Summary
Background
Relamorelin, a pentapeptide ghrelin receptor agonist, accelerated gastric emptying significantly and improved symptoms in adults with diabetic gastroparesis in phase 2 trials.
Aim
To assess the safety and tolerability of relamorelin across phase 2 trials.
Methods
Safety assessments in patients aged 18‐75 years (weight, adverse events [AEs] and laboratory tests) from two randomised, double‐blind phase 2 trials (NCT01571297, NCT02357420; results published previously) were reviewed descriptively. Analysis of covariance assessed treatment effect on glycated haemoglobin (HbA1c) and blood glucose post hoc. Phase 2a and 2b trial durations were, respectively, 4 weeks (relamorelin 10 µg once or twice daily [b.d.] or placebo b.d.) and 12 weeks (relamorelin 10, 30 or 100 µg or placebo b.d.) with 1‐ and 2‐week, single‐blind placebo run‐ins.
Results
Among 204 phase 2a and 393 phase 2b patients, respectively, 67% and 62% were female, and 88% and 89% had type 2 diabetes. Proportions of patients reporting serious AEs were similar across treatment groups, as were those with ≥1 treatment‐emergent AE (TEAE). TEAE‐related discontinuations were proportionally higher in relamorelin groups than placebo. Of 12 serious TEAEs in phase 2a, none occurred in >1 patient. In phase 2b, five serious TEAEs were reported in >1 patient, and one (100 µg) died (urosepsis), all unrelated to relamorelin. In phase 2b, increased HbA1c and fasting blood glucose levels were dose‐related (P < 0.0001 and P = 0.0043, respectively).
Conclusions
Relamorelin showed acceptable safety and tolerability in phase 2 trials. Relamorelin may elevate blood glucose: this should be managed proactively in relamorelin‐treated patients.
1. INTRODUCTION
Gastroparesis is defined as delayed gastric emptying in the absence of mechanical obstruction. Symptoms include early satiety, nausea, abdominal pain, bloating, post‐prandial fullness and vomiting, which affect 20‐40% of diabetic patients and can become debilitating with increased symptom frequency. 1 , 2 Gastroparesis can lead to malnutrition and can impair glycaemic control in diabetic patients. 3 An earlier meta‐regression study suggested that the severity of symptoms does not necessarily correlate with gastric emptying time, 4 although more recent systematic reviews and meta‐analyses of studies using optimised measurements of gastric emptying of solids over at least 3 hours show significant correlation of gastric emptying and upper gastrointestinal symptoms. 5 , 6 Hence, improvement in delayed gastric emptying rates in patients with diabetic gastroparesis can provide a clinically meaningful treatment approach.
Currently available treatment options for diabetic gastroparesis are very limited. Metoclopramide, the sole treatment currently approved for diabetic gastroparesis by the US Food and Drug Administration (FDA), is a dopamine D2 receptor antagonist as well as a serotonin type 4 (5‐HT4) receptor agonist. It acts centrally as an antiemetic (by inhibition of 5‐HT3 receptors as well as D2 receptors in the chemoreceptor trigger zone) and promotes gut motility by three mechanisms: inhibition of pre‐ and post‐synaptic D2 receptors, stimulation of pre‐synaptic excitatory 5‐HT4 receptors and antagonism of pre‐synaptic inhibition of muscarinic receptors. 7 It carries a black box warning due to the risk of irreversible tardive dyskinesia, and current FDA guidelines recommend limiting chronic treatment to 12 weeks where possible. Antiemetics are relied on for symptom relief or reduction in nausea and vomiting, while domperidone and erythromycin are used off‐label for their prokinetic properties, which can lead to some symptom relief. However, there are reports of an association between domperidone (also a dopamine D2 receptor antagonist) and cardiovascular safety concerns, 8 and erythromycin is associated with tachyphylaxis and prolongation of the QTc interval. 9 , 10 , 11 Centrally acting antidepressants, specifically tricyclic agents such as nortriptyline, are also used off‐label but have not shown efficacy in a randomised controlled trial in patients with gastroparesis. 12 The surgical option of gastric electrical stimulation (which works as a centrally acting antiemetic) was approved by the FDA on a humanitarian device exemption for severe cases, refractory to all standard treatments 13 and a combination of gastric electrical stimulation with pyloroplasty has shown promising results in a single‐arm trial. 14 Treatments based on per‐oral endoscopic myotomy are also reported to be efficacious; however, these are based on uncontrolled studies. 15 Therefore, an unmet need remains for safe and effective treatment options for patients with diabetic gastroparesis.
Relamorelin is a pentapeptide ghrelin receptor agonist with pro‐kinetic properties, which significantly accelerated gastric emptying and improved symptoms in patients with diabetic gastroparesis in phase 2 trials. 16 , 17 Ghrelin receptor agonists may stimulate gastric contractions 18 and enhance gastric emptying. However, they may also increase glycaemia, 19 , 20 in part by reducing insulin secretion, stimulating growth hormone release or enhancing carbohydrate absorption. 21 , 22 , 23
Compared with native ghrelin, relamorelin has been shown to exhibit enhanced potency and plasma stability, with a terminal half‐life in humans of approximately 4.5 hours. 17 , 24 Toxicological studies show a >750‐fold safety margin compared with clinical trial dose exposures. 17 , 24 In a small phase 1 study in diabetic patients with delayed gastric emptying (single dose, 100 µg subcutaneous relamorelin), relamorelin significantly accelerated gastric emptying time compared with placebo, and the pharmacokinetics of relamorelin were similar in diabetic patients with delayed gastric emptying and healthy volunteers (Cmax ~ 4 ng/mL in both groups). 25 Despite a higher prevalence of adverse events (AEs) in the relamorelin group, no safety concerns were highlighted, although blood glucose values measured at 120 minutes were numerically higher with relamorelin treatment compared with placebo (P = 0.07). 25
In addition to AEs relating to diabetic control, an analysis of the safety of relamorelin should also appraise major adverse cardiovascular events relating to ischaemic coronary disorders and long‐term cardiovascular safety.
The aim of this analysis was to assess the overall safety and tolerability of relamorelin in adults with diabetic gastroparesis across two phase 2 trials. Given the current unmet need for an effective diabetic gastroparesis treatment with a favourable safety profile, the safety of any new treatment in this therapy area is of particular importance. Following on from the original clinical trial publications, this article presents additional safety data from both clinical trials side by side, and explores the AE profile of relamorelin in greater depth and in the context of its mechanism of action as a ghrelin receptor agonist.
2. MATERIALS AND METHODS
2.1. Trial designs
Randomised, double‐blind, placebo‐controlled phase 2a and 2b clinical trials of subcutaneous relamorelin (NCT01571297 and NCT02357420, respectively) were conducted in patients aged 18‐75 years with diabetic gastroparesis and have been described previously. 16 , 17 Trial designs are shown in Figure 1. In each trial, patients were required to have ≥3 months’ history of gastroparesis symptoms as well as confirmed delayed gastric emptying via the gastric emptying breath test (GEBT), with vomiting (or nausea for phase 2a) in the 2‐week pre‐screening period and a glycated haemoglobin (HbA1c) value of ≤11%, among other previously described criteria.
FIGURE 1.
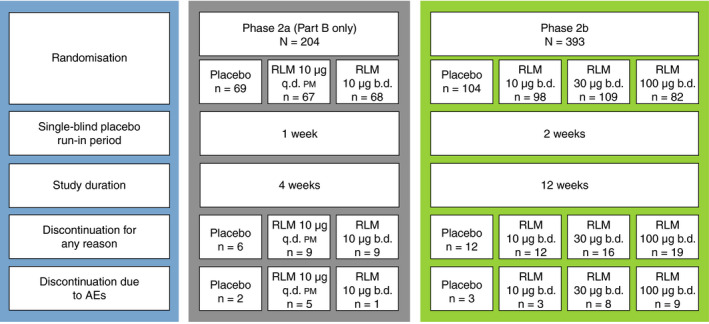
Phase 2a and 2b trial designs. AE, adverse event; b.d., twice daily; q.d. pm, once daily in the evening; RLM, relamorelin
The phase 2a trial followed an adaptive design consisting of two parts. In Part A, the optimum dose regimens and cohort size to be used in Part B were determined. Part B comprised 204 patients, of whom 69 received placebo twice daily (b.d.), 67 received relamorelin 10 µg once daily in the evening (q.d. pm) and 68 received relamorelin 10 µg b.d. The safety results presented are for Part B only (no particular safety concerns were noted in Part A).
Of 393 randomised patients in the phase 2b trial, 104 received placebo b.d., 98 received relamorelin 10 µg b.d., 109 received relamorelin 30 µg b.d. and 82 received relamorelin 100 µg b.d.
Both studies were conducted in accordance with the Declaration of Helsinki and were approved by the institutional review board. All participants in each trial gave written, informed consent. Further details of the trial designs are published elsewhere. 16 , 17
2.2. Safety assessments
Adverse events were recorded from the first screening visit through to the final study visit. Treatment‐emergent AEs (TEAEs) were defined as AEs that started on or after the first injection of double‐blind study drug, or AEs that occurred prior to the first injection but worsened in severity after the first injection. All AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 15.0, summarised by system organ class and preferred term. An event was considered serious if it resulted in death or immediate risk of death, hospitalisation, congenital anomaly or disability, or if it required medical or surgical intervention to prevent any of those outcomes, in the opinion of the investigator or sponsor. The intensity of AEs was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events. The relationship to study drug was categorised into ‘none’, ‘unlikely’, ‘possible’ or ‘probable’ by the investigator; any AEs in the latter two categories were considered treatment related.
A complete physical examination was performed at the first and final visits. Injection sites were assessed at each clinic visit. Weight was recorded at each visit; the weighing took place at approximately the same time of day, when patients were fasting, were without shoes and had empty bladders. Vital signs were measured at each visit, obtained in the sitting position following at least 5 minutes of rest. Twelve‐lead supine electrocardiograms (ECGs) were performed at all visits, following 5 minutes of rest. Measurements included QT, QTc calculated using Fridericia's formula (QTcF), QRS and PR intervals, and ventricular rate. Although the definition of ‘normal’ QTcF interval can be subjective, 26 current guidelines recommend 450 ms for men and 460 ms for women as an upper threshold for a normal range 27 ; in this study, thresholds of >450 ms and >500 ms were predefined as clinically notable, regardless of gender.
Clinical laboratory testing was performed at a central laboratory facility. Tests included blood glucose and insulin measurements, collected during the GEBT assessment days, both before the meal (fasting) and at 60, 90, 120, 180 and 240 minutes after the meal, to monitor changes in glycaemic response in the phase 2a trial. Post‐meal samples were frozen and were retained to be analysed (only if necessary) to aid in glucose homeostasis evaluation. HbA1c was measured at baseline and Day 28 in the phase 2a trial, and at screening (Day −45 to −15), Visit 7 (Day 56) and Visit 8 (Day 84) as part of the phase 2b trial.
2.3. Glycaemic monitoring
Although blood glucose was measured in both trials, proactive blood glucose monitoring and management was not routinely carried out. Any individual value that exceeded a predefined ‘potentially clinically significant’ threshold triggered a laboratory alert. For fasting blood glucose, the predefined threshold was >1.2 × upper limit of normal (ULN; ‘normal’ is patient specific in the setting of an individual patient with diabetes). However, there was no predefined threshold mandating that the value be recorded as an AE; this was at the discretion of the investigator. According to MedDRA version 15.0, ‘hyperglycaemia’ and ‘blood glucose increased’ are classified as separate terms, leading to different system organ classifications (‘metabolism and nutrition disorders’ and ‘investigations’, respectively), dependent on the language used in the investigator's report. Therefore, AEs coded as ‘blood glucose increased’ and ‘hyperglycaemia’ cannot be definitively distinguished. In addition, the reported term ‘worsening gastroparesis’ was coded using the MedDRA term ‘impaired gastric emptying’ (as the nearest match); this term did not indicate documented evidence of delayed gastric emptying, which was an inclusion criterion for participation in the study.
2.4. Statistical analysis
The safety analysis set for each trial comprised all patients who received ≥1 dose of the study drug (excluding Part A of the phase 2a trial). Descriptive statistics were used to summarise the safety data, using SAS statistical software version 9.3 (SAS Institute Inc). MedDRA version 15.0 was used to code medical history and AEs. Analysis of covariance was used post hoc to assess the effect of relamorelin treatment on HbA1c and glucose. A linear trend test was also performed on these data.
3. RESULTS
3.1. Patient demographics and healthcare characteristics
The demographics and healthcare characteristics of the phase 2a and phase 2b trial participants are presented in Table 1.
TABLE 1.
Demographics and healthcare characteristics
| Demographics and healthcare characteristics | Phase 2a (N = 204) | Phase 2b (N = 393) |
|---|---|---|
| Mean age, y | 55 | 57 |
| Female, % | 67 | 62 |
| Diabetes type, n (%) | ||
| Type 1 | 24 (11.8) | 39 (9.9) |
| Type 2 | 180 (88.2) | 351 (89.3) |
| Type 1 and type 2 | 0 | 3 (0.8) |
| BMI, kg/m2, mean (range) | 33 (19‐52) | 32 (18‐60) |
Abbreviation: BMI, body mass index.
3.2. Prevalence of TEAEs
The proportion of patients experiencing ≥1 TEAE was generally similar across treatment groups (Figure 2), with slightly higher proportions in the relamorelin 30 µg and 100 µg groups in the phase 2b trial compared with the 10 µg and placebo groups. The most commonly reported TEAEs in relamorelin‐treated patients (≥5% in any treatment group) were headache and worsening diabetes mellitus in the phase 2a trial, and hyperglycaemia (and increased blood glucose), urinary tract infection, headache, dizziness and diarrhoea in the phase 2b trial (Table 2). No clinically important injection site reactions were reported in either study.
FIGURE 2.
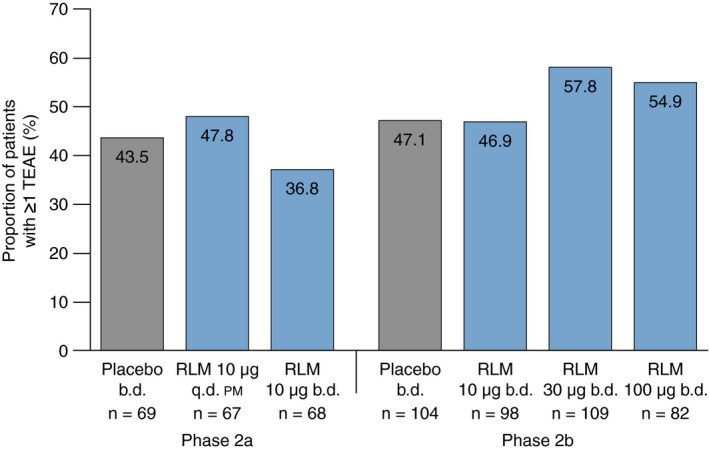
Proportion of patients in each treatment group with ≥1 TEAE. b.d., twice daily; q.d. pm, once daily in the evening; RLM, relamorelin; TEAE, treatment‐emergent adverse event
TABLE 2.
Most common TEAEs occurring in the phase 2a and phase 2b trials
| TEAEs ≥5% in any treatment group in either study, n (%) | Phase 2a | Phase 2b | |||||
|---|---|---|---|---|---|---|---|
| Placebo | Relamorelin | Placebo | Relamorelin | ||||
| b.d. (n = 69) | 10 µg q.d. pm (n = 67) | 10 µg b.d. (n = 68) | b.d. (n = 104) | 10 µg b.d. (n = 98) | 30 µg b.d. (n = 109) | 100 µg b.d. (n = 82) | |
| Constipation | 4 (5.8) | 1 (1.5) | 1 (1.5) | 3 (2.9) | 0 | 3 (2.8) | 0 |
| Diarrhoea | 2 (2.9) | 3 (4.5) | 1 (1.5) | 0 | 4 (4.1) | 7 (6.4) | 6 (7.3) |
| Dizziness | 4 (5.8) | 1 (1.5) | 1 (1.5) | 1 (1.0) | 0 | 1 (0.9) | 5 (6.1) |
| Headache | 2 (2.9) | 2 (3.0) | 5 (7.4) | 3 (2.9) | 4 (4.1) | 6 (5.5) | 2 (2.4) |
| Urinary tract infection | 4 (5.8) | 2 (3.0) | 2 (2.9) | 7 (6.7) | 7 (7.1) | 8 (7.3) | 7 (8.5) |
| TEAEs related to glycaemic control | |||||||
| Hyperglycaemia | 1 (1.4) | 2 (3.0) | 0 | 2 (1.9) | 5 (5.1) | 10 (9.2) | 10 (12.2) |
| Increased blood glucose | 1 (1.4) | 0 | 1 (1.5) | 1 (1.0) | 3 (3.1) | 4 (3.7) | 6 (7.3) |
| Diabetes mellitus | 2 (2.9) | 1 (1.5) | 4 (5.9) | 0 | 2 (2.0) | 1 (0.9) | 3 (3.7) |
Abbreviations: b.d., twice daily; q.d. pm, once daily in the evening; TEAE, treatment‐emergent adverse event.
3.3. Serious TEAEs
The incidence of serious TEAEs was generally similar between treatment groups (Table 3), although the proportion of cases was slightly higher in the 12‐week phase 2b study compared with the 4‐week phase 2a study. There were 12 serious TEAEs overall in the phase 2a trial, reported in seven patients. None of these serious TEAEs was experienced by >1 patient, and there were no discernible trends. In the phase 2b trial, there were five serious TEAEs that were experienced by >1 patient: worsening gastroparesis, which was reported in four patients (two in the placebo group and one each in the relamorelin 10 µg and 100 µg groups); unstable angina, reported in two patients in the 30 µg group; chronic obstructive pulmonary disease, reported in two patients (placebo and relamorelin 30 µg groups); diabetic ketoacidosis, reported in two patients (relamorelin 10 µg and 30 µg groups) and acute renal failure, reported in two patients (relamorelin 30 µg and 100 µg groups). None of these events was assessed as related to the study drug by the investigator. One patient in the phase 2b relamorelin 100 µg treatment group died. The death was attributed to urosepsis and was assessed by the investigator as unrelated to study drug.
TABLE 3.
Serious TEAEs or those leading to discontinuation
| TEAE summary | Phase 2a | Phase 2b | |||||
|---|---|---|---|---|---|---|---|
| Placebo | Relamorelin | Placebo | Relamorelin | ||||
| b.d. (n = 69) | 10 µg q.d. pm (n = 67) | 10 µg b.d. (n = 68) | b.d. (n = 104) | 10 µg b.d. (n = 98) | 30 µg b.d. (n = 109) | 100 µg b.d. (n = 82) | |
| Total serious TEAEs, n (%) | 2 (2.9) | 1 (1.5) | 4 (5.9) | 8 (7.7) | 7 (7.1) | 10 (9.2) | 6 (7.3) |
| TEAEs leading to study drug discontinuation, n (%) | 2 (2.9) | 5 (7.5) | 1 (1.5) | 3 (2.9) | 3 (3.1) | 8 (7.3) | 9 (11.0) |
Abbreviations: b.d., twice daily; q.d. pm, once daily in the evening; TEAE, treatment‐emergent adverse event.
3.4. TEAEs leading to discontinuation
Overall, six relamorelin‐treated (4.4%) and two placebo‐treated (2.9%) patients in the phase 2a trial discontinued the study drug due to TEAEs (Table 3). Each reason for discontinuation occurred only once, although each patient could cite multiple reasons. For the placebo‐treated patients, the reasons cited were fatigue, pneumonia, hyperhidrosis and dizziness. For relamorelin‐treated patients, reasons for discontinuation included acute myocardial infarction, vomiting, worsening diabetes, depression, nervousness, blister, nephrolithiasis and obstructive uropathy. Of 23 (5.9%) patients in the phase 2b trial who discontinued due to TEAEs, three (2.9%) were in the placebo group, and three (3.1%), eight (7.3%) and nine (11.0%) were in the relamorelin 10, 30 and 100 µg groups, respectively (Table 3); of the 20 patients who discontinued while receiving relamorelin treatment, eight discontinued due to TEAEs related to glycaemic control (zero in the placebo group).
3.5. TEAEs related to diabetic control
The proportion of patients experiencing hyperglycaemia (or increased blood glucose) or worsening of diabetes during the trials generally increased with increasing relamorelin dosage. During the phase 2b trial, the mean change from baseline to Week 12 in HbA1c levels also generally increased with relamorelin dose (P < 0.0001; Figure 3). Mean baseline HbA1c values were 7.77%, 7.44%, 7.65% and 8.15%, and mean end of study values were 7.80%, 7.94%, 8.54% and 8.84% for the placebo, relamorelin 10, 30 and 100 µg treatment groups respectively. The mean change in fasting blood glucose also increased with relamorelin dose over the study period (linear trend: P = 0.0043; Figure 4). A similar proportion of patients in each treatment group experienced a fasting blood glucose level >1.2 × ULN during the study (83.5%, 81.1%, 81.4% and 82.6% in the placebo and relamorelin 10, 30 and 100 µg groups respectively).
FIGURE 3.
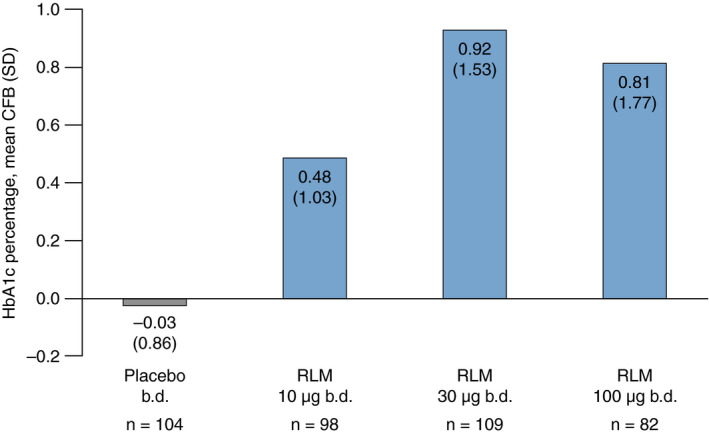
Mean change from baseline in percentage HbA1c values for each treatment group in the phase 2b trial. HbA1c values were measured in %. b.d., twice daily; CFB, change from baseline; HbA1c, glycated haemoglobin; RLM, relamorelin; SD, standard deviation
FIGURE 4.

Change from baseline of fasting blood glucose values for each treatment group in the phase 2a and phase 2b trials. To convert blood glucose values from units of mmol/L to units of mg/dL, multiply by 18.0. In phase 2a, CFB values were 4.14, 17.48 and 10.99 mg/dL for the placebo, relamorelin 10 µg q.d. pm and 10 µg b.d. groups. In phase 2b, CFB values were 25.04, 32.61, 43.24 and 56.39 mg/dL for the placebo, relamorelin 10, 30 and 100 µg groups, respectively. b.d., twice daily; CFB, change from baseline; q.d. pm, once daily in the evening; RLM, relamorelin; SD, standard deviation
Discontinuations related to glycaemic control were carefully appraised. One phase 2a patient in the relamorelin 10 µg q.d. pm group discontinued due to worsening diabetes mellitus; this was considered to be related to the study drug by the investigator. In phase 2b, eight patients discontinued due to AEs relating to glycaemic control: none in the placebo or relamorelin 10 µg groups; one due to increased blood glucose and one due to worsening diabetes mellitus in the relamorelin 30 µg group; and three due to hyperglycaemia, two due to increased blood glucose and one due to worsening diabetes mellitus in the relamorelin 100 µg group. The terminology used here conforms with the specific MedDRA terms used in the case report forms.
Diabetic ketoacidosis was reported in three relamorelin‐treated patients, one in each phase 2b relamorelin treatment arm. All three cases were assessed by the investigator to be associated with accepted risk factors for diabetic ketoacidosis, and unrelated to study drug. The case in the 10 µg group occurred 11 days after cessation of study drug, and the case in the 100 µg group was mild (not classed as a serious TEAE) and did not result in hospitalisation. The final case (in the 30 µg group) was attributed to incorrect insulin administration. Diabetic ketoacidosis was also documented in one phase 2a patient during the single‐blind placebo run‐in period; therefore, it was not considered to be treatment emergent.
The mean change in weight from baseline ranged from −0.07 kg to 0.59 kg across all treatment groups (Table 4); these small changes were not considered clinically relevant.
TABLE 4.
Mean change from baseline in weight
| Phase 2a | Phase 2b | ||||||
|---|---|---|---|---|---|---|---|
| Placebo | Relamorelin | Placebo | Relamorelin | ||||
| b.d. (n = 69) | 10 µg q.d. pm (n = 67) | 10 µg b.d. (n = 68) | b.d. (n = 104) | 10 µg b.d. (n = 98) | 30 µg b.d. (n = 109) | 100 µg b.d. (n = 82) | |
| Weight CFB a , kg, mean (SD) | −0.01 (1.6) | 0.2 (1.6) | 0.17 (2.0) | 0.25 (2.6) | −0.07 (2.7) | 0.59 (3.0) | 0.46 (2.2) |
Abbreviations: b.d., twice daily; CFB, change from baseline; q.d. pm, once daily in the evening; SD, standard deviation.
Study duration was 4 weeks for the phase 2a trial and 12 weeks for phase 2b.
3.6. TEAEs related to cardiovascular safety
Aside from the unstable angina reported in two phase 2b patients in the relamorelin 30 µg treatment group, no cardiac TEAEs occurred in ≥2% of any relamorelin treatment group. One patient in the phase 2a trial (relamorelin 10 µg q.d. pm) discontinued medication due to acute myocardial infarction (which was considered unrelated to study drug). This patient had a history of coronary artery disease, peripheral artery disease, hypertension and hyperlipidaemia. In the phase 2b trial, five relamorelin‐treated patients experienced serious cardiovascular events overall, of whom three discontinued study participation: unstable angina (30 µg group), in a patient with previous hypertension; cardio‐respiratory arrest (30 µg group), in a patient with a history of coronary artery disease, myocardial infarction and coronary artery bypass grafting (the patient was successfully resuscitated); and worsening atherosclerosis of the coronary artery (10 µg group), in a patient who had previously undergone coronary artery stent implantation. Events where the patient did not discontinue included a further case of unstable angina (30 µg group) and worsening of congestive heart failure (10 µg group). Of the five events, four were classed as unrelated or unlikely to be related to study drug, and one (worsening of congestive heart failure) was classed as possibly related.
No clinically significant changes were identified in ECGs of patients with data available at both baseline and end of study (178 phase 2a and 369 phase 2b patients). In the phase 2a trial, six (10.3%) and two (3.4%) patients who were receiving relamorelin 10 µg q.d. pm and b.d., respectively, and seven (11.5%) patients in the placebo group had a QTcF interval >450 ms on ≥1 post‐dosing time point during the study; however, no patient had a QTcF interval exceeding 500 ms at any study time point post‐dosing. In the phase 2b trial, four (4.2%), two (1.9%) and one (1.4%) patients receiving relamorelin 10, 30 or 100 µg b.d., respectively, and two (2.1%) patients in the placebo group had a QTcF interval >450 ms; one patient in the relamorelin 10 µg group and one in the relamorelin 30 µg group had a QTcF interval that exceeded 500 ms. Neither of these studies had a pre‐specified exclusion criterion relating to prolonged QTc interval. No patient in any group had a prolonged QTc interval that was reported as a TEAE and no patient with a prolonged QTc interval at either entry to or exit from the studies experienced a cardiovascular TEAE that was considered related to prolonged QTc interval.
4. DISCUSSION
These data from phase 2 trials show that relamorelin 10‐100 µg was generally well tolerated in adults with diabetic gastroparesis. The proportions of patients with TEAEs in the phase 2a trial were low and generally comparable with placebo. However, in the longer phase 2b trial, in which higher doses of relamorelin were administered, more TEAEs were observed in relamorelin‐treated patients, especially TEAEs relating to glycaemic control. Taken together with the dose‐dependent relationships observed between relamorelin and HbA1c levels or increased blood glucose, these findings suggest that the 10 µg b.d. dose is best tolerated. Importantly, this dose maintains the efficacy of relamorelin in increasing gastric emptying and improving symptoms. 16 , 17
Symptoms of gastric motor dysfunction are prevalent among diabetic patients. 1 Diabetic neuropathy, loss of interstitial cells of Cajal within the walls of the stomach, blood glucose fluctuations and psychosomatic factors may all contribute to gastric motor dysfunction by disturbing the functions of smooth muscle and enteric and extrinsic autonomic nerves, which control gastric emptying. 28 Ghrelin, the natural ligand for the growth hormone secretagogue receptor, is produced in the stomach 29 and may mediate gastric contractions and emptying through stimulation of the vagal nuclei and vagus nerve, and has been shown to promote gastric emptying in human and animal studies. It also increases appetite via the stimulating effects of ghrelin on growth hormone receptor centres in the hypothalamus. Relamorelin, with its longer plasma circulating half‐life and stability, is sixfold more potent than native human ghrelin at activating the ghrelin receptor, 24 and has been shown to accelerate gastric emptying and increase gastric antral contractility without impeding gastric accommodation or altering satiation in healthy volunteers. 18
Acute hyperglycaemia (blood glucose approximately >15 mmol/L [>270 mg/dL]) 30 is one of the known risk factors for delayed gastric emptying in diabetic patients. While no evidence has been found that improving glycaemic control leads to acceleration of gastric emptying, 31 , 32 one goal of treatment for patients with diabetes is to stabilise glucose fluctuations by improving gastric emptying, leading to better synchronisation with post‐prandial or pre‐prandial insulin dosing. Acceleration of gastric emptying—from the pro‐kinetic effects of ghrelin or an increase in post‐prandial glucose levels due to increased food intake caused by larger appetite induced by the ghrelin receptor agonist—may worsen hyperglycaemia. The effect of gastric pro‐kinetics on increased early post‐prandial blood glucose has also been observed with cisapride and erythromycin 33 , 34 ; however, these may also stimulate insulin secretion if there is a normal reserve of islet beta cells. 35 Other potential mechanisms affecting hyperglycaemia in patients treated with ghrelin agonists are inhibition of insulin secretion from islet cells of the pancreas, or enhanced insulin resistance via elevation of growth hormone levels. Long‐term treatment with oral ghrelin mimetic agents has been reported to be associated with increases in both fasting plasma glucose and HbA1c values within elderly populations. 36 , 37
Therefore, hyperglycaemia might be expected given the mechanisms of action of relamorelin, consistent with our finding that TEAEs relating to diabetic control increased with increasing relamorelin dose. Although there is evidence in healthy rodents that prolonged treatment with ghrelin for 21 days regulates plasma glucose and restores insulin to normal levels, 38 the current data suggest that proactive steps (eg self‐monitoring of blood glucose levels) should be instituted to enhance glycaemic control in the management of patients receiving treatment with ghrelin receptor agonists, and this strategy has been implemented in the ongoing phase 3 trials of relamorelin (NCT03285308; NCT03426345).
While a comparatively high proportion of patients experienced headache and urinary tract infection, the incidence of these AEs was generally similar across relamorelin and placebo treatment groups and did not generally increase with relamorelin dose. In addition, a high incidence of urinary tract infection is consistent with a diabetic, predominantly female population. 39 It is also not surprising that diarrhoea was commonly reported, as this is consistent with the pro‐kinetic effects of relamorelin, which can extend beyond gastric emptying to include the small bowel and colon. 40 This could indicate clinical applications for patients with other gastrointestinal motility disorders. 41 Although relamorelin has been shown to induce feeding and weight gain in multiple animal studies, 42 , 43 , 44 , 45 weight changes in the phase 2 trials were small and not considered clinically relevant. To date, it remains unclear whether the 10 µg dose of relamorelin increases appetite in diabetic gastroparesis patients.
Due to unpredictable gastric emptying in patients with diabetic gastroparesis, subcutaneous administration of medication may be considered more appropriate than oral delivery, as it is considered more likely that a predictable plasma level for the medication can be achieved. No clinically important injection site reactions were observed, which suggests that the subcutaneous delivery of relamorelin is well tolerated.
As there is a link between ghrelin and the cardiovascular system, 46 , 47 cardiovascular safety was of particular interest in the phase 2 trials, especially given the large proportion of obese study patients (mean body mass index >30 kg/m2 for each trial). 48 In all, there were very few serious cardiovascular TEAEs and comparatively few overall cardiovascular TEAEs, with a small number of findings of QTc prolongation on ECG. However, there is a need for careful assessment of cardiovascular risks given inconclusive findings from experimental studies. 49 , 50 , 51 , 52 , 53 Ghrelin acting through the growth hormone secretagogue receptor may regulate energy homeostasis by burning fat to generate heat. 49 Peripheral tissue effects of ghrelin on interstitial levels of glucose, glycerol and lactate have been reported, concluding that ghrelin increased insulin sensitivity. 50 There is also evidence of protective effects on the heart, such as protection against cardiac ischaemia and cardiac fibrosis, improvement in cardiac function and decreased peripheral resistance after myocardial infarction in animals and humans. 51 On the other hand, short‐term administration of ghrelin exerts direct peripheral effects on lipid metabolism, including increases in white adipose tissue mass and stimulation of lipogenesis in the liver. Ghrelin itself may increase appetite and promote adiposity by the activation of hypothalamic orexigenic neurons and stimulation of the expression of fat storage‐related proteins in adipocytes. 52 There is also evidence that ghrelin actually attenuates vascular calcification in diabetic patients who have previously undergone foot amputation. 53 Taken together, this evidence indicates that, on balance, ghrelin and its agonists would be expected to reduce the overall risk of vascular or cardiac disease. Therefore, the cardiovascular effects of relamorelin will be evaluated further in the longer‐term phase 3 trials.
There is a theoretical risk of ghrelin or ghrelin agonists having involvement in autocrine and paracrine processes, resulting in cancer progression 54 ; however, no evidence of this has been observed in human studies 55 and no cancer‐related TEAEs were associated with relamorelin in either phase 2 trial.
In contrast to 5‐HT4 and dopamine D2 receptor agonists, ghrelin receptor agonists are not associated with central nervous system‐induced movement disorders, and do not appear to carry an increased risk in terms of cardiovascular safety based on currently available data. Therefore, as long as patients’ diabetic control is monitored and appropriately managed, relamorelin may fill the unmet need for a new effective diabetic gastroparesis treatment with an acceptable safety profile.
In summary, these data demonstrate the favourable safety and tolerability profile of relamorelin 10‐100 µg in adults with diabetic gastroparesis. Given that ghrelin agonists may stimulate hyperglycaemia and that elevated blood glucose and HbA1c levels were observed in relamorelin‐treated patients with evidence of a dose‐dependent relationship, the phase 3 trials (NCT03426345, NCT03285308, NCT03383146 and NCT03420781), which will assess relamorelin 10 µg b.d. and placebo doses only, will involve closer monitoring of glycaemic parameters and proactive glycaemic management.
AUTHORSHIP
Guarantor of the article: Alexandru Iacob.
Author contributions: MC, AL and RM contributed to the study design and data acquisition; ST, LK, MBM, KB and AI contributed to the data analysis; MC, AL, RM, ST, LK, MBM, KB and AI contributed to interpretation of the data and critically revising the article for important intellectual content. MC, AL, RM, LK, MBM, KB and AI approved the final version of the manuscript; ST (deceased) approved the penultimate version.
ACKNOWLEDGEMENTS
The authors would like to remember Stavros Tourkodimitris, who passed away on 18 December 2019, after this manuscript was submitted. The authors would like to thank Harvey Schneier for his advice and contributions throughout the manuscript development. Declaration of personal interests: Michael Camilleri and Anthony Lembo have served as advisory board members and are grant recipients of Rhythm Pharmaceuticals. Michael Camilleri is currently conducting research funded by Allergan plc. Richard McCallum is a consultant for and grant recipient of Rhythm Pharmaceuticals. Lara Kemps, Matthew B. Miller, Kirk Bertelsen and Alexandru Iacob are employees of and own stock or stock options in Allergan plc. Stavros Tourkodimitris (deceased) was an employee of Allergan plc and owned stock or stock options in Allergan plc.
Camilleri M, Lembo A, McCallum R, et al. Overall safety of relamorelin in adults with diabetic gastroparesis: Analysis of phase 2a and 2b trial data. Aliment Pharmacol Ther. 2020;51:1139–1148. 10.1111/apt.15711
The Handling Editor for this article was Professor Colin Howden, and it was accepted for publication after full peer‐review.
Funding information
This study was funded by Allergan plc, Dublin, Ireland. Writing and editorial assistance was provided to the authors by Helena Cant, MChemPhys, of Complete HealthVizion, Inc, Chicago, IL, USA, and funded by Allergan plc, Dublin, Ireland. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
REFERENCES
- 1. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1589‐1591. [DOI] [PubMed] [Google Scholar]
- 2. Lacy BE, Crowell MD, Mathis C, Bauer D, Heinberg LJ. Gastroparesis: quality of life and health care utilization. J Clin Gastroenterol. 2018;52:20‐24. [DOI] [PubMed] [Google Scholar]
- 3. Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:5‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janssen P, Harris MS, Jones M, et al. The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am J Gastroenterol. 2013;108:1382‐1391. [DOI] [PubMed] [Google Scholar]
- 5. Vijayvargiya P, Jameie‐Oskooei S, Camilleri M, Chedid V, Erwin PJ, Murad MH. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta‐analysis. Gut. 2019;68:804‐813. [DOI] [PubMed] [Google Scholar]
- 6. Vijayvargiya P, Camilleri M, Chedid V, Mandawat A, Erwin PJ, Murad MH. Effects of promotility agents on gastric emptying and symptoms: a systematic review and meta‐analysis. Gastroenterology. 2019;156:1650‐1660. [DOI] [PubMed] [Google Scholar]
- 7. Lee A, Kuo B. Metoclopramide in the treatment of diabetic gastroparesis. Expert Rev Endocrinol Metab. 2010;5:653‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doggrell SA, Hancox JC. Cardiac safety concerns for domperidone, an antiemetic and prokinetic, and galactogogue medicine. Expert Opin Drug Saf. 2014;13:131‐138. [DOI] [PubMed] [Google Scholar]
- 9. Thielemans L, Depoortere I, Perret J, et al. Desensitization of the human motilin receptor by motilides. J Pharmacol Exp Ther. 2005;313:1397‐1405. [DOI] [PubMed] [Google Scholar]
- 10. Dhir R, Richter JE. Erythromycin in the short‐ and long‐term control of dyspepsia symptoms in patients with gastroparesis. J Clin Gastroenterol. 2004;38:237‐242. [DOI] [PubMed] [Google Scholar]
- 11. Hancox JC, Hasnain M, Vieweg WV, Gysel M, Methot M, Baranchuk A. Erythromycin, QTc interval prolongation, and torsade de pointes: case reports, major risk factors and illness severity. Ther Adv Infect Dis. 2014;2:47‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA. 2013;310:2640‐2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. US Food and Drug Administration . Humanitarian device exemption for gastric electrical stimulation; 2000. https://www.accessdata.fda.gov/cdrh_docs/pdf/H990014A.pdf. Accessed December 13, 2018.
- 14. Davis BR, Sarosiek I, Bashashati M, Alvarado B, McCallum RW. The long‐term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg. 2017;21:222‐227. [DOI] [PubMed] [Google Scholar]
- 15. Aghaie Meybodi M, Qumseya BJ, Shakoor D, et al. Efficacy and feasibility of G‐POEM in management of patients with refractory gastroparesis: a systematic review and meta‐analysis. Endosc Int Open. 2019;7:E322‐E329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Camilleri M, McCallum RW, Tack J, Spence SC, Gottesdiener K, Fiedorek FT. Efficacy and safety of relamorelin in diabetics with symptoms of gastroparesis: a randomized, placebo‐controlled study. Gastroenterology. 2017;153:1240‐1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lembo A, Camilleri M, McCallum R, et al. Relamorelin reduces vomiting frequency and severity and accelerates gastric emptying in adults with diabetic gastroparesis. Gastroenterology. 2016;151:87‐96. [DOI] [PubMed] [Google Scholar]
- 18. Nelson AD, Camilleri M, Acosta A, et al. Effects of ghrelin receptor agonist, relamorelin, on gastric motor functions and satiation in healthy volunteers. Neurogastroenterol Motil. 2016;28:1705‐1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zatorski H, Mosinska P, Storr M, Fichna J. Relamorelin and other ghrelin receptor agonists ‐ future options for gastroparesis, functional dyspepsia and proton pump inhibitors‐resistant non‐erosive reflux disease. J Physiol Pharmacol. 2017;68:797‐805. [PubMed] [Google Scholar]
- 20. Phillips LK, Deane AM, Jones KL, Rayner CK, Horowitz M. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol. 2015;11:112‐128. [DOI] [PubMed] [Google Scholar]
- 21. Broglio F, Arvat E, Benso A, et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86:5083‐5086. [DOI] [PubMed] [Google Scholar]
- 22. Tannenbaum GS, Epelbaum J, Bowers CY. Interrelationship between the novel peptide ghrelin and somatostatin/growth hormone‐releasing hormone in regulation of pulsatile growth hormone secretion. Endocrinology. 2003;144:967‐974. [DOI] [PubMed] [Google Scholar]
- 23. Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA. 2004;101:4679‐4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Camilleri M, Acosta A. Emerging treatments in neurogastroenterology: relamorelin: a novel gastrocolokinetic synthetic ghrelin agonist. Neurogastroenterol Motil. 2015;27:324‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shin A, Camilleri M, Busciglio I, et al. Randomized controlled phase Ib study of ghrelin agonist, RM‐131, in type 2 diabetic women with delayed gastric emptying: pharmacokinetics and pharmacodynamics. Diabetes Care. 2013;36:41‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson JN, Ackerman MJ. QTc: how long is too long? Br J Sports Med. 2009;43:657‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rautaharju PM, Surawicz B, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:982‐991. [DOI] [PubMed] [Google Scholar]
- 28. Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth‐hormone‐releasing acylated peptide from stomach. Nature. 1999;402:656‐660. [DOI] [PubMed] [Google Scholar]
- 30. Heller S, Houwing N, Kragh N, Ploug UJ, Nikolajsen A, Alleman CJ. Investigating the evidence of the real‐life impact of acute hyperglycaemia. Diabetes Ther. 2015;6:389‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Halland M, Bharucha AE. Relationship between control of glycemia and gastric emptying disturbances in diabetes mellitus. Clin Gastroenterol Hepatol. 2016;14:929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bharucha AE, Kudva Y, Basu A, et al. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol. 2015;13:466‐476.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Horowitz M, Jones KL, Harding PE, Wishart JM. Relationship between the effects of cisapride on gastric emptying and plasma glucose concentrations in diabetic gastroparesis. Digestion. 2002;65:41‐46. [DOI] [PubMed] [Google Scholar]
- 34. Parthasarathy G, Kudva YC, Low PA, Camilleri M, Basu A, Bharucha AE. Relationship between gastric emptying and diurnal glycemic control in type 1 diabetes mellitus: a randomized trial. J Clin Endocrinol Metab. 2017;102:398‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wishart JM, Horowitz M, Campbell J, Jones KL. The effect of cisapride on oral and intravenous glucose tolerance in normal subjects. J Gastroenterol Hepatol. 1997;12:795‐800. [DOI] [PubMed] [Google Scholar]
- 36. Nass R, Pezzoli SS, Oliveri MC, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149:601‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White HK, Petrie CD, Landschulz W, et al. Effects of an oral growth hormone secretagogue in older adults. J Clin Endocrinol Metab. 2009;94:1198‐1206. [DOI] [PubMed] [Google Scholar]
- 38. Goshadrou F, Kazerouni F, Mehranfard N, Sadeghi B. Chronic administration of ghrelin regulates plasma glucose and normalizes insulin levels following fasting hyperglycemia and hyperinsulinemia. Gen Comp Endocrinol. 2015;224:113‐120. [DOI] [PubMed] [Google Scholar]
- 39. de Lastours V, Foxman B. Urinary tract infection in diabetes: epidemiologic considerations. Curr Infect Dis Rep. 2014;16:389. [DOI] [PubMed] [Google Scholar]
- 40. Acosta A, Camilleri M, Kolar G, et al. Relamorelin relieves constipation and accelerates colonic transit in a Phase 2, placebo‐controlled, randomized trial. Clin Gastroenterol Hepatol. 2015;13:2312‐2319. [DOI] [PubMed] [Google Scholar]
- 41. Chedid V, Camilleri M. Relamorelin for the treatment of gastrointestinal motility disorders. Expert Opin Investig Drugs. 2017;26:1189‐1197. [DOI] [PubMed] [Google Scholar]
- 42. DeBoer MD. The use of ghrelin and ghrelin receptor agonists as a treatment for animal models of disease: efficacy and mechanism. Curr Pharm Des. 2012;18:4779‐4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. DeBoer MD, Zhu XX, Levasseur P, et al. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology. 2007;148:3004‐3012. [DOI] [PubMed] [Google Scholar]
- 44. Palus S, von Haehling S, Doehner W, et al. Effect of application route of the ghrelin analog BIM‐28131 (RM‐131) on body weight and body composition in a rat heart failure model. Int J Cardiol. 2013;168:2369‐2374. [DOI] [PubMed] [Google Scholar]
- 45. Fischer K, Finan B, Clemmensen C, van der Ploeg LH, Tschöp MH, Müller TD. The pentapeptide RM‐131 promotes food intake and adiposity in wildtype mice but not in mice lacking the ghrelin receptor. Front Nutr. 2015;1:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Virdis A, Lerman LO, Regoli F, Ghiadoni L, Lerman A, Taddei S. Human ghrelin: a gastric hormone with cardiovascular properties. Curr Pharm Des. 2016;22:52‐58. [DOI] [PubMed] [Google Scholar]
- 47. Stokes AH, Falls JG, Yoon L, et al. Integrated approach to early detection of cardiovascular toxicity induced by a ghrelin receptor agonist. Int J Toxicol. 2015;34:151‐161. [DOI] [PubMed] [Google Scholar]
- 48. Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898‐918. [DOI] [PubMed] [Google Scholar]
- 49. Sun Y. Ghrelin receptor controls obesity by fat burning. Oncotarget. 2015;6:6470‐6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vestergaard ET, Møller N, Jørgensen JOL. Acute peripheral tissue effects of ghrelin on interstitial levels of glucose, glycerol, and lactate: a microdialysis study in healthy human subjects. Am J Physiol Endocrinol Metab. 2013;304:E1273‐E1280. [DOI] [PubMed] [Google Scholar]
- 51. Mosa RMH, Zhang Z, Shao R, Deng C, Chen J, Chen C. Implications of ghrelin and hexarelin in diabetes and diabetes‐associated heart diseases. Endocrine. 2015;49:307‐323. [DOI] [PubMed] [Google Scholar]
- 52. Lv Y, Liang T, Wang G, Li Z. Ghrelin, a gastrointestinal hormone, regulates energy balance and lipid metabolism. Biosci Rep. 2018;38:BSR20181061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu S, Ye F, Li L, et al. Ghrelin attenuates vascular calcification in diabetic patients with amputation. Biomed Pharmacother. 2017;91:1053‐1064. [DOI] [PubMed] [Google Scholar]
- 54. Chopin L, Walpole C, Seim I, et al. Ghrelin and cancer. Mol Cell Endocrinol. 2011;340:65‐69. [DOI] [PubMed] [Google Scholar]
- 55. Pabalan NA, Seim I, Jarjanazi H, Chopin LK. Associations between ghrelin and ghrelin receptor polymorphisms and cancer in Caucasian populations: a meta‐analysis. BMC Genet. 2014;15:118. [DOI] [PMC free article] [PubMed] [Google Scholar]


