Abstract
The molecular basis of interleukin (IL)‐17A in driving psoriasis pathogenesis is not fully elucidated yet. To investigate the underlying mechanisms and biomarkers associated with IL‐17A and the role in psoriasis pathogenesis, over 30 serum proteins were evaluated in a study assessing the effectiveness and safety of secukinumab, where treatment was directly switched from cyclosporin A to secukinumab. Serum β‐defensin 2 (BD‐2) levels rapidly and robustly reduced following secukinumab treatment. BD‐2 levels were well‐correlated with Psoriasis Area and Severity Index (PASI) score; changes in BD‐2 levels preceded change in PASI score. Serum BD‐2, an easily measurable protein, can possibly be used as a suitable surrogate biomarker to monitor responses to IL‐17A‐targeted therapies for psoriasis in clinical practice.
Keywords: biomarker, interleukin‐17A, psoriasis, secukinumab, β‐defensin 2
Introduction
The rapid and robust improvement in psoriatic symptoms with interleukin (IL)‐17A‐targeting therapies including secukinumab, a fully human monoclonal anti‐IL‐17A antibody, enhanced the understanding of the pathogenesis of psoriasis (PsO).1, 2, 3, 4, 5 Evaluating gene expression in skin biopsy specimens from patients with PsO revealed a set of genes related to PsO, the expression of which changed following IL‐17A inhibitor therapy.6, 7 However, studies evaluating serum biomarkers in patients with PsO on IL‐17A inhibitor therapy are scarce.3, 5, 8, 9
Biologics are often used after primary or secondary failure with non‐biologic systemic treatment such as cyclosporin A (CyA). However, abrupt cessation of systemic treatment could cause disease exacerbation.10 In order to evaluate the safety and effectiveness of secukinumab in patient with PsO whose treatment was directly switched from CyA to secukinumab, a phase 4 study was conducted in Japan.11
Most studies evaluating serum biomarkers in patients with PsO on IL‐17A inhibitor therapy analyzed a limited numbers of targeted proteins. In the switching study, serum samples were collected to evaluate potential biomarkers that are associated with PsO and/or treatment. Here, we report the data of over 30 serum proteins and their correlation with PsO disease severity as evaluated by Psoriasis Area and Severity Index (PASI) before and after treatment by secukinumab.
Methods
Study design
The detailed study design and inclusion criteria have been reported previously.11 In short, this is a multicenter, single‐arm, 16‐week, open‐label phase 4 study conducted at 11 sites in Japan between June 2015 and May 2016 to assess the effectiveness and safety of secukinumab‐treated patients directly switched from CyA.
Eligible patients were aged 18 years or older and diagnosed with plaque psoriasis at least 6 months before baseline. Patients had to be taking CyA for at least 12 weeks, and should have experienced a primary or secondary inadequate response (PASI, ≥10; Investigator's Global Assessment modified 2011, ≥2 [scale, 0–4]) to the treatment.
The study protocol was approved by the institutional review board of each participating site, and the study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Eligible patients provided written informed consent.
Outcomes
In this switching study, serum samples of patients with moderate to severe PsO were collected to evaluate potential biomarkers that are associated with PsO and/or treatment.
Blood samples were collected at baseline and weeks 2 and 16. Serum chemokines (eotaxin, macrophage inflammatory protein [MIP]‐1α, MIP‐1β, eotaxin‐3, thymus and activation‐regulated chemokine [TARC], interferon‐γ‐inducible protein [IP]‐10, IL‐8 [HA], macrophage‐derived chemokine [MDC], monocyte chemoattractant protein [MCP]‐1, MCP‐4) and cytokines (granulocyte macrophage colony‐stimulating factors [GM‐CSF], tumor necrosis factor [TNF]‐α, TNF‐β, vascular endothelial growth factor [VEGF]‐A, interferon [IFN]‐γ, IL‐1α, IL‐1β, IL‐2, IL‐4, IL‐5, IL‐6, IL‐7, IL‐8, IL‐10, IL‐12/IL‐23p40, IL‐12p70, IL‐13, IL‐15, IL‐16, IL‐17A) were measured by electrochemiluminescence assay according to the manufacturer’s instruction (catalog no. K15054G; Meso Scale Discovery, Rockville, MD, USA). β‐Defensin (BD)‐2 was quantified by enzyme‐linked immunosorbent assay (ELISA) (catalog no. 100‐250‐BD‐2; Alpha Diagnostic International, San Antonio, TX, USA).
Statistical analysis
Summary of statistics for all of the tested biomarker profiles and the mean change from baseline by visit are presented. Descriptive data are presented in median (quartile range). Correlations of PsO disease and the tested biomarkers were assessed by the Pearson correlation coefficient (r‐value) using PASI score and biomarker concentration. P < 0.05 was considered statistically significant. Statistical analysis was performed using SAS Studio software 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient disposition and clinical effectiveness
Patient disposition and baseline characteristics, as well as clinical effectiveness, have been reported previously.11 All 34 patients enrolled in this study completed the required 16 weeks of treatment. The mean PASI score at baseline was 15.05. The primary end‐point of 75% improvement in PASI response (PASI‐75) at week 16 was achieved by 82.4% (n = 28) of patients receiving secukinumab.
Serum biomarkers
Serum protein levels at baseline, namely 1 day after the last dose of CyA and before the first secukinumab dose, are summarized in Table 1. The serum protein changes from baseline by visit are presented in Figure 1. Among the proteins tested, serum BD‐2 level reduced from baseline (median, 5850.00 ng/L [range, 910.00–11 000.00]) to week 2 (729.00 ng/L [451.00–2450.00]), which was sustained at week 16 (407.00 ng/L [257.00–590.00]) (Fig. 1c). A substantial increase in IL‐17A (both free and antibody‐bound forms) was seen from baseline (3.44 ng/L [2.02–5.72]) to week 2 (198.00 ng/L [111.00–264.00]), which was sustained at week 16 (112.00 ng/L [89.60–154.00]) (Fig. 1b).
Table 1.
Serum proteins at baseline
| Chemokine | Median (quartile), ng/L | Reference,12 median, pg/mL† | Cytokine | Median (quartile), ng/L | Reference,13, 14 median, pg/mL† |
|---|---|---|---|---|---|
| Eotaxin | 98.0 (74.4–142.0) | 55.9 | GM‐CSF | 0.31 (0.24–0.39) | 0.18 |
| Eotaxin‐3 | 10.1 (6.5–16.6) | 8.2 | IFN‐γ | 3.90 (2.98–5.12) | 3.77 |
| IL‐8 (HA) | 662.0 (452.0–788.0) | 575.0 | IL‐1α | 0.10 (0.03–0.15) | 1.18 |
| IP‐10 | 319.0 (239.0–404.0) | 80.9 | IL‐1β | 0 (0–0.2) | 0.16 |
| MCP‐1 | 226.5 (188.0–274.0) | 118.0 | IL‐2 | 0.27 (0.16–0.33) | 0.52 |
| MCP‐4 | 103.5 (74.0–142.0) | 44.3 | IL‐4 | 0.06 (0.03–0.07) | ND |
| MDC | 1130.0 (828.0–1590.0) | 1350.0 | IL‐5 | 0.07 (0–0.47) | 0.28 |
| MIP‐1α | 14.8 (13.7–16.4) | 37.0 | IL‐6 | 0.79 (0.56–1.40) | 0.47 |
| MIP‐1β | 106.0 (72.4–137.0) | 53.1 | IL‐7 | 25.60 (18.0–32.80) | 0.92 |
| TARC | 287.5 (178.0–412.0) | 29.1 | IL‐8 | 10.85 (8.56–13.40) | 9.61 |
| IL‐10 | 0.28 (0.20–0.36) | 0.20 | |||
| Other | Median (quartile), ng/L | Reference, 5 GM, pg / mL † | IL‐12/IL‐23p40 | 56.00 (39.80–79.00) | 53.30 |
| BD‐2 | 5850.0 (1910.0–11 000.0) | 0.2 | IL‐12p70 | 0.20 (0.11–0.25) | 0.29 |
| IL‐13 | 0.79 (0.62–1.05) | 1.65 | |||
| IL‐15 | 2.22 (1.87–2.58) | 1.29 | |||
| IL‐16 | 224.00 (188.00–328.00) | 59.90 | |||
| IL‐17A | 3.44 (2.02–5.72) | 0.93 | |||
| TNF‐α | 2.42 (1.84–2.86) | 0.36 | |||
| TNF‐β | 0.34 (0.24–0.42) | 0.15 | |||
| VEGF‐A | 90.90 (57.40–157.00) | 9.62 |
Reference values refer to assay kit product insert and published report on healthy human subjects. pg/mL is equivalent to ng/L. Different detection antibodies were used for IL‐8 measurement in chemokine and cytokine panel according to manufacturers’ instructions. BD, β‐defensin; GM, geometric mean; GM‐CSF, granulocyte macrophage colony‐stimulating factors; IFN, interferon; IL, interleukin; IP, interferon‐γ‐inducible protein; MCP, monocyte chemoattractant protein; MDC, macrophage derived chemokine; MIP, macrophage inflammatory protein; ND, non‐detectable; TARC, thymus and activation‐regulated chemokine; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Figure 1.
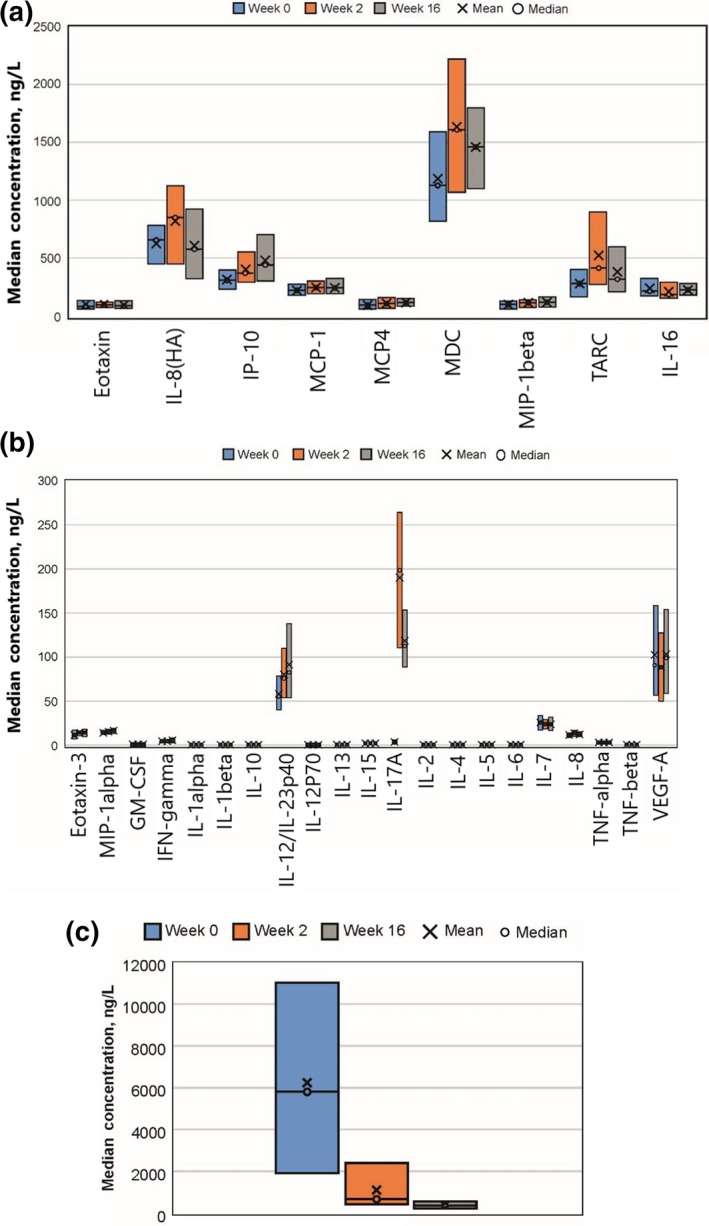
Serum chemokine, cytokine and BD‐2 levels pre‐/post‐secukinumab treatment. Serum (a) chemokine, (b) cytokine and (c) BD‐2 levels were measured in samples collected at baseline, and weeks 2 and 16. Mean (x), median (o), 25 percentile (lower line) and 75 percentile (upper line) are presented. Different detection antibodies were used for IL‐8 measurement in chemokine and cytokine panel according to the manufacturers’ instructions. BD, β‐defensin; GM‐CSF, granulocyte macrophage colony‐stimulating factors; IL, interleukin; IP, interferon‐γ‐inducible protein; MCP, monocyte chemoattractant protein; MDC, macrophage‐derived chemokine; MIP, macrophage inflammatory protein; TARC, thymus and activation‐regulated chemokine; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
At baseline, when patients’ disease severity was PASI of more than 10 despite the treatment with CyA, a positive correlation between PASI score and serum concentration was found for MDC (r = 0.48, P = 0.004), MIP‐1α (r = 0.393, P = 0.021), IL‐16 (r = 0.394, P = 0.021) and BD‐2, (r = 0.459, P = 0.006). When all time points (baseline, week 2, and week 16) were pooled for data analysis, such relationship was lost in MDC, MIP‐1α and IL‐16, whereas BD‐2 showed a stronger correlation (r = 0.767, P < 0.001) (Table 2, Fig. 2). IFN‐γ levels were inversely correlated with PASI in both baseline (r = −0.455, P = 0.007) and pooled data analysis (r = −0.275, P = 0.005), and a negative correlation was observed between serum IL‐17A level and PASI only in the pooled data analysis (r = −0.452, P < 0.001) (Table 2).
Table 2.
Correlation between PASI score and serum proteins
| Baseline (n = 34) | All time points† (n = 102) | |||
|---|---|---|---|---|
| Correlation coefficient | P | Correlation coefficient | P | |
| Chemokine | ||||
| Eotaxin | 0.006 | 0.972 | 0.002 | 0.982 |
| Eotaxin‐3 | 0.125 | 0.481 | 0.022 | 0.825 |
| IL‐8 (HA) | −0.197 | 0.263 | −0.022 | 0.83 |
| IP‐10 | 0.134 | 0.451 | −0.06 | 0.548 |
| MCP‐1 | 0.120 | 0.499 | −0.037 | 0.715 |
| MCP‐4 | −0.004 | 0.983 | 0.07 | 0.482 |
| MDC | 0.480 | 0.004 | 0.187 | 0.06 |
| MIP‐1α | 0.393 | 0.021 | 0.076 | 0.448 |
| MIP‐1β | −0.033 | 0.854 | −0.169 | 0.09 |
| TARC | 0.264 | 0.131 | 0.167 | 0.094 |
| Cytokine | ||||
| GM‐CSF | −0.173 | 0.328 | −0.117 | 0.242 |
| IFN‐γ | −0.455 | 0.007 | −0.275 | 0.005 |
| IL‐1α | −0.138 | 0.435 | 0.075 | 0.455 |
| IL‐1β | −0.143 | 0.419 | 0.07 | 0.482 |
| IL‐2 | −0.221 | 0.209 | −0.037 | 0.714 |
| IL‐4 | 0.256 | 0.143 | 0.16 | 0.109 |
| IL‐5 | −0.182 | 0.303 | −0.073 | 0.464 |
| IL‐6 | 0.231 | 0.189 | 0.167 | 0.093 |
| IL‐7 | 0.164 | 0.354 | 0.105 | 0.295 |
| IL‐8 | 0.122 | 0.491 | 0.128 | 0.199 |
| IL‐10 | −0.186 | 0.293 | 0.133 | 0.184 |
| IL‐12/IL‐23p40 | 0.104 | 0.557 | 0.009 | 0.925 |
| IL‐12p70 | −0.247 | 0.159 | −0.164 | 0.099 |
| IL‐13 | 0.141 | 0.428 | 0.046 | 0.644 |
| IL‐15 | −0.112 | 0.528 | −0.002 | 0.981 |
| IL‐16 | 0.394 | 0.021 | 0.195 | 0.05 |
| IL‐17A | 0.220 | 0.211 | −0.452 | <0.001 |
| TNF‐α | 0.329 | 0.058 | 0.076 | 0.446 |
| TNF‐β | −0.212 | 0.228 | 0.034 | 0.734 |
| VEGF‐A | 0.228 | 0.194 | 0.169 | 0.09 |
| Other | ||||
| BD‐2 | 0.459 | 0.006 | 0.767 | <0.001 |
Pearson correlation analysis presented in this table was evaluated using the baseline and pooled data of PASI scores and serum proteins observed at the three time points (baseline, week 2 and week 16). Different detection antibodies were used for IL‐8 measurement in chemokine and cytokine panel according to the manufacturers’ instructions. Bold indicates positive/negative correlation and significance at P < 0.05. BD, β‐defensin; GM‐CSF, granulocyte macrophage colony‐stimulating factors; IFN, interferon; IL, interleukin; IP, interferon‐γ‐inducible protein; MCP, monocyte chemoattractant protein; MDC, macrophage‐derived chemokine; MIP, macrophage inflammatory protein; TARC, thymus and activation‐regulated chemokine; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Figure 2.
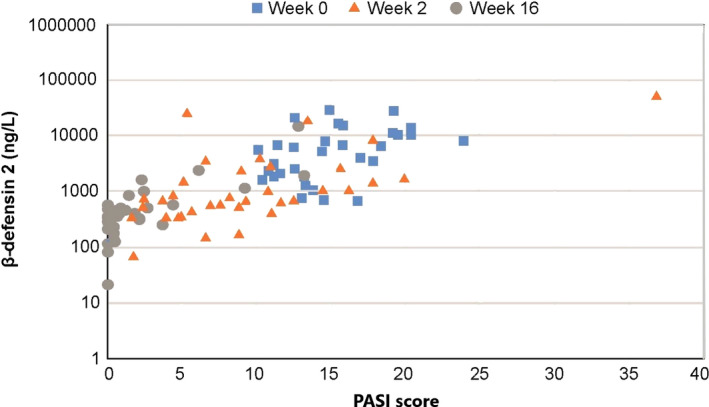
Correlation between PASI score and serum BD‐2 level. Serum samples from baseline, and weeks 2 and 16 were pooled for analysis and the correlation was assessed by Pearson correlation coefficient using PASI score and BD‐2 concentration. Data from FAS. BD, β‐defensin; FAS, full analysis set; PASI, Psoriasis Area and Severity Index.
The relative change in individual PASI score and serum BD‐2 level from baseline was plotted in Figure 3. At week 2, a remarkable decrease was observed in BD‐2 with a median percentage change of −79.5, and the decrease was greater than that of PASI (median percentage change of −47.7). Both BD‐2 and PASI levels further decreased at week 16 with median percentage change of −90.3 and −94.6, respectively. Of note, out of four patients whose PASI scores increased at week 2 from baseline (Fig. 3a), three patients exhibited a decrease in BD‐2 levels at the corresponding time point (Fig. 3b), and all of the patients subsequently showed decreased PASI scores at week 16. Inversely, one patient who retained an increased BD‐2 at week 2 continuously had an increased PASI up to week 16.
Figure 3.
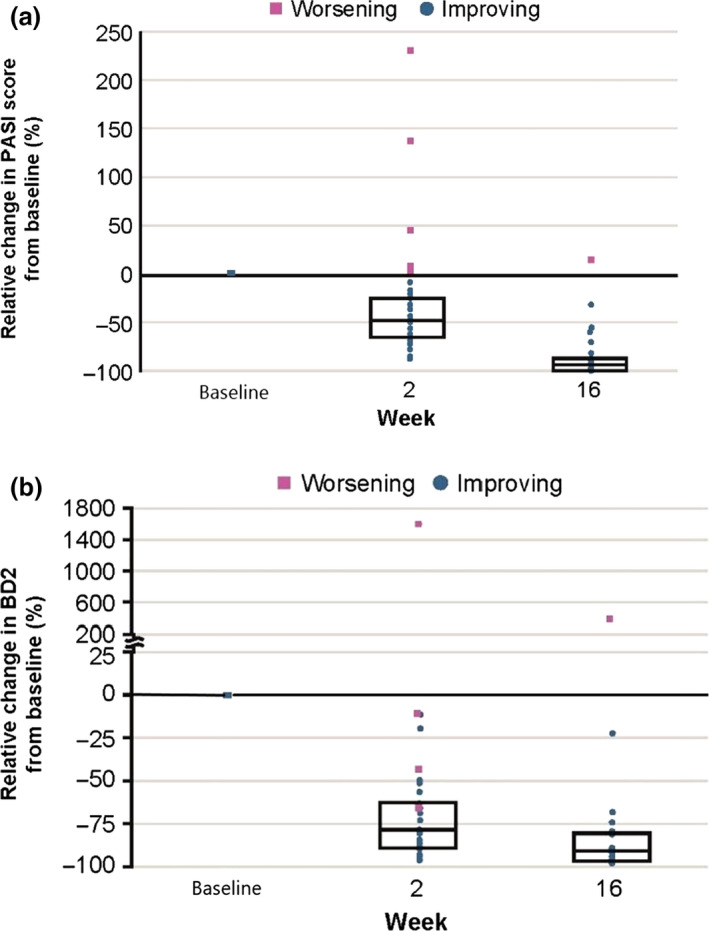
Relative change in individual PASI score from baseline and in individual serum BD‐2 level from baseline. PASI score and blood samples were collected at baseline, and weeks 2 and 16. Change of (a) PASI score and (b) serum BD‐2 at weeks 2 and 16 were evaluated relative to baseline. Data from FAS. BD, β‐defensin; FAS, full analysis set; PASI, Psoriasis Area and Severity Index.
Discussion
In the current study, treatment with secukinumab initiated immediately after discontinuing CyA led to an early improvement in PsO symptoms without CyA withdrawal‐associated relapse and was well‐tolerated without unexpected safety signals.
At baseline, when skin symptom was active even under CyA treatment, the median levels of IP‐10, TARC, IL‐16, IL‐17A, IL‐7, TNF‐α and VEGF‐A of the patients with PsO were over three‐times higher (394%, 988%, 374%, 371%, 2786%, 672% and 945%, respectively; Table 1) compared with the serum protein levels reported in healthy human subjects.12, 13, 14 Over three‐times lower protein levels were observed in IL‐1α and IL‐5 (8% and 24.7%, Table 1). The median baseline serum BD‐2 level among patients in this study was comparable with that of patients with active PsO (vs 5746 pg/mL [equivalent to ng/L], 102%) and was higher than the level in healthy volunteers (vs 82 pg/mL [equivalent to ng/L], 7134%) as previously reported by Kolbinger et al. 5
Among the proteins whose levels were elevated in patients with PsO at baseline compared with healthy human subjects, serum IL‐16 was correlated with disease activity. However, the levels of IL‐16 were relatively stable at weeks 2 and 16 when skin symptom was improved after the treatment with secukinumab. Increased IL‐17A levels were observed at weeks 2 and 16 despite the improved clinical disease activity at the corresponding time points. The increment of serum IL‐17A is likely attributable to the detection of the secukinumab‐IL‐17A complex that is eliminated slowly from circulation.15 As the current assay cannot distinguish between the free and secukinumab‐bound form of IL‐17A, it remains to be elucidated whether free IL‐17A level correlates with disease activity. Slight increase in serum TARC level was observed in week 2, which is in line with an earlier study.3 Serum IFN‐γ level at baseline in this study did not differ from the value obtained from healthy subjects (84%); however, IFN‐γ was negatively correlated with PASI at baseline and through the secukinumab treatment. The result appears to be in conflict with previous reports of a decreased mRNA expression in the PsO skin after IL‐17A inhibitor therapy,16 and a comparison of serum cytokine levels of patients with PsO to control subjects and an association of serum cytokines with PsO disease severity.17, 18 A possible influence of CyA on the cytokine/chemokine levels should be considered to interpret the difference.
β‐Defensin 2 is an antimicrobial peptide produced by epithelial cells including keratinocytes, the production of which is inducible by injury, infection or cytokines.19 In active psoriatic lesions, higher BD‐2 expression has been reported compared with the levels in atopic dermatitis and healthy skin.20 In the current study, there was a strong correlation between serum BD‐2 level and psoriatic disease activity, and the change in BD‐2 preceded the improvement in PASI score, suggesting that the suppression level of BD‐2 can predict the response to therapy. Further investigation measuring BD‐2 level at various conditions is needed to assess the possibility.
In addition to its biological antimicrobial function, BD‐2 is involved in the development of PsO by stimulating plasmacytoid dendritic cells and subsequent T‐cell activation.21, 22 In relation to the elevation of BD‐2 in psoriatic skin, Nograles et al. 23 demonstrated that human keratinocytes constitutively express IL‐17 receptors and that IL‐17A upregulated BD‐2 coding gene, DEFB4, in keratinocytes.
Moreover, in vitro data from human keratinocytes showed that IL‐17A induced NFKBIZ, which encodes a transcription co‐factor I kappa B‐zeta (IκB‐ζ), and that NFKBIZ/IκB‐ζ is required for the upregulation of IL‐17A‐induced genes including DEFB4A.24 NFKBIZ is rapidly induced by IL‐17A, but only to a lesser extent by TNF‐α stimulation in keratinocytes,24 and reduction in DEFB4 mRNA level in lesional skin was larger after anti‐IL‐17A (ixekizumab) treatment compared with after anti‐TNF‐α (etanercept) in patients with PsO,16 suggesting that IL‐17A exclusively induces expression of IκB‐ζ, which subsequently activates the promoter of DEFB4 for the production of BD‐2.
This study was conducted on a small sample size and included patients with PsO whose treatment was directly switched from CyA to secukinumab; hence, the serum protein levels of this population could have been under the influence of CyA.
Nonetheless, a rapid and robust reduction in serum BD‐2 was observed after secukinumab treatment in patients with PsO, change of which preceded the PASI score reduction. Serum BD‐2 is an easily measurable protein and could potentially be used as a specific surrogate biomarker for IL‐17A activity and to monitor responses to IL‐17A‐targeted therapies.
Conflict of Interest
Y. T., K. M., M. Y. and R. T. are employees of Novartis Pharma K.K.. A. M. has received research grants, consulting fees and/or speaker’s fees from AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, Eisai, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Nichi‐Iko, Nippon Kayaku, Novartis, Sun Pharmaceutical Industries, Taiho Pharmaceutical and Torii Pharmaceutical. M. O. has received grants for research and/or honoraria for lectures and/or advisory board from Abbvie, Boehringer‐Ingelheim, Celgene, Eisai, Eli Lilly, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe Pharma, Novartis, Pfizer, Sanofi, Taiho Pharmaceutical and Torii Pharmaceutical.
Acknowledgments
The study was funded by Novartis Pharma and Maruho. Author contributions are as follows: All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Authors also take complete responsibility for the integrity of the data and accuracy of the data analysis. The sponsor (Novartis) has performed the statistical analysis, and all authors take responsibility for the accuracy of the results. All authors reviewed and provided feedback on subsequent versions and agreed on the final version and to submit the manuscript for publication. Medical writing, editorial and other assistance: The authors would like to thank Poh Sien Ooi (Novartis) and Ashwini K. M. Kumar (Novartis Healthcare) for providing medical writing assistance in accordance with the Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). The following principle investigators are acknowledged for their support in the trial: Dr Atsuyuki Igarashi from NTT Medical Center, Tokyo, Japan; Dr Hideshi Torii from Japan Community Healthcare Organization Tokyo Yamate Medical Center, Tokyo, Japan; Dr Shinichi Imafuku from Fukuoka University Hospital, Fukuoka, Japan; Dr Mari Higashiyama from Nissay Hospital, Osaka, Japan; Dr Tadashi Terui from Nihon University Itabashi Hospital, Tokyo, Japan; Dr Takafumi Etoh from Tokyo Teishin Hospital, Tokyo, Japan; Dr Tomotaka Mabuchi from Tokai University Hospital, Kanagawa, Japan; Dr Yayoi Tada from Teikyo University Hospital, Tokyo, Japan; and Dr Yoshinori Umezawa from The Jikei University Hospital, Tokyo, Japan.
References
- 1. Langley R, Elewski B, Lebwohl M et al. Secukinumab in plaque psoriasis — results of two phase 3 trials. N Engl J Med 2014; 371(4): 326–338. [DOI] [PubMed] [Google Scholar]
- 2. Hueber W, Patel DD, Dryja T et al. Effects of AIN457, a fully human antibody to interleukin‐17A, on psoriasis, rheumatoid arthritis, and uveitis. Transl Med 2010; 2(52): 52ra72. [DOI] [PubMed] [Google Scholar]
- 3. Shibuya T, Honma M, Iinuma S, Iwasaki T, Takahashi H, Ishida‐Yamamoto A. Alteration of serum thymus and activation‐regulated chemokine level during biologic therapy for psoriasis: possibility as a marker reflecting favorable response to anti‐interleukin‐17A agents. J Dermatolog Treat 2018; 45(6): 710–714. [DOI] [PubMed] [Google Scholar]
- 4. Krueger JG, Wharton KA, Schlitt T, Suprun M. IL‐17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol 2019; 144: 750–763. [DOI] [PubMed] [Google Scholar]
- 5. Kolbinger F, Loesche C, Valentin M et al. β‐Defensin 2 is a responsive biomarker of IL‐17A – driven skin pathology in patients with psoriasis. J Allergy Clin Immunol 2017; 139(3): 923–932.e8 [DOI] [PubMed] [Google Scholar]
- 6. Dou J, Zhang L, Xie X et al. Integrative analyses reveal biological pathways and key genes in psoriasis. Br J Dermatol 2017; 177: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 7. Malakouti M, Brown GE, Wang E, Koo J, Ethan C. The role of IL‐17 in psoriasis. J Dermatolog Treat 2015; 26(1): 41–44. [DOI] [PubMed] [Google Scholar]
- 8. Nakajima H, Serada S, Fujimoto M, Naka T, Sano S. Leucine‐rich α‐2 glycoprotein is an innovative biomarker for psoriasis. J Dermatol Sci 2017; 86(2): 170–174. [DOI] [PubMed] [Google Scholar]
- 9. Honma M, Shibuya T, Iinuma S, Ishida‐Yamamoto A. Serum fatty acid‐binding protein 4 level is inversely correlated with serum thymus and activation‐regulated chemokine level in psoriatic patients achieving clear skin by biologics. J Dermatol 2018; 46(4): e116–e117. [DOI] [PubMed] [Google Scholar]
- 10. Ortiz A, Yamauchi PS. A treatment strategy for psoriasis: transitioning from systemic therapy to biologic agents. Skinmed 2006; 5(6): 285–288. [DOI] [PubMed] [Google Scholar]
- 11. Ohtsuki M, Morita A, Igarashi A et al. Secukinumab improves psoriasis symptoms in patients with inadequate response to cyclosporine A: A prospective study to evaluate direct switch. J Dermatol 2017; 44(4): 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meso Scale Discovery . MSD MULTI‐SPOT assay system package insert (K15047G). Available from URL: www.mesoscale.com. 2019. p. 1–34.
- 13. Meso Scale Discovery . MSD MULTI‐SPOT assay system package insert (K15050G). Vol. 1, Available from URL: www.mesoscale.com. 2019. p. 1–34. [Google Scholar]
- 14. Meso Scale Discovery . MSD MULTI‐SPOT assay system package insert (K15049G). Vol. 1, Available from URL: www.mesoscale.com. 2019. p. 1–34. [Google Scholar]
- 15. Bruin G, Loesche C, Nyirady J, Sander O. Population pharmacokinetic modeling of secukinumab in patients with moderate to severepPsoriasis. J Clin Pharmacol 2017; 57(7): 876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krueger J, Fretzin S, Suarez‐Farinas M et al. IL‐17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol 2012; 130(7): 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takahashi H, Tsuji H, Hashimoto Y, Iizuka H. Serum cytokines and growth factor levels in Japanese patients with psoriasis. Clin Exp Dermatol 2009; 35: 645–649. [DOI] [PubMed] [Google Scholar]
- 18. Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF‐α, IFN‐γ, IL‐6, IL‐8, IL‐12, IL‐17, and IL‐18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm 2005; 2005(5): 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schroder J, Harder È. Human beta‐defensin‐2. Int J Biochem Cell Biol 1999; 31: 645–651. [DOI] [PubMed] [Google Scholar]
- 20. Ong PY, Ohtake T, Brandt C et al. Endogenous antimicrobial peptides and skin infections in a atopic dermatitis. N Engl J Med 2002; 347(15): 1151–1160. [DOI] [PubMed] [Google Scholar]
- 21. Tewary P, De Rosa G, Sharma N et al. β ‐Defensin 2 and 3 promote the uptake of self or CpG DNA, enhance IFN‐α production by human plasmacytoid dendritic cells, and promote inflammation. J Immunol 2013; 191(2): 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nestle FO, Conrad C, Tun‐kyi A et al. Plasmacytoid predendritic cells initiate psoriasis through interferon‐ production. J Exp Med 2005; 202(1): 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nograles K, Zaba L, Guttman E et al. Th17 cytokines interleukin (IL)‐17 and IL‐22 modulate distinct inflammatory and keratinocyte‐response pathways. Br J Dermatol 2008; 159(5): 1092–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muromoto R, Hirao T, Tawa K et al. IL‐17A plays a central role in the expression of psoriasis signature genes through induction of IκB‐ζ in keratinocytes. Int Immunol 2016; 28(9): 443–452. [DOI] [PubMed] [Google Scholar]


