Abstract
Introduction
We estimated the prevalence and correlates of mild cognitive impairment (MCI) among middle-aged and older diverse Hispanics/Latinos.
Methods
Middle-aged and older diverse Hispanics/Latinos enrolled (n 5 6377; 50–86 years) in this multisite prospective cohort study were evaluated for MCI using the National Institute on Aging–Alzheimer’s Association diagnostic criteria.
Results
The overall MCI prevalence was 9.8%, which varied between Hispanic/Latino groups. Older age, high cardiovascular disease (CVD) risk, and elevated depressive symptoms were signify-cant correlates of MCI prevalence. Apolipoprotein E4 (APOE) and APOE2 were not significantly associated with MCI.
Discussion
MCI prevalence varied among Hispanic/Latino backgrounds, but not as widely as reported in the previous studies. CVD risk and depressive symptoms were associated with increased MCI, whereas APOE4 was not, suggesting alternative etiologies for MCI among diverse Hispanics/Latinos. Our findings suggest that mitigating CVD risk factors may offer important pathways to understanding and reducing MCI and possibly dementia among diverse Hispanics/Latinos.
Keywords: Epidemiology, Mild cognitive impairment, Alzheimer’s disease, Dementia, Neuroepidemiology, Cognitive function, Cognitive decline, Neuropsychology, Hispanics, Latinos, Hispanics/Latinos, Population neuroscience
1. Introduction
Hispanics/Latinos (henceforth Latinos) represent nearly one-fifth of the United States and 40% of its two most popu-lous states, California and Texas [1]. By 2060, the census projects that Latinos (In this study, we refer only to Latinos with backgrounds from Spanish-speaking regions in North and South America) will represent nearly one-third of Americans and that the Latino elderly population (>65 years) will nearly quadruple [2]. Over the next40 years, Latinos are projected to have the largest increase in Alzheimer’s disease and related dementia (ADRD) cases [3], based on prevalence estimates ranging from 21% among US Caribbean (Dominican, Puerto Rican) to 5% among Mainland Latinos (Mexican and Central Americans) from two large and inde-pendent studies in New York and northern California [4,5]. Additionally, the differences between Caribbean and Mainland Latinos for cognitive impairment were 35.2% and 4.8%, respectively [4,5]. These extant prevalence estimates are 20 years old and have been the extent of our understanding of Latino ADRD epidemiology to date.
Latinos share regional forms of the Spanish language, but their cultural histories, genetic ancestries, and health profiles are diverse [6,7], which have important but understudied and poorly understood implications for Latino health in general and age-related neurodegenerative disorders specifically. Striking differences in ADRD prevalence have been reported between African admixed Caribbean Latinos and Mainland Latinos of mostly Amerindian ancestry [4,5]. Furthermore, risks for ADRD may vary by continental ancestry. Associations between apolipoprotein E (APOE) 4 and ADRD, an important genetic risk among whites, have shown little to no relationship with ADRD in Caribbean Latinos and mixed associations among Mainland Latinos [5,8,9]. Highly prevalent cardiovascular disease (CVD) risks (e.g., diabetes) vary among Latino backgrounds, which may contribute to excess MCI and ADRD among diverse Latinos [10]. Moreover, understanding and preventing CVD would likely open opportunities to prevent excess cognitive decline and dementia by reducing stroke risk [11].
Latinos are an important and growing part of American society, which will only increase in significance in the coming decades. There are major gaps in Latino dementia research, which form scientific barriers to the field and US public health, and updated Latino dementia information is needed. Additionally, new biomarker and genomic tools expand our understanding of neurodegenerative processes and provide insights into disease prevention and therapeutic targets. It will be vital to know precisely how these tools inform current understanding of dementia disease processes among diverse Latinos. Thus, the goal of this study is to clarify the prevalence and correlates of MCI among understudied and unstudied diverse Latinos. We also sought to replicate previous research by dividing Latinos into 3 groups of Caribbean, Mainland, and Cuban backgrounds.
2. Methods
2.1. Study design
The Study of Latinos–Investigation of Neurocognitive Aging (SOL-INCA) is an ancillary study of the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). HCHS/SOL and SOL-INCA study designs and rationales are available elsewhere [12,13]. HCHS/SOL is a multisite, population-based, prospective cohort study of CVD risks among Latinos (Baseline years 2008–2011). HCHS/SOL survey data collection procedures were implemented to yield representative estimates of diverse Latinos in four targeted US metropolitan areas: Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA. Each field center enrolled about 4000 eligible, self-identified Latino adults (18–74 years old; N = 16,415). Biospecimens (e.g., blood) were assayed for CVD risk factors (e.g., triglycerides) and stored for later studies. Genetic data were collected only from participants consenting for genetic testing. Detailed HCHS/SOL sampling methods have been published elsewhere and on the HCHS/SOL website: https://sites.cscc.unc.edu/hchs/.
Baseline cognitive testing at HCHS/SOL visit 1 (base-line) included only middle-aged and older (45–74 years) participants who were oversampled (n = 9714) in the cohort. The Neurocognitive Reading Center trained and supervised bicultural/bilingual technicians who administered the brief cognitive battery, which included 4 tests: (1) six-item screener (SIS; mental status) [14]; (2) Brief-Spanish English verbal learning test (B-SEVLT; verbal episodic learning and memory) [15]; (3) word fluency (WF) [16]; and (4) digit symbol subtest (DSS; processing speed, executive function) [17]. Of all eligible participants, only 59 (<1%) did not participate because of health limitations and/or refusals.
SOL-INCA cognitive tests were administered to HCHS/ SOL participants who returned for visit 2, which occurred on an average of 7 years after visit 1. We expanded the cognitive battery to derive an MCI research diagnosis based on the National Institute on Aging-Alzheimer’s Association (NIA-AA) criteria [18]. In addition to visit 1 tests, we included the Trail Making Test (TMT, parts A&B, executive function) and NIH Toolbox Picture Vocabulary Test (PVT; general premorbid cognitive function), self-reported cognitive decline (Everyday Cognition-12; eCog12) and instrumental activities of daily living (a measure of functional impairment) [19,20]. The PVT was used to assess premorbid cognitive function because these scores remain stable with age and in later neurodegenerative stages, and to control for potential educational quality test biases [21]. At HCHS/SOL visit 2, the Coordinating Center identified 7420 potentially eligible participants for the SOL-INCA. Inclusion criteria were (1) visit 2 completion (aged 50 years and older at visit 2) and (2) visit 1 neurocognitive testing completion. Of this group, 222 were determined to be ineligible, 569 were eligible but refused to participate, and 6377 were eligible and agreed to participate. The overall response rate for the SOL-INCA of eligible participants was 88.7%. Eligible participants return-ing for SOL-INCA had largely similar baseline characteristics compared with those who were not included in the study (Supplementary Table 1). Furthermore, to guard against any possible biases by sample attrition, the HCHS/SOL Coordinating Center generated study-specific calibrated probability weights that were used in our analyses to adjust for nonre-sponse and allow generalization of estimates to the HCHS/ SOL metropolitan area target populations aged 50 years and older.
MCI diagnostic criteria in SOL-INCA were operational-ized and implemented to generate four NIA-AA criteria: (1) any cognitive score in the mildly impaired range (i.e., from 21 to 22 SD) compared with SOL-INCA internal robust norms adjusted for age, education, sex, and PVT scores; (2) significant cognitive decline (≥−0.055 SD/year) from visit 1; (3) self-reported cognitive decline (eCog12); and (4) no or minimum functional (instrumental activities of daily living) impairment [18]. AD biomarkers (e.g., amyloid β [A β]) were unavailable in the SOL-INCA. We included both cognitive impairment and significant cognitive decline to reduce false positive bias. Participants with severe cognitive impairment (below −2 SD relative to SOL-INCA robust norms and with significant functional impairment) were not included in these MCI prevalence estimates.
We examined MCI in the six HCHS/SOL backgrounds (Central American, Cuban, Dominican, Mexican, Puerto Ri-can, and South American). Latinos are genetically admixed, and previously published HCHS/SOL GWAS results indicate that there are 3 major admixture groups representing African, Amerindian, and European continental ancestries [6]. To apply HCHS/SOL ancestry findings and, to some extent, replicate previous studies, we also generated three groups: (1) Caribbean (Dominican, Puerto Rican), (2) Mainland (Mexican, Central and South American), and (3) Cuban [4,5]. However, it should be noted that previous Latino dementia studies did not include South Americans and Cubans [4,5].
Cardiovascular risk was calculated based on the participants’ visit 2 Framingham cardiovascular risk score (FCRS) composed of age, total cholesterol (mg/dL), high-density lipoprotein (HDL), cholesterol (mg/dL), systolic blood pressure (based on the average of three readings in mm Hg), blood pressure medication use, current smoking status, and diabetes [22,23]. FCRSs in the range of 0– 100% were divided by 10, when modeled to allow direct interpretation of relative risks in 10% FCRS CVD risk increments.
APOE genotyping was conducted on the SOL-INCA participants who consented to genetic data collection (Supplementary Fig. 1). The distribution of allele frequencies varied by Latino background, as previously reported [24]. We used a binary classification that groups those with 1 or more APOE4 allele versus no APOE4 allele. Additionally, a three-category indicator that separately clas-sifies APOE2 allele carriers (≥1 or 0) was generated and tested in sensitivity models. All analyses adjusting for APOE4 were restricted to the subpopulation consenting for genetic data collection. Descriptive characteristics comparing differences between those that provided consent and those that opted out are presented in Supplementary Table 2.
Multivariable model covariables included sex, age (50– 59 years; 60–69 years; and 70 + years), education (<12 years, 12 years, >12 years), and depressive symptoms at HCHS/SOL visit 2 (Center for Epidemiologic Studies Depression scale-10; CESD-10) [25]. In additional ana-lyses, we considered age measured continuously (in years) and dichotomously (<65 years and 65 + years) to allow comparison of findings of this report with those of previous reports. We also considered education measured continuously and generated estimates of MCI prevalence over the number of years of education and at education levels that better reflects the distribution of educational achieve-ment in this cohort.
The analytic sample included 6377 enrolled participants aged 50–86 years at HCHS/SOL V2. A flow chart detailing the SOL-INCA sample and exclusion criteria is provided in Supplementary Fig. 1. Given our interest in providing MCI estimates for specific Latino groups, we excluded participants (n = 120) who reported mixed Latino backgrounds and participants (n = 14) who did not provide background information. We excluded participants (n = 103) with missing cognitive data needed to classify MCI. Additionally, we excluded participants (n = 85) who met criteria for sus-pected severe cognitive impairment (<2 SD below the normative mean on any cognitive domain and functional impairment). For multivariable modeling, we also excluded individuals (n = 174) with missing values on any of the covariates of interest. The analytic sample size was 5881. The excluded participants had similar age, sex, and Latino background distributions relative to those included in the analytic sample.
2.2. Statistical analyses
First, we provide descriptive statistics to characterize the full sample (Table 1) and the MCI group (Table 2) by a specific Latino background. Second, we profile differences in demographic, cardiovascular, behavioral, and genetic risk for cognitively normal and MCI groups. Third, we use survey logistic models to test the associations between MCI classification and Latino background groupings. We fit two models (Table 3) to sequentially derive (1) crude and (2) sex-, age-, education-, FCRS-, and CESD-10-adjusted odds ratios and their 95% confidence intervals (CI). We fit an additional model to independently adjust for ≥1 APOE4 (Table 4). For all the tested models, we generated and plotted post hoc estimates of crude and adjusted marginal probabilities and their 95% CIs to facilitate visualization of associations between the model covariates and MCI classification. Fig. 1 details the crude prevalence estimates. Supplementary Figs. 2 and 3 include estimates for the additional operationalizations of age and education described in the above sections. Fig. 2 provides the adjusted estimates for all model covariates of interest. Supplementary Fig. 4 provides these estimates using the classified Latino groups. All analyses incorporated the HCHS/SOL and SOL-INCA sampling design including stratification, clustering, and probability weights using the Stata statistical software (v.15.1, StataCorp, College Station, TX) survey functionalities.
Table 1.
Characteristics by Hispanic/Latino backgrounds in the Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA)
| Dominican | Central American | Cuban | Mexican | Puerto Rican | South American | Total | P value | |
|---|---|---|---|---|---|---|---|---|
| Age (years), % (SE) | ||||||||
| 50–59 | 43.97 (2.69) | 40.83 (2.74) | 33.33 (2.26) | 44.38 (1.85) | 33.99 (1.90) | 39.10 (3.40) | 39.24 (0.99) | .000 |
| 60–69 | 36.64 (2.81) | 38.54 (2.72) | 33.58 (2.28) | 36.73 (1.53) | 36.66 (2.03) | 37.17 (2.93) | 36.05 (0.97) | |
| 70+ | 19.39 (2.52) | 20.63 (2.99) | 33.10 (2.74) | 18.89 (1.51) | 29.36 (2.00) | 23.73 (3.42) | 24.71 (1.04) | |
| Education (years), % (SE) | ||||||||
| <12 | 45.22 (2.83) | 42.61 (2.64) | 22.77 (2.02) | 48.05 (1.89) | 41.57 (2.04) | 24.92 (3.00) | 38.48 (1.08) | .000 |
| 12 | 20.13 (2.11) | 19.47 (2.25) | 25.48 (1.54) | 20.14 (1.41) | 22.59 (1.73) | 18.55 (2.35) | 21.78 (0.76) | |
| >12 | 34.65 (2.51) | 37.92 (2.57) | 51.75 (2.10) | 31.82 (1.82) | 35.84 (2.14) | 56.53 (3.44) | 39.74 (1.02) | |
| Sex, % (SE) | ||||||||
| Female | 60.32 (2.48) | 60.99 (2.82) | 48.59 (1.85) | 56.33 (1.58) | 52.98 (2.13) | 56.38 (2.91) | 54.53 (0.88) | .000 |
| Framingham cardiovascular risk score (FCRS), % (SE) | ||||||||
| <10% | 32.05 (2.34) | 31.42 (2.47) | 20.94 (1.39) | 34.14 (1.48) | 22.93 (1.46) | 36.01 (3.11) | 28.60 (0.78) | .000 |
| 10–<20% | 31.33 (2.28) | 27.48 (2.19) | 29.74 (2.13) | 35.30 (1.55) | 31.67 (1.93) | 35.18 (3.16) | 32.28 (0.89) | |
| 20+% | 36.62 (2.63) | 41.10 (2.54) | 49.32 (2.20) | 30.56 (1.50) | 45.40 (1.94) | 28.81 (3.21) | 39.12 (0.97) | |
| APOE4 status, % (SE) | ||||||||
| Any 4 | 29.41 (2.44) | 21.14 (3.04) | 21.57 (1.74) | 19.60 (1.54) | 25.18 (2.02) | 16.64 (2.70) | 22.12 (0.86) | .006 |
| Age (years), Mean (SD) | 62.11 (7.68) | 62.66 (8.99) | 64.61 (6.88) | 62.05 (8.22) | 64.26 (8.46) | 63.29 (9.55) | 63.19 (8.18) | .000 |
| FCRS (r = 0%–100%), Mean (SD) | 19.0 (15.0) | 20.0 (17.0) | 25.0 (14.0) | 18.0 (15.0) | 22.0 (15.0) | 17.0 (15.0) | 21.0 (16.0) | .000 |
| CESD-10 (r = 0–30), Mean (SD) | 7.00 (6.14) | 6.16 (7.07) | 6.58 (5.30) | 5.65 (5.67) | 8.28 (6.63) | 6.04 (7.02) | 6.51 (6.14) | .000 |
Abbreviations: SD, standard deviation; SE, standard error; CESD, Center for Epidemiologic Studies Depression Scale; APOE4, apolipoprotein E4.
Table 2.
Mild cognitive impairment participants’ characteristics between Hispanic/Latino backgrounds
| Dominican | Central American | Cuban | Mexican | Puerto Rican | South American | Total | P value | |
|---|---|---|---|---|---|---|---|---|
| Age (years), % (SE) | ||||||||
| 50–59 | 32.08 (7.81) | 37.78 (7.31) | 23.66 (6.05) | 28.68 (4.27) | 28.64 (5.50) | 32.57 (8.88) | 28.90 (2.59) | .960 |
| 60–69 | 35.45 (6.97) | 26.73 (6.37) | 38.05 (6.31) | 35.80 (4.86) | 33.00 (5.37) | 27.15 (8.67) | 34.42 (2.74) | |
| 70+ | 32.47 (8.38) | 35.49 (9.52) | 38.29 (7.79) | 35.52 (5.86) | 38.36 (6.57) | 40.28 (10.94) | 36.68 (3.26) | |
| Education (years), % (SE) | ||||||||
| <12 | 47.52 (7.93) | 60.47 (7.73) | 28.03 (5.61) | 59.92 (5.15) | 56.00 (6.09) | 26.18 (10.55) | 49.30 (2.82) | .002 |
| 12 | 15.25 (6.34) | 11.63 (4.93) | 23.55 (5.79) | 17.19 (3.68) | 19.48 (4.97) | 18.60 (8.45) | 18.46 (2.19) | |
| >12 | 37.23 (8.08) | 27.91 (5.84) | 48.42 (6.43) | 22.89 (4.68) | 24.52 (5.42) | 55.21 (11.44) | 32.24 (2.88) | |
| Sex, % (SE) | ||||||||
| Female | 68.66 (8.55) | 62.47 (7.47) | 62.19 (6.44) | 50.61 (5.47) | 61.44 (6.35) | 57.94 (9.90) | 58.51 (3.03) | .411 |
| Framingham cardiovascular risk score (FCRS), % (SE) | ||||||||
| <10% | 23.16 (6.62) | 17.82 (4.58) | 13.13 (3.63) | 22.30 (3.79) | 8.38 (2.05) | 43.27 (10.57) | 18.21 (1.79) | .017 |
| 10–<20% | 35.03 (7.03) | 25.21 (5.88) | 28.17 (6.85) | 33.34 (4.71) | 35.95 (6.19) | 34.97 (9.22) | 32.33 (2.69) | |
| 20+% | 41.81 (7.66) | 56.97 (7.45) | 58.70 (6.96) | 44.35 (5.68) | 55.67 (6.37) | 21.76 (7.98) | 49.46 (2.98) | |
| APOE4 Status, % (SE) | ||||||||
| Any 4 | 24.87 (8.58) | 28.53 (10.85) | 23.76 (6.91) | 21.05 (5.82) | 25.30 (6.32) | 14.75 (7.06) | 23.45 (2.96) | .947 |
| Age (years), Mean (SD) | 65.19 (8.20) | 65.19 (10.51) | 66.23 (6.64) | 66.07 (9.15) | 66.63 (9.04) | 65.56 (9.93) | 66.04 (8.73) | .97 |
| FCRS (r = 0%–100%), Mean (SD) | 22.0 (14.0) | 24.0 (16.0) | 29.0 (15.0) | 25.0 (20.0) | 26.0 (16.0) | 18.0 (18.0) | 25.0 (18.0) | .09 |
| CESD-10 (r = 0–30), Mean (SD) | 9.32 (6.85) | 7.68 (8.07) | 9.38 (5.83) | 7.52 (6.43) | 12.00 (6.31) | 10.60 (9.25) | 9.21 (6.94) | <.0001 |
Abbreviations: SD, standard deviation; SE, standard error; CESD, Center for Epidemiologic Studies Depression; APOE4, Apolipoprotein E4.
Results are based on data from Hispanics/Latinos aged 50–86 years in the Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA).
Table 3.
Multivariable association between Hispanic/Latino backgrounds and MCI status
| M1 |
M2 |
|
|---|---|---|
| OR [95% CI] | OR [95% CI] | |
| Background (ref: Cubans) | ||
| Dominican | 1.22 [0.78; 1.92] | 1.37 [0.86; 2.18] |
| Central American | 1.38 [0.89; 2.15] | 1.58* [1.00; 2.48] |
| Cuban | 1.00 [1.00; 1.00] | 1.00 [1.00; 1.00] |
| Mexican | 1.20 [0.84; 1.72] | 1.55* [1.06; 2.28] |
| Puerto Rican | 1.69† [1.16; 2.45] | 1.61* [1.10; 2.36] |
| South American | 1.21 [0.72; 2.02] | 1.60 [0.96; 2.64] |
| Sex (ref: Female) | ||
| Male | 0.77 [0.56; 1.05] | |
| Age (ref: 50–59) | ||
| 60–69 | 1.17 [0.86; 1.58] | |
| 70+ | 1.65* [1.09; 2.49] | |
| Education, years (12 ref) | ||
| <12 | 1.28 [0.93; 1.75] | |
| >12 | 0.98 [0.68; 1.42] | |
| Framingham cardiovascular risk score | 1.21‡ [1.09; 1.34] | |
| CESD-10 (r = 0–30) | 1.07‡ [1.05; 1.09] |
NOTE. M1 is a crude model; M2 adjusts for sex, age, education; Framingham cardiovascular risk score, and CESD-10.
Odds ratios for the Framingham Cardiovascular Risk Score are for a 10% increase in the score.
Results are based on data from Hispanics/Latinos aged 50–86 years in the Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA).
Abbreviation: CESD, Center for Epidemiologic Studies Depression Scale.
P < .05.
P < .01.
P < .001.
Table 4.
Associations between FCRS, depressive symptoms, and APOE4 and MCI status among Hispanics/Latinos aged 50–86 years in the SOL-INCA
| M1 |
M2 |
|
|---|---|---|
| OR [95% CI] | OR [95% CI] | |
| Background (ref: Cubans) | ||
| Dominican | 1.24 [0.74; 2.09] | 1.35 [0.79; 2.31] |
| Central American | 1.73* [1.03; 2.89] | 1.99* [1.17; 3.39] |
| Cuban | 1.00 [1.00; 1.00] | 1.00 [1.00; 1.00] |
| Mexican | 1.20 [0.77; 1.87] | 1.55 [0.97; 2.47] |
| Puerto Rican | 1.68* [1.09; 2.60] | 1.63* [1.05; 2.53] |
| South American | 1.13 [0.63; 2.04] | 1.51 [0.84; 2.70] |
| Age (ref: 50–59) | ||
| 60–69 | 1.16 [0.83; 1.61] | |
| 70+ | 1.57 [0.97; 2.56] | |
| Education, years (12 ref) | ||
| <12 | 1.30 [0.89; 1.90] | |
| >12 | 1.04 [0.67; 1.62] | |
| Sex (ref: Female) | ||
| Male | 0.76 [0.52; 1.11] | |
| Framingham cardiovascular risk score | 1.22† [1.08; 1.38] | |
| CESD-10 | 1.06‡ [1.04; 1.09] | |
| APOE-status (No 4s ref) | ||
| Any 4 | 1.06 [0.74; 1.51] |
NOTE. M1 is a crude model. M2 adjusts for Latino/Hispanic background, age, sex, and education, Framingham cardiovascular risk, CESD-10, and Apolipoprotein E4 (APOE4).
Odds ratios for the Framingham cardiovascular risk score are for a 10% increase in the score.
Abbreviation: CESD, Center for Epidemiologic Studies Depression Scale.
P < .05.
P < .01.
P < .001.
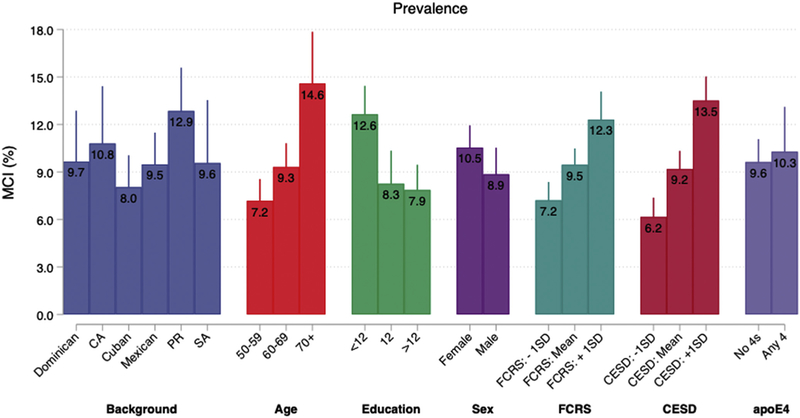
Fig. 1.
Prevalence of mild cognitive impairment (MCI; unadjusted) between backgrounds, demographic, cardiovascular, and behavioral factors, and APOE4 status in the Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA). Abbreviations: CA, Central American; PR, Puerto Rican; SA, South American; SD, standard deviation; FCRS, Framingham cardiovascular risk score; CESD, Center for Epidemiologic Studies Depression Scale (CESD-10); APOE4, apolipoprotein E4.
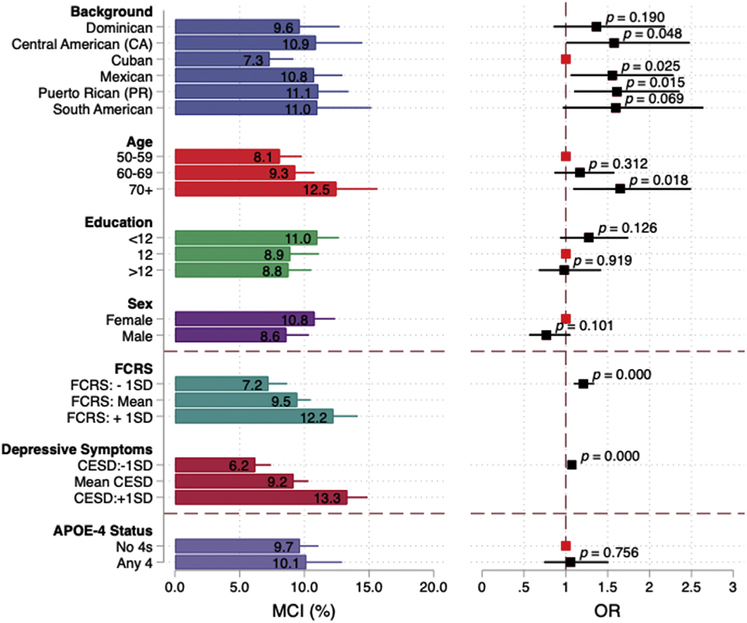
Fig. 2.
Adjusted prevalence of mild cognitive impairment (MCI) and odds ratios (ORs) between Hispanic/Latino backgrounds, demographic, Framingham cardiovascular risk score, depressive symptoms, and APOE4. Findings from the Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA). Note 1: Estimates are based on multivariable models that include Latino background, age, education, sex, Framingham cardiovascular risk score, and CESD-10. Note 2: Odds ratios for the Framingham cardiovascular risk score are for a 10% increase in the score. Abbreviations: SD, standard deviation; FCRS, Framingham cardiovascular risk score; CESD, Center for Epidemiologic Studies Depression Scale (CESD-10); APOE4, apolipoprotein E4.
3. Results
We present the SOL-INCA target population characteristics in Table 1 and stratified characteristics for the MCI subpopulation in Table 2. The average age of the target population was 63 ± 8 years, about a quarter of the popular-tion were aged 70+ years, 55% were female, and 40% had >12 years of education. High proportions of participants met criteria for intermediate (32.3%) or high (39.1%) CVD risk based on the FCRS. The prevalence of any APOE4 was 22%, and the average CESD score was 6.6 ± 6.2. Mexicans, Dominicans, and Central Americans were slightly younger and more likely to have ≤12 years of education. APOE4 prevalence was higher among Dominicans and Puerto Ricans, and FCRS were higher in Cubans and Puerto Ricans.
Overall, 9.8% of diverse middle-aged and older Latinos met NIA-AA research diagnostic criteria for MCI. The prevalence varied between Latino backgrounds with Puerto Ricans having the highest MCI prevalence (12.9%) and Cubans having the lowest (8.0%). MCI prevalence among Central Americans, Dominicans, South Americans, and Mexicans were 10.8%, 9.7%, 9.6%, and 9.5%, respectively (Fig. 1).
MCI was higher among participants older than 70 years (14.6% vs. 7.2% among 50–59 years; P< 0001) and those with <12 years of education (13.2% vs. 8.3% among more than 12 years of education; P = .0001), high FCRS (12.4% [≥1 SD mean] vs. 6.4% among low [≤1 SD mean]; P <.0001), and high depressive symptoms (14.2% vs. 6.3% among low symptoms [−1 SD below mean]; P <.0001) (Fig. 1). MCI prevalence did not significantly vary by sex, APOE4, or APOE2 genotypes.
In the MCI subpopulation, Latino groups had similar age, sex, and APOE4 genotype distributions (Table 2). Education level, CVD risk, and depression scores varied in Latino groups. Those with Mexican (60%) and Central American (60.5%) backgrounds were less likely to have completed a high school education. Cubans (58.7%), Central Americans (57%), and Puerto Ricans (55.7%) had higher cardiovascular risk (FCRS ≥20%; risk for an event within 10 years), and Puerto Ricans and South Americans had higher average CESD scores.
Multivariable results and marginal estimates are presented in Table 3 and Fig. 2. In our fully adjusted model, Cubans (7.3%) had consistently lower MCI prevalence relative to Puerto Ricans (△ = −3.8%; P = .015), Mexicans (△=−3.4%; P = .025), South Americans (△=−3.7%; P = .069), and Central Americans (△=−3.6%; P =.048). Older age (≥70 years), higher FCRS, and higher CESD scores were also significantly associated with higher risk for MCI. Sex, educational attainment, and APOE genotype were not significantly associated with MCI. Group differences remained quantitatively and qualitatively unchanged after adjustments for APOE (Table 4).
4. Discussion
Among diverse middle-aged and older Latinos, the overall MCI prevalence was 9.8%. The prevalence varied between Latino backgrounds (Fig. 1) and was higher among Puerto Ricans and lower in Cubans. MCI was associated with higher CVD risk, which suggests that CVD is an important contributor to cognitive decline and impairment in Latinos. It also suggests that reducing CVD risk potentially could reduce MCI and dementia risk as well in this important and rapidly growing and aging US population [11]. Elevated depressive symptoms were also associated with higher risk of MCI, which has been reported in the previous studies [26]. High Aβ levels have been also linked to incident depressive symptoms in cognitively normal older adults [27]. This raises questions about MCI in relation to CVD, Aβ, and effect-tive changes co-occurring in the early stages of demen-tia. The natural history of MCI and progression to dementia in any population is unclear, but it is largely unknown among diverse Latinos in the United States. The SOL-INCA is a younger cohort, which affords opportunities to discover factors that can contribute to and prevent dementia in this important but understudied population.
MCI was higher among Caribbean than Mainland Latinos in the SOL-INCA, particularly among Puerto Ricans, but the differences were not as large as previously reported [4,5]. One explanation for differences between studies is that previous findings come from two independent studies with differing cognitive protocols compared with the single methodology used in the SOL-INCA. Another explanation is that different diagnostic criteria (e.g., cognitive impairment no dementia) were used in the previous study, which could yield different MCI estimates. MCI rates in the SOL-INCA were similar to a recent study of Mexican-origin Latinos in rural and urban Texas, which also used the NIA-AA MCI diagnostic criteria [5]. Additionally, the SOL-INCA MCI prevalence estimates were similar to African Americans and whites of comparable age groups in the Atherosclerosis Risk in Communities-Neurocognitive study (ARIC-NCS) [28]. The SOL-INCA provides updated MCI prevalence estimates using present diagnostic criteria in a single multisite study of diverse middle-aged and older Latinos [18]. To our knowledge, this is the first comparative large study of MCI in diverse and representative Latinos.
The two major risk factors for MCI were cardiovascular risk and elevated depressive symptoms. We found that for each 10% increase in the FCRS, the odds of MCI increased by 21%. This is important because nearly 40% of the total SOL-INCA sample was in the FCRS high-risk group and this proportion increased to 57% among Latinos who met MCI criteria. Higher depressive symptoms were also associated with MCI prevalence. Depressive symptoms are commonly seen in poststroke patients, and 63% of middle-aged and older patients have evidence of asymptomatic brain infarction [29]. Together, this evidence suggests that the depressive symptoms found to be associated MCI could be the behavioral manifestations of vascular brain injury and not necessarily affective in nature. Our results highlight the potential for mitigating cognitive disease burden by reducing these modifiable factors in this higher risk population.
Low education is a risk factor for MCI and ADRD reported in previous studies comparing whites with popular-tions facing socioeconomic and health disparities [4]. In the SOL-INCA, the socioeconomic conditions between diverse Latino groups were generally balanced, which, we suggest, allowed us to strip away most of the “noise” of socioeconomic imbalances to better see salient health-related factors associated with cognitive decline and MCI. Women had slightly higher MCI rates than men, but were not significantly different. The SOL-INCA con-sists of a younger cohort than most dementia studies of adults older than 65 years of age in which women are over-represented secondary to early male mortality. As such, our middle-aged and older cohort would be less susceptible to bias due to selective mortality of men. Additionally, the HCHS/SOL representative sampling of target areas is less susceptible to recruitment sampling bias. Nevertheless, it remains to be seen if participants with low education and female participants go on to convert MCI to dementia at an increased rate.
We previously reported in SOL-INCA that APOE2 and APOE4 varied between Latino backgrounds with Dominicans having the highest prevalence of APOE2 and APOE4 alleles [24]. In this study, we did not see strong associations between MCI and APOE2 and APOE4 genotypes among diverse Latino backgrounds. APOE2 is relatively uncommon among Mainland Amerindian-admixed populations, and our study may have been underpowered to detect significant APOE2 protective effect differences between Latino backgrounds. Our APOE4 findings replicate previous studies that showed little to no association between APOE4 genotype and MCI among diverse Latinos, regardless of the background [5,8,9]. Our findings suggest that the eti-ology of MCI may be different among African- and Amerindian-admixed Latinos as compared with persons of European ancestry. Although we anticipated some relationship between APOE4 and MCI among Cubans who have higher degrees of European ancestry [6], such differences were not found in the SOL-INCA. This may be related to European colonists originating from southern Europe, which is a region with low APOE4 frequencies and different admixture patterns compared with northern Europeans [30].
This study had several strengths and limitations. One of the strengths was our inclusion of both cognitive impairment and significant cognitive decline, which are both NIA-AA MCI diagnostic criteria [18]. This was done to limit identification of false-positive MCI cases in this sample of diverse Latinos with low education levels. Pre-vious research has demonstrated that estimating premorbid function reduces false-positive cases associated with educational quality [21]. Although there may be some lost sensitivity for detecting MCI cases, we anticipate that our research diagnostic approach will improve identification of persons who will convert from MCI to demen-tia. Additionally, omitting the cognitive decline diagnostic criterion effectively doubled our MCI prevalence estimates to levels seen in other research [4]. First, the PVT overcomes major challenges in assessing Spanish speakers’ premorbid cognition (i.e., reading level) because Spanish-language pronunciations are regular. Second, we leveraged the HCHS/SOL sampling procedures that enabled the SOL-INCA to make population-level inferences to targeted metropolitan areas. Our study is somewhat limited by our brief cognitive assessment battery, which relied on the participant’s self-reported subjective cognitive decline and functional status. In the SOL-INCA, we used our mental status (SIS) to construct our robust normative sample; however, SIS was not used as a measure of cognitive decline. Although optional in NIA-AA criteria, the SOL-INCA did not include informant reports. Including infor-mants in the SOL-INCA to corroborate participants’ self-reports could have improved case identifications. Third, the SOL-INCA cohort is younger than that of older studies of cognitive aging and disorders among persons aged 65 years and older, which could explain the low MCI rates reported by us. It should be noted that our MCI findings among SOL-INCA participants aged 70–80 years are comparable with those of a recent study of Mexican Americans in Texas and in ARIC-NCS [9]. Fourth, a sizable number of Latinos did not consent to genetic testing, which reduced the sample size of APOE analyses and statistical power to detect APOE associations with MCI. Nevertheless, our sensitivity analyses suggested that our APOE results would not have changed. Fifth, this study lacked AD biomarkers to examine the extent of preclinical AD. Finally, we relied on the FCRS for estimating CVD risk; however, the FCRS has not been validated in diverse Latinos.
5. Conclusions
MCI varied among Latino backgrounds, but less so than in previous studies. APOE4 was not significantly associated with increased risk for MCI, which suggests that alternative etiologic factors may contribute to MCI among diverse Hispanics/Latinos. We found that high CVD risk and depressive symptoms were associated with increased MCI prevalence. Both conditions are modifiable, which suggests pathways for improving healthy brain aging and reducing Latino and national ADRD burden.
Supplementary Material
RESEARCH IN CONTEXT.
Systematic review: Latinos are a diverse group and represent nearly 20% of the US. Currently, little to nothing is known about mild cognitive impairment (MCI) in diverse Latino backgrounds (e.g., Central Americans). In this multisite, prospective cohort study of diverse middle-aged and older Latinos (N = 6377), we found that 9.8% met MCI criteria, and MCI prevalence significantly varied by age among Latino groups.
Interpretation: Cardiovascular disease risk and depressive symptoms were significant correlates of MCI prevalence.
Future direction: Cardiovascular disease and depressive symptoms are modifiable risk factors for MCI among diverse Latinos that afford opportunities for reducing dementia-disease burden in this large, rapidly growing, but understudied population facing health disparities.
Acknowledgments
The authors thank the staff and participants of HCHS/SOL and SOL-INCA for their important contributions. Investigators website - http://www.cscc.unc.edu/hchs/.
Funding/Support: This work is supported by the National Institute on Aging (R01AG048642, RF1AG054548, RF1AG061022, and R21AG056952). Dr. González also receives additional support from P30AG005131 and P30AG059299. The Hispanic Community Health Study/Study of Latinos was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institution-Office of Dietary Supplements.
Role of Funding Source: This work was supported by the National Institute on Aging and National Heart, Lung, and Blood Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Financial Disclosures: The authors report no conflicts of interest that could inappropriately influence, or be perceived to influence, this work.
Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jalz.2019.08.202.
References
- [1].Census. American Community Survey. 2017. Available at: http://www.census.gov/programs-surveys/acs Accessed October 11, 2019.
- [2].Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060 Population Estimates and Projec-tions. Current Population Reports. US Census Bureau; 2015. p. 25–1143. [Google Scholar]
- [3].Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, et al. Racial and ethnic estimates of Alzheimer’s disease and related demen-tias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement 2019;15:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EH, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry 1999;14:481–93. [PubMed] [Google Scholar]
- [5].Haan MN, Mungas DM, González HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc 2003;51:169–77. [DOI] [PubMed] [Google Scholar]
- [6].Conomos MP, Laurie CA, Stilp AM, Gogarten SM, McHugh CP, Nelson SC, et al. Genetic diversity and association studies in US Hispanic/Latino populations: applications in the Hispanic Community Health Study/Study of Latinos. Am J Hum Genet 2016;98:165–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].González HM, Tarraf W, Rodríguez CJ, Gallo LC, Sacco RL, Talavera GA, et al. Cardiovascular health among diverse Hispanics/ Latinos: Hispanic Community Health Study/Study of Latinos (HCHS/SOL) results. Am Heart J 2016;176:134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA 1998;279:751–5. [DOI] [PubMed] [Google Scholar]
- [9].O’Bryant SE, Johnson L, Reisch J, Edwards M, Hall J, Barber R, et al. Risk factors for mild cognitive impairment among Mexican Americans. Alzheimers Dement 2013;9:622–631.e621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schneiderman N, Llabre M, Cowie CC, Barnhart J, Carnethon M, Gallo LC, et al. Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic Community Health Study/ Study of Latinos (HCHS/SOL). Diabetes Care 2014;37:2233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hachinski V, Einhäupl K, Ganten D, Alladi S, Brayne, Stephan BC, et al. Preventing dementia by preventing stroke: the Berlin Manifesto. Alzheimer’s Dement 2019;15:961–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].LaVange LM, Kalsbeek WD, Sorlie PD, Aviles-Santa SM, Kaplan RC, Barnhart J,et al. SampledesignandcohortselectionintheHispanicCom-munity Health Study/Study of Latinos. Ann Epidemiol 2010;20:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010;20:629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential sub-jects for clinical research. Med Care 2002;40:771–81. [DOI] [PubMed] [Google Scholar]
- [15].González HM, Mungas D, Reed BR, Marshall S, Haan MN. A new verbal learning and memory test for English- and Spanish-speaking older people. J Int Neuropsychol Soc 2001;7:544–55. [DOI] [PubMed] [Google Scholar]
- [16].Lezak M, Howieson DB, Loring DW. Neuropsychological Assess-ment New York: Oxford University Press; 2004. p. 545–6. [Google Scholar]
- [17].Wechsler D WAIS-R Manual. San Antonio, TX: Psychological Cor-poration; 1981. [Google Scholar]
- [18].Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, et al. The measurement of everyday cognition (ECog): scale develop-ment and psychometric properties. Neuropsychology 2008;22:531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fillenbaum GG. Multidimensional Functional Assessment of Older Adults: The Duke Older Americans Resources and Services Procedures 1988. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. p. 7–11. [Google Scholar]
- [21].Manly JJ, Byrd DA, Touradji P, Stern Y. Acculturation, reading level, and neuropsychological test performance among African American el-ders. Appl Neuropsychol 2004;11:37–46. [DOI] [PubMed] [Google Scholar]
- [22].Kannel WB, McGee D, Gordon T. A general cardiovascular risk pro-file: the Framingham Study. Am J Cardiol 1976;38:46–51. [DOI] [PubMed] [Google Scholar]
- [23].D’agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardio vascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–53. [DOI] [PubMed] [Google Scholar]
- [24].González HM, Tarraf W, Jian X, Vásquez PM, Kaplan R, Thyagarajan B, et al. Apolipoprotein E genotypes among diverse middle-aged and older Latinos: Study of Latinos-Investigation of Neurocognitive Aging results (HCHS/SOL). Scientific Rep 2018;8:17578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wassertheil-Smoller S, Arredondo EM, Cai J, Castaneda SF, Choca JP, Gallo LC, et al. Depression, anxiety, antidepressant use, and cardiovascular disease among Hispanic men and women of different national backgrounds: results from the Hispanic Community Health Study/ Study of Latinos . Ann Epidemiol 2014;24:822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. JAMA Psychiatry 2006;63:273–9. [DOI] [PubMed] [Google Scholar]
- [27].Harrington KD, Gould E, Lim YY, Ames D, Pietrzak RH, Rembach A, et al. Amyloid burden and incident depressive symptoms in cognitively normal older adults. Int J Geriatr Psychiatry 2017;32:455–63. [DOI] [PubMed] [Google Scholar]
- [28].Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, et al. Mild cognitive impairment and dementia prevalence: the atherosclerosis risk in communities neurocognitive study. Alzheimers Dement 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vermeer SE, Den Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke 2003;34:392–6. [DOI] [PubMed] [Google Scholar]
- [30].Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Ann Hum Genet 1999; 63:301–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.