Abstract
Cigarette smoke (CS) is a major risk factor for the development of lung cancer and chronic obstructive pulmonary disease (COPD). Epithelial-mesenchymal transition (EMT) commonly coexists in lung cancer and COPD. CS triggers many factors including matrix metalloproteinases (MMPs) production, contributing to EMT progression in the lungs. Here, how Shp2 signaling regulates the CS-induced MMP-9 production and EMT progression were investigated in mouse lungs and in pulmonary epithelial cell cultures (NCI-H292) found CS induced MMP-9 production, EMT progression (increased vimentin and α-SMA; decreased E-cadherin) and collagen deposition in lung tissues; cigarette smoke extract (CSE) induced MMP-9 production and EMT-related phenotypes in NCI-H292 cells, which were partially prevented by Shp2 KO/KD or Shp2 inhibition. The CSE exposure induced EMT phenotypes were suppressed by MMP-9 inhibition. Recombinant MMP-9 induced EMT, which was prevented by MMP-9 inhibition or Shp2 KD/inhibition. Mechanistically, CS and CSE exposure resulted in ERK1/2, JNK and Smad2/3 phosphorylation, which were suppressed by Shp2 KO/KD/inhibition. Consequentially, the CSE exposure-induced MMP-9 production and EMT progression were suppressed by ERK1/2, JNK and Smad2/3 inhibitors. Thus, CS induced MMP-9 production and EMT resulted from activation of Shp2/ERK1/2/JNK/Smad2/3 signaling pathways. Our study contributes to the underlying mechanisms of pulmonary epithelial structural changes in response to CS, which may provide novel therapeutic solutions for treating associated diseases, such as COPD and lung cancer.
Introduction
Smoking can cause lung cancer, which commonly coexists with chronic obstructive pulmonary disease (COPD) [1, 2]. The people with COPD are much more of developing lung cancer than those without COPD, and are worse treatment expectations after diagnosis and treatment. Lung cancer and COPD are closely related and may share common characteristics, such as an underlying genetic predisposition, epithelial and endothelial cell plasticity, dysfunctional inflammatory mechanisms including the deposition of excessive extracellular matrix, angiogenesis, susceptibility to DNA damage and cellular mutagenesis [2–5]. The epithelial mesenchymal transition (EMT) is a highly plastic process in which epithelial cells change into a mesenchymal phenotype, and it has been found to be associated with an invasive or metastatic phenotype during cancer progression [2–4]. The active compounds found in cigarette smoke, such as nicotine and reactive oxygen species (ROS), can induce inflammation and EMT through various signaling pathways [6–8]. Cigarette smoking induces a higher expression of vimentin and other mesenchymal markers and a decrease in E-cadherin expression, which are key indicators of EMT production [2–5]. Several key matrix metalloproteinases (MMPs) and signaling pathways such as MMP-9 production that drive an EMT are also aberrantly activated in lung cancer [2, 9, 10]. We previously showed that cigarette smoke extract (CSE) and cigarette smoke (CS) induced oxidative stress, inflammation, EMT and fibrosis in a lung cancer cell culture and in the lungs of mice through the activation of SH2 domain-containing phosphatase (Shp) 2 and Rac1 signaling pathways, respectively, which further activate MMP-9 production [11–13]. Recently, studies further demonstrated that pulmonary epithelial cells induced by cigarette smoke release MMP-2 and MMP-9, which contribute to the progressions of EMT [14]. Among all MMPs, MMP-9 is thought to play a key role in mediating EMT by tissue remodeling through the degradation of basement membrane collagens and extracellular matrix proteins in COPD and lung cancer patients [2, 9, 10]. MMP-9 level is elevated in peripheral blood, bronchoalveolar lavage fluid (BALF) and exhaled breath condenses in COPD and lung cancer patients [15, 16]. The activity of MMP-9 in non-small cell lung cancer (NSCLC) was positively correlated with advanced T category and distant metastasis. Moreover, the meta-analysis revealed that over-expression of MMP-9 in tissue was a risk factor of advanced T category, tumor stage and poor outcome [17]. However, its role in mediating airway damage and remodeling is still controversial, given that constitutional knockout (KO) of MMP-9 in mice does not affect the pathological outcomes of CS-induced emphysema [18]. MMP-9 is produced mainly by macrophages and neutrophils [18], but also by epithelial cells, mast cells, and fibroblasts in the lung [19, 20]. It has been reported that pulmonary macrophages in COPD patients express similar level of MMP-9 compared to normal controls [18]. Further, stimulation of human airway epithelial cells by TGF-β1, a key proinflammatory factor contributing to the generation of EMT and COPD, resulted in MMP-9 elevation [11], indicating pulmonary epithelial cells could be a significant source of MMP-9 production in COPD and lung cancer. Adding to the complexity, the activity, besides the absolute amount of MMP-9, is proposed to be essential for determining its function in COPD [19]. Studies showed controversial results regarding the relationship between the activity of MMP-9 and lung function measured using the Tiffeneau-Pinelli index (FEV1/FEV ratio) [21, 22], suggesting the urgent need to understand the mechanisms underlying MMP-9 expression, activation and its regulation of airway remodeling in COPD.
The non-receptor protein-tyrosine phosphatase (PTP) Shp2 is encoded by the proto-oncogene PTPN11 and is a ubiquitously expressed key regulator of cell signaling, which regulates physiological and pathological processes, including cell development, growth, inflammation, chemotaxis, oxidative stress, and the Ras/Raf/Erk, PI3K/Akt, and JAK/STAT pathways and immune checkpoint receptors [23]. Our group previously demonstrated that CS stimulates pulmonary epithelial cells to release IL-8 through Shp2 activation [11]. Selective inhibition or conditional knockout (KO) of Shp2 in lung epithelia reduced airway inflammation in a CS-induced mouse model [11]. In the same experiments, we noticed that inhibition or conditional KO of Shp2 in lung epithelia ameliorated small airway epithelia lesion, which led us consider the role of epithelial Shp2 in regulating small airway fibrosis during COPD and its underlying mechanisms. Given that Shp2 promotes tumor EMT that is concurrent with the elevation of MMP-9 secretion [24, 25], we hypothesized that CS-induces Shp2 activation in lung epithelial cells promotes MMP-9 production, and contributes to the progression of COPD-related EMT.
In this work, we employed a mouse model of CS-induced COPD, and a pulmonary epithelial cell culture model of cigarette smoke extract (CSE)-induced EMT to study the expression of MMP-9 during CS exposure, as well as the role of MMP-9 in regulating EMT. We found MMP-9 was upregulated following CS or CSE exposure in both in vivo and in vitro models at least partially through the activation of the Shp2/ERK1/2/JNK/Smad pathway. Selective inhibition of Shp2 or Shp2 KO in lung epithelial cells significantly suppressed the expression of MMP-9 activity, which prevented the CS exposure induced EMT progression. Our study contributes to understanding the underlying mechanisms of pulmonary epithelial structural remodeling, which may provide novel therapeutic solutions for treating its related diseases, such as COPD and lung cancer.
Materials and methods
Reagents
Phenylhydrazonopyrazolone sulfonate 1 (PHPS1) was obtained from Sigma-Aldrich (St. Louis, MO). SB-3CT was obtained from Selleckchem (Houston, TX). RPMI 1640, FBS, penicillin, and streptomycin were obtained from HyClone (Logan, UT). TRIzol reagents were purchased from Takara Bio, Dalian, China. All PCR primers were purchased from Shanghai Bioengineering (Shanghai, China). ERK, p-ERK, Shp2, p-Shp2, Smad2, p-Smad2, Smad3, p-Smad3, JNK, P-JNK, P38, p-P38, MMP-9, MMP-2, E-cadherin, vimentin, α-SMA and β-actin primary antibody were from Cell Signaling Technology (Danvers, MA), and Shp2 primary antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). MMP-9 and TGF-β1 ELISA kits were from Boster (Wuhang, China). Lipofectamine LTX was from Invitrogen (Carlsbad, CA).
Animals and handling
C57BL/6 mice (Laboratory Animal Center of Zhejiang University, Hangzhou, China; certificate no. SCXK 2007–0029) weighing 20 ± 2 g were used. All animals were housed in Plexiglas cages, kept on a 12/12-h light–dark cycle and received food and water ad libitum in temperature- and humidity-controlled rooms. To investigate the treatment effects of PHPS1 on airway inflammation and fibrosis, mice were pretreated with PHPS1 through an intraperitoneal injection (i.p) at 3 mg/kg. PHPS1 was dissolved in saline with 0.5% DMSO 0.5 h before daily CS exposure. Substitutively, an equal volume of saline with 0.5% DMSO was administered to control animals without PHPS1 treatment. Animals were placed in a customized plastic box for CS exposure.
Endogenous ablation of the Shp2 enzyme in lung epithelia was generated using a combined genetic strategy as previously described [11]. Shp2-floxed mouse was a kind gift from Dr. Gen-Sheng Feng at the Department of Pathology, School of Medicine, University of California and Dr. Yuehai Ke at the Zhejiang University. Briefly, Shp2f/f mice (C57BL/6 background) were mated with SP-C-rtTAtg/− and (tetO)7CMV-Cretg/− transgenic mice (C57BL/6 background) to generate SP-C-rtTA/(tetO)7-Cre/Shp2f/f triple transgenic mice. Transgenic mice and non-deleted littermate mice (control) were used for the experiments. To induce Cre recombinase in transgenic mice, 4-week-old mice were fed with the drinking water containing 2 mg/ml doxycycline (Sigma-Aldrich) for 7 d. To validate Shp2 KO in lung epithelia, a forward primer (5′-CAGTTGCAACTTTCTTACCTC-3′) in intron 3 and a reverse primer (5′-GCAGGAGACTGCAGCTCAGTGATG-3′) in intron 4 were used.
Cell culture and treatment
NCI-H292 cells, a human pulmonary epithelial cell line, were obtained from the Cell Bank, Chinese Academy of Sciences. The cells were maintained in RPMI 1640 containing 10% FBS at 37 °C in the presence of 5% CO2. The cells were starved with serum free medium (SFM) for 6 h before any stimulation.
Preparation of cigarette smoke extract
Research-grade cigarettes (3R4F) were obtained from the Kentucky Tobacco Research Council (University of Kentucky). The composition of 3R4F research-grade cigarettes was as follows: total particulate matter, 10.9 mg per cigarette; tar, 9.4 mg per cigarette; and nicotine, 0.726 mg per cigarette. Cigarette smoke extract (CSE) was prepared by bubbling smoke from three cigarettes into 30 ml PBS. CSE was standardized by measuring the absorbance at a wavelength of 320 nm. After filtering through a 0.45-μm filter, CSE was frozen in aliquots and stored at − 80 °C. An aliquot of CSE was thawed immediately before use.
CS exposure
The mice were exposed to CS generated from research-grade cigarettes (3R4F; University of Kentucky, Lexington, KY) in smoking chambers as previously reported [11]. Briefly, mice were exposed to 12 cigarettes (control mice were exposed to laboratory air) each day for consecutive 20 days. Lung tissues were collected 18 h after the last CS exposure.
Shp2 siRNA preparation and transfection
Shp2-specific siRNA interference was performed as previously reported [11]. Sequences of the oligonucleotides are as follows: 5′-GAACAUCACGGCAAUUAAUU-3′; 5′-GAACACUGGUGAUUACUAUUU-3′. The cells were cultured in 24-well plates for 24 h. The Shp2-specific or control siRNA was transfected into NCI-H292 cells using Lipofectamine LTX (Invitrogen) according to the manufacturer’s instructions. Immunoblotting analysis was used to validate Shp2 silencing by siRNA at 72 h after transfection.
Histology
The left lungs were preserved in 10% formalin and sectioned within paraffin-embedded blocks for histological examination. Masson’s trichrome was performed to visualize tissue and reveal collagen deposition. For immunohistochemical staining, lung sections were stained with the first antibody of Shp2 (Santa Cruz Biotechnology), MMP-9 (Abcam), vimentin, α-SMA and S100A4 (Cell Signaling Technology), all using a 1:200 dilution rate, then the accordant secondary antibody with the Streptavidin-Biotin Complex kit was applied (Boster Bio-engineering Ltd. Co., Wuhan, China). Staining was quantified using Image Pro 6.1 software. All analyses were performed in a blind fashion.
Double immunostaining for MMP-9 and vimentin was performed using Envision labeled polymer reagent and a biotin-streptavidin method. First, sections were stained for MMP-9 by incubation with anti-MMP-9 antibody and then Envision reagent as described in the previous section. The bound antibody was visualized with the substrate diaminobenzidine. After the first and secondary antibodies were removed from tissue, the sections were incubated with anti-vimentin antibody for 30 min at room temperature, followed by streptavidin conjugated with alkaline phosphatase for another 30 min. Alkaline phosphatase activity was visualized with naphthol AS-BI phosphate (Sigma, St Louis, MO, USA). We compared the double immunostaining for epithelial cell-specific antigen MMP-9, and for mesenchymal antigen vimentin, in the alveolar epithelium covering the airway and lung tissues foci in CS-exposed lung tissues and also in airway and lung tissues foci in control tissues. The percentage of epithelial cells expressing vimentin was calculated by examining five airway and lung tissues foci in CS-exposed lung tissues, and 5 randomly selected five airway and lung tissues foci in control tissues. Furthermore, we also evaluated the percentages of positive cells in airway and lung tissues foci that retained the expression of vimentin, compared with control tissues.
ELISA assay
The right lungs of the experimental mice were collected and cut into small cubes. The lung tissues (100 ± 5 mg) from each mouse were homogenized in 1 ml ice-cold PBS buffer (pH 7.4). The lung homogenates were centrifuged at 2000×g at 4 °C for 20 min. The supernatants were acidified with 1 N HCl followed by subsequent neutralization with 1.2 N NaOH/0.5 M HEPES and then measured with a mouse MMP-9 ELISA kit (Boster, Wuhan, China) according to the manufacturer’s instruction.
NCI-H292 cells were plated in 24-well plates, then were exposed to CSE in the presence or absence of PHPS1 and siRNA interfering for 24 h. The supernatants were collected, centrifuged at 2000×g for 20 min at 4 °C, then were measured for MMP-9 using a human MMP-9 ELISA kit (Boster, Wuhan, China) according to the manufacturer’s instruction.
RNA isolation and quantitative PCR
The methods for RNA isolation and qPCR were described previously [12]. Briefly, total RNA from the right lung or NCI-H292 cells was extracted with TRIzol according to the manufacturer’s instruction. The PCR primers were checked against the basic local alignment search tool for selectivity. β-actin were used as an internal control for NCI-H292 cells and mouse lung homogenates. Real-time PCR cycling was conducted using a 7500 Real-Time PCR System (Applied Biosystems, Carlsbad, CA) with the following system: 2 × SYBR qPCR Premix or SYBR Premix EX Taq; 0.2 μM forward and reverse primers; 50 ng cDNA. The program was conducted as following: a denaturation step at 95 °C for 40 s and 40 cycles of 95 °C for 10 s, 58 °C for 30 s, and 72 °C for 34 s. Post-PCR melting curves confirmed the specificity of single-target amplification. Sample absorbency was assessed at 497 nm. The mRNA levels were calculated using the 2-ΔΔCt method (relative) [26], normalized to β-actin.
Western blot
The lung tissues were homogenized in radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl; 50 mM Tris, pH 8.0, 1% Triton X-100, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS) (Biyuntian Biotechnology, Haimen, China), and were supplemented with protease and phosphatase inhibitors. NCI-H292 cells were seeded into a six-well plate at 3 × 105 cells per well. After treatment, the cells were washed three times with ice-cold PBS and lysed in 100 μL of RIPA buffer containing 1% PMSF (Haoxin Biotechnology, Hangzhou, China). The protein concentration of lung tissues or NCI-H292 cells was measured by the BCA Protein Assay Kit (cwbiotech, Beijing, China). Total Protein (30 μg) was separated on 10% SDS-polyacrylamide gels by electrophoresis at 80 V for 1.5 h and then transferred to 0.45-μm nitrocellulose membranes at 70 V for 2 h. The membranes were blocked by 5% fat-free milk, and the blots were incubated with antibodies specific for Shp2 (Santa Cruz Biotechnology), MMP-9 (Abcam), p-Shp2, vimentin, α-SMA, E-cadherin, ERK1/2, p-ERK1/2, JNK, p-JNK, Smad2, p-Smad2, Smad3, p-Smad3, P38, p-P38 (Cell Signaling Technology) or β-actin (Sigma-Aldrich) overnight at 4 °C. Afterwards, the blots were incubated with the accordant fluorescent secondary antibodies (LI-COR) for 2 h at room temperature. Immunoreactive bands were visualized by a two-color infrared imaging system (Odyssey - LI-COR, Lincoln, NE).
Gelatin zymography
To investigate the effects of PHPS1 and Shp2 KO on pro-MMP-9 production and MMP-9 activity, 100 mg lung tissue samples per mouse were homogenized in 1 ml ice-cold PBS buffer (pH 7.4), and centrifuged at 2000×g at 4 °C for 20 min. The supernatants were determined by gelatin zymography, using Gelatin Zymo Electrophoresis Kit (Genmed Scientifics, Arlington, MA), according to the manufacturer’s instructions.
Immunofluorescence analysis
NCI-H292 cells were seeded in twelve-well plates containing glass cover slips overnight. NCI-H292 cells were treated with PHPS1, siRNA or SB-3CT (a MMP-2 and MMP-9 selective inhibitor) for 30 min. Subsequently, the cells were stimulated with human recombinant MMP-9 for 48 h, then washed three times with PBS. Then, they were fixed in 4% paraformaldehyde for 30 min, permeabilized in 0.4% Triton X-100 for 10 min, washed three times with PBS, and blocked with 5% bovine serum albumin (BSA) for 30 min at 37 °C. Next, the samples were incubated with primary antibody specific for E-cadherin or α-SMA (Cell Signaling Technology) at 4 °C overnight, followed by incubation with a CY-3-conjugated goat anti-rabbit secondary antibody (Biotech Well) for 2 h at 37 °C and DAPI (Biotech Well) for 5 min at room temperature. Cells were visualized using fluorescence microscopy (Leica Microsystems, Wetzlar, Germany).
Statistical analysis
The GraphPad prism V5.0 software was used for statistical analyses. The data are presented as the mean ± S.E.M. The differences between the mean values of multiple groups were analyzed by one-way analysis of variance, followed by the Student-Newman-Keuls test. Statistical significance was set at P < 0.05.
Results
CS or CSE induces MMP-9 production and Shp2 overexpression in the lungs and in NCI-H292 cells
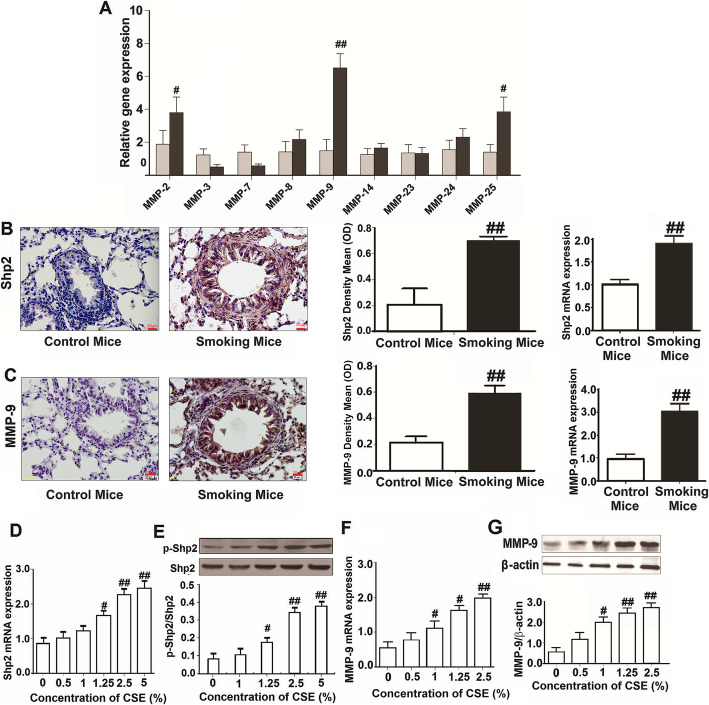
Various studies have implicated increased expression of MMP-9 in the pathogenesis of both COPD and lung cancer [27]. Our group previously demonstrated that CS stimulates pulmonary epithelial cells to release IL-8 through Shp2 activation, and preliminarily found that CS significantly elevated MMP-9 protein and mRNA expression in mouse lungs [11]. In this study, we first screening examined using a cell model, and found that the effects of CSE on a panel of MMPs mRNA expression in respond to 2.5% CSE exposure, where we found an elevation of MMP-9, MMP-2 and MMP-25 mRNA expression by Q-PCR assay (Fig. 1a). Moreover, in the CS-exposed mice model, we further proved that CS significantly elevated Shp2 and MMP-9 protein and mRNA expression in mouse lungs by ELISA and Q-PCR assay (Fig. 1b and c). The immunohistochemistry results showed both Shp2 and MMP-9 were upregulated in the lungs (Fig. 1b and c). Consistently, Shp2 mRNA and MMP-9 mRNA and protein expression in NCI-H292 cells were also upregulated with 24 h CSE exposure stimulation in a concentration-dependent manner (Fig. 1d, e and f). In addition, CSE exposure induced the phosphorylation of Shp2 at the phosphor site (Y-542) in a concentration dependent manner in NCI-H292 cells (Fig. 1e).
Fig. 1.
CS and CSE elevates the expression of Shp2 and MMP-9 in lung tissues and pulmonary epithelial cells, respectively, n = 6 per group. a CSE elevates the mRNA expression of MMP-2, MMP-9 and MMP-25 in NCI-H292 cells. b and c Shp2 and MMP-9 protein (immunohistochemistry) and mRNA expression (Q-PCR) are elevated in lung tissues from the smoking mice (exposed to CS for 20 days), n = 8 mice/per group. Scale bar = 20 μm; ##p < 0.01, compared with control. d CSE elevates the mRNA expression of Shp2 in a concentration-dependent manner in pulmonary epithelial cells (NCI-H292 cells). n = 3 per group. e CSE activates the Shp2 phosphorylation in a concentration-dependent manner in NCI-H292 cells. n = 3 per group. f CSE elevates the mRNA expression of MMP-9 in a concentration-dependent manner in NCI-H292 cells. n = 3 per group. g CSE elevates the protein expression of MMP-9 in a concentration-dependent manner in NCI-H292 cells. n = 3 per group. At least three independent experiments in vitro were completed for each group assessment. Data are presented as the mean ± SEM. #p < 0.05, ##p < 0.01, compared with control, one-way ANOVA followed by the Student-Newman-Keuls test
Knockout of Shp2 in lung epithelia or pharmacological inhibition of Shp2 alleviates the CS-induced MMP-9 and vimentin upregulation
Next, we investigated whether Shp2 expression or its activation regulates MMP-9 production in lung tissues in the CS-exposed mice. We generated lung epithelial Shp2 KO mice [11]. Shp2 deletion in the lungs was confirmed by immunoblotting analysis (Fig. 2a), which showed 72.5% reduction of Shp2 expression, indicating the lung epithelia is the predominant contributor of Shp2 expression. Interestingly, Shp2 KO also reduced MMP-9 expression below control levels (Fig. 2a).
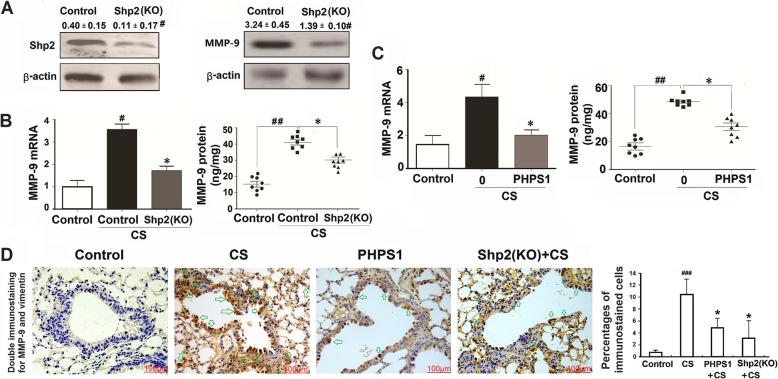
Fig. 2.
Genetic ablation of Shp2 in lung epithelial cells or pharmacological inhibition of Shp2 suppresses the CS-induced MMP-9 expression in mice. a Genetic ablation of Shp2 in lung epithelial cells significantly decreases Shp2 and MMP-9 protein expression in lung tissues. n = 8 per group, #p < 0.05 compared with control (WT mice). b Shp2 knockout in lung epithelial cells reversed the CS-induced MMP-9 mRNA and protein elevation in lung tissues. n = 8 per group, #p < 0.05 compared with control (WT mice), *p < 0.05 compared with control with CS (WT mice with CS exposure). c Shp2 inhibitor - PHPS1 (3 mg/kg, i.p daily injection) treatment reduces the CS-induced MMP-9 mRNA and protein elevation in lung tissues. N = 8 per group, #p < 0.05, ##p < 0.01 compared with control (WT mice), *p < 0.05 compared with control with CS (WT mice with CS exposure). d Double immunostaining confirms Shp2 knockout in lung epithelial cells or us of the Shp2 inhibitor reduces the CS-induced the mesenchymal marker vimentin elevation in lung tissues. n = 8 per group, ##p < 0.01 compared with control (WT mice), *p < 0.05 compared with control with CS (WT mice with CS exposure). Data are presented as the mean ± SEM. Scale bar = 100 μm. Statistical significance is determined by one-way ANOVA followed by the Student-Newman-Keuls test
To assess the potential role of Shp2 in regulating MMP-9 production, we examined the effects of the Shp2 KO or pharmacological inhibition (PHPS1 at daily 3 mg/kg i.p. injection) during the 20 days consecutive with CS exposure on induced MMP-9 mRNA and protein expression in the lungs. The Shp2 KO significantly reduced the CS exposure induced MMP-9 mRNA expression and MMP-9 protein level by 56.3 and 41.2% respectively (Fig. 2b). Shp2 inhibition by PHPS1, acting on all cell types in the lungs, also suppressed the CS exposure-induced MMP-9 mRNA expression and MMP-9 protein level by 52.4 and 42.3% respectively (Fig. 2c).
To confirm that express epithelial-specific marker antigens also express a mesenchymal marker antigen in CS-exposed lung tissues, we performed double immunostaining. The epithelial cells were positive for vimentin. Thus, vimentin was chosen as a mesenchymal antigen for double immunostaining. The assay demonstrated co-expression of MMP-9 (as light brown in the nucleus) and vimentin (as dark brown in the cytoplasm) in epithelial cells in airway and lung tissues (Fig. 2d). These results at least in part suggested that some of the EMT cells in airway and lung tissues may originate from the epithelial cells. Moreover, the immunohistochemical results of MMP-9 and vimentin co-expression of in airway and lung sections showed that both the Shp2 KO and PHPS-1 application significantly reduced the CS exposure-induced vimentin expression by 59.6 and 68.2% respectively (Fig. 2d).
Genetic knockout of Shp2 in lung epithelia or pharmacological inhibition of Shp2 attenuates collagen deposition and EMT progression in the CS-exposed lung tissues
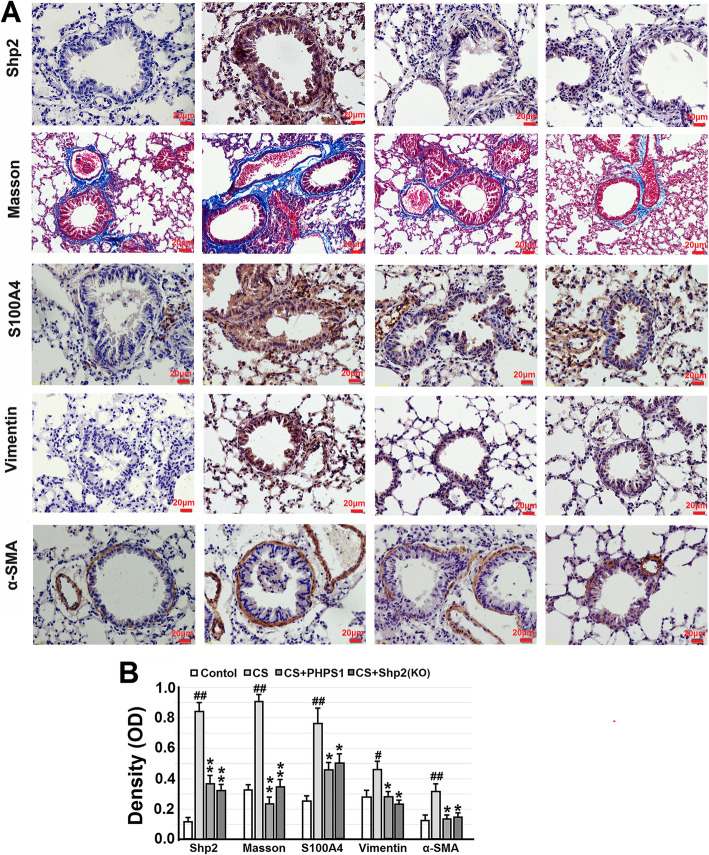
We next evaluated the effects of the Shp2 KO and PHPS1 on the CS-induced collagen deposition. Lung sections were assessed using Masson’s staining and immunohistochemistry of S100A4, a member of the S100 calcium-binding protein family, has been identified in a subpopulation of lung macrophages and promotes lung fibrosis [28]. CS exposed mice exhibited a significant pathological fibrotic manifestation, including increased number of pulmonary interstitial cells (fibroblasts), thickening of the alveolar walls, focal damage of alveolar structure, granulomatous lesions in the alveolar space, distorted pulmonary architecture, and accumulated collagen deposition (blue staining) in the alveolar space and the submucosa of small airways. Shp2 KO or treatment with 3 mg/kg PHPS1 significantly attenuated collagen deposition (Fig. 3a and b). Further, we employed immunohistochemistry to assess the effects of the Shp2 KO and PHPS1 treatment on the CS-induced EMT phenotypes. We found CS exposure significantly increased the expression of vimentin and α-SMA (α-smooth muscle actin is mainly expressed in airway smooth muscle rather than epithelial cells), which was concurrent with the expression of Shp2 and collagen deposition (Fig. 3a and b), suggesting the development of collagen deposition may be resulted from EMT progressions. The Shp2 KO and PHPS1 almost abolished the CS-induced expression of vimentin and α-SMA, along with the reduction of Shp2 expression.
Fig. 3.
Genetic ablation of Shp2 in lung epithelial cells or pharmacological inhibition of Shp2 ameliorates collagen deposition and the expression of EMT-related proteins in lung tissues from the CS-exposed mice. a Representative images show that Shp2 knockout in lung epithelial cells or Shp2 inhibitor – PHPS1 (3 mg/kg, daily i.p injection) significantly reverses the CS-induced collagen deposition (Masson’s trichrome staining – blue color) and immunohistochemical signal (brown color) of Shp2, S100A4, vimentin and α-SMA in lung tissue sections. b The optical density value of Masson’s trichrome staining and immunohistochemical staining are quantified. Data are presented as the mean ± SEM. n = 8 per group. Scale bar = 20 μm. #p < 0.05, ##p < 0.01, compared with control (WT mice), *p < 0.05, **p < 0.01 compared with control (WT mice) with CS exposure, one-way ANOVA followed by the Student-Newman-Keuls test
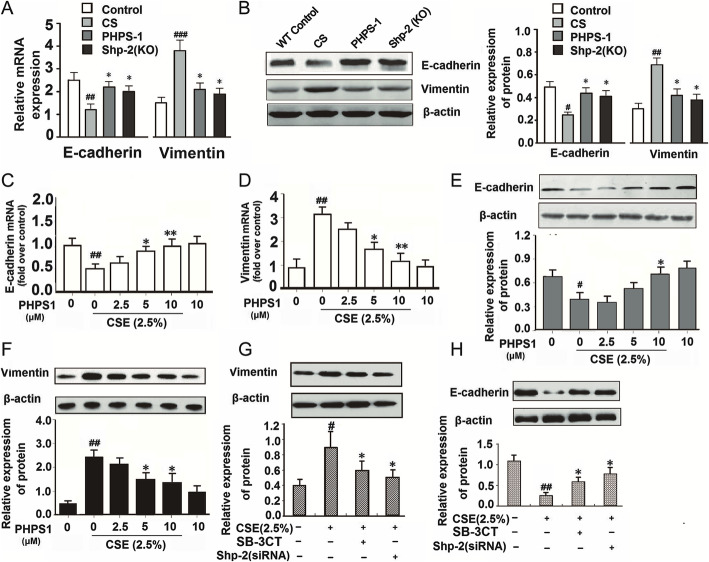
Next, we examined the effects of the Shp2 KO and PHPS1 on the CS-induced alterations of E-cadherin and vimentin mRNA and protein expression in lung tissues. Consistent with the immunohistochemical results and previous reports [29], lung tissues exposed to CS exhibited reduced E-cadherin and increased vimentin mRNA and protein expression (Fig. 4a and b), suggesting they were undergoing EMT progression. Shp2 KO and pharmacological inhibition both partially reversed the changes of E-cadherin and vimentin mRNA and protein expression (Fig. 4a and b), indicating an amelioration of the CS-induced EMT progression in lung tissues by Shp2 inhibition. Meanwhile, we also examined the effects of Shp2 knockdown or pharmacological inhibition on the CSE-induced EMT progression in NCI-H292 cells. Consistent with the in vivo results, 2.5% CSE exposure significantly decreased E-cadherin mRNA and protein expression, and increased vimentin mRNA and protein expression (Fig. 4c and d). Pharmacological inhibition of Shp2 by PHPS1 reversed the CSE exposure-induced changes of E-cadherin and vimentin mRNA and protein expression in a concentration dependent manner (Fig. 4e and f). Shp2 knockdown by siRNA also partially reversed the CSE exposure-induced EMT-related changes (Fig. 4g and h) in NCI-H292 cells, as did MMP-9 inhibition by SB-3CT (Fig. 4g and h).
Fig. 4.
Genetic ablation of Shp2 in lung epithelial cells or pharmacological inhibition of Shp2 alleviates the CS- and CSE-induced EMT phenotypes in lung tissues and NCI-H292 cells. a-b Shp2 knockout in lung epithelial cells or Shp2 inhibitor – PHPS1 (3 mg/kg, i.p injection) reduces the CS-induced changes of E-cadherin and vimentin mRNA and protein expression in lung tissues. n = 8 mice/per group. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with control (WT mice), *p < 0.05 compared with control (WT mice) with CS exposure. c-e Shp2 inhibitor – PHPS1 alleviates the CSE exposure induced changes of E-cadherin and vimentin mRNA and protein expression in a concentration-dependent manner in NCI-H292 cells. n = 3 per group. #p < 0.05, ##p < 0.01 compared with control (WT mice), *p < 0.05, **p < 0.01 compared with control (WT mice) with CS exposure. f–h) Shp2 knockdown or MMP-9 inhibitor – SB-3CT (1 μM) alleviates the 2.5% CSE exposure induced changes of E-cadherin and vimentin protein expression in NCI-H292 cells. n = 3 per group. #p < 0.05, ##p < 0.01, compared with control; *p < 0.05 compared with 2.5% CSE exposure. At least three independent experiments in vitro were completed for each group assessment. Data are presented as the mean ± SEM. Statistical significance is determined by one-way ANOVA followed by the Student-Newman-Keuls test
Genetic knockout of Shp2 in lung epithelia or pharmacological inhibition of Shp2 reduces pro-MMP-9 levels and inhibits MMP-9 activity in the CS-exposed mice
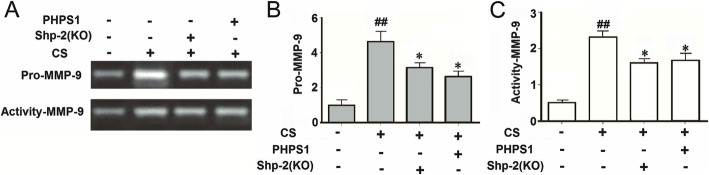
The pulmonary epithelial cells or neutrophils express high levels of MMP-9, stored in the cytoplasmic granules (“specific” and “gelatinase” types) as the inactive form of pro-MMP-9 (92 kDa). CS stimulation can induce extracellular release of granule content, and the proteolytic conversion of pro-MMP-9 to the catalytically active form [30, 31]. Here, we investigated the effects of Shp2 KO and pharmacological inhibition on the CS exposure-induced pro-MMP-9 and active MMP-9 production in lung tissues. We first confirmed that CS exposure significantly increased pro-MMP-9 (4.1-fold) and active MMP-9 (4.6-fold) production assessed by gelatin zymography. Shp2 KO and pharmacological inhibition by PHPS1 partially reduced the production of both pro-MMP-9 and active MMP-9 in the CS-exposed lung tissues (Fig. 5a, b and c).
Fig. 5.
Genetic ablation of Shp2 in lung epithelial cells or pharmacological inhibition of Shp2 alleviates the CS- and CSE-induced pro-MMP-9 (gelatinase B) expression and MMP-9 activity in lung tissues. a-c Genetic ablation of Shp2 in lung epithelial cells or Shp2 inhibitor – PHPS1 (3 mg/kg, i.p injection) alleviates the CS-induced pro-MMP-9 (gelatinase B) expression and MMP-9 activity in lung tissues, assessed by gelatin zymography. n = 8 mice/per group. #p < 0.05, ##p < 0.01, compared with control (WT mice), *p < 0.05, **p < 0.01 compared with control (WT mice) with CS exposure. Data are presented as the mean ± SEM. Statistical significance is determined by one-way ANOVA followed by the Student-Newman-Keuls test
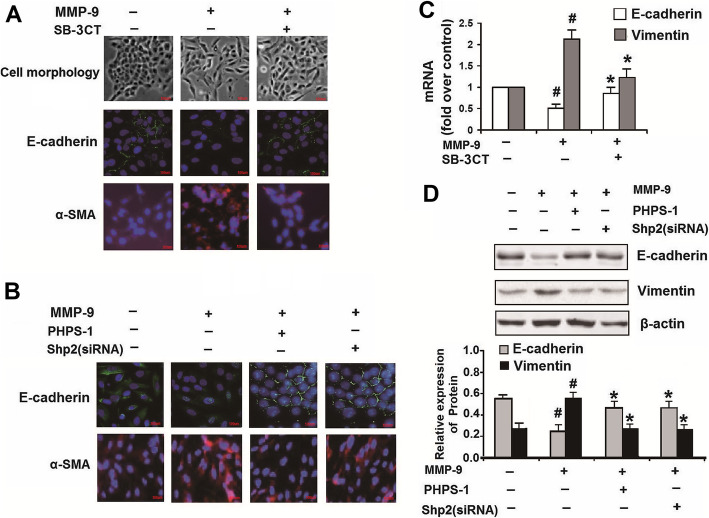
Shp2 inhibition/knockdown or MMP-9 inhibition attenuates human recombinant MMP-9-induced EMT in NCI-H292 cells
In the above experiments, we demonstrated that CS exposure promoted MMP-9 production in lung tissues. It has been reported that MMP-9 itself could induce EMT progression in epithelial cells [14]. Indeed, we found 2 μg/ml human recombinant MMP-9 induced a fibroblast-like morphology with cellular elongation and reduction of cell-cell contacts. Along with a reduction of E-cadherin and an increase in α-SMA expression in NCI-H292 cells, while the untreated cells exhibited a pebble-like shape and displayed cell-cell contacts consistent with an epithelial morphology (Fig. 6a). MMP-9 inhibition by SB-3CT (1 μM) prevented the changes of morphology and EMT (increased E-cadherin and decreased α-SMA) induced by human recombinant MMP-9 treatment (Fig. 6a and c). Interestingly, Shp2 KD by siRNA or inhibition by PHPS1 both prevented the human recombinant MMP-9-induced expression of EMT-indicators by elevation of E-cadherin, and reduction of vimentin and α-SMA protein expression in NCI-H292 cells (Fig. 6b and d). Therefore, aside from the regulation of MMP-9 production, Shp2 signaling also positively regulates the MMP-9-induced EMT progression.
Fig. 6.
MMP-9 inhibition, Shp2 inhibition or Shp2 knockdown suppresses the expression of EMT-related factors induced by recombinant MMP-9 in NCI-H292 cells. a NCI-H292 cells with no treatment exhibit a pebble-like shape and display cell-cell contacts consistent with an epithelial morphology. The cells treated with human recombinant MMP-9 (2 μg/ml, 48 h) exhibit a fibroblast-like morphology with cellular elongation and reduction of cell-cell contacts. SB-3CT (1 μM) prevents the MMP-9-induced cellular changes and preserves normal epithelial morphology. The cells treated with recombinant MMP-9 for 48 h exhibit weaker expression of E-cadherin and stronger expression of α-SMA, compared with control. MMP-9 inhibition by SB-3CT (1 μM) alleviates the recombinant MMP-9 induced changes of E-cadherin and α-SMA expression. Scale bar = 100 μm. b Pharmacological inhibition or Shp2 knock down reverses the recombinant MMP-9 induced changes of E-cadherin and α-SMA expression. Scale bar = 100 μm. c MMP-9 inhibition by SB-3CT (1 μM) prevents the recombinant MMP-9 (2 μg/ml) induced decreases in E-cadherin expression and increases in α-SMA mRNA expression assessed by real-time PCR. n = 3 per group. #p < 0.05 compared with control (no treatment); *p < 0.05 compared with cells treated with recombinant MMP-9. d Shp2 inhibition by PHPS1 (10 μM) or knock down by siRNA prevents the recombinant MMP-9 (2 μg/ml) induced decreases in E-cadherin expression and increases in α-SMA protein expression assessed by western blot. Data are expressed as mean ± SEM of three independent experiments. n = 3 per group. #p < 0.05 compared with control (no treatment); *p < 0.05 compared with cells treated with recombinant MMP-9. At least three independent experiments were completed for each group assessment. Data are presented as the mean ± SEM. Statistical significance is determined by one-way ANOVA followed by the Student-Newman-Keuls test
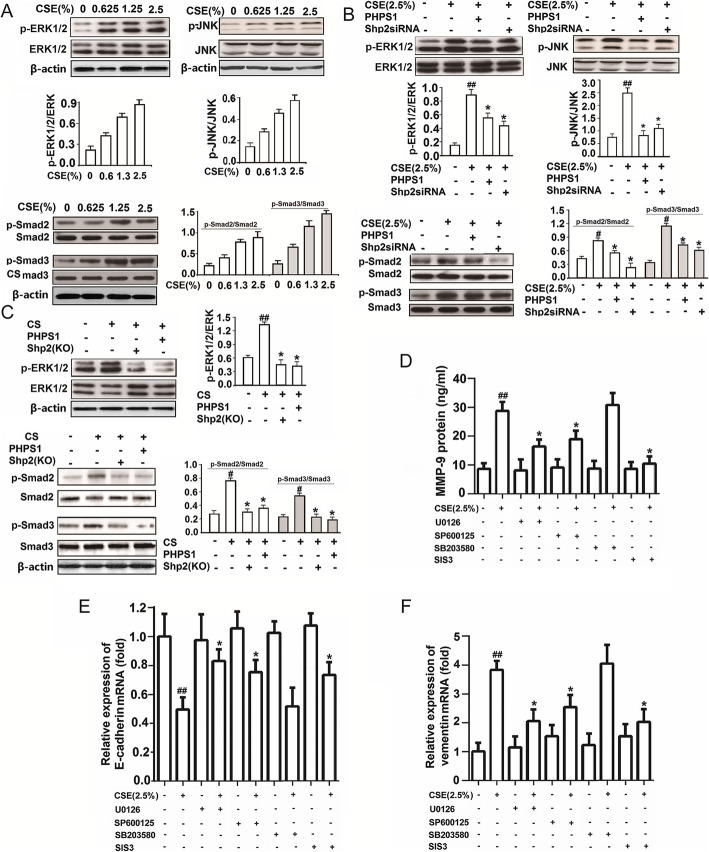
Shp2 signaling regulates MMP-9 production and EMT progression through ERK1/2/JNK/Smad signaling pathway in the CS-exposed mice and CSE-exposed NCI-H292 cells
Last, we wanted to explore the molecular mechanisms underlying the Shp2 regulation of MMP-9 production and EMT progressions. We first validated that 24 h CSE (0.625, 1.25 and 2.5%) exposure concentration-dependently induced the phosphorylation of ERK1/2, JNK and Smad2/3 (Fig. 7a), which were all partially or completely inhibited by Shp2 knockdown (siRNA) or pharmacological inhibition (PHPS1) in NCI-H292 cells (Fig. 7b). Consistently, CS exposure induced the phosphorylation of ERK1/2 and Smad2/3 in lung tissues, which were completely abolished by Shp2 KO or pharmacological inhibition (3 mg/kg PHPS1) (Fig. 7c). These in vitro and in vivo results indicate that the CS exposure induced EMT-related signaling pathway was predominately regulated by Shp2 activation. To confirm these signaling pathways also contributed to the MMP-9 production and EMT progression, we screened the effects of ERK1/2 inhibitor - U0126, JNK inhibitor - SP600125, Smad2/3 inhibitor - SIS3 and p38 inhibitor - SB203580 on the 2.5% CSE-induced MMP-9 protein expression, E-cadherin and vimentin mRNA expression in NCI-H292 cells. We showed that treatment with U0126, SP600125 and SIS3, but not the p38 inhibitor SB203580, significantly reduced CSE-induced MMP-9 protein levels in the supernatant of NCI-H292 cells by ELISA assay (Fig. 7d) and partially reversed the CSE-induced changes of E-cadherin and vimentin mRNA expression in the NCI-H292 cells by Q-PCR assay (Fig. 7e and f), indicating Shp2 signaling positively regulated MMP-9 production and EMT progressions through JNK/ERK1/2/Smad2/3 signaling pathways.
Fig. 7.
Genetic ablation of Shp2 in lung epithelial cells or pharmacological inhibition of Shp2 suppresses the CS- and CSE-induced expression of MMP-9 through MAPK/JNK/Smad signaling in lung tissues and in NCI-H292 cells. a Fifteen minutes of CSE incubation with indicated concentration induces phosphorylation of ERK1/2, JNK and Smad2/3 in a concentration dependent manner in NCI-H292 cells. n = 3 per group. b Shp2 inhibition by PHPS1 or knock down by siRNA attenuates the CSE-induced phosphorylation of ERK1/2, JNK and Smad2/3 in NCI-H292 cells. n = 3 per group. #p < 0.05, ##p < 0.01, compared with control (no treatment); *p < 0.05 compared with cells with CSE exposure. c Shp2 knock out in lung epithelial cells or inhibition by PHPS1 attenuates the CS-induced phosphorylation of ERK/1/2 and Smad2/3 in lung tissues. n = 3 per group. #p < 0.05, ##p < 0.01, compared with control (no treatment); *p < 0.05 compared with cells with CSE exposure. d-f ERK1/2 inhibitor U0126 (10 μM), JNK inhibitor SB600152 (10 μM), P38MAPK inhibitor SB203580 (10 μM) and Smad2/3 inhibitor SIS3 (10 μM) attenuates the 24 h CSE (2.5%) induced MMP-9 protein production and vimentin mRNA expression, and reverses the 24 h CSE (2.5%) induced E-cadherin mRNA decreases in NCI-H292 cells. n = 3 per group. #p < 0.05, ##p < 0.01, compared with control (no treatment); *p < 0.05 compared with cells with CSE exposure. At least three independent experiments in vitro were completed for each group assessment. Data are presented as the mean ± SEM. Statistical significance is determined by one-way ANOVA followed by the Student-Newman-Keuls test
Discussion
Here, we studied the mechanisms underlying elevated MMP-9 production and EMT progression following CS exposure in mouse lungs and CSE exposure in pulmonary epithelial cells. We demonstrated that CS exposure induced MMP-9 production and upregulation of its active form in lung tissues, which were concurrent with Shp2 overexpression. Consistently, CSE exposure to pulmonary epithelial cells induced MMP-9 production and Shp2 upregulation and phosphorylation. Shp2 KO in lung epithelial cells or Shp2 inhibition prevented the CS exposure induced MMP-9 production and EMT progression in mouse lung tissues, which aligned with effects of Shp2 KD or inhibition on the CSE exposure-induced MMP-9 production and EMT progression in pulmonary epithelial cells. Interestingly, direct application of human recombinant MMP-9 also induced EMT progressions through Shp2 activation in pulmonary epithelial cells, which were suppressed by Shp2 KD or Shp2/MMP-9 inhibition.
EMT is an active process in both the small and large airways of smokers and patients with COPD [9, 32]. In clinic, Sohal and colleagues observed that EMT-related changes were associated with increased hypervascularity of the underlying reticular basement membrane (Rbm) in the large airways, MMP-9 leads to Rbm splitting which is a core structural marker of EMT [33, 34], representing a typical active type-3 EMT process, considered as precursor to malignant conditions and metastasis [5]. In this experimental model, we also tried to find this phenomenon, but unfortunately we did not observe a significant Rbm splitting. We analyze the reasons that may be this model differs from human COPD, and cannot fully simulate human COPD. Another possibility is that the model in this study has not been smoking for long enough.
CS exposure is believed to induce the secretion of MMPs from epithelial, endothelial, fibroblasts and inflammatory cells, such as polymorphonuclear neutrophil, mast cells and macrophages [31, 35, 36]. Here, we validated that CSE exposure induced MMP-9 production in pulmonary epithelial cells. In addition, KO of Shp2 in lung epithelial cells robustly suppressed MMP-9 production and activity induced by CS exposure, indicating lung epithelial cells are a significant source of MMP-9 production following CS exposure. In this study, we found MMP-9 production in pulmonary epithelial cells, which was consistent with the TGF-β1 induced MMP-9 production in other cells, including human corneal epithelial cells [36], keratinocytes [37], odontoblasts [38], human lung fibroblasts [39] and oral tumor cells [40]. We also provided evidence that CS exposure induced MMP-9 production depends upon Shp2/ERK1/2/JNK/Smad2/3 pathways, which is consistent with TGF-β1 induced MMP-9 production in human corneal epithelial cells [36]. Recently we found the Shp2 KO or inhibition suppressed the CS exposure-induced TGF-β1 production in lung tissues (Data not shown). Thus, the suppression of the CS exposure induced MMP-9 production in the lungs in Shp2 KO or PHPS1 treated mice was likely due to lower TGF-β1 production and less TGF-β1 activated MAPK and Smad signaling pathways. Moreover, we found that Shp2 KO or inhibition not only suppressed the CS exposure-induced MMP-9 gene transcription, but also inhibited the production of the pro-MMP-9 (the active form of MMP-9), which is essential for determining the role of MMP-9 in lung diseases [19].
MMP-9 has gelatinolytic, elastolytic and collagenolytic activity, thus playing a key role in extracellular matrix turnover, mainly by cleavage of extracellular matrix components. In addition, MMP-9 may also modulate the activity of various biological factors, including other proteinases (e.g., MMP-13), their inhibitors (e.g., α1-antitrypsin) or cytokines (e.g., IL-1, VEGF) [19]. In this study, we showed that direct application of recombinant MMP-9 induced EMT phenotypes in pulmonary epithelial cells, consistent with the findings from glomerular endothelial cells [41]. Particularly, we found a downregulation of E-cadherin and an upregulation of α-SMA which are thought to play a key role during early steps of invasion and metastasis during EMT progression [42]. EMT was assessed by vimentin and α-SMA production of immunohistochemical assay, we found an evidence of the EMT consist with pathological changes which included a distinct collagen deposits in the submucosa and around the airway, and a thicken mucosal layer, using Masson’s staining and immunohistochemistry of S100A4 assay (Fig. 3). That are the typical representative of small airway fibrosis and airway remodeling. Shp2 KO or inhibition, along with MMP-9 inhibition, suppressed the recombinant MMP-9 induced EMT, by preventing the decreases in E-cadherin and increases in α-SMA expression. It could be attributed to less TGF-β1 production, less MMP-9 production, or their synergistic effects following suppression of Shp2 signaling. In addition, Shp2 has been shown to mediate focal adhesion kinase (FAK) dephosphorylation, which is required for down-regulation of E-cadherin [43]. Further, Shp2 was shown to positively regulate TGF-β1-induced EMT in A549 cells – another lung epithelial cell line [24]. Thus, reduced EMT induced by CS could also be attributed to the direct suppression of Shp2-related signaling pathways in epithelial cells.
In this study, we showed that Shp2 signaling positively regulated the CS exposure induced EMT progression in pulmonary epithelial cells, indicating Shp2 signaling played a critical role in regulating small airway fibrosis, which results in airways with significant functional impairments in COPD [44, 45]. We previously showed that Shp2 signaling also positively regulates the CS exposure induced inflammation, particularly meditating IL-8 release from pulmonary epithelial cells via activation of Shp2-regualted epidermal growth factor receptor/Grb-2-associated binders/MAPK signaling [11]. Thus, both the CS exposure induced pulmonary inflammation and remodeling are positively regulated by Shp2/MAPK signaling. There is little agreement on whether the airway remodeling is a consequence of the inflammation, or rather exists as a distinct phenomenon [19]. CS exposure induced inflammatory responses following the production of pro-inflammatory agents may promote the development of chronic forms of inflammation, with damage and metaplasia of the respiratory epithelium [19]. Moreover, cytokines and chemokines may be converted by MMP-9 into active forms, including pro-IL-1β and IL-8 [46]. Targeting either mechanism independently or both airway remodeling and inflammation is a putative therapy to treat CS exposure related diseases, such as COPD and lung cancer [5]. Evidence for this approach includes a 3-month combined treatment of fluticasone with salmeterol which decreased MMP-9 and IL-8 levels in the serum, likely contributing to the clinical benefits seen in the COPD patients [47]. Therefore, reducing MMP-9 and IL-8 production by suppression of Shp2 signaling may be preventing both pulmonary remodeling and inflammation in the diseases associated with CS, making Shp2 inhibition a putative therapy for COPD and lung cancer [48].
Conclusions
We present the first study demonstrating that the MMP-9 production and EMT progressions induced by CS exposure in vivo or CSE exposure in vitro were through Shp2/ERK1/2/JNK/Smad2/3 signaling pathways; pharmacological inhibition of these signaling pathways markedly suppressed the MMP-9 production and EMT progression. Thus, we discovered novel molecular mechanisms underlying the regulation of MMP-9 production and EMT progression in CS exposed mice and CSE exposed pulmonary epithelial cells, providing the framework for developing novel therapeutic solutions for treating CS related diseases, such as COPD and lung cancer.
Acknowledgments
We thank Dr. G.S. Feng (University of California, San Diego, CA, USA) and Dr. Yuehai Ke (Zhejiang University, Hangzhou, China) for Shp2f/f mice and J.A. Whitsett (University of Cincinnati, Cincinnati, OH, USA) and Ning Wen (Nankai University, Tianjin, China) for the SP-C-rtTAtg/2 and (tetO)7CMV-Cretg/2 transgenic mice. We thank Steven Connor at the York University in Canada for editing the language of the manuscript.
Abbreviations
- EMT
Epithelial mesenchymal transition
- COPD
Chronic obstructive pulmonary disease
- CS
Cigarette smoke
- CSE
Cigarette smoke extract
- JNK
Jun N-terminal kinase
- ERK
Extracellular regulated protein kinase
- TGF-β1
Transforming growth factor beta-1
- MMP
Matrix metalloproteinase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium-bromide
- FBS
Fetal bovine serum
- PBS
Phosphate-buffered saline
- DMSO
Dimethylsulfoxide
- EdU
5-ethynyl-2′-deoxyuridine
- i.p.
Intraperitoneal injection
- KO
Knock-out
- KD
Knock down
- FAK
Focal adhesion kinase
- Rbm
Reticular basement membrane
Authors’ contributions
YL and YG designed and performed experiments, as well as analyzed the data. JS, YJ, WZ and HS assisted in performing the animal experiments. JJ and JZ performed some of the in vitro experiments. YS and QS provided resources. QX and YX designed the study, supervised the overall project, and wrote the manuscript. The authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81872876 and 81573439).
Availability of data and materials
The analyzed datasets generated during the study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was carried out in accordance with the recommendations of Guide for the Care and Use of Laboratory Animals of Zhejiang University, Zhejiang University Animal Care and Use Committee. The protocol was approved by the Zhejiang University Animal Care and Use Committee.
Competing interests
The authors have no financial conflicts of interest.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1186/s12931-023-02506-2
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ya-nan Liu and Yan Guan contributed equally to this work.
Change history
8/24/2022
A Correction to this paper has been published: 10.1186/s12931-022-02121-7
Change history
8/11/2023
This article has been retracted. Please see the Retraction Notice for more detail: 10.1186/s12931-023-02506-2
Contributor Information
Qiang-min Xie, Email: xieqm@zju.edu.cn.
Yicheng Xie, Email: ycxie@zju.edu.cn.
References
- 1.Barnes J, Adcock I.M. Chronic obstructive pulmonary disease and lung cancer: a lethal association. Am J Respir Crit Care Med 2011;184:866–867. [DOI] [PubMed]
- 2.Sohal SS, Mahmood MQ, Walters EH. Clinical significance of epithelial mesenchymal transition (EMT) in chronic obstructive pulmonary disease (COPD): potential target for prevention of airway fibrosis and lung cancer. Clin Transl Med. 2014;3:33. doi: 10.1186/s40169-014-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohal SS, Ward C, Danial W, Wood-Baker R, Walters EH. Recent advances in understanding inflammation and remodeling in the airways in chronic obstructive pulmonary disease. Expert Rev Respir Med. 2013;7:275–288. doi: 10.1586/ers.13.26. [DOI] [PubMed] [Google Scholar]
- 4.Sohal SS, Walters EH. Role of epithelial mesenchymal transition (EMT) in chronic obstructive pulmonary disease (COPD) Respir Res. 2013;14:120. doi: 10.1186/1465-9921-14-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eapen MS, Hansbro PM, Larsson-Callerfelt AK, Jolly MK, Myers S, Sharma P, Jones B, Rahman MA, Markos J, Chia C, Larby J, Haug G, Hardikar A, Weber HC, Mabeza G, Cavalheri V, Khor YH, McDonald CF, Sohal SS. Chronic obstructive pulmonary disease and lung cancer: underlying pathophysiology and new therapeutic modalities. Drugs. 2018;78:1717–1740. doi: 10.1007/s40265-018-1001-8. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C, Ding XP, Zhao QN, Yang XJ, An SM, Wang H, Xu L, Zhu L, Chen HZ. Role of α7-nicotinic acetylcholine receptor in nicotine-induced invasion and epithelial-to-mesenchymal transition in human non-small cell lung cancer cells. Oncotarget. 2016;7:59199–59208. doi: 10.18632/oncotarget.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milara J, Peiró T, Serrano A, Cortijo J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax. 2013;68:410–420. doi: 10.1136/thoraxjnl-2012-201761. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee R, Chatterjee J. ROS and oncogenesis with special reference to EMT and stemness. Eur J Cell Biol. 2020;99:151073. doi: 10.1016/j.ejcb.2020.151073. [DOI] [PubMed] [Google Scholar]
- 9.Sohal SS, Reid D, Soltani A, Ward C, Weston S, Muller HK, Wood-Baker R, Walters EH. Reticular basement membrane fragmentation and potential epithelial mesenchymal transition is exaggerated in the airways of smokers with chronic obstructive pulmonary disease. Respirology. 2010;15:930–938. doi: 10.1111/j.1440-1843.2010.01808.x. [DOI] [PubMed] [Google Scholar]
- 10.Sohal SS, Reid D, Soltani A, Ward C, Weston S, Muller HK, Wood-Baker R, Walters EH. Evaluation of epithelial mesenchymal transition in patients with chronic obstructive pulmonary disease. Respir Res. 2011;12:130. doi: 10.1186/1465-9921-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li FF, Shen J, Shen HJ, Zhang X, Cao R, Zhang Y, Qui Q, Lin XX, Xie YC, Zhang LH, Jia YL, Dong XW, Jiang JX, Bao MJ, Zhang S, Ma WJ, Wu XM, Shen H, Xie QM, Ke Y. Shp2 plays an important role in acute cigarett smoke-mediated lung inflammation. J Immunol. 2012;189:3159–3167. doi: 10.4049/jimmunol.1200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang JX, Zhang SJ, Shen HJ, Guan Y, Liu Q, Zhao W, Jia YL, Shen J, Yan XF, Xie QM. Rac1 signaling regulates cigarette smoke-induced inflammation in the lung via the Erk1/2 MAPK and STAT3 pathways. Biochim Biophys Acta. 1863;2017:1778–1788. doi: 10.1016/j.bbadis.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Shen HJ, Sun YH, Zhang SJ, Jiang JX, Dong XW, Jia YL, Shen J, Guan Y, Zhang LH, Li FF, Lin XX, Wu XM, Xie QM, Yan XF. Cigarette smoke-induced alveolar epithelial-mesenchymal transition is mediated by Rac1 activation. Biochim Biophys Acta. 1840;2014:1838–1849. doi: 10.1016/j.bbagen.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 14.Agraval H, Yadav UCS. MMP-2 and MMP-9 mediate cigarette smoke extract-induced epithelial-mesenchymal transition in airway epithelial cells via EGFR/Akt/GSK3β/β-catenin pathway: amelioration by fisetin. Chem Biol Interact. 2019;314:108846. doi: 10.1016/j.cbi.2019.108846. [DOI] [PubMed] [Google Scholar]
- 15.Bugdayci G, Kaplan T, Sezer S, Turhan T, Koca Y, Kocer B, Yildirim E. Matrix metalloproteinase-9 in broncho-alveolar lavage fluid of patients with non-small cell lung cancer. Exp Oncol. 2006;28:169–171. [PubMed] [Google Scholar]
- 16.Patyk I, Rybacki C, Kalicka A, Rzeszotarska A, Korsak J, Chciałowski A. Simvastatin therapy and bronchoalveolar lavage fluid biomarkers in chronic obstructive pulmonary disease. Adv Exp Med Biol. 2019;1150:43–52. doi: 10.1007/5584_2018_272. [DOI] [PubMed] [Google Scholar]
- 17.Gong L, Wu D, Zou J, Chen J, Chen L, Chen Y, Ni C, Yuan H. Prognostic impact of serum and tissue MMP-9 in non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7:18458–18468. doi: 10.18632/oncotarget.7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkinson JJ, Lutey BA, Suzuki Y, Toennies HM, Kelley DG, Kobayashi DK, Ijem WG, Deslee G, Moore CH, Jacobs ME, Conradi SH, Gierada DS, Pierce RA, Betsuyaku T, Senior RM. The role of matrix metalloproteinase-9 in cigarette smoke-induced emphysema. Am J Respir Crit Care Med. 2011;183:876–884. doi: 10.1164/rccm.201005-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grzela K, Litwiniuk M, Zagorska W, Grzela T. Airway remodeling in chronic obstructive pulmonary disease and asthma: the role of matrix metalloproteinase-9. Arch Immunol Ther Exp. 2016;64:47–55. doi: 10.1007/s00005-015-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royer PJ, Henrio K, Pain M, Loy J, Roux A, Tissot A, Lacoste P, Pison C, Brouard S, Magnan A. COLT consortium. TLR3 promotes MMP-9 production in primary human airway epithelial cells through Wnt/β-catenin signaling. Respir Res. 2017;18:208. doi: 10.1186/s12931-017-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowrey GE, Henderson N, Blakey JD, Corne JM, Johnson SR. MMP-9 protein level does not reflect overall MMP activity in the airways of patients with COPD. Respir Med. 2008;102:845–851. doi: 10.1016/j.rmed.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Culpitt SV, Rogers DF, Traves SL, Barnes PJ, Donnelly LE. Sputum matrix metalloproteases: comparison between chronic obstructive pulmonary disease and asthma. Respir Med. 2005;99:703–710. doi: 10.1016/j.rmed.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Tajan M, de Rocca SA, Valet P, Edouard T, Yart A. SHP2 sails from physiology to pathology. Eur J Med Genet. 2015;58:509–525. doi: 10.1016/j.ejmg.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Wang L, Zhao Q, Liu Y, He L, Xu Q, Sun X, Teng L, Cheng H, Ke Y. SHP2 positively regulates TGFβ1-induced epithelial-mesenchymal transition modulated by its novel interacting protein Hook1. J Biol Chem. 2014;289:34152–34160. doi: 10.1074/jbc.M113.546077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang FM, Liu HQ, Liu SR, Tang SP, Yang L, Feng GS. SHP-2 promoting migrationand metastasis of MCF-7 with loss of E-cadherin, dephosphorylation of FAK and secretion of MMP-9 induced by IL-1beta in vivo and in vitro. Breast Cancer Res Treat. 2005;89:5–14. doi: 10.1007/s10549-004-1002-z. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Navratilova Z, Kolek V, Petrek M. Matrix metalloproteinases and their inhibitors in chronic obstructive pulmonary disease. Arch Immunol Ther Exp. 2016;64:177–193. doi: 10.1007/s00005-015-0375-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Ohno S, Steer B, Klee S, Staab-Weijnitz CA, Wagner D, Lehmann M, Stoeger T, Königshoff M, Adler H. S100a4 is secreted by alternatively activated alveolar macrophages and promotes activation of lung fibroblasts in pulmonary fibrosis. Front Immunol. 2018;9:1216. doi: 10.3389/fimmu.2018.01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang B, Guan Y, Shen HJ, Zhang LH, Jiang JX, Dong XW, Shen HH, Xie QM. Akt/PKB signaling regulates cigarette smoke-induced pulmonary epithelial-mesenchymal transition. Lung Cancer. 2018;122:44–53. doi: 10.1016/j.lungcan.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Fortin M, D'Anjou H, Higgins ME, Gougeon J, Aubé P, Moktefi K, Mouissi S, Séguin S, Séguin R, Renzi PM, Paquet L, Ferrari N. A multi-target antisense approach against PDE4 and PDE7 reduces smoke-induced lung inflammation in mice. Respir Res. 2009;10:39. doi: 10.1186/1465-9921-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Rojas-Quintero J, Wilder J, Tesfaigzi Y, Zhang D, Owen CA. Tissue inhibitor of metalloproteinase-1 promotes polymorphonuclear neutrophil (PMN) pericellular proteolysis by anchoring matrix metalloproteinase-8 and -9 to pmn surfaces. J Immunol. 2019;202:3267–3281. doi: 10.4049/jimmunol.1801466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmood MQ, Sohal SS, Shukla SD, Ward C, Hardikar A, Noor WD, Muller HK, Knight DA, Walters EH. Epithelial mesenchymal transition in smokers: large versus small airways and relation to airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2015;10:1515–1524. doi: 10.2147/COPD.S81032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sohal SS. Epithelial and endothelial cell plasticity in chronic obstructive pulmonary disease (COPD) Respir Investig. 2017;55:104–113. doi: 10.1016/j.resinv.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Sohal SS, Soltani A, Reid D, Ward C, Wills KE, Muller HK, Walters EH. A randomized controlled trial of inhaled corticosteroids (ICS) on markers of epithelial-mesenchymal transition (EMT) in large airway samples in COPD: an exploratory proof of concept study. Int J Chron Obstruct Pulmon Dis. 2014;9:533–542. doi: 10.2147/COPD.S63911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abel M, Vliagoftis H. Mast cell-fibroblast interactions induce matrix metalloproteinase-9 release from fibroblasts: role for IgE-mediated mast cell activation. J Immunol. 2008;180:3543–3550. doi: 10.4049/jimmunol.180.5.3543. [DOI] [PubMed] [Google Scholar]
- 36.Wright JL, Tai H, Wang R, Wang X, Churg A. Cigarette smoke upregulates pulmonary vascular matrix metalloproteinases via TNF-alpha signaling. Am J Physiol Lung Cell Mol Physiol. 2007;292:L125–L133. doi: 10.1152/ajplung.00539.2005. [DOI] [PubMed] [Google Scholar]
- 37.Kim HS, Luo L, Pflugfelder SC, Li DQ. Doxycycline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:840–8. [DOI] [PubMed]
- 38.Salo T, Lyons JG, Rahemtulla F, Birkedal-hansen H, Larjava H. Transforming growth factor-beta 1 up-regulates type IV collagenase expression in cultured human keratinocytes. J Biol Chem. 1991;266:11436–11441. doi: 10.1016/S0021-9258(18)98977-5. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi T, Hattori S, Shinkai H. Matrix Metalloproteinases-2 and -9 are secreted from human fibroblasts. Acta Derm Venereol. 2003;83:105–7. [DOI] [PubMed]
- 40.Palosaari H, Cj P, Larmas M, Tja L, Salo T, Larmas M, Edwards DR. Expression profile of matrix metalloproteinases ( MMPs ) and tissue inhibitors of MMPs in mature human odontoblasts and pulp tissue. Eur J Oral Sci. 2003;20:117–27. [DOI] [PubMed]
- 41.Zhao Y, Qiao X, Wang L, Tan TK, Zhao H, Zhang Y, Zhang J, Rao P, Cao Q, Wang Y, Wang Y, Wang YM, Lee VW, Alexander SI, Harris DC, Zheng G. Matrix metalloproteinase 9 induces endothelial-mesenchymal transition via notch activation in human kidney glomerular endothelial cells. BMC Cell Biol. 2016;17:21. [DOI] [PMC free article] [PubMed]
- 42.Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014;69:760–765. doi: 10.1136/thoraxjnl-2013-204608. [DOI] [PubMed] [Google Scholar]
- 43.Avizienyte E, Wyke AW, Jones RJ, McLean GW, Westhoff MA, Brunton VG, Frame MC. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat Cell Biol. 2002;4:632–638. doi: 10.1038/ncb829. [DOI] [PubMed] [Google Scholar]
- 44.Barnes PJ. Small airway fibrosis in COPD. Int J Biochem Cell Biol. 2019;116:105598. doi: 10.1016/j.biocel.2019.105598. [DOI] [PubMed] [Google Scholar]
- 45.Jones RL, Noble PB, Elliot JG, James AL. Airway remodelling in COPD: It’s not asthma! Respirology. 2016;21:1347–1356. doi: 10.1111/resp.12841. [DOI] [PubMed] [Google Scholar]
- 46.Opdenakker G, Van den Steen PE, Van Damme J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol. 2001;22:571–579. doi: 10.1016/S1471-4906(01)02023-3. [DOI] [PubMed] [Google Scholar]
- 47.Perng W, Tao W, Su C, Tsai C, Lee C. Anti-inflammatory effects of salmeterol/fluticasone, tiotropium/fluticasone or tiotropium in COPD. Eur Respir J. 2009;33:778–784. doi: 10.1183/09031936.00115308. [DOI] [PubMed] [Google Scholar]
- 48.Frankson R, Yu ZH, Bai Y, Li Q, Zhang RY, Zhang ZY. Therapeutic targeting of oncogenic tyrosine phosphatases. Cancer Res. 2017;77:5701–5705. doi: 10.1158/0008-5472.CAN-17-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed datasets generated during the study are available from the corresponding author on reasonable request.