Summary
Background
Both tioguanine and low‐dose thiopurines combined with allopurinol (LDTA) can be considered for the treatment of inflammatory bowel disease (IBD) when conventional thiopurines fail due to adverse events.
Aim
To compare the safety of tioguanine and LDTA in IBD patients.
Methods
Inflammatory bowel disease patients who failed conventional thiopurines due to adverse events and initiated LDTA in standard care were identified in the prospective ICC Registry. IBD patients who failed conventional thiopurines due to adverse events and initiated tioguanine were enrolled in three university hospitals. Patients on concomitant biologicals were excluded. The primary outcome was discontinuation of therapy due to adverse events. Secondary outcomes included: safety outcomes and surgery‐, biological‐ and corticosteroid‐free clinical remission (physician global assessment = 0) after 104 weeks. Both multiple logistic regression and propensity score matching were used to correct for confounders.
Results
In total, 182 IBD patients treated with tioguanine (n = 94) or LDTA (n = 88) were included with a median follow‐up of 104 weeks (IQR 91‐104). Of these, 19% (tioguanine: 20%, LDTA: 18%) of patients discontinued therapy due to adverse events. After adjusting for confounders, there were no differences in terms of discontinuation rate due to adverse events (OR 0.50, 95% CI 0.15‐1.68, P = 0.26), adverse events (OR 0.89, 95% CI 0.44‐1.81, P = 0.75), infections (OR 1.05, 95% CI 0.40‐2.73, P = 0.93), hospitalisations (OR 2.00, 95% CI 0.64‐6.23, P = 0.23) or clinical remission (OR 0.74, 95%CI 0.33‐1.68, P = 0.48). All results are comparable with the propensity score matched cohort.
Conclusion
Nineteen percent of IBD patients with prior failure to conventional thiopurines due to adverse events discontinued therapy with tioguanine or LDTA due to adverse events. Either therapy may be considered before escalating to biological therapy.
1. INTRODUCTION
Conventional thiopurines, consisting of azathioprine and mercaptopurine are a cornerstone maintenance treatment for inflammatory bowel disease (IBD) patients. This is evidenced by cumulative thiopurine exposure rates of 70% in the first 5 years of treatment in population based cohorts. 1 Yet, up to 43% of patients develop adverse events or intolerance during treatment resulting in discontinuation or alteration of treatment. 2
Adverse events can in part be explained by the metabolism of thiopurines. Azathioprine and mercaptopurine are pro‐drugs and undergo complex metabolism. Simplified, azathioprine is non‐enzymatically converted to mercaptopurine and mercaptopurine is converted through three main pathways: firstly, mercaptopurine can be phosphorylated by inosine monophosphate dehydrogenase to 6‐tioguanine nucleotides (6‐TGN). These 6‐TGN levels correlate with the efficacy of thiopurines but high levels of 6‐TGN are also related to myelotoxicity. 3 Secondly, mercaptopurine can be methylated by the polymorphic enzyme thiopurine methyltransferase (TPMT) to 6‐methylmercaptopurine (6‐MMP) of which the level is related to adverse events, especially hepatotoxicity. 4 Thirdly, mercaptopurine can be catabolised to thiouric acid by xanthine oxidase. Up to 20% of patients preferentially metabolise thiopurines to produce high levels of 6‐MMP and low levels of 6‐TGN. 5 These so‐called ‘thiopurine hypermethylators’ or ‘shunters’ are usually refractory to standard doses of thiopurines and are likely to develop adverse events.
When patients are intolerant to conventional thiopurines, two actions can be considered within the thiopurine spectrum. First, allopurinol, a xanthine oxidase inhibitor, can be added to redirect the thiopurine metabolism towards 6‐TGN formation resulting in increased concentration of 6‐TGN and reduced 6‐MMP levels. Although this method has shown clinical benefits, both azathioprine/mercaptopurine and allopurinol independently carry hypersensitivity and dose‐related toxicities. 6 , 7 Second, conventional thiopurines can be replaced by the non‐conventional thiopurine‐drug, tioguanine. Tioguanine is metabolised in fewer steps towards the effective 6‐TGN metabolite, bypassing multiple intermediate metabolites which are associated with the majority of adverse events. 3 However, the use of tioguanine in IBD was initially discouraged when high doses of tioguanine (>40 mg/day) were associated with the development of nodular regenerative hyperplasia of the liver (NRH). 8 , 9 , 10 Recent studies with reduced dosing (0.2‐0.3 mg/kg) have shown no increased risk for NRH in patients using tioguanine when compared to thiopurine‐naïve IBD patients and demonstrated a favourable safety profile. 11 , 12 Consequently, tioguanine has been conditionally licensed as IBD maintenance treatment in the Netherlands since 2015. 13
Currently, both low dose thiopurine and allopurinol (LDTA) and tioguanine can be used when conventional thiopurines fail but it is currently unknown whether these treatment options are comparable in terms of safety and effectiveness. The objective of this study was to determine the comparative safety and effectiveness of LDTA and tioguanine in IBD patients who previously failed conventional thiopurines using multiple regression models with correction for confounders and propensity score matching.
2. METHODS
2.1. Study design and patient population
In this multicenter cohort study, LDTA patients of ≥16 years with prior failure to conventional thiopurines due to adverse events and without biological treatment at baseline were identified in the prospective, multicenter Dutch ‘Initiative on Crohn and Colitis’ (ICC) Registry. The ICC Registry was developed to determine the effectiveness, safety and usage of specific IBD treatments in the Netherlands, as previously described. 14 , 15 In short, IBD patients initiating LDTA were included in four tertiary referral centres and one teaching hospital and were followed for 2 years with a predefined follow‐up schedule of outpatient visits designed to closely follow regular care (week 0, 12, 24, 52, and 104). Second, tioguanine patients of ≥16 years, with prior failure to conventional thiopurines due to adverse events and without biological treatment at baseline were selected from prospectively maintained local databases in three tertiary referral centres. The registered visits were scheduled at initiation of therapy (baseline) and on weeks 24, 52, and 104 or until the medication was discontinued. Patients with prior treatment with either LDTA or tioguanine were excluded in this study. The decision to prescribe either tioguanine or LDTA following adverse events on conventional thiopurines was based on the local physician's experience and preference. Of note, in the Netherlands tioguanine therapy is formally (yet conditionally) approved for the treatment of IBD after failure of azathioprine or mercaptopurine.
2.2. Outcomes and definitions
The primary outcome of this study was the proportion of patients discontinuing treatment within 104 weeks of treatment due to adverse events. Secondary outcomes included: number of medication‐related adverse events, infections and disease‐related hospitalisations per 100 patient years, clinical remission, biochemical remission and discontinuation rate. Adverse events were classified as unrelated, possibly‐related, probably‐related or reason for discontinuing treatment. Infections were classified as moderate: oral antibiotics or anti‐viral medication needed, or severe: hospitalisation or intravenous administrated antibiotics or anti‐viral medication needed. Both treatment‐related adverse events and infections were classified according to the Common Terminology Criteria for Adverse Events (CTCAE) (version 5.0, released November 27, 2017). 16 Clinical remission was based on the physician global assessment without the need for corticosteroids, biologicals or surgery. Biochemical remission was defined as a C‐reactive protein (CRP) concentration of ≤5 ml/L with a faecal calprotectin level ≤250 µg/g when available. Discontinuation was categorised as follows: lack of primary response, loss of response, adverse events, malignancy, pregnancy, long‐term remission, or at request of patient. Patients who discontinued treatment were classified as non‐responders in determining the effectiveness outcomes. Only patients who discontinued treatment due to long‐term remission or pregnancy were considered censored cases at timepoints after the discontinuation.
2.3. Statistical methods
Patients were analysed on an intention‐to‐treat basis. Continuous variables were presented as means with standard deviations (SD) or as medians with interquartile ranges (IQR) depending on the normality of the underlying distribution. Continuous variables were consequently compared using the independent T‐test or Mann‐Whitney U test. Categorical variables were presented as percentages and compared by using the chi‐squared test. To adjust for confounding two different types of analyses were used. First, multiple logistic regression was used to assess the association between treatment (tioguanine or LDTA) and outcomes of interest and to correct for potential confounders a priori agreed upon. These confounders were selected based on an assumed association on either the clinical outcomes or disease severity. The variables included: type of disease (Crohn's disease or ulcerative colitis/IBD‐undefined), disease duration, complicated disease (stricturing or penetrating behaviour for Crohn's disease and pancolitis at diagnosis for ulcerative colitis), and clinical and biochemical disease activity at baseline. To account for differences in follow‐up duration in the safety analyses, follow‐up duration was added as variable in the regression model. Second, to create a sensitivity cohort, propensity score matching (1:1 nearest‐neighbour, without replacement, caliper 0.2) was used to create two cohorts of matched patients with evenly distributed variables at baseline. A propensity score is the conditional probability of receiving either LDTA or tioguanine given the observed covariates and is obtained by using a non‐parsimonious logistic regression model based on the selected variables. The variables used for the propensity score matched cohorts were: type of disease (Crohn's disease or ulcerative colitis), disease duration, complicated disease (stricturing or penetrating behaviour for Crohn's disease and pancolitis at diagnosis for ulcerative colitis), clinical and biochemical disease activity at baseline, corticosteroid use at baseline, and perianal disease at baseline. To assess differences in drug survival a cox regression analysis was used. A two‐sided P value of 0.05 or less was considered statistically significant. All data analyses were performed using ibm spss Statistics for Windows, version 24.0 (IBM Corp).
2.4. Ethical consideration
The study was reviewed and approved by the Committee on Research Involving Human Subjects at the Radboudumc (Institutional Review Board: 4076).
3. RESULTS
3.1. Baseline characteristics
A total of 182 IBD patients with adverse events to conventional thiopurines and a subsequent switch to tioguanine (n = 94) or LDTA (n = 88) were included in this study. Baseline characteristics are presented in Tables 1 and 2. There were fewer Crohn's disease patients treated with tioguanine when compared to LDTA (58.5% vs 71.6% P = 0.050) and these Crohn's disease patients had less often a stricturing disease phenotype (7.3% vs 23.0%, P = 0.025). The tioguanine patients had a higher CRP at baseline (4 mg/L [IQR: 0.9‐14.0] vs 1.9 mg/L [0.4‐6.0]) and more often concomitant corticosteroids at baseline (31.9% vs 14.0%, P = 0.005). The median dose of tioguanine at initiation of therapy was 20 mg (IQR: 20‐20) (0.27 mg/kg [IQR: 0.22‐0.32]), for LDTA this was: 100 mg allopurinol with either 50 mg azathioprine (IQR: 50‐50) (0.67 mg/kg [IQR: 0.54‐0.75]; n = 45) or 25 mg mercaptopurine (IQR: 25‐25) (0.35 mg/kg [IQR: 0.28‐0.38]; n = 41).
Table 1.
Baseline characteristics of tioguanine‐ and low dose thiopurine and allopurinol‐treated IBD patients
| Tioguanine (n = 94) | LDTA (n = 88) | P value | |
|---|---|---|---|
| Age a , median (IQR) | 40.3 (28.1‐54.1) | 42.6 (27.1‐57.1) | 0.911 |
| Gender—male, N (%) | 37 (39.4) | 25 (28.4) | 0.119 |
| Current smoker, N (%) | 16 (17.0) | 23 (26.1) | 0.134 |
| Disease duration (y), median (IQR) | 5.0 (1.3‐13.9) | 6.2 (1.1‐16.7) | 0.456 |
| Treatment duration, median (IQR) | 91.0 (37.3‐104.4) | 95.0 (35.9‐103.9) | 0.576 |
| Follow‐up duration in weeks, median (IQR) | 104.0 (91.0‐104.4) | 104.0 (97.7‐104.0) | 0.336 |
| Type of disease (Crohn's disease) N (%) | 55 (58.5) | 63 (71.6) | 0.050 |
| Crohn's disease location, N (%) | 0.117 | ||
| Ileum | 26 (47.3) | 34 (55.7) | |
| Colon | 8 (14.5) | 14 (23.0) | |
| Ileum and colon | 21 (38.2) | 13 (21.3) | |
| Upper GI involvement, N (%) | 3 (5.5) | 4 (6.6) | 0.803 |
| Ulcerative colitis disease location, N (%) | 0.746 | ||
| Proctitis | 2 (5.7) | 1 (4.0) | |
| Left‐sided | 12 (34.3) | 11 (44.0) | |
| Pancolitis | 20 (57.1) | 13 (52.0) | |
| Unknown | 1 (2.9) | ||
| Disease behaviour, N (%) | 0.010 | ||
| Inflammatory disease | 46 (83.6) | 36 (57.1) | |
| Stricturing disease | 4 (7.3) | 18 (28.6) | |
| Penetrating disease | 5 (9.1) | 8 (12.7) | |
| Unknown | 0 (0.0) | 1 (1.6) | |
| Peri‐anal disease | 8 (14.5) | 4 (6.3) | 0.152 |
| Prior intestinal resections | 18 (19.6) | 26 (29.5) | 0.119 |
| Prior peri‐anal interventions | 4 (6.9) | 2 (3.2) | 0.261 |
| Prior biological therapy use | 21 (22.3) | 14 (15.9) | 0.271 |
| Clinical disease activity, N (%) | 0.082 | ||
| Remission | 14 (15.1) | 22 (25.9) | |
| Mild disease | 48 (51.6) | 47 (55.3) | |
| Moderate disease | 30 (32.3) | 16 (18.8) | |
| Unknown | 1 (1.1) | ||
| C‐reactive protein (mg/L) | 4 (0.9‐14.0) | 1.9 (0.4‐6.0) | 0.006 |
| Concomitant medication, N (%) | 0.004 | ||
| No concomitant medication | 64 (68.1) | 76 (86.4) | |
| Corticosteroids | 30 (31.9) | 12 (13.6) | |
| Corticosteroids range, mg (IQR) | 22.5 (9.0‐31.3) | 20.0 (12.5‐36.3) | |
Abbreviations: IQR, interquartile range; LDTA, low‐dose thiopurine and allopurinol; N, number of patients.
Table 2.
Number of adverse events leading to discontinuation of prior conventional thiopurines
| Azathioprine (N) | Mercaptopurine (N) | Total (N) | |
|---|---|---|---|
| Gastrointestinal disorders | 50 | 42 | 92 |
| Nausea | 21 | 21 | 42 |
| Abdominal pain | 9 | 8 | 17 |
| Vomiting | 9 | 7 | 16 |
| Pancreatitis | 11 | 4 | 15 |
| Oral dysesthesia | 0 | 1 | 1 |
| Diarrhoea | 0 | 1 | 1 |
| Investigations | 25 | 19 | 44 |
| Liver function test increased a | 13 | 10 | 23 |
| White blood cell decreased | 5 | 5 | 10 |
| Alanine aminotransferase increased | 3 | 1 | 4 |
| Pancytopenia b | 1 | 2 | 3 |
| Aspartate aminotransferase increased | 2 | 0 | 2 |
| GGT increased | 0 | 1 | 1 |
| Weight gain | 1 | 0 | 1 |
| General disorders and administration site conditions | 13 | 12 | 25 |
| Malaise | 4 | 6 | 10 |
| Fever | 6 | 3 | 9 |
| Flu like symptoms | 3 | 0 | 3 |
| Edema limbs | 0 | 2 | 2 |
| Fatigue | 0 | 1 | 1 |
| Skin and subcutaneous tissue disorders | 8 | 8 | 16 |
| Rash (undefined) c | 4 | 5 | 9 |
| Alopecia | 2 | 2 | 4 |
| Pruritus | 1 | 1 | 2 |
| Eczema | 1 | 0 | 1 |
| Musculoskeletal and connective tissue disorders | 8 | 6 | 14 |
| Arthralgia | 5 | 2 | 7 |
| Back pain | 1 | 2 | 3 |
| Bone pain | 2 | 0 | 2 |
| Myalgia | 0 | 2 | 2 |
| Nervous system disorders | 6 | 6 | 12 |
| Headache | 3 | 3 | 6 |
| Hypersomnia | 3 | 3 | 6 |
| Infections and infestations | 0 | 2 | 2 |
| Upper respiratory infection | 0 | 2 | 2 |
| Epstein‐Barr virus infection reactivation | 0 | 1 | 1 |
| Folliculitis | 1 | 0 | 1 |
| Psychiatric disorders | 1 | 2 | 3 |
| Irritability | 1 | 1 | 2 |
| Insomnia | 0 | 1 | 1 |
| Other | 5 | 1 | 6 |
| Anemia (blood and lymphatic system disorders) | 4 | 1 | 5 |
| Hematomas (vascular disorders) | 2 | 0 | 2 |
| Unknown | 3 | 1 | 4 |
| Total | 121 | 100 | 221 |
Pooled adverse events according to Common Terminology Criteria for Adverse Events (CTCAE v5.0) of prior conventional thiopurine therapy (azathioprine or mercaptopurine). Some patients experienced multiple adverse events.
Increased value of ≥1 of the following: Alanine aminotransferase, aspartate aminotransferase, GGT, alkaline phosphatase, bilirubin.
Pancytopenia is defined as a decreased value of ≥1 of the following: Hemoglobin, white blood cells, thrombocytes.
Any skin condition involving erythema or other visual skin changes not specified in CTCAE.
3.2. Safety
A total of 35 patients (19.2%) discontinued tioguanine or LDTA treatment due to adverse events during 104 weeks of follow‐up (tioguanine: n = 19 [20.2%], LDTA: n = 16 [18.2%]) (Table 3). The most common adverse events were myelotoxicity and gastrointestinal complications such as nausea and stomach ache. Type of treatment was not associated with discontinuing medication due to adverse events in the unadjusted (odds ratio (OR): 0.877 95%CI: 0.419‐1.838, P = 0.728) or adjusted analyses (OR: 0.496 95% CI: 0.146‐1.680, P = 0.260). The median treatment duration until discontinuation due to adverse events was 9.4 weeks (IQR: 2.1‐29.1).
Table 3.
Infections and adverse events during tioguanine or LDTA treatment
| Tioguanine, n = 94 (132.0 patient years) | LDTA, n = 88 (119.8 patient years) | |
|---|---|---|
| Possibly related | 21 (15.9 per 100 patient years) | 23 (19.2 per 100 patient years) |
| Cutaneous lesions | 1 | 11 |
| Arthralgia | 7 | 6 |
| Gastrointestinal | 2 | 3 |
| Hair loss | 4 | — |
| Headache | 2 | — |
| Pruritus | 2 | — |
| Vertigo | — | 1 |
| Mood disorder | 1 | — |
| Fatigue | 1 | — |
| Insomnia | 1 | — |
| Other | — | 2 |
| Probably related | 6 (4.5 per 100 patient years) | 5 (4.2 per 100 patient years) |
| Cutaneous lesions | 2 | 1 |
| Hair loss | 1 | 1 |
| Malaise | — | 1 |
| Gastrointestinal | 1 | — |
| Transient tingling sensation | 1 | — |
| Hypersensitivity sunlight | 1 | — |
| Myelotoxicity | — | 2 |
| Adverse event requiring discontinuation | 19 (14.4 per 100 patient years) | 16 (13.4 per 100 patient years) |
| Gastrointestinal | 8 | 4 |
| Myelotoxicity | 3 | 5 |
| Pancreatitis | 3 | — |
| Arthralgia | 3 | — |
| Recurrent infections | — | 1 |
| Psychiatric disorder | — | 1 |
| Sunlight sensitivity | 1 | — |
| Vertigo | 1 | — |
| Other | 5 | |
| Moderate infections | 8 (6.1 per 100 patient years) | 12 (10.0 per 100 patient years) |
| Upper respiratory | 3 | 3 |
| Urinary tract | 3 | 2 |
| Gastrointestinal | — | 3 |
| Pneumonia | 1 | 2 |
| Cutaneous lesions | 1 | — |
| Soft tissue | — | 1 |
| Other | — | 1 |
| Severe infections | 5 (3.8 per 100 patient years) | 6 (5.0 per 100 patient years) |
| Gastrointestinal | 2 | 4 |
| Pneumonia | 2 | 2 |
| Neutropenic fever | 1 | — |
| Hospitalisation | 5 (3.8 per 100 patient years) | 9 (7.5 per 100 patient years) |
Number and details of adverse events during treatment of IBD patients with tioguanine or low dose thiopurine and allopurinol. Infections were classified as: mild infections: no antibiotics or anti‐viral medication; moderate infections: oral antibiotics or anti‐viral medication; severe infections: hospitalisation or intravenously administrated antibiotics or anti‐viral medication.
Ninety adverse events (possibly, probably or reason for discontinuation) occurred during follow‐up (tioguanine: n = 46, 34.8 per 100 patient years, LDTA: n = 44, 36.7 per 100 patient years; Table 3). Type of treatment was not associated with more adverse events in the unadjusted (OR: 0.921 95% CI: 0.505‐1.680, P = 0.788) or adjusted analyses (OR: 0.888 95% CI: 0.435‐1.812, P = 0.745). There were no cases of NRH or (signs of) non‐cirrhotic portal hypertension reported.
Thirty‐one moderate or severe infections occurred during follow‐up (tioguanine: n = 13, 9.8 per 100 patient years, LDTA: n = 18, 15.0 per 100 patient years; Table 3). Type of treatment was not associated with a higher infection rate in the unadjusted (OR: 1.308 95% CI: 0.553‐3.095, P = 0.541) or adjusted analyses (OR: 1.047 95% CI: 0.402‐2.728, P = 0.926). In both cohorts, when adverse events and infections are combined, 10 severe adverse events (CTCAE ≥grade 3) occurred (Table 4).
Table 4.
Severe adverse events according to CTCAE
| Tioguanine, n = 94 (132.0 patient years) | LDTA, n = 88 (119.8 patient years) | |
|---|---|---|
| Grade 3 | 9 (6.8 per 100 patient years) | 10 (8.3 per 100 patient years) |
| Myelotoxicity | 1 | 4 |
| Pancreatitis | 3 | ‐ |
| Gastrointestinal infection | 2 | 4 |
| Pneumonia | 2 | 2 |
| Neutropenic fever | 1 | ‐ |
| Grade 4 | 1 (0.8 per 100 patient years) | 0 (0 per 100 patient years) |
| Myelotoxicity | 1 | ‐ |
Pooled severe adverse events and severe infections according to Common Terminology Criteria for Adverse Events (CTCAE v5.0) of tioguanine‐ and low dose thiopurine and allopurinol—treated IBD patients. Grade 3: severe or medically significant but not immediately life‐threatening; hospitalisation or prolongation of hospitalisation indicated; disabling; limiting self‐care activities of daily living. Grade 4: life‐threatening consequences; urgent intervention indicated (LDTA: low‐dose thiopurine and allopurinol).
Fourteen IBD‐ and medication‐related hospitalisations occurred during follow‐up (tioguanine: n = 5, 3.8 per 100 patient years, LDTA: n = 9, 7.5 per 100 patient years; Table 3). LDTA treatment was not associated with hospitalisations in the unadjusted analysis (OR: 2.028, 95% CI: 0.652‐6.305, P = 0.222). Due to the limited number of events, only disease activity at baseline was used in the adjusted analyses (OR: 1.996, 95%CI: 0.640‐6.229, P = 0.234).
3.3. Effectiveness
Only patients with active clinical disease (physician global assessment ≥1) at baseline were included in the clinical effectiveness analyses (tioguanine: n = 80, LDTA: n = 66). Unadjusted surgery‐, biological‐ and corticosteroid‐free clinical remission rates in tioguanine‐ and LDTA‐treated patients at week 24, 52 and 104 were: 31.3% (n = 25/80) vs 28.8% (n = 19/66) P = 0.747, 39.2% (n = 31/79) vs 36.9% (n = 24/65) P = 0.776, and 33.3% (n = 23/69) vs 22.6% (n = 14/62) P = 0.172, respectively. Type of treatment was not associated with surgery‐, biological‐ and corticosteroid‐free clinical remission in the unadjusted (OR: 0.583, 95% CI 0.268‐1.270, P = 0.174) or adjusted analyses (OR: 0.743, 95% CI 0.328‐1.684, P = 0.477) after 104 weeks of treatment. There was no statistically significant difference between biochemical remission rates after 104 weeks of treatment in the unadjusted (OR: 1.082, 95% CI 0.514‐2.277, P = 0.835) or adjusted (OR: 0.999, 95% CI 0.454‐2.199, P = 0.997) analyses. Escalation to biological treatment was comparable between the treatments (tioguanine 23.8% vs LDTA 28.8% P = 0.49).
3.4. Discontinuation
In total 33 tioguanine‐ and 30 LDTA‐treated patients discontinued treatment after a median treatment duration of 28.0 weeks (IQR: 4.1‐52.9) and 22.6 weeks (IQR: 6.8‐58.1), respectively (Table 5). For both treatments, adverse events were the main reason for discontinuation (57.6% and 53.3%, respectively). The proportion of patients still using treatment after 104 weeks was 57.9% for tioguanine and 62.7% for LDTA (P = 0.743; Figure 1). The type of treatment was not statistically significant associated with discontinuation (HR: 0.964, 95% CI: 0.588‐1.580, P = 0.883). After 104 weeks of treatment, 51.1% (n: 48/94) of tioguanine‐ and 51.1% (n: 45/88) of LDTA‐treated patients continued treatment with second‐line thiopurines without biological treatment. One patient discontinued LDTA treatment due to pregnancy.
Table 5.
Discontinuation
| Tioguanine (n = 33) | LDTA (n = 30) | |
|---|---|---|
| Treatment duration—weeks, median (IQR) | 28.0 (4.1‐52.9) | 22.6 (6.8‐58.1) |
| Reason discontinuation, N (%) | ||
| No response | 5 (15.2) | 1 (3.3) |
| Loss of response | — | 2 (6.7) |
| Adverse events | 19 (57.6) | 16 (53.3) |
| Malignancy | — | 1 (3.3) |
| Pregnancy | — | 1 (3.3) |
| Stable remission | 2 (6.1) | 2 (6.7) |
| Patients request | 4 (12.1) | 3 (10.0) |
| Other | 3 (9.1) | 1 (3.3) |
| Unknown | — | 3 (10.0) |
Discontinuation visit of tioguanine‐ and low‐dose thiopurine and allopurinol‐treated IBD patients.
Abbreviation: LDTA, low‐dose thiopurine and allopurinol.
Figure 1.
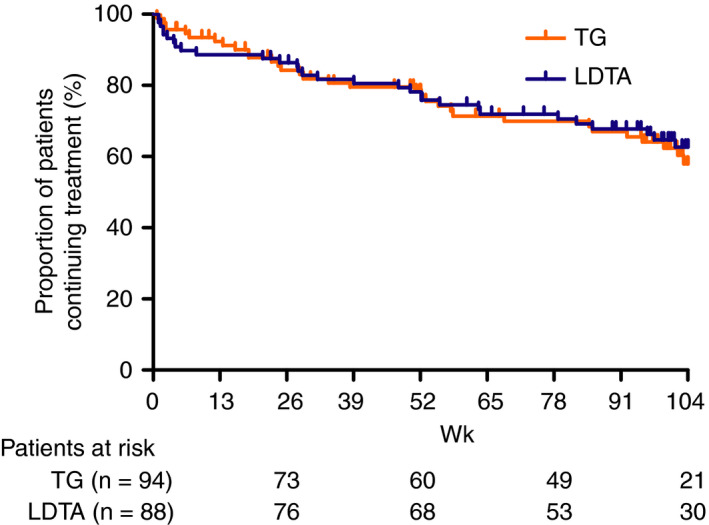
Unadjusted cumulative drug survival of tioguanine‐ and low dose thiopurine and allopurinol‐ (LDTA) treated IBD patients after 104 weeks of follow‐up
3.5. Propensity score matched cohort
In the propensity score matched cohort patients were matched on: type of disease (Crohn's disease or ulcerative colitis), disease duration, complicated disease (stricturing or penetrating behaviour for Crohn's disease and pancolitis at diagnosis for ulcerative colitis), clinical and biochemical disease activity at baseline, corticosteroid use at baseline and perianal disease at baseline. There was no difference in baseline characteristics as depicted in Table 6. There were no differences in safety outcomes between the treatments: Adverse event resulting in discontinuation (OR: 0.823, 95% CI: 0.256‐2.648, P = 0.743), total adverse events (OR: 0.794, 95% CI 0.355‐1.775, P = 0.574), infections (OR: 1.306, 95% CI: 0.433‐3.944, P = 0.636) and hospitalisations (OR: 1.449, 95% CI 0.435‐4.830, P = 0.546). There was also no difference in effectiveness outcomes: biological and corticosteroid‐free clinical remission (OR: 0.883, 95% CI: 0.356‐2.189, P = 0.788) and biochemical remission (OR: 0.871, 95% CI: 0.363‐2.089, P = 0.757).
Table 6.
Baseline characteristics of propensity score matched cohort
| Tioguanine (n = 64) | LDTA (n = 64) | P value | |
|---|---|---|---|
| Age a , median (IQR) | 39.2 (27.4‐53.5) | 38.8 (26.5‐57.8) | 0.773 |
| Sex—male, N (%) | 26 (40.6) | 22 (34.4) | 0.465 |
| Current smoker, N (%) | 11 (17.2) | 17 (26.6) | 0.200 |
| Disease duration in years Median (IQR) | 5.0 (1.3‐12.1) | 5.5 (1.2‐16.3) | 0.705 |
| Treatment duration, median (IQR) | 95.1 (47.1‐104.4) | 85.1 (35.9‐104.1) | 0.388 |
| Follow‐up duration in weeks, median (IQR) | 104.0 (97.8‐104.4) | 104.0 (96.4‐104.1) | 0.323 |
| Type of disease (Crohn's disease), N (%) | 43 (67.2) | 40 (62.5) | 0.663 |
| Crohn's disease disease location, N (%) | 0.125 | ||
| Ileum | 22 (51.2) | 19 (48.7) | |
| Colon | 6 (14.0) | 12 (30.8) | |
| Ileum and colon | 15 (34.9) | 8 (20.5) | |
| Upper GI involvement, N (%) | 2 (4.7) | 3 (7.7) | 0.565 |
| Ulcerative colitis disease location, N (%) | 0.489 | ||
| Proctitis | 1 (4.8) | 1 (4.2) | |
| Left‐sided | 6 (28.6) | 11 (45.8) | |
| Pancolitis | 14 (66.7) | 12 (50.0) | |
| Unknown | — | — | |
| Disease behaviour, N (%) | 0.320 | ||
| Inflammatory disease | 35 (81.4) | 30 (75.0) | |
| Stricturing disease | 4 (9.3) | 8 (20.0) | |
| Penetrating disease | 4 (9.3) | 2 (5.0) | |
| Unknown | — | — | |
| Peri‐anal disease, N (%) | 4 (9.3) | 3 (7.5) | 0.768 |
| Prior intestinal resections, N (%) | 15 (23.4) | 11 (17.2) | 0.331 |
| Prior peri‐anal interventions, N (%) | 3 (7.0) | 1 (2.5) | 0.341 |
| Prior biological therapy use, N (%) | 13 (20.3) | 9 (14.1) | 0.349 |
| Clinical disease activity, N (%) | 0.588 | ||
| Remission | 10 (15.6) | 15 (23.4) | |
| Mild disease | 36 (56.3) | 36 (56.3) | |
| Moderate disease | 16 (25.0) | 11 (17.2) | |
| Unknown | 2 (3.1) | 2 (3.1) | |
| C‐reactive protein (mg/L), Median (IQR) | 4.0 (0.0‐11.8) | 2.0 (0.3‐6.0) | 0.119 |
| Fecal calprotectin (µg/g), Median (IQR) | 227 (168‐409) | 107 (21‐1100) | 0.201 |
| Concomitant medication, N (%) | 1.000 | ||
| No concomitant medication, N (%) | 52 (81.3) | 52 (81.3) | |
| Corticosteroids, N (%) | 12 (18.8) | 12 (18.8) | |
| Corticosteroids range, N (%) | 20 (6‐25) | 20 (13‐36) | |
Baseline characteristics of propensity score matched cohort of IBD patients treated with tioguanine or low dose thiopurine and allopurinol. Variables included: type of inflammatory bowel disease, disease duration, complicated disease (stricturing or penetrating disease for Crohn's disease, pancolitis for ulcerative colitis), peri‐anal disease at baseline, clinical and biochemical disease activity at baseline, corticosteroids at baseline.
Abbreviations: IQR, interquartile range; LDTA, low‐dose thiopurine and allopurinol; N, number of patients.
4. DISCUSSION
In this comparative effectiveness study we compared clinical outcomes between patients treated with tioguanine or LDTA after failure of conventional thiopurines due to adverse events. The discontinuation rate due to adverse events was relatively low (19%) for both treatments after 104 weeks of treatment. Moreover, after correction for confounders by both multiple logistic regression and propensity score matching, we observed no differences in terms of safety or effectiveness between the treatments.
The discontinuation rate due to adverse events of tioguanine (20%)‐ and LDTA (18%)‐treated IBD patients who previously failed conventional thiopurines is comparable to other real‐life cohorts. For LDTA, a prospective (n = 74) and retrospective (n = 89) cohort of LDTA patients with prior failure to conventional thiopurines showed a discontinuation rate of 15% and 18% due to adverse events, respectively. 17 , 18 A retrospective cohort study of tioguanine‐patients conducted in the Netherlands showed a lower rate of adverse events requiring discontinuation (11%). 19 However, in a systematic review of 353 tioguanine‐treated patients with median follow‐up between 3 and 22 months, approximately 20% discontinued treatment, mostly due to adverse events. 20 Of note, a large proportion of patients in this systematic review was treated with ≥40 mg a day. Taking into account that all patients in our study discontinued conventional thiopurines due to adverse events, a relative low proportion of patients discontinued second‐line thiopurine treatment due to adverse events.
The total rate of adverse events was relatively low for both tioguanine‐ (35 per 100 patient years) and LDTA‐ (37 per 100 patient years) treated patients. Simsek et al showed in a retrospective cohort of 274 tioguanine‐treated patient with 1567 patient‐years of follow‐up an adverse events rate of 12 per 100 patient years. 19 However, in this cohort, the median treatment duration was 51 months (IQR 36‐89). Since most adverse events occur in the first months of thiopurine treatment, the longer follow‐up duration compared to our study (median 24 months; IQR: 22‐24) could have resulted in a lower rate. 21 Further comparison of adverse events with other cohorts is difficult since not the number of adverse events but the number of patients having adverse events is reported.
In this study, we did not encounter a diagnosis of NRH of the liver in both the tioguanine‐ and LDTA‐treated cohort. Previously published studies reported that the prevalence of NRH is relatively low when treated with lower doses of tioguanine (±0.3 mg/kg) and the average dose of tioguanine in our cohort was 0.27 mg/kg (IQR: 0.22‐0.32). 11 , 22 Even though we did not systematically evaluate all patients by liver biopsy, no signs or symptoms for indicating portal hypertension were observed in both cohorts. The most common adverse events in our cohort were cutaneous lesions and arthralgia. Ten patients (11%) in both cohorts developed severe adverse events such as myelotoxicity and pancreatitis. Since adverse events requiring discontinuation occur most often in the first months of treatment (9.4 weeks; IQR: 2.1‐29.1), strict follow‐up especially in the first months of treatment, comparable to conventional thiopurines, remains needed. 23
When safety between tioguanine and LDTA is comparable, the effectiveness of therapy is another important selection criterion to decide on subsequent therapy after failure of conventional thiopurines. The effectiveness of tioguanine and LDTA was comparable after 2 years of treatment in our cohort (tioguanine: 33% vs LDTA: 23% P = 0.172 in surgery‐, biological‐ and corticosteroid‐free clinical remission). In literature, a number of cohorts have reported a sustained effect of tioguanine ranging between 22% and 60% after 12 months of treatment. 19 For LDTA, a randomised controlled trial (n = 73) has shown that LDTA achieved a corticosteroid‐free clinical remission rate of 53% after 24 weeks. 24 A retrospective cohort (n = 77) showed a drug survival of 65% after 60 months of treatment, however, in this study, 44% was already in corticosteroid‐free clinical remission at baseline. 25 The large differences between the effectiveness outcomes in different cohorts could partly be explained by variation in the number of patients in remission at baseline, outcome measurements and statistical analyses. In our study, we only selected patients with clinical disease activity at baseline to measure and compare the effectiveness outcomes and no differences between the two treatments were found.
In this study, we assessed the comparative safety and effectiveness of tioguanine and LDTA after failure of conventional thiopurines. We found no differences between the treatments indicating that both therapies can be considered when conventional thiopurines fail. Importantly, safety including adverse events, infections and hospitalisations did not show differences between therapies within a follow‐up period of 2 years. Several factors can be considered when deciding the type of therapy after conventional thiopurine failure. The advantage of tioguanine is that only one pill has to be administered which may improve treatment adherence and avoids the toxicity of a second drug such as allopurinol. In contrast, therapy with LDTA is supported by more clinical evidence that may guide the interpretation of the levels of metabolites associated with effectiveness and safety. For tioguanine more research is needed on this subject. Simsek et al described that a 6‐TGN concentration of >682 pmol/8 × 108 red blood cell count was associated with clinical effectiveness in tioguanine‐treated patients, however, other studies are needed to confirm these findings. 19 Furthermore, no information is available on 6‐TGN levels which correlate with tioguanine‐associated toxicity. In our study, 51% of patients showed sustained clinical benefit of both second‐line thiopurine therapies and avoided escalation to biological therapy, indicating that both tioguanine and LDTA are valid options to consider before escalation to more expensive biological treatments or surgery.
Physicians should be aware that LDTA strategy carries the risk of developing myelotoxicity, due to skewing towards 6‐TGN. 3 Previous studies showed 4% myelotoxicity in LDTA treatment after conventional thiopurines (n = 221) while this is not clearly identified for tioguanine therapy (0.2‐0.3 mg/kg). 10 , 26 In our study, we observed myelotoxicity in 5.7% (n = 5) of patients starting LDTA, compared to 3.2% (n = 3) of patients starting tioguanine. One and zero of these patients stopped conventional thiopurine for myelotoxicity, respectively. Patients who experienced myelotoxicity on conventional therapy were allowed enrolment in our study (n = 18) as this preceding effect was less likely related to 6‐TGN levels given the skewed metabolism towards 6‐MMP, resulting in relatively low 6‐TGN levels in these patients. The thiopurine dose reduction after the addition of allopurinol may even allow for reducing the risk of myelosuppression in these patients, despite an increase in 6‐TGN levels. When initiating either strategy, frequent blood counts during the initiation of therapy should be applied to allow relatively safe implementation.
With the arrival of new treatments with different mechanism of action (vedolizumab, ustekinumab and tofacitinib) and cheaper anti‐TNF treatment due to biosimilars, IBD‐experts and guidelines recommend switching to biological treatment when conventional thiopurines fail. 27 This recommendation holds truth for specific patients in selected countries, but many other patients may benefit from optimising thiopurine therapy first rather than switching to biologicals for several reasons. First, the clinical experience with thiopurines exceeds 50 years and the risk of common and rare adverse events have been reported extensively. 28 , 29 This is not yet the case for many of the recently approved new IBD therapies. Second, costs of biologicals are high, and these costs dominate the budget for the treatment of inflammatory diseases. This is problematic for the majority of patients living in newly industrialised countries, both insured and uninsured, since biological therapy is often not funded in these countries. 30 With the already observed rise in the incidence of IBD in the newly industrialised countries this becomes even more relevant for the near future. Thiopurines remain relatively cheap and provide an affordable therapeutic option. For example, azathioprine 200 mg per day costs between 180 and 280 euro per year in the Netherlands. 30 Third, approximately two‐thirds of patients treated with anti‐TNF fail this treatment over time due to primary or secondary loss of response or adverse events. 31 , 32 Although there is limited data for vedolizumab, ustekinumab and tofacitinib on this topic, real‐world data show, especially for Crohn's disease, a high rate of loss of response between the first and third year for vedolizumab. 15 , 33 Therefore, optimising thiopurine therapy before switching to biologicals remains important to preserve treatment options for generally young patients with a life‐long chronic incurable disease. Fourth, patients who tolerate thiopurines (if needed after therapeutic drug monitoring/dose optimisation) often remain in remission for years and the drug survival (after the first 6 months) is high. Subsequently, the likelihood of loss of response (absence of antibody formation) or toxicity during the maintenance phase is low. In patients who do develop a flare despite thiopurine treatment, an anti‐TNF based strategy is often applied with continuation of thiopurine as co‐treatment. 34 , 35
Strengths of our study include the large cohort of patients with prior failure to conventional thiopurines due to adverse events, subsequently treated with second‐line thiopurines and the classification of severity of adverse events based on the validated CTCAE. Furthermore, by using both a multiple logistic regression and propensity score matching we reduced the influence of confounders on the outcomes. There are also limitations in this study that need to be acknowledged. We performed a comparative effectiveness analysis without the preferred randomised study design. Therefore, it is possible that unknown confounders have influenced the results. However, we did adjust for important factors widely recognised for being associated with disease severity, a refractory phenotype or safety with two different types of analyses. Since there are currently no randomised studies ongoing or being planned, comparison of observational cohorts with correction for confounders is the best available option to address the selection of therapy after thiopurine failure. Another limitation is the absence of metabolite levels in tioguanine‐treated patients, 6‐TGN comparisons could therefore not be made. Although patients were followed for 2 years, this follow‐up duration may be insufficient to observe long‐term adverse events such as lymphoma and non‐melanoma skin cancer. Lastly, to compare the effectiveness of both treatments we used surgery‐, biological‐ and corticosteroid‐free clinical remission based on the physician global assessment instead of the preferred endoscopy outcomes.
To conclude, both tioguanine and LDTA are relatively safe options when conventional thiopurines fail due to intolerance. The rate of adverse events, infections and hospitalisations was relatively low and a substantial and comparable proportion of patients showed clinical benefit from therapy after 104 weeks of treatment. Therefore, based on this study, both tioguanine and LDTA are valid therapeutic options when conventional thiopurines fail due to adverse events. This strategy may provide an additional therapeutic option in selected IBD patients before escalating to biological treatment.
AUTHORSHIP
Guarantor of the article: Frank Hoentjen.
Author contributions: No additional writing assistance was used for this manuscript. VB, ES, MS, GD, MP, NB and FH contributed to the design of the study. All authors collected data, VB and FH analysed the data. VB, ES and FH drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors have approved the final version of this manuscript.
ACKNOWLEDGEMENT
Declaration of personal interests: VBC Biemans, E. Savelkoul, RY Gabriëls have no conflicts of interest to declare. G. Dijkstra unrestricted research grants from Abbvie and Takeda. Advisory boards for Mundipharma and Pharmacosmos. Received speakers fees from Abbvie, Takeda and Janssen Pharmaceuticals. M. Simsek has received an unrestricted research grant from Teva Pharma BV. NKH de Boer has served as a speaker for AbbVie, Takeda and MSD. He has served as consultant and principal investigator for Takeda and TEVA Pharma BV He has received (unrestricted) research grants from Dr Falk, TEVA Pharma BV, and Takeda. MJ Pierik has served on advisory boards, or as speaker or consultant for Abbvie, Janssen‐Cilag, MSD, Takeda, Ferring, Dr Falk, and Sandoz and has received unrestricted grants from, Janssen‐Cilag, Abbvie and Takeda outside the submitted work. RL West has served as a speaker for Takeda. She has served as principal investigator for Abbvie, Ferring and Janssen. She has received (unrestricted) research grants from Janssen and Abbvie. F. Hoentjen has served on advisory boards, or as speaker or consultant for Abbvie, Celgene, Janssen‐Cilag, MSD, Takeda, Celltrion, Teva, Sandoz and Dr Falk, and has received unrestricted grants from Dr Falk, Janssen‐Cilag, Abbvie.
Biemans VBC, Savelkoul E, Gabriëls RY, et al. A comparative analysis of tioguanine versus low‐dose thiopurines combined with allopurinol in inflammatory bowel disease patients. Aliment Pharmacol Ther. 2020;51:1076–1086. 10.1111/apt.15730
Vince BC Biemans and Edo Savelkoul shared authorship.
The Handling Editor for this article was Professor Roy Pounder, and it was accepted for publication after full peer‐review.
Funding information
No funding has been received for this specific study. Data have been generated as part of routine work of the participating organisations.
REFERENCES
- 1. Jeuring SFG, van den Heuvel TRA, Liu LYL, et al. Improvements in the long‐term outcome of Crohn's disease over the past two decades and the relation to changes in medical management: results from the population‐based IBDSL cohort. Am J Gastroenterol. 2017;112:325‐336. [DOI] [PubMed] [Google Scholar]
- 2. Macaluso FS, Renna S, Maida M, et al. Tolerability profile of thiopurines in inflammatory bowel disease: a prospective experience. Scand J Gastroenterol. 2017;52:981‐987. [DOI] [PubMed] [Google Scholar]
- 3. Osterman MT, Kundu R, Lichtenstein GR, et al. Association of 6‐thioguanine nucleotide levels and inflammatory bowel disease activity: a meta‐analysis. Gastroenterology. 2006;130:1047‐1053. [DOI] [PubMed] [Google Scholar]
- 4. Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6‐mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705‐713. [DOI] [PubMed] [Google Scholar]
- 5. Blaker PA, Arenas‐Hernandez M, Marinaki AM, et al. The pharmacogenetic basis of individual variation in thiopurine metabolism. Per Med. 2012;9:707‐725. [DOI] [PubMed] [Google Scholar]
- 6. Ryu HJ, Song R, Kim HW, et al. Clinical risk factors for adverse events in allopurinol users. J Clin Pharmacol. 2013;53:211‐216. [DOI] [PubMed] [Google Scholar]
- 7. Chaparro M, Ordás I, Cabré E, et al. Safety of thiopurine therapy in inflammatory bowel disease: long‐term follow‐up study of 3931 patients. Inflamm Bowel Dis. 2013;19:1404‐1410. [DOI] [PubMed] [Google Scholar]
- 8. Seiderer J, Zech CJ, Reinisch W, et al. A multicenter assessment of liver toxicity by MRI and biopsy in IBD patients on 6‐thioguanine. J Hepatol. 2005;43:303‐309. [DOI] [PubMed] [Google Scholar]
- 9. Toksvang LN, Schmidt MS, Arup S, et al. Hepatotoxicity during 6‐thioguanine treatment in inflammatory bowel disease and childhood acute lymphoblastic leukaemia: a systematic review. PLoS ONE. 2019;14:e0212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bayoumy AB, Simsek M, Seinen ML, et al. The continuous rediscovery and the benefit‐risk ratio of thioguanine, a comprehensive review. Expert Opin Drug Metab Toxicol. 2020;16:1‐13. [DOI] [PubMed] [Google Scholar]
- 11. van Asseldonk DP, Jharap B, Verheij J, et al. The prevalence of nodular regenerative hyperplasia in inflammatory bowel disease patients treated with thioguanine is not associated with clinically significant liver disease. Inflamm Bowel Dis. 2016;22:2112‐2120. [DOI] [PubMed] [Google Scholar]
- 12. De Boer NK, Tuynman H, Bloemena E, et al. Histopathology of liver biopsies from a thiopurine‐naive inflammatory bowel disease cohort: prevalence of nodular regenerative hyperplasia. Scand J Gastroenterol. 2008;43:604‐608. [DOI] [PubMed] [Google Scholar]
- 13. Simsek M, Meijer B, van Bodegraven AA, et al. Finding hidden treasures in old drugs: the challenges and importance of licensing generics. Drug Discov Today. 2018;23:17‐21. [DOI] [PubMed] [Google Scholar]
- 14. Biemans VBC, van der Meulen ‐ de Jong AE, van der Woude CJ, et al. Ustekinumab for Crohn's disease: results of the ICC Registry, a nationwide prospective observational cohort study. J Crohns Colitis. 2020;14:33‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Biemans VBC, van der Woude J, Dijkstra G, et al. Vedolizumab for Inflammatory Bowel Disease: two year results of the ICC Registry, a nationwide prospective observational cohort study. Clin Pharmacol Ther. 2019. [Epub ahead of print]. 10.1002/cpt.1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trotti A, Colevas A, Setser A, et al. CTCAE v3. 0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176‐181. [DOI] [PubMed] [Google Scholar]
- 17. Vasudevan A, Beswick L, Friedman AB, et al. Low‐dose thiopurine with allopurinol co‐therapy overcomes thiopurine intolerance and allows thiopurine continuation in inflammatory bowel disease. Dig Liver Dis. 2018;50:682‐688. [DOI] [PubMed] [Google Scholar]
- 18. Pavlidis P, Stamoulos P, Abdulrehman A, et al. Long‐term safety and efficacy of low‐dose azathioprine and allopurinol cotherapy in inflammatory bowel disease: a large observational study. Inflamm Bowel Dis. 2016;22:1639‐1646. [DOI] [PubMed] [Google Scholar]
- 19. Simsek M, Deben DS, Horjus CS, et al. Sustained effectiveness, safety and therapeutic drug monitoring of tioguanine in a cohort of 274 IBD patients intolerant for conventional therapies. Aliment Pharmacol Ther. 2019;50:54‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meijer B, Mulder CJJ, Peters GJ, et al. Efficacy of thioguanine treatment in inflammatory bowel disease: a systematic review. World J Gastroenterol. 2016;22:9012‐9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hindorf U, Lindqvist M, Hildebrand H, et al. Adverse events leading to modification of therapy in a large cohort of patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24:331‐342. [DOI] [PubMed] [Google Scholar]
- 22. van Asseldonk DP, Simsek M, de Boer NKH, et al. Limited relevance and progression of histological alterations in the liver during thioguanine therapy in inflammatory bowel disease patients. Scand J Gastroenterol. 2019;54:753‐760. [DOI] [PubMed] [Google Scholar]
- 23. de Boer N, Reinisch W, Teml A, et al. 6‐Thioguanine treatment in inflammatory bowel disease: a critical appraisal by a European 6‐TG working party. Digestion. 2006;73:25‐31. [DOI] [PubMed] [Google Scholar]
- 24. Friedman AB, Brown SJ, Bampton P, et al. Randomised clinical trial: efficacy, safety and dosage of adjunctive allopurinol in azathioprine/mercaptopurine nonresponders (AAA Study). Aliment Pharmacol Ther. 2018;47:1092‐1102. [DOI] [PubMed] [Google Scholar]
- 25. Hoentjen F, Seinen ML, Hanauer SB, et al. Safety and effectiveness of long‐term allopurinol‐thiopurine maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:363‐369. [DOI] [PubMed] [Google Scholar]
- 26. Kreijne JE, de Veer RC, de Boer NK, et al. Real‐life study of safety of thiopurine‐allopurinol combination therapy in inflammatory bowel disease: myelotoxicity and hepatotoxicity rarely affect maintenance treatment. Aliment Pharmacol Ther. 2019;50:407‐415. [DOI] [PubMed] [Google Scholar]
- 27. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn's disease: medical treatment. J Crohns Colitis. 2020;14:4‐22. [DOI] [PubMed] [Google Scholar]
- 28. Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50:485‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peyrin–Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621‐1628. [DOI] [PubMed] [Google Scholar]
- 30. de Boer NKH, Ahuja V, Almer S, et al. Thiopurine therapy in inflammatory bowel diseases: making new friends should not mean losing old ones. Gastroenterology. 2019;156:11‐14. [DOI] [PubMed] [Google Scholar]
- 31. Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn's disease: a review. Am J Gastroenterol. 2009;104:760‐767. [DOI] [PubMed] [Google Scholar]
- 32. Billioud V, Sandborn WJ, Peyrin‐Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn's disease: a systematic review. Am J Gastroenterol. 2011;106:674‐684. [DOI] [PubMed] [Google Scholar]
- 33. Amiot A, Serrero M, Peyrin‐Biroulet L, et al. Three‐year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multi‐centre cohort study. Aliment Pharmacol Ther. 2019;50:40‐53. [DOI] [PubMed] [Google Scholar]
- 34. Roblin X, Williet N, Boschetti G, et al. Addition of azathioprine to the switch of anti‐TNF in patients with IBD in clinical relapse with undetectable anti‐TNF trough levels and antidrug antibodies: a prospective randomised trial. Gut. 2020. [Epub ahead of print]. 10.1136/gutjnl-2019-319758 [DOI] [PubMed] [Google Scholar]
- 35. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383‐1395. [DOI] [PubMed] [Google Scholar]


