel, gastroschisis, intra‐abdominal bowel diameter, mesenteric artery, ultrasound
CONTRIBUTION —
What are the novel findings of this work?
This prospective longitudinal study showed that increased fetal intra‐abdominal bowel diameter measured on at least three occasions can differentiate between simple and complex (atresia, volvulus, perforation or necrosis of the bowel) gastroschisis.
What are the clinical implications of this work?
Children born with complex gastroschisis have a higher morbidity than do children with simple gastroschisis. This study identifies ultrasound markers that may help to identify those fetuses that are at risk of a complicated neonatal period.
INTRODUCTION
Gastroschisis is diagnosed antenatally in over 90% of cases1. Although for liveborn infants with gastroschisis the rate of survival to initial hospital discharge is good (> 90%)2–6, the risk of intrauterine death (IUD) is still 7.5 times higher than in the normal population7 and morbidity occurs in 30% of affected liveborns. The condition of the bowel at birth is an important prognostic factor for neonatal outcome8. Compared with gastroschisis cases without additional intestinal abnormalities (simple gastroschisis), children born with complex gastroschisis (i.e. involving atresia, volvulus, perforation or necrosis of the bowel) have an increased risk of mortality and morbidity, and of prolonged hospitalization, long‐term use of parenteral nutrition, additional ventilation days, need for multiple surgical procedures and postoperative complications9–11.
The antenatal prediction of intestinal complications in infants with gastroschisis could identify those cases that might benefit from obstetric intervention, such as preterm induction of labor and improved parental counseling. In addition, prenatal identification of bowel atresia would help the surgeon to plan a timely repair; at present, bowel atresia is missed at the first surgery in about 40% of cases because significant individual bowel loops cannot be identified12–14.
Numerous attempts have been made to correlate antenatal ultrasound findings with neonatal outcomes in pregnancies with fetal gastroschisis. Reports are conflicting because of the small size of the study populations, retrospective study design and non‐standardized methods and timing of ultrasound examinations15, 16. A recent meta‐analysis, based mainly on retrospective studies, showed that intra‐abdominal bowel dilatation and polyhydramnios are the best ultrasound markers for bowel atresia; however, definitions of bowel dilatation and polyhydramnios were not given16.
We conducted a prospective longitudinal multicenter study of pregnancies with isolated fetal gastroschisis that underwent fetal ultrasound assessment and surveillance according to a standardized protocol, in order to identify antenatal ultrasound markers that could differentiate between complex and simple gastroschisis. In addition to bowel dilatation and polyhydramnios, we documented fetal biometry, including abdominal circumference (AC), and the blood flow pattern of the superior mesenteric artery (SMA) as potential markers for complex gastroschisis.
METHODS
In The Netherlands, all pregnancies diagnosed with fetal gastroschisis are referred to one of seven university medical centers with a pediatric surgery department. This prospective nationwide longitudinal observational cohort study was conducted at all seven centers between 2010 and 2015. Pregnancies with fetal gastroschisis confirmed by ultrasound and with no suspicion of other extragastrointestinal congenital disorder that could potentially influence the outcome were eligible for participation. Cases found to have major extragastrointestinal congenital abnormalities (non‐isolated gastroschisis) at any time, pre‐ or postnatally, were excluded post hoc. The study protocol was approved by the Medical Review Ethics Committee of each participating center.
Ultrasound examination
As part of the Dutch healthcare system, all pregnancies undergo a fetal anomaly scan at 18–22 weeks' gestation to detect structural anomalies. After obtaining informed consent from the parents to participate in the study, all pregnancies with gastroschisis underwent ultrasound follow‐up evaluations at 24, 28, 30, 32, 34, 35 and 36 weeks. During each examination, fetal biometry and amniotic fluid index were evaluated and the pulsatility index (PI) of the umbilical artery (UA), SMA‐PI and bowel diameter were measured. All examinations were carried out by trained ultrasonographers using a GE Voluson 730 or E8 (GE Healthcare, Zipf, Austria) ultrasound machine, with a 4–8‐MHz transabdominal transducer.
Polyhydramnios was defined as an amniotic fluid index ≥ 24 cm17. Intra‐ and extra‐abdominal bowel diameters were measured at the short axis of the bowel lumen (inner to inner wall) of the most dilated bowel segment. Intra‐abdominal SMA velocity measurements were obtained in a sagittal or axial plane of the fetal abdomen after its origin from the aorta, just above the renal arteries (with an angle of insonation below 30°)18. The extra‐abdominal SMA was identified and its flow velocity measured directly distally to the abdominal wall defect.
In both simple‐ and complex‐gastroschisis cases, the AC and estimated fetal weight (EFW) were measured and the values were expressed as Z‐scores and compared with well‐established reference data19, 20. The occurrence of polyhydramnios during pregnancy was compared between cases with simple and those with complex gastroschisis.
The intra‐ and extra‐abdominal bowel diameters of both the simple‐ and complex‐gastroschisis cases were compared with the longitudinal reference curves for normal fetal colon diameters (based on 39 uncomplicated pregnancies at one of the participating units)21.
The raw SMA‐PI measurements in simple‐ and complex‐gastroschisis cases were compared with the reference ranges for fetal SMA‐PI described by Ebbing et al.18 and expressed as Z‐scores.
Fetal monitoring and labor
Cardiotocographic surveillance was performed from 34 weeks' gestation at least twice a week in a home‐monitoring or outpatient setting until delivery. Delivery was planned from 37 weeks onwards by induction of labor22. Cesarean delivery was performed only for obstetric reasons, such as fetal distress or failure to progress in labor.
Birth weight was expressed as a Z‐score according to Dutch reference charts, adjusted for parity, sex and gestational age at birth. Small‐for‐gestational age was defined as a neonate with a birth weight < 10th percentile based on The Netherlands Perinatal Registry data/ Dutch reference curves23.
Neonatal care
Primary operative abdominal‐wall repair of gastroschisis was attempted in all cases based on the condition of the child, the volume of exteriorized viscera and the judgment of the surgeon. If the viscera could not be reduced primarily, a silo bag was placed and elective closure of the abdominal wall was planned in subsequent days. Gastroschisis cases were categorized as simple or complex based on the condition of the gastrointestinal tract at birth. The presence of atresia, volvulus, necrosis or perforation of the bowel at birth was defined as complex gastroschisis9, 24. The primary outcome was simple or complex gastroschisis at birth.
Statistical analysis
Statistical analysis was performed using the statistical software package SPSS version 23 (SPSS Inc., Chicago, IL, USA). Results are summarized as n (%) for categorical variables and mean ± SD or median (range) for continuous variables. The normality of the continuous variables was assessed using the Kolmogorov–Smirnov test and Q–Q plot. Comparison of clinical characteristics between the two study groups (simple and complex gastroschisis) was performed using standard statistical tests. The Mann–Whitney U‐test was used to compare continuous data with skewed distribution and the unpaired t‐test was used for comparison of variables for which the assumption of normal distribution was retained. Comparison of categorical variables was performed using either the chi‐square test or Fisher's exact test, as appropriate. The Yates' continuity of correction factor was added to the chi‐square test when testing two variables each with two categories (2 × 2 contingency table). Linear mixed modeling was performed to analyze the regression of serial measurements over time, for the biometric variables (Z‐scores), intestinal intra‐ and extra‐abdominal diameters and PI values of the UA and SMA (Z‐scores). Models with linear and quadratic components of gestational age (centered at 20 weeks) were explored and compared using the Bayesian information criterion. If significant differences in ultrasound parameters were found between the simple‐ and complex‐gastroschisis cases, logistic regression analysis was performed to estimate the strength of the association between different ultrasound parameters and outcome; P < 0.05 was considered statistically significant.
RESULTS
During the study period, 131 pregnancies were diagnosed with fetal gastroschisis in The Netherlands. Of these, 27 (20.6%) were excluded from the study: one pregnancy resulted in IUD before 20 weeks' gestation, 12 couples opted for termination of the pregnancy and 14 couples did not want to participate. Therefore, 104 pregnancies with isolated fetal gastroschisis were included in the study, comprising 103 singleton pregnancies and one dichorionic twin pregnancy with one affected fetus. Four of the pregnancies resulted in IUD; apart from severe growth restriction, no other causes of IUD were identified (Table S1).
Table 1 shows the maternal characteristics and perinatal outcomes of the remaining 100 pregnancies with liveborn neonates, according to whether they were diagnosed with simple (n = 81; 81%) or complex (n = 19; 19%) gastroschisis. There were no significant differences between the two groups with respect to maternal characteristics. Children with complex gastroschisis were born on average 1 week earlier (P = 0.02) and more often after spontaneous onset of delivery (P = 0.003) than were those with simple gastroschisis. All other perinatal outcomes were not significantly different between the two groups.
Table 1.
Maternal characteristics and perinatal outcomes of 100 liveborn infants with gastroschisis, according to whether they were diagnosed with simple or complex gastroschisis
| Parameter | Simple gastroschisis (n = 81) | Complex gastroschisis (n = 19) | P |
|---|---|---|---|
| Maternal characteristic | |||
| Maternal age (years) | 26.3 ± 5.5 | 26.9 ± 4.9 | 0.54 |
| Nulliparous | 57 (70.4) | 15 (78.9) | 0.58 |
| Body mass index (kg/m2) a | 22.9 ± 3.7 | 22.4 ± 3.3 | 0.59 |
| Smoker in first trimester b | 21 (29.2) | 6 (33.3) | 0.78 |
| Perinatal outcome | |||
| GA at birth (weeks) | 36.9 (31.9–38.3) | 36.0 (32.3–37.6) | 0.02 |
| Onset of delivery | 0.003 | ||
| Spontaneous | 21 (25.9) | 11 (57.9) | |
| Induction | 48 (59.3) | 3 (15.8) | |
| Cesarean section | 12 (14.8) | 5 (26.3) | |
| Mode of delivery | 0.05 | ||
| Spontaneous vaginal | 59 (72.8) | 11 (57.9) | |
| Instrumental vaginal | 2 (2.5) | 3 (15.8) | |
| Cesarean section | 20 (24.7) | 5 (26.3) | |
| Birth weight (g) | 2500 ± 464 | 2372 ± 403 | 0.27 |
| Birth weight < 10th percentile | 12 (14.8) | 4 (21.1) | 0.50 |
| Male gender | 41 (50.6) | 12 (63.2) | 0.32 |
| Primary closure c | 52 (65.0) | 11 (57.9) | 0.56 |
| Repeat surgery after defect closure | 35 (43.2) | 17 (89.5) | < 0.001 |
| Bowel condition at birth | |||
| Atresia | — | 18 (94.7) | |
| Antenatal volvulus | — | 1 (5.3) | |
| Necrosis | — | 3 (15.8) | |
| Perforation | — | 3 (15.8) | |
| Intestinal complications* | 2 (2.5) | 5 (26.3) | 0.0025 |
| Non‐intestinal complications | |||
| Cholestatic icterus | 23 (28.4) | 14 (73.7) | < 0.001 |
| Line sepsis | 27 (33.3) | 12 (63.2) | 0.021 |
| Wound infections | 9 (11.1) | 4 (21.1) | 0.26 |
| Respiratory problems | 14 (17.3) | 3 (15.8) | 1.00 |
| Neurological problems | 10 (12.3) | 1 (5.3) | 0.69 |
| Mortality | 1 (1.2) | 2 (10.5) | 0.09 |
| Time to full enteral feeding (days) d † | 27 (8–183) | 90 (13–236) | < 0.001 |
| Length of hospital stay (days) e , ‡ | 37 (12–155) | 99 (31–203) | < 0.001 |
Data are given as mean ± SD, median (range) or n (%). Data available for:
74 patients in simple‐gastroschisis group;
72 patients in simple‐gastroschisis group and 18 in complex‐gastroschisis group;
80 patients in simple‐gastroschisis group;
80 patients in simple‐gastroschisis group and 17 in complex‐gastroschisis group (three cases who died during hospital stay were not included);
78 patients in simple‐gastroschisis group and 16 in complex‐gastroschisis group (three cases who died during hospital stay were not included).
Necrotizing enterocolitis, postnatal bowel perforation, postnatal bowel stricture.
Three patients in simple‐gastroschisis group and five in complex‐gastroschisis group were discharged home with parenteral nutrition, and discharge date was defined as time to full enteral feeding.
Information for length of hospital stay was missing for three patients (two in simple‐gastroschisis group and one in complex‐gastroschisis group) who were transferred to regional hospital.
GA, gestational age.
The most common additional gastrointestinal disorder was bowel atresia, accounting for 94.7% (18/19) of cases with complex gastroschisis. The remaining case had perforation of the proximal jejunum without atresia. Six cases with complex gastroschisis had more than one additional intestinal disorder. Minor additional congenital abnormalities were unilateral clubfoot in one child and hydronephrosis in six children.
Three postnatal deaths occurred: one (1.2%) in the simple‐gastroschisis group and two (10.5%) in the complex‐gastroschisis group (P = 0.09). The case with simple gastroschisis died 48 days after birth owing to respiratory insufficiency and severe hydrocephalus after a subdural hematoma. One case with complex gastroschisis died 128 days after birth owing to multiorgan failure after repeat operations for small‐bowel perforation and sepsis. The second case with complex gastroschisis died 254 days after delivery because of persistent sepsis caused by perforation of the duodenum after adhesiolysis.
Median time to full enteral feeding and length of hospital stay were three times longer in the complex‐gastroschisis group than in the simple‐gastroschisis group (both P < 0.001). The incidence of common neonatal respiratory and neurological complications was similar between the two groups, but fetuses with complex gastroschisis more often needed repeat surgical interventions (P < 0.001), as expected.
Fetal ultrasound evaluation
The mean number of serial measurements per fetus and the mean gestational age at first and last examination were similar between the two groups for biometry and most Doppler variables evaluated (Table S2).
Polyhydramnios occurred in six cases (7.4%) with simple gastroschisis and in three (15.8%) with complex gastroschisis (P = 0.37). Neonatal mortality was significantly higher in cases with polyhydramnios (22.2% (2/9; one case with simple and one with complex gastroschisis)) than in those without (1.1% (1/90; one case with complex gastroschisis)) (P = 0.02).
Both simple‐ and complex‐gastroschisis cases had a smaller AC and lower EFW than did normal controls (Figure 1), but the model‐predicted trajectories for AC and EFW did not differ between the simple‐ and complex‐gastroschisis groups (Table S3).
Figure 1.
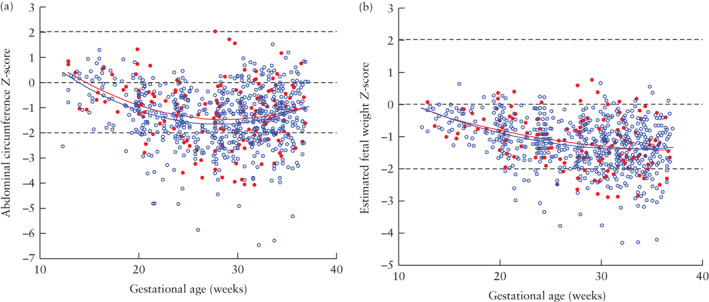
Distribution of serial measurements of abdominal circumference (a) and estimated fetal weight (b), expressed as Z‐scores, in 19 fetuses with complex ( ) and 81 with simple (
) and 81 with simple ( ) gastroschisis, presented relative to reference lines (mean ± 2SD) for normal population (
) gastroschisis, presented relative to reference lines (mean ± 2SD) for normal population ( ). Model‐predicted median curves, based on linear mixed modeling, are shown for simple‐gastroschisis (
). Model‐predicted median curves, based on linear mixed modeling, are shown for simple‐gastroschisis ( ) and complex‐gastroschisis (
) and complex‐gastroschisis ( ) groups.
) groups.
The distribution of the UA‐PI measurements of gastroschisis cases was comparable to that of normal reference ranges and did not differ between fetuses with simple and those with complex gastroschisis (data not shown).
In fetuses with simple gastroschisis, the intra‐abdominal bowel diameter was similar to the colon diameter in normal fetuses , but the extra‐abdominal bowel diameter was generally larger (Figure 2). Compared with normal controls, fetuses with complex gastroschisis had larger intra‐ and extra‐abdominal bowel diameters, with the intra‐abdominal bowel diameter exceeding the 97.7th percentile of controls in just over half of the cases and the extra‐abdominal bowel diameter exceeding the 97.7th percentile in over 70% of third‐trimester measurements. Cases with complex gastroschisis had larger intra‐ and extra‐abdominal bowel diameters than did those with simple gastroschisis (P < 0.001 and P < 0.005, respectively; Table S3), resulting in different model‐predicted trajectories (Figure 2).
Figure 2.
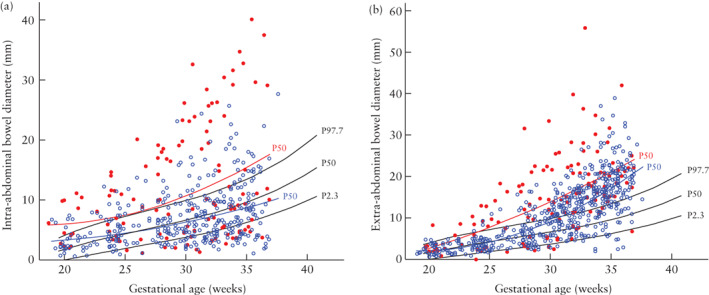
Distribution of serial measurements of intra‐abdominal (a) and extra‐abdominal (b) bowel diameter in 19 fetuses with complex ( ) and 81 with simple (
) and 81 with simple ( ) gastroschisis. Model‐predicted 50th percentile (P50) curves, calculated based on linear mixed modeling, are shown for simple‐gastroschisis (
) gastroschisis. Model‐predicted 50th percentile (P50) curves, calculated based on linear mixed modeling, are shown for simple‐gastroschisis ( ) and complex‐gastroschisis (
) and complex‐gastroschisis ( ) groups. Reference curves (2.3rd (P2.3), 50th (P50) and 97.7th (P97.7) percentiles) for colon diameter in normal fetuses are also shown (
) groups. Reference curves (2.3rd (P2.3), 50th (P50) and 97.7th (P97.7) percentiles) for colon diameter in normal fetuses are also shown ( ).
).
There were only a few complex‐gastroschisis cases with consistently large intra‐ and/or extra‐abdominal bowel diameters, while in the rest of the cases, the bowel diameters varied over time, tending to normalize near term. This is illustrated in Figure 3c, which shows the individual trajectories of the intra‐abdominal bowel diameter for all complex‐gastroschisis cases. Intra‐abdominal bowel diameter ≥ 97.7th percentile on at least two or three occasions, not necessarily successive ones, during a fetus's development was predictive of complex gastroschisis (Table 2). Statistical significance was reached for the occurrence of at least three intra‐abdominal bowel measurements ≥ 97.7th percentile, with an odds ratio (OR) of 4.39 (95% CI, 1.46–13.21) (P = 0.009), relative risk of 1.56 (95% CI, 1.02–2.10) (P = 0.006), sensitivity of 40.9%, specificity of 86.4% and positive and negative predictive values of 50.0% and 81.4%, respectively.
Figure 3.
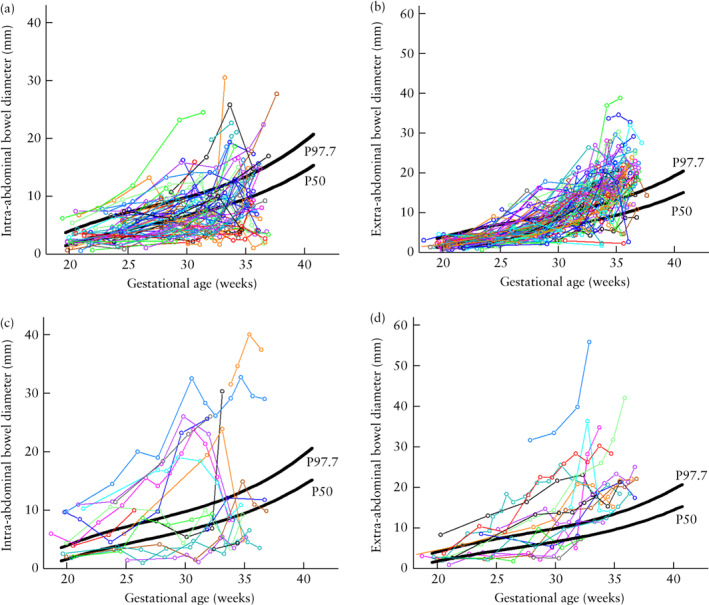
Individual trajectories, according to gestational age, of intra‐abdominal (a,c) and extra‐abdominal (b,d) bowel diameter measurements in 81 fetuses with simple (a,b) and 19 fetuses with complex (c,d) gastroschisis. Reference curves (50th (P50) and 97.7th (P97.7) percentiles) for colon diameter in normal fetuses are shown ( ).
).
Table 2.
Potential predictors of complex gastroschisis
| Variable | β (SE) |
Odds ratio (95% CI) |
P | n | Nagelkerke R 2 |
|---|---|---|---|---|---|
| Polyhydramnios | 0.84 (0.76) | 2.31 (0.52–10.24) | 0.27 | 99 | 0.018 |
| IA bowel diameter ≥ 97.7th percentile | |||||
| ≥ 1 event per fetus | 0.94 (0.61) | 2.56 (0.77–8.46) | 0.12 | 93 | 0.044 |
| ≥ 2 events per fetus | 0.98 (0.54) | 2.66 (0.93–7.64) | 0.07 | 90 | 0.058 |
| ≥ 3 events per fetus | 1.48 (0.56) | 4.39 (1.46–13.21) | 0.009 | 89 | 0.117 |
| Final measurement | 0.02 (0.56) | 1.02 (0.34–3.05) | 0.97 | 91 | 0.001 |
| EA bowel diameter ≥ 97.7th percentile | |||||
| ≥ 1 event per fetus | 0.09 (0.83) | 1.09 (0.22–5.53) | 0.92 | 98 | 0.001 |
| ≥ 2 events per fetus | –0.47 (0.65) | 0.63 (0.18–2.23) | 0.47 | 97 | 0.008 |
| ≥ 3 events per fetus | 0.17 (0.62) | 1.19 (0.35–4.03) | 0.78 | 97 | 0.001 |
| Final measurement | –0.19 (0.59) | 0.83 (0.26–2.61) | 0.75 | 98 | 0.002 |
| IA SMA‐PI ≤ 2.3rd percentile | |||||
| ≥ 1 event per fetus | 0.24 (0.70) | 1.27 (0.32–4.95) | 0.74 | 92 | 0.002 |
| ≥ 2 events per fetus | 0.28 (0.61) | 1.33 (0.41–4.36) | 0.64 | 87 | 0.004 |
| ≥ 3 events per fetus | 0.65 (0.66) | 1.92 (0.53–6.96) | 0.32 | 76 | 0.023 |
| EA SMA‐PI ≤ 2.3rd percentile | |||||
| ≥ 1 event per fetus | –0.05 (0.84) | 0.96 (0.19–4.94) | 0.96 | 93 | 0.001 |
| ≥ 2 events per fetus | 0.10 (0.71) | 1.10 (0.28–4.41) | 0.89 | 85 | 0.001 |
| ≥ 3 events per fetus | 0.41 (0.66) | 1.51 (0.41–5.54) | 0.53 | 77 | 0.009 |
EA, extra‐abdominal; IA, intra‐abdominal; PI, pulsatility index; SMA, superior mesenteric artery.
Individual fetal trajectories for the extra‐abdominal bowel diameter of complex‐gastroschisis cases were analyzed in a similar way, but did not show significant results (Table 2, Figure 3d). For comparison, the individual trajectories for intra‐ and extra‐abdominal bowel diameters in fetuses with simple gastroschisis are shown in Figures 3a and 3b.
Doppler measurements of intra‐ and extra‐abdominal SMA‐PI in fetuses with complex and those with simple gastroschisis are shown in Figure 4. Overall, 83% of intra‐abdominal and 89% of extra‐abdominal SMA‐PI measurements were below the median (50th percentile) of the normal reference ranges. The lowest PI values were found for the extra‐abdominal SMA. Simple‐ and complex‐gastroschisis cases had similar developmental trajectories for the intra‐ and extra‐abdominal SMA‐PI measurements (Figure 4, Table S3). Intra‐ and extra‐abdominal SMA‐PI measurements were not predictive of complex gastroschisis (Table 2). There was no significant relationship between SMA‐PI and the degree of bowel dilatation (data not shown).
Figure 4.
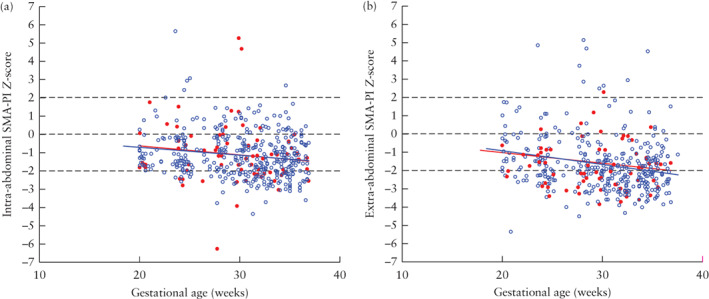
Distribution of serial measurements of intra‐abdominal superior mesenteric artery pulsatility index (SMA‐PI) (a) and extra‐abdominal SMA‐PI (b), expressed as Z‐scores, in 19 fetuses with complex ( ) and 81 with simple (
) and 81 with simple ( ) gastroschisis, presented relative to reference lines (mean ± 2SD) for normal population (
) gastroschisis, presented relative to reference lines (mean ± 2SD) for normal population ( ). Model‐predicted median curves, based on linear mixed modeling, are shown for simple‐gastroschisis (
). Model‐predicted median curves, based on linear mixed modeling, are shown for simple‐gastroschisis ( ) and complex‐gastroschisis (
) and complex‐gastroschisis ( ) groups.
) groups.
DISCUSSION
This is the largest prospective longitudinal study to date to investigate the predictive value of antenatal ultrasound markers for complex gastroschisis. The study confirms that complex fetal gastroschisis is associated with higher morbidity than is simple gastroschisis. Although both intra‐ and extra‐abdominal bowel diameters were larger in complex‐gastroschisis cases than in fetuses with simple gastroschisis, antenatal prediction using this marker remained difficult, given the large overlap in values between the simple‐ and complex‐gastroschisis cases and fluctuations in the measured bowel diameters between successive examinations in the same fetus. If the intra‐abdominal bowel diameter is above the 97.7th percentile (adjusted for gestational age) in three or more measurements during pregnancy, the likelihood of having complex gastroschisis is significantly increased for this fetus.
Compared with normal fetuses, a significantly lower flow resistance in the SMA was observed in both simple‐ and complex‐gastroschisis cases, but we were unable to differentiate between the two groups using this marker. AC, EFW, UA‐PI and amniotic fluid volume (polyhydramnios) were also not useful in discriminating between simple and complex gastroschisis. In the overall study cohort (simple and complex cases) polyhydramnios was associated with neonatal mortality but not with neonatal morbidity, although the small number of cases precludes a definitive conclusion.
Similarly to our findings, a recent meta‐analysis on prenatal ultrasound variables and their association with outcomes in gastroschisis showed that prenatal intra‐abdominal bowel dilatation was associated with bowel atresia (OR, 5.48 (95% CI, 3.1–9.8))16. However, in the studies included in this meta‐analysis (eight retrospective and one prospective study), the cut‐off values for abnormal intra‐abdominal bowel diameter either were not stated11, 25, 26 or varied widely from 6 to 18 mm27–32, and gestational age at scanning was not reported consistently. This hinders the use of this parameter in clinical practice. Hijkoop et al.33 demonstrated a significant association between extra‐abdominal bowel dilatation at 30 weeks' gestation and complex gastroschisis with a high negative predictive value (88%) but also a low positive predictive value (40%).
In our study, the occurrence of intra‐abdominal bowel diameter measurements above the 97.7th percentile (adjusted for gestational age) on at least three occasions was significantly different between simple‐ and complex‐gastroschisis cases. The specificity of this marker was high (86.4%); however, there was a considerable degree of overlap between simple and complex gastroschisis cases, rendering the sensitivity of the marker low (40.9%). Therefore its usefulness as a screening marker for detecting complex gastroschisis remains modest.
In search of potential markers for vascular obstruction, a putative cause of intestinal damage, we studied the PI of the SMA and found that this was on average significantly lower in fetuses with gastroschisis than in normal controls, especially with advancing gestation. It may be hypothesized that this decreased vascular resistance may be caused by vasodilatation as a consequence of progressive chronic inflammation of the extra‐abdominal bowel. This may, in turn, lead to leakage of albumin from the vessel wall and eventually to hypovolemia due to reduced serum protein concentration, and hypoperfusion of the exposed bowel34.
Our results do not support the theory that vascular constriction at the level of the abdominal wall is a cause of intestinal damage in fetal gastroschisis35, 36. Only three previous studies have studied SMA Doppler velocimetry in fetal gastroschisis. Martillotti et al.37 performed a retrospective study in which they found a higher incidence of perturbed mesenteric circulation in cases with complex gastroschisis; however, the measurement method and the definition of abnormal Doppler were not described. Volumenie et al.38 analyzed the influence of amnioinfusion on Doppler velocimetry of the SMA in 17 cases of gastroschisis and reported a significant positive correlation between the extra‐abdominal PI before amnioinfusion and maximal bowel dilatation (r = 0.54) and length of stay in the neonatal intensive care unit; no other correlations with neonatal outcome were found. Abuhamad et al.39 conducted a prospective longitudinal study to determine whether Doppler velocimetry of the SMA could predict adverse neonatal outcome in 25 infants with gastroschisis. They found that about 50% of cases had SMA‐PI below the normal range and there was no difference between cases with simple and those with complex gastroschisis, which is in line with our findings. Blood‐flow velocimetry of both the intra‐ and extra‐abdominal SMA was unable to differentiate between good and poor neonatal outcomes, which is also in agreement with our findings. It seems that Doppler velocimetry of the SMA is not a useful parameter for differentiating between simple and complex gastroschisis cases and does not seem to be related to neonatal morbidity.
In keeping with other studies, we also found no relationship between complex gastroschisis or adverse outcome and either the AC or the EFW during gestation16.
Fetuses with an obstruction of the proximal small bowel generally have polyhydramnios40. Several studies have found a relationship between polyhydramnios and fetal bowel obstruction in gastroschisis cases. In a meta‐analysis by D'Antonio et al.16, based on five studies, polyhydramnios was associated with bowel atresia, but the definition of polyhydramnios differed between the included studies. We did not find a relationship between polyhydramnios and bowel atresia, but polyhydramnios occurred in two of the three cases that resulted in neonatal death. However, the small number of cases prevents a firm conclusion from being drawn.
The major strengths of this national cohort study are its prospective and longitudinal design, and that all gastroschisis cases were evaluated prenatally and managed postnatally according to a standardized protocol. This enabled documentation of the individual trends in prenatal ultrasound markers, and detailed collection of postnatal outcomes, in a large sample of pregnancies. Another advantage of this study was the use of universal, objective and contemporary primary outcomes for risk‐categorization according to Molik et al.9, making it possible to reproduce the study.
One of the limitations of our study is that some data were missing. However, the missing values were randomly distributed with no differences between simple and complex gastroschisis cases. Therefore, the data are still representative of our entire gastroschisis population. We also did not try to distinguish the colon from the small bowel. In gastroschisis, the normal intestinal anatomic markers of the different bowel portions are missing and the haustra of the colon cannot be recognized. This prevents a clear differentiation being made between the small and large bowel in these cases. One could speculate that the clinical consequences of a dilated colon may be less serious than those of a dilated small bowel, knowing that atresia of the colon seldom occurs in gastroschisis12.
Not all ultrasound markers previously described in the literature were assessed in this study. As such, gastric dilatation or abnormal stomach size may be a prognostic marker for neonatal death16, and absence of a lumen in the extra‐abdominal loops may be a sign of complex (or in particular closing) gastroschisis37.
In conclusion, this study confirms the importance of classifying cases of gastroschisis into simple and complex for prediction of the outcome, which is poorer in the latter group. This large prospective longitudinal study indicates that intra‐abdominal bowel dilatation may be a useful prenatal marker in differentiating between simple and complex gastroschisis. The repeat presence of severely increased intra‐abdominal bowel diameter can reliably identify cases with complex gastroschisis; however, the predictive value of this marker seems to be limited.
FLAMINGO (FetaL Abdominal Markers Identified by ultrasound to predict Neonatal Gastroschisis Outcome) Study Working Group:
C. J. Bax, Department of Obstetrics and Gynecology, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam Reproduction and Development Research Institute, Amsterdam, The Netherlands.
R. van Baren, Department of Pediatric Surgery, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
H. A. A. Brouwers, Department of Neonatology, Division Woman and Baby, University Medical Center Utrecht, Utrecht, The Netherlands.
P. H. Dijk, Department of Neonatology Beatrix Children's Hospital, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
A. H. van Kaam, Department of Neonatology, Emma Children's Hospital, Amsterdam University Medical Center, Amsterdam, The Netherlands.
C. Koopman‐Esseboom, Department of Neonatology, Division Woman and Baby, University Medical Center Utrecht, Utrecht, The Netherlands.
E. Sikkel, Department of Obstetrics and Gynecology, Radboud University Medical Center Nijmegen, Amalia Children's Hospital, Nijmegen, The Netherlands
M. C. Haak, Department of Obstetrics and Gynaecology, Leiden University Medical Center, Leiden, The Netherlands
A. F. van Heijst, Department of Neonatology, Radboud University Medical Center Nijmegen, Nijmegen, The Netherlands
M. A. van der Hoeven, Department of Neonatology, Maastricht University Medical Center, Maastricht, The Netherlands
E. L. van Heurn, Pediatric Surgical Center of Amsterdam, Emma Children's Hospital Amsterdam University Medical Center, Amsterdam, The Netherlands
C. Sleeboom, Pediatric Surgical Center of Amsterdam, Emma Children's Hospital Amsterdam University Medical Center, Amsterdam, The Netherlands
M. M. van Weissenbruch, Department of Neonatology, Emma Children's Hospital, Amsterdam University Medical Center, Amsterdam, The Netherlands.
C. Willekes, Department of Obstetrics and Gynaecology, Maastricht University Medical Center, Maastricht, The Netherlands
Supporting information
Table S1 Characteristics of four pregnancies with gastroschisis that resulted in intrauterine fetal death
Table S2 Number of measurements and gestational age at first and last measurement of biometric and Doppler variables in 100 liveborn infants diagnosed with gastroschisis, according to whether it was simple or complex
Table S3 Model estimates for abdominal circumference and estimated fetal weight Z‐scores, intra‐ and extra‐abdominal bowel diameters and superior mesenteric artery pulsatility index Z‐scores
Contributor Information
C. C. M. M. Lap, Email: c.c.m.m.lap@umcutrecht.nl.
The FLAMINGO Study Working Group:
C. J. Bax, R. van Baren, H. A. A. Brouwers, P. H. Dijk, A. H. van Kaam, C. Koopman‐Esseboom, E. Sikkel, M. C. Haak, A. F. van Heijst, A. F. van der Hoeven, E. L. van Heurn, C. Sleeboom, M. M. van Weissenbruch, and C. Willekes
REFERENCES
- 1. Garne E, Loane M, Dolk H, De Vigan C, Scarano G, Tucker D, Stoll C, Gener B, Pierini A, Nelen V, Rosch C, Gillerot Y et al Prenatal diagnosis of severe structural congenital malformations in Europe. Ultrasound Obstet Gynecol 2005; 25: 6–11. [DOI] [PubMed] [Google Scholar]
- 2. Bradnock TJ, Marven S, Owen A, Johnson P, Kurinczuk JJ, Spark P, Draper ES, Knight M. Gastroschisis: one year outcomes from national cohort study. BMJ 2011; 343: d6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fillingham A, Rankin J. Prevalence, prenatal diagnosis and survival of gastroschisis. Prenat Diagn 2008; 28: 1232–1237. [DOI] [PubMed] [Google Scholar]
- 4. Abdel‐Latif ME, Bolisetty S, Abeywardana S, Lui K; Australian and New Zealand Neonatal Network . Mode of delivery and neonatal survival of infants with gastroschisis in Australia and New Zealand. J Pediatr Surg 2008; 43: 1685–1690. [DOI] [PubMed] [Google Scholar]
- 5. Salihu HM, Emusu D, Aliyu ZY, Pierre‐Louis BJ, Druschel CM, Kirby RS. Mode of delivery and neonatal survival of infants with isolated gastroschisis. Obstet Gynecol 2004; 104: 678–683. [DOI] [PubMed] [Google Scholar]
- 6. Lap CC, Brizot ML, Pistorius LR, Kramer WLM, Teeuwen IB, Eijkemans MJC, Brouwers HAA, Pajkrt E, van Kaam AH, Adama van Scheltema PN, Eggink AJ, van Heijst AF et al Outcome of isolated gastroschisis; an international study, systematic review and meta‐analysis. Early Hum Dev 2016; 103: 209–218. [DOI] [PubMed] [Google Scholar]
- 7. South AP, Stutey KM, Meinzen‐Derr J. Metaanalysis of the prevalence of intrauterine fetal demise in gastroschisis. Am J Obstet Gynecol 2013; 209: 114.e1–13. [DOI] [PubMed] [Google Scholar]
- 8. Cowan KN, Puligandla PS, Laberge JM, Skarsgard ED, Bouchard S, Yanchar N, Kim P, Lee S, McMillan D, von Dadelszen P; Canadian Pediatric Surgery Network . The gastroschisis prognostic score: Reliable outcome prediction in gastroschisis. J Pediatr Surg 2012; 47: 1111–1117. [DOI] [PubMed] [Google Scholar]
- 9. Molik KA, Gingalewski CA, West KW, Rescorla FJ, Scherer LR, Engum SA, Grosfeld JL. Gastroschisis: A plea for risk categorization. J Pediatr Surg 2001; 36: 51–55. [DOI] [PubMed] [Google Scholar]
- 10. Bergholz R, Boettcher M, Reinshagen K, Wenke K. Complex gastroschisis is a different entity to simple gastroschisis affecting morbidity and mortality – a systematic review and meta‐analysis. J Pediatr Surg 2014; 49: 1527–1532. [DOI] [PubMed] [Google Scholar]
- 11. Emil S, Canvasser N, Chen T, Friedrich E, Su W. Contemporary 2‐year outcomes of complex gastroschisis. J Pediatr Surg 2012; 47: 1521–1528. [DOI] [PubMed] [Google Scholar]
- 12. Snyder CL, Miller KA, Sharp RJ, Murphy JP, Andrews WA, Holcomb GW 3rd, Gittes GK, Ashcraft KW. Management of intestinal atresia in patients with gastroschisis. J Pediatr Surg 2001; 36: 1542–1545. [DOI] [PubMed] [Google Scholar]
- 13. Phillips JD, Raval MV, Redden C, Weiner TM. Gastroschisis, atresia, dysmotility: surgical treatment strategies for a distinct clinical entity. J Pediatr Surg 2008; 43: 2208–2212. [DOI] [PubMed] [Google Scholar]
- 14. Kronfli R, Bradnock TJ, Sabharwal A. Intestinal atresia in association with gastroschisis: a 26‐year review. Pediatr Surg Int 2010; 26: 891–894. [DOI] [PubMed] [Google Scholar]
- 15. Page R, Ferraro ZM, Moretti F, Kee Fung KF. Gastroschisis: antenatal sonographic predictors of adverse neonatal outcome. J Pregnancy 2014; 2014: 239406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D'Antonio F, Virgone C, Rizzo G, Khalil A, Baud D, Cohen‐Overbeek TE, Kuleva M, Salomon LJ, Flacco ME, Manzoli L, Giuliani S. Prenatal risk factors and outcomes in gastroschisis: a meta‐analysis. Pediatrics 2015; 136: e159–169. [DOI] [PubMed] [Google Scholar]
- 17.American College of Obstetricians and Gynecologists . ACOG practice bulletin no. 101: Ultrasonography in pregnancy. Obstet Gynecol 2009; 113: 451–461. [DOI] [PubMed] [Google Scholar]
- 18. Ebbing C, Rasmussen S, Godfrey KM, Hanson MA, Kiserud T. Fetal superior mesenteric artery: longitudinal reference ranges and evidence of regulatory link to portal liver circulation. Early Hum Dev 2009; 85: 207–213. [DOI] [PubMed] [Google Scholar]
- 19. Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements – a prospective study. Am J Obstet Gynecol 1985; 151: 333–337. [DOI] [PubMed] [Google Scholar]
- 20. Verburg BO, Steegers EA, De Ridder M, Snijders RJ, Smith E, Hofman A, Moll HA, Jaddoe VW, Witteman JC. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population‐based cohort study. Ultrasound Obstet Gynecol 2008; 31: 388–396. [DOI] [PubMed] [Google Scholar]
- 21. Lap CC, Voskuilen CS, Pistorius LR, Mulder EJH, Visser GHA, Manten GTR. Reference curves for the normal fetal small bowel and colon diameters; their usefulness in fetuses with suspected dilated bowel. J Matern Fetal Neonatal Med 2020; 33: 633–638. [DOI] [PubMed] [Google Scholar]
- 22. Sparks TN, Shaffer BL, Page J, Caughey AB. Gastroschisis: mortality risks with each additional week of expectant management. Am J Obstet Gynecol 2017; 216: 66.e1–66.e7. [DOI] [PubMed] [Google Scholar]
- 23. Visser GH, Eilers PH, Elferink‐Stinkens PM, Merkus HM, Wit JM. New Dutch reference curves for birthweight by gestational age. Early Hum Dev 2009; 85: 737–744. [DOI] [PubMed] [Google Scholar]
- 24. Abdullah F, Arnold MA, Nabaweesi R, Fischer AC, Colombani PM, Anderson KD, Lau H, Chang DC. Gastroschisis in the United States 1988–2003: analysis and risk categorization of 4344 patients. J Perinatol 2007; 27: 50–55. [DOI] [PubMed] [Google Scholar]
- 25. Huh NG, Hirose S, Goldstein RB. Prenatal intraabdominal bowel dilation is associated with postnatal gastrointestinal complications in fetuses with gastroschisis. Am J Obstet Gynecol 2010; 202: 396.e1–396.e6. [DOI] [PubMed] [Google Scholar]
- 26. Brantberg A, Blaas HG, Salvesen KA, Haugen SE, Eik‐Nes SH. Surveillance and outcome of fetuses with gastroschisis. Ultrasound Obstet Gynecol 2004; 23: 4–13. [DOI] [PubMed] [Google Scholar]
- 27. Contro E, Fratelli N, Okoye B, Papageorghiou A, Thilaganathan B, Bhide A. Prenatal ultrasound in the prediction of bowel obstruction in infants with gastroschisis. Ultrasound Obstet Gynecol 2010; 35: 702–707. [DOI] [PubMed] [Google Scholar]
- 28. Kuleva M, Khen‐Dunlop N, Dumez Y, Ville Y, Salomon LJ. Is complex gastroschisis predictable by prenatal ultrasound? BJOG 2012; 119: 102–109. [DOI] [PubMed] [Google Scholar]
- 29. Goetzinger KR, Tuuli MG, Longman RE, Huster KM, Odibo AO, Cahill AG. Sonographic predictors of postnatal bowel atresia in fetal gastroschisis. Ultrasound Obstet Gynecol 2013; 4: 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghionzoli M, James CP, David AL, Shah D, Tan AW, Iskaros J, Drake DP, Curry JI, Kiely EM, Cross K, Eaton S, De Coppi P, Pierro A. Gastroschisis with intestinal atresia – predictive value of antenatal diagnosis and outcome of postnatal treatment. J Pediatr Surg 2012; 47: 322–328. [DOI] [PubMed] [Google Scholar]
- 31. Mears AL, Sadiq JM, Impey L, Lakhoo K. Antenatal bowel dilatation in gastroschisis: a bad sign? Pediatr Surg Int 2010; 26: 581–588. [DOI] [PubMed] [Google Scholar]
- 32. Nick AM, Bruner JP, Moses R, Yang EY, Scott TA. Second‐trimester intra‐abdominal bowel dilation in fetuses with gastroschisis predicts neonatal bowel atresia. Ultrasound Obstet Gynecol 2006; 28: 821–825. [DOI] [PubMed] [Google Scholar]
- 33. Hijkoop A, IJsselstijn H, Wijnen RMH, Tibboel D, Rosmalen JV, Cohen‐Overbeek TE. Prenatal markers and longitudinal follow‐up in simple and complex gastroschisis. Arch Dis Child Fetal Neonatal Ed 2018; 103: F126–F131. [DOI] [PubMed] [Google Scholar]
- 34. Carroll SG, Kuo PY, Kyle PM, Soothill PW. Fetal protein loss in gastroschisis as an explanation of associated morbidity. Am J Obstet Gynecol 2001; 184: 1297–1301. [DOI] [PubMed] [Google Scholar]
- 35. Langer JC, Bell JG, Castillo RO, Crombleholme TM, Longaker MT, Duncan BW, Bradley SM, Finkbeiner WE, Verrier ED, Harrison MR. Etiology of intestinal damage in gastroschisis, II. Timing and reversibility of histological changes, mucosal function, and contractility. J Pediatr Surg 1990; 25: 1122–1126. [DOI] [PubMed] [Google Scholar]
- 36. Langer JC, Longaker MT, Crombleholme TM, Bond SJ, Finkbeiner WE, Rudolph CA, Verrier ED, Harrison MR. Etiology of intestinal damage in gastroschisis. I: Effects of amniotic fluid exposure and bowel constriction in a fetal lamb model. J Pediatr Surg 1989; 24: 992–997. [DOI] [PubMed] [Google Scholar]
- 37. Martillotti G, Boucoiran I, Damphousse A, Grignon A, Dube E, Moussa A, Bouchard S, Morin L. Predicting perinatal outcome from prenatal ultrasound characteristics in pregnancies complicated by gastroschisis. Fetal Diagn Ther 2016; 39: 279–286. [DOI] [PubMed] [Google Scholar]
- 38. Volumenie JL, de Lagausie P, Guibourdenche J, Oury JF, Vuillard E, Saizou C, Luton D. Improvement of mesenteric superior artery Doppler velocimetry by amnio‐infusion in fetal gastroschisis. Prenat Diagn 2001; 21: 1171–1174. [DOI] [PubMed] [Google Scholar]
- 39. Abuhamad AZ, Mari G, Cortina RM, Croitoru DP, Evans AT. Superior mesenteric artery Doppler velocimetry and ultrasonographic assessment of fetal bowel in gastroschisis: a prospective longitudinal study. Am J Obstet Gynecol 1997; 176: 985–990. [DOI] [PubMed] [Google Scholar]
- 40. Virgone C, D'Antonio F, Khalil A, Jonh R, Manzoli L, Giuliani S. Accuracy of prenatal ultrasound in detecting jejunal and ileal atresia: systematic review and meta‐analysis. Ultrasound Obstet Gynecol 2015; 45: 523–529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Characteristics of four pregnancies with gastroschisis that resulted in intrauterine fetal death
Table S2 Number of measurements and gestational age at first and last measurement of biometric and Doppler variables in 100 liveborn infants diagnosed with gastroschisis, according to whether it was simple or complex
Table S3 Model estimates for abdominal circumference and estimated fetal weight Z‐scores, intra‐ and extra‐abdominal bowel diameters and superior mesenteric artery pulsatility index Z‐scores


