Abstract
Background
The choice of treatment in laryngeal cancer is mainly based on tumor stage, post‐treatment morbidity and quality of life. Biological tumor markers might also be of potential clinical relevance.
Objective of the review
The aim was to systematically review the value of published biological tumor markers to predict local control in laryngeal cancer patients treated with definitive radiotherapy.
Type of Review
Systematic review.
Search strategy
PubMed, Embase, Cochrane Library.
Evaluation Method
A literature search was performed using multiple terms for laryngeal cancer, radiotherapy, biological markers, detection methods and local control or survival. Studies regarding the relation between biological tumor markers and local control or survival in laryngeal cancer patients primarily treated with radiotherapy were included. Markers were clustered on biological function. Quality of all studies was assessed. Study selection, data extraction and quality assessment was performed by two independent reviewers.
Results
A total of 52 studies out of 618 manuscripts, concerning 118 markers, were included. EGFR and P53 showed consistent evidence for not being predictive of local control after primary radiotherapy, whereas proliferation markers (ie high Ki‐67 expression) showed some, but no consistent, evidence for being predictive of better local control. Other clusters of markers (markers involved in angiogenesis and hypoxia, apoptosis markers, cell cycle, COX‐2 and DNA characteristics) showed no consistent evidence towards being predictors of local control after primary radiotherapy.
Conclusions
Cell proliferation could be of potential interest for predicting local control after primary radiotherapy in laryngeal cancer patients, whereas EGFR and p53 are not predictive in contrast to some previous analyses. Large diversity in research methods is found between studies, which results in contradictory outcomes. Future studies need to be more standardised and well described according to the REMARK criteria in order to have better insight into which biomarkers can be used as predictors of local control after primary radiotherapy.
Keywords: biomarkers, cell Proliferation, laryngeal Neoplasms, prognosis, radiotherapy, treatment outcome
Key points.
This systematic review presents an overview of the value of all published biological tumor markers to predict local control in laryngeal cancer patients treated with definitive radiotherapy.
The data show that cell proliferation could be of potential interest for predicting local control in laryngeal cancer patients treated with primary radiotherapy.
EGFR and p53 are not predictive of local control after primary radiotherapy in laryngeal cancer patients.
1. INTRODUCTION
Worldwide, laryngeal cancer is diagnosed in 157 000 patients and is responsible for 83 000 deaths each year. 1 Treatment of laryngeal cancer is nowadays highly focused on laryngeal preservation, retaining the main functions of the of the larynx, speech, breathing and swallowing, with primary radiotherapy as one of the most important treatment modalities. Despite relatively early diagnosis and efforts to improve treatments, overall survival rates have not significantly improved over the last 30 years. 2 Currently, the choice of treatment in laryngeal cancer is mainly based on tumor stage, post‐treatment morbidity and quality of life. 3 Besides these clinicopathological and patient factors, biological tumor markers might be of potential clinical relevance. The aim of this systematic review was to identify predictive biological tumor markers that are relevant for the outcome of primary radiotherapy in laryngeal cancer. The main endpoint for outcome after primary radiotherapy was local control. Most common definitions for local control were as follows: time to local disease‐free survival and presence of local recurrence within two years.
2. METHODS
2.1. Search strategy
A literature search was performed in PubMed, Embase, and in the Cochrane Library on 19 July 2018, to identify studies on predictive biological tumor markers in laryngeal cancer patients primarily treated by radiotherapy. The search was updated on 23 May 2019. An extensive search was performed using keywords as well as free search terms on the following items: a) laryngeal cancer; b) radiotherapy or chemoradiation; c) biological tumor markers or common detection methods used in prognostic marker studies; and d) prognosis or response. The final search strategy used is shown in Table S1.
2.2. Selection criteria
A title, abstract and finally manuscript selection was performed, using the following exclusion criteria: a) non‐English articles; b) tumor site other than larynx; c) treatment modalities other than primary radiotherapy; d) markers not studied in primary tumor tissue, for example serum tumor markers; e) no biological tumor marker studied; f) outcome other than radiotherapy response or locoregional recurrence reported; g) cell lines or xenograft models; and h) non‐original research articles, for example reviews, case reports. Title, abstract and manuscript selection, as well as data extraction and quality assessment, was performed by two independent researchers (MGN and EAK).
2.3. Data extraction
From the papers included in this systematic review, the following data were extracted and recorded in a predefined database: a) year of publication; b) number of patients; c) retrospective or prospective patient selection; d) patient's characteristics: age, gender, stage and location; e) details on radiotherapy; f) details on the assay used: assay method, cut‐offs, number of positive and negative tumors; and g) results of the analysis to evaluate the relation between the marker and outcome of radiotherapy. This could be either results of survival analysis for local disease‐free survival (log‐rank analysis and Cox regression) or, in case of matched‐control study results of the chi‐square test, logistic regression analysis, t‐test or Mann‐Whitney U test. Biological tumor markers were clustered on biological function.
2.4. Study quality
Study quality assessment was based on the REMARK (REporting recommendations for tumor MARKer prognostic studies) criteria from McShane et al 4 A comparable quality measurement form was used previously in a systematic review. 5 In short, the following items were explored: 1) four out of six of the following patient and tumor characteristics were described: age, gender, tumor location, T‐stage, N‐stage and differentiation grade; 2) the study reported inclusion and exclusion criteria; 3) the radiotherapy treatment schedule was well described; 4) definition of the study endpoint was given; 5) the relation between marker and outcome was sufficiently described; 6) a rational for number of patient tested was given; 7.1) the assay used to measure biomarker expression was sufficiently described, 7.2) as well as interpretation of the assay; 8) the follow‐up time of patients in the study was described; 9) the study reported how many patients were available for statistical analysis; and 10) limitations of the study were described. A maximum of 11 points could be allocated to an article. To compare quality scores, the Mann‐Whitney U test was used.
2.5. Data analysis
Results of the analysis describing the relation between a marker and local control after radiotherapy were compared. P‐values of <.05 were considered statistically significant. P‐values of <.10 were considered as a trend (explicitly stated).
A biological tumor marker was considered as a potential marker for local control if >50% of the studies investigating the marker described a relation with either poor or good local control after radiotherapy. In case the study results were sufficiently described in the manuscript, but only a P‐value was presented, the Odds ratio (OR) and 95% confidence interval (CI) were calculated (explicitly stated). 6 This enabled us to compare results of various studies reporting on the same biological tumor marker better. Also, to uniformly present the data, “positive” expression is used as indicator; in case a manuscript presented the results with “negative” expression as indicator, we inverted the OR or Hazard Ratio (HR) and 95%CI.
2.6. Data availability statement
The data that support the findings of this study are available from the corresponding author upon request.
3. RESULTS
In total, 400 (English n = 359) manuscripts were identified in PubMed and 435 (English n = 427) in Embase. No Cochrane review was available. After removal of duplicate publications, 651 were further analysed. Figure 1 shows a flow chart of selection process. Finally, 52 studies describing 118 markers could be included. Remarkably, all of the included studies were identified in PubMed, and no additional were found in Embase.
Figure 1.
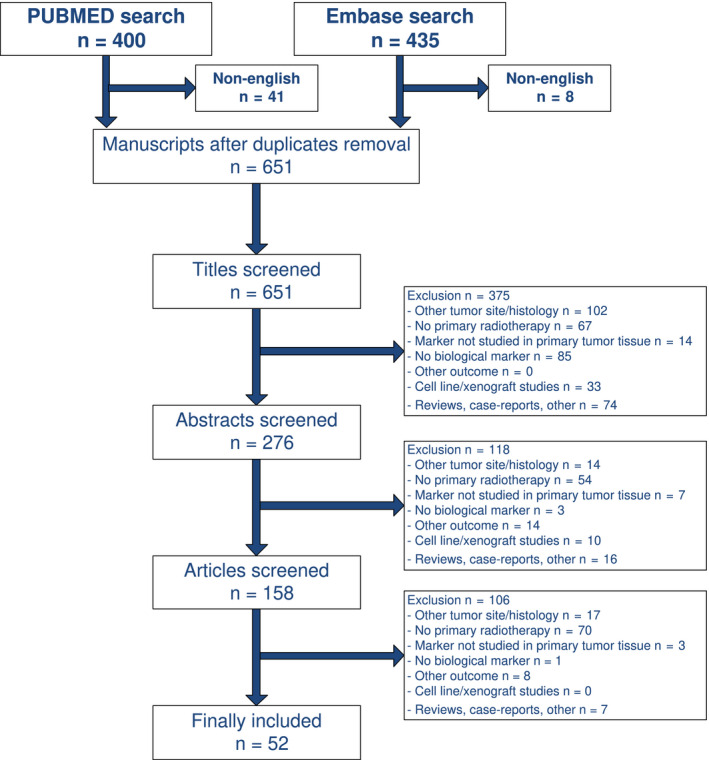
Flow chart of selection process
3.1. Study characteristics and study quality
In total, 52 studies concerning 118 biological tumor markers were included. Studies had been published between 1987 and 2017 (median 2007). A maximum of 13 markers was investigated by one study. The median number of patients per study included was 60 (range 21‐281). The median quality score was 6 out of 11 (range 3‐10). A low number of studies reported inclusion and exclusion criteria, and none of the studies gave a rational for the number of patients analysed (power analysis). This is due to the retrospective design of most studies. Patient characteristics and details of the assay used were generally described well (50 of 52 studies). Studies published from 2007 (median 7, range 4‐10) were of better quality than studies published before 2007 (median 6, range 3‐8) (P = .03).
3.2. Proliferation
Ki‐67, a nuclear protein involved in cell proliferation, was described eleven times (Table 1). In five, high Ki‐67 proliferation index was related to better local control after radiotherapy (two out of five showed a trend, studies with low number of patients), either in univariate or multivariate analysis. 7 , 8 , 9 , 10 , 11 In four, no relation with local control was found. 12 , 13 , 14 , 15 Also, in two other papers an inverse correlation was found, high Ki‐67 expression being a predictor for poor local control. 16 , 17 Interestingly, in the paper of Sakata et al the association with local recurrence was only found in patients treated by accelerated radiotherapy, not in the group treated by conventional radiotherapy. 17 Another marker for proliferation is Proliferating Cell Nuclear Antigen (PCNA). Munck‐Wikland et al showed that non‐recurrent tumors had higher levels of PCNA expression (Table 1), 18 again indicating that high proliferation contributes to good outcome after radiotherapy.
Table 1.
Proliferation markers in relation to outcome after primary radiotherapy in laryngeal cancer patients
|
Author Year |
Marker | Method | Cut‐off | N | Stage | Side | Treatment | Outcome, definition |
Univariate HR/OR (95% CI) |
Multivariate HR/OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kwon 2015 12 | Ki‐67 | IHC | >50% | 42 | T1‐2 | Larynx | RT | Residual tumor < 0.5 yrs | = 2.16 (0.40‐11.80) | |
| Rademakers 2015 13 | Ki‐67 | IHC | >10% nuclear | 128 | T2‐4N0‐+ | Larynx | ART | Time to local recurrence | = | |
| Nichols 2012 16 | Ki‐67 | IHC | >10% nuclear | 75 | T1‐2 | Glottic | RT | Time to local recurrence | ↓ 3.37 (1.14‐9.86) | ↓ 4.86 (1.58‐15.00) |
| Wildeman 2009 14 | Ki‐67 | IHC‐TMA | OR per 10% increase, nuclear | 59 | T1‐3N0‐3 | Larynx | RT | Local recurrence < 2 yrs | = 0.71 (0.44‐1.15) | |
| Rafferty 2008 7 | Ki‐67 | IHC | >50% Continuous, nuclear | 50 | T2N0 | Larynx | RT | Local recurrence |
↑ = |
= |
| Ahmed 2008 8 | Ki‐67 | IHC |
>10% nuclear Continuous, nuclear |
24 | T1‐2 | Glottic | RT | Persistence or local recurrence |
↑ 0 (0‐∞)* (trend) ↑ |
|
| Cho 2004 10 | Ki‐67 | IHC‐TMA | >10% nuclear | 123 | T1‐2N0 | Larynx | RT | Time to local recurrence | = 0.47 (0.18 −1.23)* | ↑ 0.17 (0.06‐0.49)* |
| Condon 2002 15 | Ki‐67 | IHC | >20% nuclear | 21 | T1‐2N0 | Glottic | RT | Local recurrence < 1 yrs | = 1.94 (0.32‐11.8)* | |
| Motamed 2001 9 | Ki‐67 | IHC | Continuous, nuclear | 28 | T1aN0 | Glottic | n.s. | Radioresistance, n.s. | ↑ (trend) | |
| Sakata 2000 17 | Ki‐67 | IHC* | ≥50% |
130 51 79 |
T1‐2N0 | Glottic |
RT/ART RT ART |
Local recurrence |
↓ 2.66 (1.17‐6.08)* = 1.32 (0.40‐4.38)* ↓ 5.11 (1.53‐17.04)* |
|
| Kropveld 1998 11 | Ki‐67 | IHC | Continuous | 36 | T2N0‐2 | Larynx | RT | Locoregional recurrence | ↑ | |
| Munck 1993 18 | PCNA | IHC | Continuous, nuclear | 28 | T1N0 | Glottic | RT | Recurrent or persistent, n.s. | ↑ |
*, (Subgroup) analysis performed by authors of this systematic review; “=”, No relation with outcome; ↑, Positivity of marker associated with good outcome; ↓, Positivity of marker associated with poor outcome; ART, accelerated radiotherapy; CI, confidence interval; HR, hazard ratio; IHC, immunohistochemistry; n.s., not specified; OR, odds ratio; RT, conventional radiotherapy; TMA, tissue microarray; yrs, years.
3.3. Angiogenesis and hypoxia
Eight studies investigating five angiogenesis or hypoxia associated markers were included (Table S2). Hypoxia‐inducible factor 1α (HIF‐1α) was found to be a predictor of poor outcome after radiotherapy in two out of five studies. 12 , 14 , 19 , 20 , 21 Hypoxia also induces CA‐IX expression in tumor cells. Two out of six studies investigating CA‐IX showed that positive expression predicted poor local control after radiotherapy. 12 , 14 , 19 , 20 , 21 , 22 Glucose transporter 1 (Glut‐1), osteopontin (OPN) and vascular endothelial growth factor (VEGF) were not associated with outcome after radiotherapy. 12 , 19 , 21 , 23 , 24 Finally, a 26‐gene hypoxia signature was not associated with local control. 25
3.4. Apoptosis
Apoptotic marker BCL‐2 was studied most extensively and expression was associated with local recurrence in two out of seven studies. 14 , 15 , 16 , 20 , 26 , 27 , 28 Bxl‐xL showed a relation with poor local control in one study, 14 , 27 while Bax was associated with favourable outcome in one out of three studies. 15 , 27 , 28 Bak, Survivin and BAG‐1 were studied once, and only BAG‐1 showed a trend towards poor local control. 27 , 29 (Table S3).
3.5. P53
The human tumor suppressor gene p53 was investigated extensively; fourteen studies found no relation with local control. 8 , 9 , 10 , 11 , 14 , 15 , 23 , 28 , 30 , 31 , 32 , 33 , 34 , 35 Only Narayana et al found poor local control in glottic carcinomas 36 (Table S4). Overall suggesting p53 not as a relevant marker for local control after radiotherapy.
3.6. Cell cycle
FADD was not associated with local control, whereas phosphorylated FADD only showed a relation with better local control in glottic tumors, but not in supraglottic tumors. 37 , 38 Cyclin D1 showed some contradictory results; three out of five studies did not show a relation, while Yoo et al found cyclin D1 to be a predictor for better local control and Chang et al for poor local control after radiotherapy. 7 , 14 , 37 , 39 , 40 P16, P21 (both three studies) and p27 showed no relation with local control, as well as retinoblastoma gene (Rb) 14 , 15 , 30 , 32 , 41 (Table S5).
3.7. COX‐2
Cyclooxygenase‐2 (COX‐2) was described four times (Table S6), two studies showed a relation between positive expression and poor local control, in either univariate of multivariate analysis, 10 , 42 whereas two others did not. 12 , 14
3.8. EGFR
The epidermal growth factor receptor (EGFR) is a well‐known and extensively studied biological tumor marker. Protein expression of EGFR in relation to local control after radiotherapy in laryngeal cancer patients has been studied in ten independent studies (Table S7). 7 , 8 , 14 , 16 , 23 , 26 , 39 , 43 , 44 , 45 Only Miyaguchi et al found a relation between positivity of EGFR and recurrence, 45 thereby concluding EGFR is not a relevant marker.
3.9. DNA content
Seven, mostly older studies (6 of 7 ≤1995) investigated DNA ploidy and compared diploid with aneuploid tumors. Three found no relation with local control, while Toffoli et al found that diploid tumors showed poor local control and three others found a relation with better local control after radiotherapy (Table S8). 18 , 46 , 47 , 48 , 49 , 50 , 51
3.10. Genome‐wide expression profiling
Instead of analysing individual, mostly well‐known and frequently investigated biological tumor markers, it is also possible to perform genome wide expression profiling for example by using mRNA expression microarrays. One study using gene expression data to discover markers for radiotherapy outcome in laryngeal cancer was identified. De Jong et al studied 52 laryngeal cancer patients with or without a local recurrence after radiotherapy. 52 Expression of the stem cell‐associated marker CD44 showed a relation with local recurrence (HR 20.2, 95%CI 3.4‐172.3), and this was confirmed by immunohistochemistry for CD44 in an independent set of 76 laryngeal cancer patients (HR 15.2, P < .01). However, gene sets defining hypoxia, radiosensitivity or proliferation did not correlate with local recurrence.
3.11. Micro‐RNAs
Micro‐RNAs were investigated by two (Table S9). Maia et al found miR‐296‐5p associated with poor locoregional control and de Jong et al found miR‐203 as predictor of good local control after radiotherapy. 53 , 54
3.12. Miscellaneous biological tumor markers
An overview of the predictive value of other markers is given in Table S10. All of these markers were studied once. ATM, LOH at 9p21, LOH at 3p21, BCCIP, Cortactin, ERCC1 and EPOR were not predictive of outcome,, 12 , 15 , 21 , 28 , 31 , 55 whereas EpCAM/BerEP4, IGF‐1R, HDAC1, NF‐κB, β1 integrin and TGF‐α were associated with poor local control after radiotherapy. 26 , 30 , 44 , 56 , 57 , 58
4. DISCUSSION
4.1. Summary of main results
In this systematic review, a comprehensive overview of studies reporting on biological tumor markers in relation to local control after primary radiotherapy in laryngeal cancer patients is presented. None of the investigated markers or clusters of markers showed consistent evidence for a positive or negative relation with local control. There is some evidence for Ki‐67/proliferation towards better local control (Table 1). Interestingly, two well‐known markers in HNSCC, EGFR and P53, showed consistent evidence for not being predictive of local control after primary radiotherapy.
4.2. Overall completeness, quality and applicability of evidence
An extensive search has been performed to identify all published markers for local control after radiotherapy in laryngeal cancer. As found in this systematic review study quality varies among studies, almost all studies are retrospective cohorts, some do not report details on radiotherapy schedule, or outcome chosen, while others inadequately describe the interpretation of the assay used for detection of the biological tumor marker. In 2005, the REMARK criteria were published to encourage transparent and complete reporting in prognostic marker studies. 4 However, also after introduction of REMARK, prognostic biological marker studies are still poorly reported. 59 Remarkably, studies included in this systematic review did have a slightly better quality after 2006 than before. Adequate reporting is essential for drawing conclusions from a systematic review and should be emphasised for new publications.
Despite the fact that this systematic review included studies on relatively homogeneous patient populations (eg only laryngeal cancer; only patients treated with primary radiotherapy), it was still difficult to compare studies analysing the same biological tumor marker, because of multiple factors. First, most studies used small, heterogeneous patient populations. Some studies include both glottic and supraglottic tumors, others evaluate those separately, with sometimes different outcomes. For example, pFADD positivity was associated with better local control in glottic, but not in supraglottic laryngeal cancer patients. 37 , 38 Glottic and supraglottic tumors often present with different symptoms, at different stages. 60 , 61 , 62 Therefore, supra‐ and glottic tumors might be regarded as distinct entities. Although we only included primary radiotherapeutically treated patients, different treatment schedules (conventional vs. accelerated) could have influenced the results, as for Ki‐67 and EGFR. Furthermore, the number of patients is low (median number of patients is 60) and a rationale for the number of included patients is not given. There are differences in methodology, such as different antibodies used, different cut‐offs used for a marker, often without giving a rationale for the cut‐off used. Finally, different definitions of outcome after radiotherapy are used such a local recurrence within 6 months while others use local recurrence within 5 years, where can be discussed if a local recurrence after 5 years should be considered as a recurrence or as a second primary tumor.
4.3. Potential biases in review
The most important potential bias in this review is publication bias, especially research with negative results might have not been published.
4.4. Comparison with other reviews
Also for other tumor sides, there is no consistent evidence for the predictive value of Ki‐67 for radiation response, though Ki‐67 positivity is likely to be a poor prognostic factor for breast and cervical cancer, irrespectively of treatment modality. 63 , 64
A review by Bossi et al, shows the same results for EGFR in other HNSCC tumor sites. 65 However, a relation with EGFR positivity and poor outcome after primary radiotherapy was found in some large groups of HNSCC patient treated with an accelerated radiotherapy schedule. 65
Previous p53 research showed p53 mutations as predictor of poor local control after primary radiotherapy in a variety of tumor sites, including HNSCC. 66 A potential reason that we did not find a relation might be that in the studies included in our analysis, p53 was mostly studied by immunohistochemistry, not by mutational analysis. Research has shown that immunohistochemical staining is not always strongly correlated with mutational status, but optimisation, standardisation and validation of p53 immunohistochemistry could reliably predict p53 mutations. 67
5. IMPLICATIONS FOR CLINICAL PRACTICE AND RESEARCH
Biological tumor markers that are strongly predictive of response to primary radiotherapy could be helpful in selecting patients who are in need for additional (or intensified) treatment or alternative treatment modalities such as surgery. Biological tumor markers could also contribute to identify new potential targets for therapeutic intervention. We found some evidence for Ki‐67/proliferation being predictive of better local control after primary radiotherapy, whereas EGFR and P53 showed consistent evidence for not being predictive of local control after primary radiotherapy. Cell proliferation is one of the most essential biological processes in oncogenesis. 68 Ki‐67 is present in all the active phases of the cell cycle (G1, G2, M and mitosis), but absent in resting cells (G0), 69 suggesting a role for Ki‐67 as a potential predictor of outcome after radiotherapy, as radiotherapy affects dividing cells. However, the predictive value of Ki‐67 for response to primary radiotherapy in laryngeal cancer is currently unclear and Ki‐67 assessment methods lack standardisation, thereby preventing clinical implementation. More research in well‐defined study populations is needed to elucidate the potential predictive role of Ki‐67 in outcome after primary radiotherapy and thereby potential application.
ETHICAL CONSIDERATIONS
For this systematic review, there are no potential conflicts of interest to declare. For writing this systematic review, no Institutional Review Board approval was needed. Study selection, data extraction and quality assessment was performed by two independent reviewers and attention was paid to not include duplicate published data.
Supporting information
Supplementary Material
Noordhuis MG, Kop EA, van der Vegt B, et al. Biological tumor markers associated with local control after primary radiotherapy in laryngeal cancer: A systematic review. Clin Otolaryngol. 2020;45:486–494. 10.1111/coa.13540
Noordhuis and Kop contributed equally.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;5:359. [DOI] [PubMed] [Google Scholar]
- 2. Rachet B, Quinn MJ, Cooper N, Coleman MP. Survival from cancer of the larynx in England and Wales up to 2001. Br J Cancer. 2008;99(S1):S35–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Back G, Sood S. The management of early laryngeal cancer: options for patients and therapists. Curr Opin Otolaryngol Head Neck Surg. 2005;2:85‐91. [DOI] [PubMed] [Google Scholar]
- 4. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM and Statistics Subcommittee of the NCI‐EORTC Working Group on Cancer Diagnostics . REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;4:387‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noordhuis MG, Eijsink JJ, Roossink F, et al. Prognostic cell biological markers in cervical cancer patients primarily treated with (chemo)radiation: a systematic review. Int J Radiat Oncol Biol Phys. 2011;2:325‐334. [DOI] [PubMed] [Google Scholar]
- 6. Altman DG. Practical statistics for medical research. London: Chapman & Hall; 1991. [Google Scholar]
- 7. Rafferty M, Helliwell TR, Husband DJ, Fenton J, Jones TM, Jones AS. Expression of cell cycle associated proteins influences radiocurability of T2N0 squamous cell carcinoma of the larynx. Oral Oncol. 2008;10:975‐981. [DOI] [PubMed] [Google Scholar]
- 8. Ahmed WA, Suzuki K, Imaeda Y, Horibe Y. Ki‐67, p53 and epidermal growth factor receptor expression in early glottic cancer involving the anterior commissure treated with radiotherapy. Auris Nasus Larynx. 2008;2:213‐219. [DOI] [PubMed] [Google Scholar]
- 9. Motamed M, Banerjee AR, Bradley PJ, Powe D. MIB‐1 and p53 expression in radiotherapy‐resistant T1aN0M0 glottic squamous cell carcinoma. Clin Otolaryngol Allied Sci. 2001;3:227‐230. [DOI] [PubMed] [Google Scholar]
- 10. Cho EI, Kowalski DP, Sasaki CT, Haffty BG. Tissue microarray analysis reveals prognostic significance of COX‐2 expression for local relapse in T1–2N0 larynx cancer treated with primary radiation therapy. Laryngoscope. 2004;114(11):2001–2008. [DOI] [PubMed] [Google Scholar]
- 11. Kropveld A, Slootweg PJ, Blankenstein MA, Terhaard CH, Hordijk GJ. Ki‐67 and p53 in T2 laryngeal cancer. Laryngoscope. 1998;10:1548‐1552. [DOI] [PubMed] [Google Scholar]
- 12. Kwon OJ, Park JJ, Ko GH, et al. HIF‐1α and CA‐IX as predictors of locoregional control for determining the optimal treatment modality for early‐stage laryngeal carcinoma. Head Neck. 2015;4:505‐510. [DOI] [PubMed] [Google Scholar]
- 13. Rademakers SE, Hoogsteen IJ, Rijken PF, et al. Prognostic value of the proliferation marker Ki‐67 in laryngeal carcinoma: Results of the Accelerated Radiotherapy with Carbogen Breathing and Nicotinamide phase III randomized trial. Head Neck. 2015;2:171‐176. [DOI] [PubMed] [Google Scholar]
- 14. Wildeman MAM, Gibcus JH, Hauptmann M, et al. Radiotherapy in laryngeal carcinoma: Can a panel of 13 markers predict response? Laryngoscope. 2009;2:316‐322. [DOI] [PubMed] [Google Scholar]
- 15. Condon LT, Ashman JN, Ell SR, Stafford ND, Greenman J, Cawkwell L. Overexpression of Bcl‐2 in squamous cell carcinoma of the larynx: a marker of radioresistance. Int J Cancer. 2002;4:472‐475. [DOI] [PubMed] [Google Scholar]
- 16. Nichols AC, Whelan F, Basmaji J, et al. Ki‐67 expression predicts radiotherapy failure in early glottic cancer. J Otolaryngol Head Neck Surg. 2012;2:124‐130. [PubMed] [Google Scholar]
- 17. Sakata K, Oouchi A, Nagakura H, et al. Accelerated radiotherapy for T1, 2 glottic carcinoma: Analysis of results with KI‐67 index. Int J Radiat Oncol Biol Phys. 2000;1:81‐88. [DOI] [PubMed] [Google Scholar]
- 18. Munck‐Wikland E, Fernberg J, Kuylenstierna R, Lindholm J, Auer G. Proliferating cell nuclear antigen (PCNA) expression and nuclear DNA content in predicting recurrence after radiotherapy of early glottic cancer. EUR. J Cancer Part B Oral Oncol. 1993;1:75‐79. [DOI] [PubMed] [Google Scholar]
- 19. Wachters JE, Schrijvers M, Slagter‐Menkema L, et al. Prognostic value of hypoxiamarkers HIF‐1a, CA IX and OPN in T1–T2 laryngeal carcinomas treated with primarily RT. Radiother. 2011;Oncol:S42. [DOI] [PubMed] [Google Scholar]
- 20. Douglas CM, Bernstein JM, Ormston VE, et al. Lack of prognostic effect of carbonic anhydrase‐9, hypoxia inducible factor‐1α and Bcl‐2 in 286 patients with early squamous cell carcinoma of the Glottic larynx treated with radiotherapy. Clin Oncol. 2013;1:59‐65. [DOI] [PubMed] [Google Scholar]
- 21. Schrijvers ML, van der Laan BF, de Bock GH, et al. Overexpression of intrinsic hypoxia markers HIF1alpha and CA‐IX predict for local recurrence in stage T1–T2 glottic laryngeal carcinoma treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2008;1:161‐169. [DOI] [PubMed] [Google Scholar]
- 22. Rademakers SE, Hoogsteen IJ, Rijken PF, et al. Pattern of CAIX expression is prognostic for outcome and predicts response to ARCON in patients with laryngeal cancer treated in a phase III randomized trial. Radiother Oncol. 2013;3:517‐522. [DOI] [PubMed] [Google Scholar]
- 23. Parikh RR, Yang Q, Haffty BG. Prognostic significance of vascular endothelial growth factor protein levels in T1–2 NO laryngeal cancer treated with primary radiation therapy. Cancer. 2007;3:566‐573. [DOI] [PubMed] [Google Scholar]
- 24. Homer JJ, Greenman J, Stafford ND. The expression of vascular endothelial growth factor (VEGF) and VEGF‐C in early laryngeal cancer: relationship with radioresistance. Clin Otolaryngol Allied Sci. 2001;6:498‐504. [DOI] [PubMed] [Google Scholar]
- 25. Eustace A, Mani N, Span PN, et al. A 26‐gene hypoxia signature predicts benefit from hypoxia‐modifying therapy in laryngeal cancer but not bladder cancer. Clin Cancer Res. 2013;17:4879‐4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoshida K, Sasaki R, Nishimura H, et al. Nuclear factor‐κB expression as a novel marker of radioresistance in early‐stage laryngeal cancer. Head Neck. 2010;5:646‐655. [DOI] [PubMed] [Google Scholar]
- 27. Nix P, Cawkwell L, Patmore H, Greenman J, Stafford N. Bcl‐2 expression predicts radiotherapy failure in laryngeal cancer. Br J Cancer. 2005;12:2185‐2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ogawa T, Shiga K, Tateda M, et al. Protein expression of p53 and Bcl‐2 has a strong correlation with radiation resistance of laryngeal squamous cell carcinoma but does not predict the radiation failure before treatment. Oncol Rep. 2003;5:1461‐1466. [PubMed] [Google Scholar]
- 29. Yamauchi H, Adachi M, Sakata K, et al. Nuclear BAG‐1 localization and the risk of recurrence after radiation therapy in laryngeal carcinomas. Cancer Lett. 2001;1:103‐110. [DOI] [PubMed] [Google Scholar]
- 30. Murakami N, Mori T, Yoshimoto S, et al. Expression of EpCAM and prognosis in early‐stage glottic cancer treated by radiotherapy. Laryngoscope. 2014;11:E436. [DOI] [PubMed] [Google Scholar]
- 31. Rewari A, Lu H, Parikh R, Yang Q, Shen Z, Haffty BG. BCCIP as a prognostic marker for radiotherapy of laryngeal cancer. Radiother Oncol. 2009;2:183‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saunders ME, MacKenzie R, Shipman R, Fransen E, Gilbert R, Jordan RC. Patterns of p53 gene mutations in head and neck cancer: full‐length gene sequencing and results of primary radiotherapy. Clin Cancer Res. 1999;9:2455‐2463. [PubMed] [Google Scholar]
- 33. Pai HH, Rochon L, Clark B, Black M, Shenouda G. Overexpression of p53 protein does not predict local‐regional control or survival in patients with early‐stage squamous cell carcinoma of the glottic larynx treated with radiotherapy. Int J Radiat Oncol Biol Phys. 1998;1:37‐42. [DOI] [PubMed] [Google Scholar]
- 34. Tan LK, Ogden GR. P53 Over‐Expression in Laryngeal Carcinoma is Not Predictive of Response to Radiotherapy. Oral Oncol. 1997;3:177‐181. [DOI] [PubMed] [Google Scholar]
- 35. Kokoska MS, Piccirillo JF, El‐Mofty SK, Emami B, Haughey BH, Scholnick SB. Prognostic significance of clinical factors and p53 expression in patients with glottic carcinoma treated with radiation therapy. Cancer. 1996;8:1693‐1700. [PubMed] [Google Scholar]
- 36. Narayana A, Vaughan ATM, Kathuria S, Fisher SG, Walter SA, Reddy SP. P53 overexpression is associated with bulky tumor and poor local control in T1 glottic cancer. Int J Radiat Oncol Biol Phys. 2000;1:21‐26. [DOI] [PubMed] [Google Scholar]
- 37. Schrijvers ML, Pattje WJ, Slagter‐Menkema L, et al. FADD expression as a prognosticator in early‐stage glottic squamous cell carcinoma of the larynx treated primarily with radiotherapy. Int J Radiat Oncol Biol Phys. 2012;4:1220‐1226. [DOI] [PubMed] [Google Scholar]
- 38. Wachters JE, Schrijvers ML, Slagter‐Menkema L, et al. Phosphorylated FADD is not prognostic for local control in T1–T2 supraglottic laryngeal carcinoma treated with radiotherapy. Laryngoscope. 2017;9:E307. [DOI] [PubMed] [Google Scholar]
- 39. Chang AR, Wu HG, Park CI, Jun YK, Kim CW. Expression of epidermal growth factor receptor and cyclin D1 in pretreatment biopsies as a predictive factor of radiotherapy efficacy in early glottic cancer. Head Neck. 2008;7:852‐857. [DOI] [PubMed] [Google Scholar]
- 40. Yoo SS, Carter D, Turner BC, et al. Prognostic significance of cyclin D1 protein levels in early‐stage larynx cancer treated with primary radiation. Int J Cancer. 2000;1:22‐28. [DOI] [PubMed] [Google Scholar]
- 41. Rafferty M, Walker C, Husband D, Helliwell T, Fenton J, Jones A. Retinoblastoma gene abnormalities in early laryngeal cancer. Eur Arch Oto‐Rhino‐Laryngol SUPPL. 2008;1(S83):S87. [DOI] [PubMed] [Google Scholar]
- 42. Nix P, Lind M, Greenman J, Stafford N, Cawkwell L. Expression of Cox‐2 protein in radioresistant laryngeal cancer. Ann Oncol. 2004;5:797‐801. [DOI] [PubMed] [Google Scholar]
- 43. Nijkamp MM, Span PN, Terhaard CHJ, et al. Epidermal growth factor receptor expression in laryngeal cancer predicts the effect of hypoxia modification as an additive to accelerated radiotherapy in a randomised controlled trial. Eur J Cancer. 2013;15:3202‐3209. [DOI] [PubMed] [Google Scholar]
- 44. Wen Q, Miwa T, Yoshizaki T, Nagayama I, Furukawa M, Nishijima H. Prognostic value of EGFR and TGF‐α in early laryngeal cancer treated with radiotherapy. Laryngoscope. 1996;7:884‐888. [DOI] [PubMed] [Google Scholar]
- 45. Miyaguchi M, Olofsson J, Hellquist HB. Expression of epidermal growth factor receptor in glottic carcinoma and its relation to recurrence after radiotherapy. Clin Otolaryngol Allied Sci. 1991;5:466‐469. [DOI] [PubMed] [Google Scholar]
- 46. Ii N, Fuwa N, Ando M, Itoh Y, Nomoto Y, Takeda K. DNA ploidy analysis performed prospectively using fresh tumor samples in early glottic carcinoma treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2002;2:415‐419. [DOI] [PubMed] [Google Scholar]
- 47. Stern Y, Aronson M, Shpitzer T, et al. Significance of DNA ploidy in the treatment of T1 glottic carcinoma. Arch Otolaryngol Head Neck Surg. 1995;9:1003‐1005. [DOI] [PubMed] [Google Scholar]
- 48. Toffoli G, Franchin G, Barzan L, et al. Brief report: prognostic importance of cellular DNA content in T1–2 N0 laryngeal squamous cell carcinomas treated with radiotherapy. Laryngoscope. 1995;6:649‐652. [DOI] [PubMed] [Google Scholar]
- 49. Westerbeek HA, Mooi WJ, Hilgers FJ, Baris G, Begg AC, Balm AJ. Ploidy status and the response of T1 glottic carcinoma to radiotherapy. Clin Otolaryngol Allied Sci. 1993;2:98‐101. [DOI] [PubMed] [Google Scholar]
- 50. Walter MA, Peters GE, Peiper SC. Predicting radioresistance in early glottic squamous cell carcinoma by DNA content. Ann Otol Rhinol Laryngol. 1991;7:523‐526. [DOI] [PubMed] [Google Scholar]
- 51. Franzen G, Olofsson J, Klintenberg C, Brunk U. Prognostic value of malignancy grading and DNA measurements in small glottic carcinomas. ORL J Otorhinolaryngol Relat Spec. 1987;2:73‐80. [DOI] [PubMed] [Google Scholar]
- 52. De Jong MC, Pramana J, Van Der Wal JE, et al. CD44 expression predicts local recurrence after radiotherapy in larynx cancer. Clin Cancer Res. 2010;21:5329‐5338. [DOI] [PubMed] [Google Scholar]
- 53. Maia D, de Carvalho AC, Horst MA, Carvalho AL, Scapulatempo‐Neto C, Vettore AL. Expression of miR‐296‐5p as predictive marker for radiotherapy resistance in early‐stage laryngeal carcinoma. Journal of Translational Medicine. 2015;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Jong MC, Ten Hoeve JJ, Grenman R, et al. Pretreatment microRNA expression impacting on epithelial‐to‐mesenchymal transition predicts intrinsic radiosensitivity in head and neck cancer cell lines and patients. Clin Cancer Res. 2015;24:5630‐5638. [DOI] [PubMed] [Google Scholar]
- 55. Johung KL, Rewari A, Wu H, Contessa J, Haffty B, Decker R. Role of excision repair cross complementation 1 expression as a prognostic marker for response to radiotherapy in early stage laryngeal cancer. Int J Radiat Oncol Biol Phys. 2010;3:S664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Matsumoto F, Ohba S, Fujimaki M, Ikeda K. The value of insulin‐like growth factor‐1 receptor for predicting early glottic carcinoma response to radiotherapy. Auris Nasus Larynx. 2016;4:440‐445. [DOI] [PubMed] [Google Scholar]
- 57. Zhao R, Chen K, Cao J, Yu H, Tian L, Liu M. A correlation analysis between HDAC1 over‐expression and clinical features of laryngeal squamous cell carcinoma. Acta Otolaryngol. 2016;2:172‐176. [DOI] [PubMed] [Google Scholar]
- 58. Choi S, Cho K, Nam S, Lee S, Kang J, Kim SY. Clinical significance of β1 integrin expression as a prediction marker for radiotherapy in early glottic carcinoma. Laryngoscope. 2006;7:1228‐1231. [DOI] [PubMed] [Google Scholar]
- 59. Sekula P, Mallett S, Altman DG, Sauerbrei W. Did the reporting of prognostic studies of tumour markers improve since the introduction of REMARK guideline? A comparison of reporting in published articles. PLoS ONE. 2017;6:e0178531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brandstorp‐Boesen J, Sorum Falk R, Boysen M, Brondbo K. Impact of stage, management and recurrence on survival rates in laryngeal cancer. PLoS ONE. 2017;7:e0179371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Raitiola H, Pukander J, Laippala P. Glottic and supraglottic laryngeal carcinoma: differences in epidemiology, clinical characteristics and prognosis. Acta Otolaryngol. 1999;7:847‐851. [DOI] [PubMed] [Google Scholar]
- 62. Hirvikoski P, Virtaniemi J, Kumpulainen E, Johansson R, Kosma VM. Supraglottic and glottic carcinomas. Clinically and biologically distinct entities? Eur J Cancer. 2002;13:1717‐1723. [DOI] [PubMed] [Google Scholar]
- 63. Pan D, Wei K, Ling Y, Su S, Zhu M, Chen G. The prognostic role of Ki‐67/MIB‐1 in cervical cancer: a systematic review with meta‐analysis. Med Sci Monit. 2015;882‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Azambuja E, Cardoso F, de Castro G, et al. Ki‐67 as prognostic marker in early breast cancer: a meta‐analysis of published studies involving 12,155 patients. Br. J. Cancer. 2007;10:1504‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bossi P, Resteghini C, Paielli N, Licitra L, Pilotti S, Perrone F. Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget. 2016;45:74362‐74379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cuddihy AR, Bristow RG. The p53 protein family and radiation sensitivity: Yes or no? Cancer Metastasis Rev. 2004;3–4:237‐257. [DOI] [PubMed] [Google Scholar]
- 67. Kobel M, Piskorz AM, Lee S, et al. Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J Pathol Clin Res. 2016;4:247‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van Diest PJ, Brugal G, Baak JP. Proliferation markers in tumours: interpretation and clinical value. J. Clin. Pathol. 1998;10:716‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation‐associated human nuclear antigen defined by the monoclonal antibody Ki‐67. J. Immunol. 1984;4:1710‐1715. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


