Abstract
Background
Capecitabine plus oxaliplatin (XELOX) as adjuvant therapy for gastric cancer (GC) reduces cancer recurrence and improves survival. S‐1 plus oxaliplatin (SOX) is well‐tolerated and effective against advanced GC, and also be used widely in adjuvant treatment. However, data comparing SOX and XELOX as adjuvant treatments are lacking.
Method
Data on treatment modalities, adverse events, recurrence and metastasis were collected from 180 patients with stage II and III GC, who received SOX or XELOX after D2 gastrectomy between January 2012 and December 2015, and analyzed retrospectively. The primary endpoint was 3‐year disease‐free survival (DFS) rate.
Results
Median follow was 52.9 months; 3‐year DFS rate and overall survival (OS) rate were 75.2% and 67.6% (P = 0.359) and 81.2% and 83.3% (P = 0.77) in the SOX and XELOX groups, respectively. There was no significant difference in peritoneal metastasis rates in the SOX and XELOX groups (8.6% vs 15%, respectively; P = 0.232). Compound recurrent disease was associated with significantly shorter OS. Multivariate analysis identified metastatic lymph node ratio (LNR) as an independent prognostic factor for OS (P = 0.036; hazard ratio = 2.875; 95% confidence interval, 1.069–7.729); the LNR ≥17% group had inferior 3‐year OS rate to the LNR <17% group (P = 0.001). The incidence of grades 3 and 4 adverse events was similar in both groups; however, grade ≥2 hand–foot syndrome was significantly less frequent in the SOX group (P = 0.01).
Conclusion
SOX has similar survival benefits to XELOX and is well‐tolerated in Chinese patients with GC following D2 gastrectomy.
Keywords: adjuvant chemotherapy, gastric cancer, SOX, XELOX
1. INTRODUCTION
Gastric cancer (GC) is the fifth most common cancer and the third leading cause of cancer death in the world, with over 1 000 000 new cases and 783 000 deaths in 2018. 1 The incidence is remarkably high in East Asian countries, including China, Japan and Korea. 2 In China, GC is the second most frequently diagnosed cancer, and the total number of GC cases account for approximately 44.1% of the global number. 1 , 3 Complete resection with D2 lymphadenectomy is accepted as the standard treatment for operable GC. 4 , 5 Nevertheless, approximately 40%–65% patients experience recurrence after curative resection. 6 Unlike patients with GC in Japan and Korea, approximately 60% of those in China are diagnosed with locally advanced disease; therefore, it is crucial to improve the prognosis for patients with stage II–III GC following surgery. According to a meta‐analysis conducted by the Global Advanced/Adjuvant Stomach Tumor Research International Collaboration (GASTRIC) group, adjuvant chemotherapy, based on fluorouracil regimens, leads to significantly superior outcomes with reduced recurrence. 7 In the CLASSIC study, the efficacy and safety of capecitabine plus oxaliplatin (XELOX) were evaluated as adjuvant treatments for patients with stage II–III GC. Five‐year disease‐free survival (DFS) rate was remarkably improved by XELOX chemotherapy compared with surgery alone (68% vs. 53%; hazard ratio [HR] = 0.58; 95% confidence interval [CI)], 0.47–0.72; P < 0.001). Furthermore, XELOX therapy also led to an increase in 5‐year overall survival (OS) rate from 69% to 78% (HR = 0.66; 95% CI, 0.51–0.85; P = 0.0015). 8 Currently, XELOX is recommended as the standard adjuvant chemotherapy after curative D2 gastrectomy worldwide. Moreover, in recent years, numerous clinical trials have been conducted to explore optimal adjuvant chemotherapies, with more powerful therapeutic effects and lower toxicity.
S‐1 is an oral anticancer FU analogue containing tegafur, gimeracil and potassium oxonate, with advantages over capecitabine in terms of reducing the incidence of toxicities, such as hand–foot syndrome. 9 Further, S‐1 was demonstrated to confer clear survival benefits for advanced GC in Asia when used in combination with cisplatin. S‐1 plus oxaliplatin (SOX), a common regimen for treatment of advanced GC in Asia, is as effective as S‐1 plus cisplatin (SP), according to several reports. 10 , 11 , 12 Moreover, the results of a phase III study, ACTS‐GC showed that 1 year of S‐1 monotherapy was superior for reducing relapse and improving 5‐year OS rate from 61.1% to 71.7%, compared with observation after surgery (HR = 0.669; 95% CI, 0.540–0.828). 13 , 14 And S‐1 also showed a tendency to reduce peritoneal metastasis. Nevertheless, S‐1 did not provide a similar benefit for patients with stage IIIB GC, as for those with stage II and IIIA disease. 15 S1 monotherapy seemed to be not enough for these patients with later stage. Hence, theoretically, SOX may be an effective treatment for patients with GC following D2 gastrectomy.
In China, SOX has been widely used as an adjuvant treatment for gastric cancer after D2 gastrectomy; however, current studies have almost always focused on the evaluation of SOX efficacy and safety, whereas comparisons of the therapeutic effects of SOX and XELOX in the Chinese population remain limited. In this study, clinical data from 180 patients with stage II–III GC was collected to compare the efficacy and safety of SOX and XELOX as adjuvant therapies. We also analyzed clinical features, recurrence patterns and prognostic factors in this cohort.
2. MATERIALS AND METHODS
2.1. Patients
Data were collected from patients with GC or gastroesophageal junction cancer, who received gastrectomy and completed adjuvant chemotherapy at the National Cancer Center/Cancer Hospital Chinese Academy of Medical Sciences and Peking Union Medical College from January 2012 to December 2015. Eligible patients underwent D2 gastrectomy with R0 resection, had stage II or III disease, based on the American Joint Committee on Cancer (AJCC) staging system (7th edition), had pathologically confirmed adenocarcinoma, and completed at least four cycles of SOX or XELOX as adjuvant chemotherapy. Patients in whom distant metastasis was identified during surgery, or who developed recurrence within 8 weeks after surgery, or received other adjuvant chemotherapy or who underwent preoperative chemotherapy, were excluded.
SOX treatment comprised oral S‐1 (80, 100 or 120 mg daily, according to body surface area in two separate doses on days 1–14 of each cycle) plus intravenous oxaliplatin (130 mg/m2 on day 1 of each cycle) every 3 weeks. XELOX treatment included at least four cycles of oral capecitabine (1000 mg/m2 twice daily on days 1–14 of each cycle) plus intravenous oxaliplatin (130 mg/m2 on day 1 of each cycle) every 3 weeks.
The clinical characteristics of patients, including pathological findings (pathological types, Lauren type, Bormann type, vascular invasion, lymph node involvement, Epstein–Barr virus infection status and HER2 amplification status); TNM stage, based on the AJCC (7th edition) staging system; treatment modalities (type of gastrectomy, adjuvant chemotherapy and adverse events) and information on recurrence and metastasis, were collected retrospectively. MLN refers to the number of metastatic lymph nodes, whereas the metastatic lymph node ratio (LNR) is the ratio of positive lymph nodes relative to the total number of lymph nodes examined. Recurrence information was based on radiologic or histological examination data. Patterns of recurrence were classified as local recurrence (including anastomotic recurrence and regional lymph node metastasis), distant metastasis (comprising distant nodal and organ metastasis) and peritoneal metastasis. Single, combined and triple recurrence patterns refer to one, two or three incidences of recurrence since first relapse, respectively. DFS was defined as the time from the date of curative operation to the date of recurrence, metastasis or death. OS was defined as the time from the date of the D2 gastrectomy to the date of death from any cause. Adverse events were estimated using the Common Terminology Criteria for Adverse Events 4.0. The primary endpoint was 3‐year DFS rate. Secondary endpoints included 3‐year OS rate and safety.
2.2. Statistical analyses
Statistical analyses were carried out using SPSS version 20.0. χ2 and independent t tests were used to evaluate the significance of differences between patients undergoing SOX and XELOX therapies. DFS and OS were calculated using the Kaplan–Meier method and differences in survival rate analyzed by Log‐rank test. Multivariate analysis was performed using a Cox proportional hazards model. A P value < 0.05 was considered significant. Cut‐off values for MLN and LNR were evaluated by receiver‐operating characteristic curve analysis.
The study was approved by the institutional review boards of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
3. RESULTS
3.1. Patient clinical characteristics
Patient and tumor characteristics are shown in Table 1. A total of 727 patients underwent D2 gastrectomy and received adjuvant chemotherapy in our institution from January 2012 to December 2015. Patients were excluded because they were diagnosed with stage I or IV disease (n = 111), were diagnosed with nonadenocarcinoma (n = 14), had gastric stump carcinoma (n = 10) or had second primary tumors (n = 8). Further, 138 patients underwent adjuvant chemotherapy other than SOX or XELOX, including three‐drug cytotoxic regimens, based on paclitaxel, platinum and fluoropyrimidine (n = 68); cisplatin and S‐1 (n = 7) and regimens containing epirubicin or other drugs (n = 63). Other patients were excluded because of incomplete treatment data (n = 266, including incomplete data of adverse events and receiving treatment in other hospitals after several cycles). Finally, 180 patients were enrolled. The median age at diagnosis of the 180 included patients was 55 years (range 25–81 years), and there were 126 (70%) males and 54 (30%) females. The majority of patients (n = 121) had stage III disease, whereas 59 patients (32.78%) had stage II disease. Patients with stage IIIC disease accounted for 30.56%. Most patients had T4 (51.67%) or N3 (43.89%), and were assumed to be at high risk of recurrence.
Table 1.
Patients and tumor characteristics
| Characteristics | All N = 180 | % | SOX N = 140 | % | XELOX N = 40 | % | P value |
|---|---|---|---|---|---|---|---|
| Age | 0.326 | ||||||
| ≤50 | 60 | 33.3% | 51 | 36.4% | 9 | 22.5% | |
| >50 | 120 | 66.7% | 89 | 63.6% | 31 | 77.5% | |
| 50 < age ≤60 | 60 | 33.3% | 47 | 33.6% | 13 | 32.5% | |
| 60 < age ≤70 | 50 | 27.8% | 36 | 25.7% | 14 | 35% | |
| >70 | 10 | 5.6% | 6 | 4.3% | 4 | 10% | |
| Gender | 1 | ||||||
| Male | 126 | 70% | 98 | 70.0% | 28 | 70% | |
| Female | 54 | 30% | 42 | 30.0% | 12 | 30% | |
| Type of gastrectomy | 0.712 | ||||||
| Total Gastrectomy | 39 | 21.7% | 30 | 21.4% | 9 | 22.5% | |
| Distal gastrectomy | 132 | 73.3% | 102 | 72.9% | 30 | 75% | |
| Proximate gastrectomy | 9 | 5% | 8 | 5.7% | 1 | 2.5% | |
| Lauren type | 0.997 | ||||||
| Intestinal | 40 | 22.2% | 31 | 22.1% | 9 | 22.5% | |
| Diffuse | 83 | 46.1% | 65 | 46.4% | 18 | 45% | |
| Mixed | 52 | 28.9% | 40 | 28.6% | 12 | 30% | |
| Unknown | 5 | 2.8% | 4 | 2.9% | 1 | 2.5% | |
| Bormann type | 0.160 | ||||||
| Type I | 4 | 2.2% | 2 | 1.4% | 2 | 5% | |
| Type II | 31 | 17.2% | 27 | 19.3% | 4 | 10% | |
| Type III | 93 | 51.7% | 73 | 52.1% | 20 | 50% | |
| Type IV | 21 | 11.7% | 13 | 9.3% | 8 | 20% | |
| Unknown | 31 | 12.8% | 25 | 17.9% | 6 | 15% | |
| Differentiation | 0.938 | ||||||
| Poor | 115 | 63.9% | 89 | 63.6% | 26 | 65% | |
| Moderately and poor | 36 | 20% | 27 | 19.3% | 9 | 22.5% | |
| Moderately | 20 | 11.1% | 17 | 12.1 % | 3 | 7.5% | |
| Well | 4 | 2.2% | 3 | 2.1% | 1 | 2.5% | |
| Unknown | 5 | 2.8% | 4 | 2.9% | 1 | 2.5% | |
| Vessel tumor emboli | 0.750 | ||||||
| Yes | 95 | 52.8% | 73 | 52.1% | 22 | 55% | |
| No | 85 | 47.2% | 67 | 47.9% | 18 | 45% | |
| Nerve invasion | 0.019 | ||||||
| Yes | 106 | 58.9% | 76 | 54.3% | 30 | 75% | |
| No | 74 | 41.1% | 64 | 45.7% | 10 | 25% | |
| HER2 | 168 | 130 | 38 | 0.220 | |||
| Negative | 156 | 92.9% | 119 | 91.5% | 37 | 97.4% | |
| Positive | 12 | 7.1% | 11 | 8.5% | 1 | 2.6% | |
| EBER | 75 | 57 | 18 | 0.429 | |||
| Negative | 70 | 93.3% | 52 | 91.2% | 18 | 100% | |
| Positive | 3 | 4.0% | 3 | 5.3% | 0 | 0% | |
| Uncertainty | 2 | 2.7% | 2 | 3.5% | 0 | 0% | |
| T stage | 0.014 | ||||||
| T1a | 0 | 0.0% | 0 | 0% | 0 | 0% | |
| T1b | 8 | 4.4% | 7 | 5.0% | 1 | 2.5% | |
| T2 | 16 | 8.9% | 15 | 10.7% | 1 | 2.5% | |
| T3 | 63 | 35% | 53 | 37.9% | 10 | 25% | |
| T4a | 89 | 49.4% | 64 | 45.7% | 25 | 62.5% | |
| T4b | 4 | 2.2% | 1 | 0.7% | 3 | 7.5% | |
| N stage | 0.605 | ||||||
| N0 | 24 | 13.3% | 18 | 12.9% | 6 | 15% | |
| N1 | 31 | 17.2% | 27 | 19.3% | 4 | 10% | |
| N2 | 46 | 25.6% | 33 | 23.6% | 13 | 32.5% | |
| N3a | 35 | 19.4% | 27 | 19.3% | 8 | 20% | |
| N3b | 44 | 24.4% | 35 | 25% | 9 | 22.5% | |
| TNM stage | 0.235 (stage II vs. stage III) | ||||||
| II | 59 | 32.8% | 49 | 35% | 10 | 25% | |
| II A | 25 | 13.9% | 21 | 15% | 4 | 10% | |
| II B | 34 | 18.9% | 28 | 20% | 6 | 15% | |
| III | 121 | 67.2% | 91 | 65% | 30 | 75% | |
| III A | 28 | 15.6% | 26 | 18.6% | 2 | 5% | |
| III B | 38 | 21.1% | 24 | 17.1% | 14 | 35% | |
| III C | 55 | 30.6 % | 41 | 29.3% | 14 | 35% |
Finally, SOX was administered to 140 patients, whereas 40 received the XELOX regimen as adjuvant treatment; no significant differences in clinical characteristics were observed between these two treatment groups.
3.2. Treatment outcomes
By December 2018, median follow‐up duration was 52.9 (8.5–90) months. Patients in both groups accepted a median of six treatment cycles and 29.9% of patients in the SOX group developed recurrence, versus 40.5% of those in the XELOX group, which was not a significant difference (P = 0.229). Patients who received SOX adjuvant chemotherapy had a similar DFS and OS to those who underwent XELOX: 1‐year DFS rate in the SOX and XELOX groups were 91.5% and 86.5% (P = 0.374), whereas 3‐year DFS rate were 75.2% and 67.6% (P = 0.359), respectively (Figure 1A). One‐year OS rate was 97.7% in the SOX group and 97.2% in the XELOX group (P = 0.855), whereas 3‐year OS rate were 81.2% and 83.3% (P = 0.77), respectively (Figure 1B). For patients with stage IIIC disease, who were at higher risk of recurrence, 1‐year OS rate were 97.4% and 91.7% (P = 0.38) and 3‐year OS rate were 65.8% and 83.3% (P = 0.248) in the SOX and XELOX groups, respectively.
Figure 1.
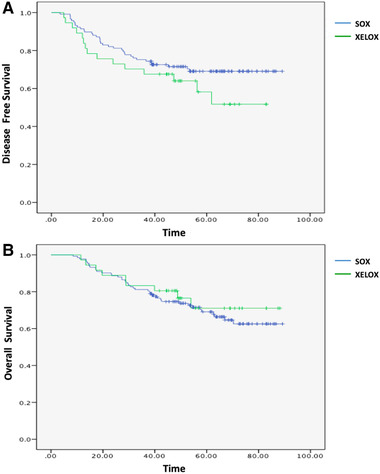
(A) DFS in the SOX and XELOX groups. (B) OS in the SOX and XELOX groups [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Patterns of recurrence
Patterns of disease recurrence are detailed in Table 2 and OS for patients with different patterns of recurrence are shown in Table 3. Distant metastases (20.1%) were more common than local recurrence (10%). Disease progressed more rapidly in patients with peritoneal metastasis, with a median survival after recurrence of 11 months. Compared with the XELOX group, patients in the SOX group had fewer peritoneal metastasis, with an incidence of 8.6% versus 15% (P = 0.232). Further, patients with local relapse had prolonged median survival of 23 months after recurrence, compared with 10.2 months for those with distant metastasis (P = 0.575). Patients who suffered single (57%), combined (36%) or triple (7%) recurrence had median OS of 39, 39.9 and 17.6 months, respectively (P = 0.242); hence, those with multiple recurrence appeared to have shorter OS.
Table 2.
The patterns of recurrence
| Site | The number of patients, N = 180 | SOX, N = 140 | XELOX, N = 40 | P |
|---|---|---|---|---|
| Anastomotic recurrence | 10 (5.6%) | 8 (5.7%) | 2 (5.0%) | 0.862 |
| Regional lymph node metastasis | 8 (4.4%) | 6 (4.3%) | 2 (5.0%) | 0.847 |
| Distant nodal metastasis | 10 (5.6%) | 8 (5.7%) | 2 (5.0%) | 0.862 |
| Distant organ metastasis | 26 (14.5%) | 19 (13.6%) | 7 (17.5%) | 0.533 |
| Peritoneal metastasis | 18 (10.0%) | 12 (8.6%) | 6 (15.0)% | 0.232 |
Table 3.
The overall survival of different patterns of recurrence
| Recurrence pattern | OS (month) | P value |
|---|---|---|
| Single recurrence pattern | 39 | P = 0.242 |
| Double recurrence pattern | 39.9 | |
| Triple recurrence pattern | 17.6 |
3.4. Survival and prognostic factors
An MLN cut‐off value of 7 (AUC = 0.762; 95% CI, 0.681–0.844) resulted in sensitivity of 70.6% and specificity of 73.7%, while the optimal cut‐off point for LNR was estimated at 17% (AUC = 0.745; 95% CI, 0.66–0.83), with a sensitivity of 72.5% and a specificity of 66.9%. More MLN or higher LNR was associated with a significantly increased risk of recurrence and reduced survival (Figure 2). Three‐year DFS rate in the MLN < 7 and ≥ 7 groups were 82.4% and 60.3% (P = 0.002), whereas 3‐year OS rate were 89.5% vs 71.6% (P = 0.003), respectively. For patients in the LNR <17% and ≥17% groups, the 3‐year DFS rate were 84.3% versus 58.5% (P < 0.001), whereas 3‐year OS rate were 90.3% versus 71.1% (P = 0.001). In subgroup analysis of the MLN ≥7 group, 3‐year OS rate did not differ significantly (68.3% vs 81.2%, respectively; P = 0.311), regardless of whether patients received SOX or XELOX. Similarly, for patients with LNR ≥17%, 3‐year OS rate were 65.2% and 86.7% respectively (P = 0.103), with no significant difference between the two regimens
Figure 2.
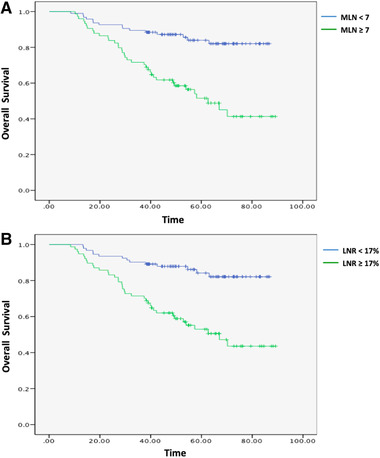
(A) Comparison of OS in patients with MLN <7 and ≥ 7. (B) Comparison of OS in patients with LNR <17% and ≥17% [Color figure can be viewed at wileyonlinelibrary.com]
By univariate analysis, T stage (P = 0.011; HR= 2.1; 95% CI, 1.181–3.734), N stage (P < 0.01; HR = 3.452; 95% CI, 1.907–6.248), and LNR (P < 0.01; HR = 3.839; 95% CI, 2.073–7.109) were identified as significant prognostic factors for OS; however, in multivariate analysis only LNR was finally detected as an independent prognostic factor (P = 0.036; HR = 2.875; 95% CI, 1.069–7.729).
3.5. Safety and toxicity
Both SOX and XELOX chemotherapy regimens were well‐tolerated, and there was no significant difference between them. Grade 3 or 4 adverse events (AEs) occurred in 42.1% of patients receiving SOX, which was lower than that in their counterparts undergoing XELOX treatment, who had an AE incidence of 45% (P = 0.747); however, the difference was not significant. No treatment related deaths were reported. The most common AEs were hematologic and gastrointestinal toxicity. The proportions of patients experiencing grade ≥3 thrombocytopenia (11.4% vs 10%; P = 0.8), neutropenia (22.1% vs 22.5%; P = 0.962) and leukopenia (3.6% vs 2.5%; P = 0.793) was similar in the SOX and XELOX groups. Further, grade ≥3 vomiting was experienced by 4.3% of the patients in the SOX arm and 10% of those in the XELOX group (P = 0.164). Other reported grade ≥2 AEs were transaminase disorder (2.9% vs 7.5%; P = 0.18), hyperbilirubinemia (1.4% vs 0%; P = 0.447) and peripheral neuropathy (2.1% vs 2.5%; P = 0.893). In particular, grade ≥2 hand–foot syndrome was more frequent in the XELOX group (7.5% of patients) than the SOX group (0.7% of patients; P = 0.01; Table 4). Grade 3 or 4 adverse events reported by ≥10% of the patients.
Table 4.
Grade 3 or 4 adverse events reported by ≥10% of the patients
| SOX | XELOX | |||
|---|---|---|---|---|
| All grades | Grade 3 or 4 | All grades | Grade 3 or 4 | |
| Leukopenia | 44 (31.4%) | 5 (3.6%) | 9 (22.5%) | 1 (2.5%) |
| Neutropenia | 51 (36.4%) | 31 (22.1%) | 13 (32.5%) | 9 (22.5%) |
| Thrombocytopenia | 36 (25%) | 16 (11.4%) | 11 (27.5%) | 4 (10%) |
| Vomiting | 21 (15%) | 6 (4.3%) | 9 (22.5%) | 4 (10%) |
| Nausea | 56 (40%) | 0 (0%) | 17 (42.5%) | 0 (0%) |
| Decreased appetite | 39 (27.9%) | 3 (2.1%) | 13 (32.5%) | 0 (0%) |
| Fatigue | 30 (21.4%) | 0 (0%) | 10(25%) | 0 (0%) |
| Elevated AST/ALT level | 15 (10.7%) | 0 (0%) | 7 (17.5%) | 0 (0%) |
| Peripheral neuropathy | 31 (21.4%) | 1 (0.7%) | 4 (10%) | 0 (0%) |
4. DISCUSSION
In this study, patients receiving both SOX and XELOX regimens achieved excellent 3‐year DFS and OS rate, comparable to those reported by other clinical trials. No significant difference in survival was observed between patients administered these two regimens. In a phase III study, SOX was administered to 54 Chinese patients with GC following D2 gastrectomy, with reported 3‐year DFS and OS rate of 75.9% and 85.2%, 16 similar to those observed in this study (75.2% and 81.2%). Furthermore, similar survival outcomes were observed in this study for patients receiving XELOX to those reported from the previous phase III CLASSIC study; with 3‐year DFS rate of 67.6% versus 74%, 3‐year OS rate of 83.3% versus 83%, respectively in this study and CLASSIC study. 8 These results suggest that SOX is as effective as XELOX in an adjuvant setting and may be another acceptable therapy choice for patients with GC.
In China, reported 5‐year OS rate for stage III GC range from 29% to 53%. 17 In our study, higher LNR was associated with an increased risk of recurrence and inferior prognosis. Patients with LNR ≥ 17% had much poorer 3‐year DFS rate and 3‐year OS rate, relative to those with LNR < 17%. The majority of these patients had stage IIIC disease and still had poor prognosis. The efficacy of S‐1 adjuvant monotherapy for treatment of patients with stage III GC was less satisfactory than for those with earlier stage disease. In the ACTS‐GC study, S‐1 did not confer a significant survival benefit for patients with stage IIIB gastric cancer compared with placebo. Further, median 5‐year OS rate was only 50.2% for patients with stage IIIB disease in the S‐1 group. 14 Whether double chemotherapy regimens, such as XELOX and SOX, can improve survival of patients with locally advanced GC, relative to treatment with S‐1 alone, remains uncertain. In a retrospective investigation conducted in Korea, patients with stage III GC in the XELOX group demonstrated no improvement in survival compared with the S‐1 group; however, subgroup analysis suggested that XELOX may be associated with a better 3‐year OS rate for patients with stage IIIC GC (55.2% vs 39%; HR = 0.5; 95% CI, 0.23–1.10; P = 0.075). 18 In the ARTIST 2 study, 3‐year DFS rate in the S‐1 group was shorter than that in the SOX group for patients with stage II and III GC with lymph node metastasis (64% vs 78%; HR = 0.617; P = 0.0157); 19 however, its effects on OS are yet to be reported. In this study, the 3‐year OS rate of patients with stage IIIC disease in the SOX group was 65.8%, which was higher than that of 59.1% reported for patients with locally advanced disease receiving S‐1 monotherapy in the ACTS‐GC study. 14 Hence, SOX is a potential option for treatment of locally advanced GC after D2 resection; however, further research is required.
The peritoneum is a common site of metastasis in patients with GC and peritoneal metastasis is associated with a poor prognosis of 3–4 months median survival without treatment. 20 In our study, compared with XELOX, SOX was associated with decreased peritoneal recurrence (8.6% vs 15%); however, the difference was not significant. In the ACTS‐GC study, S‐1 significantly reduced peritoneal metastasis, which occurred in 18.9% of the patients without treatment after surgery relative to 14.6% in the S‐1 group. 14 Whether S‐1‐based treatment can prevent peritoneal metastasis requires verification in prospective studies. Intraperitoneal chemotherapy may help to prevent intraabdominal metastasis; 21 however, further research is required.
In this study, patients who presented with a triple recurrence pattern had the worst survival and LNR was an independent prognostic indicator. Patients with an LNR value of ≥17% had significantly lower median 3‐year DFS rate (58.5%) than that of patients with LNR <17% (84.3%; P < 0.001).
In previous studies, grade 3 or 4 AEs were reported in 47%–56% of patients receiving the XELOX regimen. 8 , 18 Further, according to Ren et al, 38.6% of patients receiving SOX suffered from AEs of the same grades. 22 In our study, both SOX and XELOX appeared to be well‐tolerated in Chinese patients, with similar occurrence of grade 3 or 4 AEs in these two groups (42.1% vs 45%), respectively. In both groups, grade ≥3 neutropenia was detected in approximately 22% of patients, similar to previous reports of 22.6%–26% for patients administered SOX 16 , 22 and 22%–35% for XELOX. 8 , 18 No more thrombocytopenia was found in the SOX group that than in the XELOX group, which was a primary reason for discontinuation of chemotherapy in previous studies. 16 , 23 In addition, SOX was associated with a significantly lower incidence of hand–foot syndrome (0.7% vs. 7.5%), which may indicate an improvement in quality of life.
The limitations of this study include its retrospective nature and the small sample size in the XELOX group. Longer follow‐up is required to acquire 5‐year DFS and OS data. Overall, the data from this study indicate that SOX is as effective as XELOX, in terms of 3‐year DFS rate and OS rate, as an adjuvant treatment for GC after D2 resection and showed favorable tolerability, with a lower incidence of hand–foot syndrome. Further prospective clinical trials are required to comprehensively evaluate the efficacy and safety of SOX as an adjuvant treatment for GC in Asia.
Jiang Z, Sun Y, Zhang W, Cui C, Yang L, Zhou A. Comparison of S‐1 plus oxaliplatin (SOX) and capecitabine plus oxaliplatin (XELOX) as adjuvant chemotherapies for stage II and III gastric cancer after D2 resection: A single center retrospective study. Asia-Pac J Clin Oncol. 2020;16:180–186. 10.1111/ajco.13321
A retrospective comparison of S‐1 plus oxaliplatin and capecitabine plus oxaliplatin as adjuvant chemotherapies for stage II and III gastric cancer after D2 resection in Chinese patients.
SOX has similar survival benefits to XELOX and is well‐tolerated in Chinese patients with GC following D2 gastrectomy. There was no significant difference in peritoneal metastasis rates in these two groups. Metastatic lymph node ratio was an independent prognostic factor for OS.
The clinical data were collected after informed consent and approval of the Ethical Committee of Cancer Hospital Chinese Academy of Medical Science.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: gLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Kamangar F, Dores GM, Anderson WF, et al. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. [DOI] [PubMed] [Google Scholar]
- 3. Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China in 2013: An analysis based on urbanization level. Chin J Cancer Res. 2017;29(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park JM, Kim YH. Current approaches to gastric cancer in Korea. Gastrointest Cancer Res. 2008;2:137–144. [PMC free article] [PubMed] [Google Scholar]
- 6. Gee DW, Rattner DW. Management of gastroesophageal tumors. Oncologist. 2007;12:175‐185. [DOI] [PubMed] [Google Scholar]
- 7. G A S T R I C (Global Advanced /Adjuvant Stomach Tumor Research International Collaboration) Group , Paoletti X, Oba K, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta‐analysis. JAMA. 2010;303(17):1729–1737. [DOI] [PubMed] [Google Scholar]
- 8. Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open‐label, randomized controlled trial. Lancet. 2012;379:315–321. [DOI] [PubMed] [Google Scholar]
- 9. Lee JL, Kang YK, Kang HJ, et al. A randomised multicentre phase II trial of capecitabine vs S‐1 as first‐line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer. 2008;99:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park I, Lee JL, Ryu MH, et al. Phase I/II and pharmacokinetic study of S‐1 and oxaliplatin in previously untreated advanced gastric cancer. Cancer Chemother Pharmacol. 2010;65:473–480. [DOI] [PubMed] [Google Scholar]
- 11. Koizumi W, Takiuchi H, Yamada Y, et al. Phase II study of oxaliplatin plus S‐1 as first‐line treatment for advanced gastric cancer (G‐SOX study). Ann Oncol. 2010;21:1001–1005. [DOI] [PubMed] [Google Scholar]
- 12. Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S‐1 with cisplatin plus S‐1 in chemotherapy‐naive patients with advanced gastric cancer. Ann Oncol. 2015;26:141–148. [DOI] [PubMed] [Google Scholar]
- 13. Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S‐1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. [DOI] [PubMed] [Google Scholar]
- 14. Sasako M, Sakuramoto S, Katai H, et al. Five‐year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S‐1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–4393. [DOI] [PubMed] [Google Scholar]
- 15. Kochi M, Fujii M, Kanamori N, et al. Effect of gastrectomy on the pharmacokinetics of S‐1, an oral fluoropirimidine, in resectable gastric cancer patients. Cancer Chemother Pharmacol. 2007;60:693–701. [DOI] [PubMed] [Google Scholar]
- 16. Wang G, Zhao J, Song Y, et al. Phase II study of adjuvant chemotherapy with S1 plus oxaliplatin for Chinese patients with gastric cancer. BMC Cancer. 2018;18:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shu P, Qin J, Shen K, et al. The IGCA staging system is more accurate than AJCC7 system in stratifying survival of patients with gastric cancer in stage III. BMC Cancer. 2017;17:238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cho JH, Lim JY, Cho JY. Comparison of capecitabine and oxaliplatin with S‐1 as adjuvant chemotherapy in stage III gastric cancer after D2 gastrectomy. PLoS ONE. 2017;12(10):e0186362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park SH, ARTIST 2: interim results of a phase III trial involving adjuvant chemotherapy and/or chemoradiotherapy after D2‐gastrectomy in stage II/III gastric cancer (GC). 2019. ASCO Abstract 4001.
- 20. Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non‐gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88(2):358–363. [DOI] [PubMed] [Google Scholar]
- 21. Shi C, Yang B, Chen Q, et al. Retrospective analysis of adjuvant intraperitoneal chemotherapy effect prognosis of resectable gastric cancer. Oncology 2011;80(5–6):289–295 [DOI] [PubMed] [Google Scholar]
- 22. Ren D‐F, Zheng F‐C, Zhao J‐H, et al. Adjuvant chemotherapy with S‐1 plus oxaliplatin improves survival of patients with gastric cancer after D2 gastrectomy: A multicenter propensity score‐matched study. World J Clin Cases 2018;6(10):373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shitara K, Chin K, Yoshikawa T, et al. Phase II study of adjuvant chemotherapy of S‐1 plus oxaliplatin for patients with stage III gastric cancer after D2 gastrectomy. Gastric Cancer. 2017;20(1):175–181. [DOI] [PubMed] [Google Scholar]


