Abstract
Background
Hidradenitis suppurativa (HS) is a chronic skin disease characterized by inflammatory lesions that flare unpredictably. The impact of weekly adalimumab (ADAew) on HS flare is not well‐characterized.
Objective
To evaluate the impact of disease flare on health‐related quality of life (HRQOL) in moderate‐to‐severe HS patients and to determine the effect of ADAew on disease flare using integrated data from two phase 3 trials over 36 weeks.
Methods
In period A (12 weeks), Dermatology Life Quality Index (DLQI) score change from baseline was compared in patients who flared and those who did not, regardless of treatment. The proportion of patients experiencing flare, duration of flare and time to flare was evaluated for ADAew vs. placebo (PBO). In period B (24 weeks), proportion of patients experiencing flare who received continuous ADAew treatment through 36 weeks was assessed.
Results
HRQOL was markedly improved among those who did not experience flare. In period A, the proportion of patients who experienced flare was significantly lower with ADAew vs. PBO (12.3% vs. 35.3%, P < 0.001). ADAew patients also had longer time to first flare (101 days vs. 57 days; P < 0.001) and shorter flare duration (18.9 days vs. 32.0 days, respectively; P = 0.001) vs. PBO. Through 36 weeks of treatment, 20.2% of ADAew patients flared, and for those who achieved at least a partial clinical response to ADAew at 12 weeks, only 5.7% flared.
Conclusions
Flare reduction is an important measure in HS that correlates with clinically meaningful improvement in HRQOL. ADAew reduces HS flare through 12 and subsequent 36 weeks of treatment.
Introduction
Hidradenitis suppurativa (HS) is a chronic, painful and debilitating skin disease of the hair follicle. It usually begins in young adulthood and is characterized by recurrent inflammatory abscesses and nodules in inverse body areas.1, 2, 3 Prevalence estimates of HS vary between 0.1% in the United States and 1–2% in European countries, depending on how and where data are collected.1, 2, 3 While the exact underlying cause remains unknown, HS does have a genetic link in approximately one‐third of patients4 and is strongly correlated with smoking and high body mass index.5, 6, 7
The clinical course of HS is highly variable6, 8, 9 with skin lesions flaring throughout the natural course of the disease.10 The timing and magnitude of disease flares in HS cannot be accurately predicted, though premenstrual flare is commonly reported.6 Many patients with HS also experience prodromal symptoms, such as fatigue, malaise, headache, nausea, skin erythema, paresthesia and itching prior to HS flare.11
Flare has been recognized as an important outcome by HS patients as well as clinicians even though its effect on quality of life unknown. In a rigorous international Delphi exercise to define the key outcomes for clinical trials in HS, experts and patients from 19 countries across four continents reached consensus regarding inclusion of flare frequency and duration in the core domain set.12 As treatment for HS depends on clinical staging as well as frequency and duration of flares, HS clinical trials should evaluate a medication's effect on flares in addition to clinical response to demonstrate efficacy.10, 12
Adalimumab (ADA), a monoclonal antibody targeting tumour necrosis factor‐alpha, is approved for the treatment of HS. However, the effect of ADA on HS flares has not been studied. By integrating data from the two phase 3 studies of ADA in HS (PIONEER I and II), we first evaluated the impact of HS disease flare on patients’ health‐related quality of life (HRQOL) to demonstrate the clinical importance of flare. We then assessed the proportion of patients experiencing flare, time to flare and duration of flare in those treated with weekly ADA (ADAew) or placebo (PBO) over 12 weeks. To understand the longer‐term impact of ADA on flare, we evaluated the flare incidence over the entire 36‐week period in patients receiving continuous ADAew.
Materials and methods
Patients
Eligible patients were men and non‐pregnant women aged 18 years or older with a diagnosis of HS ≥ 1 year prior to baseline who had stable disease for at least 2 months before screening. Patients had lesions in ≥ 2 distinct body areas (Hurley Stage II or III in at least one area), total abscess and inflammatory nodule (AN) count ≥ 3 at baseline, and an inadequate response to a ≥ 3‐month trial of oral antibiotics to treat HS.
Patients were excluded if they received prior treatment with ADA or another anti‐tumour necrosis factor therapy, had a draining fistula count > 20 at baseline or had received oral antibiotics for HS ≤ 28 days before baseline (PIONEER I only) or analgesics for HS‐related pain or prescription topical treatment for HS ≤ 14 days prior to baseline.13
Study design and treatment
This analysis integrated data from two phase 3 studies (PIONEER I [NCT01468207] and II [NCT01468233])13 and the initial portion of the open‐label extension (OLE) (NCT01635764) that paralleled the 36 weeks of the controlled portion of the PIONEER trials for patients who escaped early.14 The PIONEER I and II trials had similar study designs (Fig. 1), with each having two double‐blind periods (a 12‐week period A and 24‐week period B). During period A, patients were randomized 1:1 to receive ADA 160 mg at week 0, 80 mg at week 2, and 40 mg every week or matching PBO starting at week 4. In period B, patients taking ADA from period A were re‐randomized to receive ADAew, ADA every other week (ADAeow), or PBO, while PBO patients from period A were switched to ADAew (PIONEER I) or continued with PBO (PIONEER II) from weeks 12 to 36. The primary endpoint at week 12 was HS clinical response (HiSCR), a validated assessment of HS treatment efficacy.15, 16 HiSCR is defined as a ≥ 50% improvement in total AN count, with no increase in abscess and no increase in draining fistula counts relative to baseline. While all patients were eligible to enter the OLE after completing period B, patients were rescued into the OLE during period B if they lost response or experienced a worsening or absence of improvement (see Fig. 1 for definitions).
Figure 1.
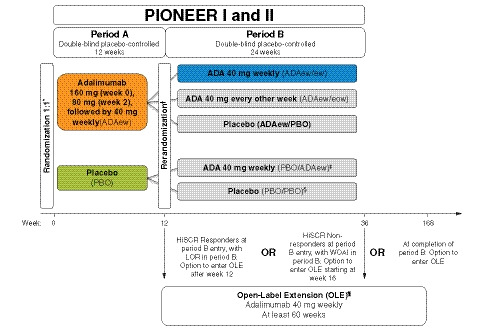
PIONEER study schematic. *Stratified by baseline Hurley Stage II vs. III (PIONEER I & II) and baseline concomitant antibiotic use (PIONEER II). Rerandomization at entry to period B, stratified by week 12 HiSCR status and BL Hurley Stage II vs. III. ‡PIONEER I only (160 mg week 12, 80 mg week 14, 40 mg from week 16). §PIONEER II only. ¶OLE entry criteria: completed PIONEER I or II or met prespecified escape criteria (LOR or WOAI in period B). LOR was defined as a count greater than the average AN count at baseline and at week 12. Patients who achieved HiSCR at week 12 and experienced LOR during period B were discontinued from the study and were eligible to enter the OLE and receive open‐label ADAew. WOAI was defined as AN count greater than or equal to baseline AN count at two consecutive visits (excluding week 12) occurring ≥ 14 days apart. Patients who did not achieve HiSCR at week 12 continued in period B through at least week 16 (and up to week 36). At or after week 16, patients who experienced WOAI were discontinued from the study and were eligible to enter the OLE study to receive open‐label ADAew. ADA, adalimumab; AN, sum of inflammatory nodules and abscesses; BL, baseline; ew, every week; eow, every other week; HiSCR, hidradenitis suppurativa clinical response; LOR, loss of response; OLE, open‐label extension; PBO, placebo; WOAI, worsening or absence of improvement.
Assessments
Flare assessment
HS disease flare was defined a priori as a ≥ 25% increase in the total AN count with a minimum increase of 2 AN relative to baseline. This measure was developed with the intent to approximate flare as closely as possible to the real‐world clinical presentation, as there is no currently available objective and measurable definition for flare in HS.
To understand the impact of flare on patients’ HRQOL, change in Dermatology Life Quality Index (DLQI) score from baseline was evaluated in those who experienced flare vs. those who did not, regardless of treatment.17 The following efficacy assessments were completed in period A for ADAew vs. PBO: the proportion of patients who experienced flare, duration of flare (calculated from the day that flare was first observed at scheduled visit to the day prior to the observation that flare was no longer present), and time to flare. In period B, the proportion of patients on continuous ADAew through 36 weeks who flared was assessed.
As described earlier, patients who experienced either a loss of response or a worsening or lack of improvement during period B escaped early into the OLE to receive ADAew. As expected, many of the patients on PBO in period B were rescued into the OLE once they met the escape criteria and before experiencing a protocol‐defined flare. Therefore, there were not enough patients on PBO remaining in period B who were experiencing disease flare to make a robust comparison vs. ADAew through 36 weeks.
Statistical analysis
Efficacy variables were assessed in the following patient populations: (i) the intent‐to‐treat period A population, defined as all patients who were randomized at baseline (week 0); and (ii) the intent‐to‐treat period B ADAew/ew population, defined as patients randomized to ADAew in period A and re‐randomized to ADAew in period B (including those who escaped period B early due to loss of response or worsening or absence of improvement and continued ADAew treatment in the OLE).
The Cochran–Mantel–Haenszel test was used to analyse the treatment difference in the proportion of patients who experienced flare in period A, adjusting for study, baseline Hurley stage and concomitant use of antibiotics at baseline; 95% confidence interval (CI) was calculated using the extended Mantel–Haenszel statistic. Analysis of covariance was used to analyse the treatment difference in the number of days on flare in period A, with strata (study, baseline Hurley stage and concomitant use of baseline antibiotics) and treatment in the model. Kaplan–Meier plot plus log‐rank test and corresponding summary statistics were used to analyse the treatment difference in time to flare in period A. Fisher's exact test was used to analyse the treatment difference in adverse events of worsening HS in period A. Missing data were handled by non‐responder imputation analysis for categorical data and last‐observation‐carried‐forward analysis for continuous data. Descriptive analyses were conducted by treatment sequence group.
Results
Patients
A total of 633 patients were included in period A (316 in the ADAew group and 317 in the PBO group). A total of 99 patients were randomized to ADAew in period A and were re‐randomized to ADAew in period B (Table 1). Patient demographics and baseline characteristics were generally similar between groups. There were approximately two times as many women as men, and the proportion of patients classified as having Hurley stages II and III was similar within groups. The overall number of lesions at baseline was also similar between groups.
Table 1.
Patient demographics and baseline characteristics (ITT population)
| Characteristic | Period A | Period B | |
|---|---|---|---|
| ADAew (n = 316) | PBO (n = 317) | ADAew/ADAew (n = 99) | |
| Age, years | 35.5 (10.4) | 37.0 (11.8) | 34.8 (10.2) |
| Mean (SD) | |||
| Sex, n (%) | |||
| Female | 199 (63.0) | 218 (68.8) | 66 (66.7) |
| Male | 117 (37.0) | 99 (31.2) | 33 (33.3) |
| Race, n (%) | |||
| White | 259 (82.0) | 248 (78.2) | 90 (90.9) |
| Black | 42 (13.3) | 49 (15.5) | 6 (6.1) |
| Other | 15 (4.7) | 20 (6.3) | 3 (3.0) |
| BMI, kg/m2 | N = 315 | N = 315 | 32.1 (6.9) |
| Mean (SD) | 32.2 (7.6) | 33.7 (8.0) | |
| Hurley Stage, n (%) | |||
| II | 166 (52.5) | 170 (53.6) | 49 (49.5) |
| III | 150 (47.5) | 147 (46.4) | 50 (50.5) |
| Duration of HS, years | 11.3 (8.8) | 11.7 (9.1) | 11.8 (8.8) |
| Mean (SD) | |||
| Lesion counts, mean (SD) | |||
| AN | 12.4 (10.3) | 13.1 (13.0) | 12.1 (10.1) |
| Abscess | 2.4 (3.1) | 2.6 (3.5) | 2.0 (2.6) |
| Inflammatory nodule | 10.0 (9.2) | 10.5 (11.9) | 10.1 (9.4) |
| Draining fistula | 3.8 (4.7) | 3.8 (4.8) | 3.6 (4.2) |
| Modified Sartorius Score | |||
| Mean (SD) | 128.6 (109.9) | 134.6 (93.2) | 137.3 (152.1) |
| Range | 18–1093 | 20–531 | 20–1093 |
| Current nicotine user, n (%) | 186 (58.9) | 201 (64.0) | 59 (59.6) |
ADAew, weekly adalimumab; AN, sum of inflammatory nodules and abscesses; BMI, body mass index; HS, hidradenitis suppurativa; hs‐CRP, high‐sensitivity C‐reactive protein; PBO, placebo; SD, standard deviation.
Efficacy assessments
Impact of flare on quality of life
Quality of life at week 12, as assessed by DLQI, was significantly improved from baseline in patients who did not experience flare compared with those who did, with a least‐squares mean (95% CI) difference of 3.9 (−4.8 vs. −0.9 for no flares vs. flares; P < 0.001) (Fig. 2a). The proportion of patients who achieved DLQI scores of 0–1 at week 12 was significantly higher for patients who did not experience flare compared with patients who did (P = 0.009; Fig. 2b).
Figure 2.
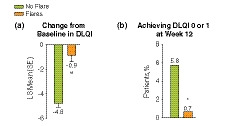
DLQI during period A among patients who experienced flares compared with patients who did not experience a flare. (a) Least‐square mean (SE) change from baseline in DLQI; (b) proportion of patients who achieved DLQI score 0–1 at week 12. DLQI, Dermatology Life Quality Index; SE, standard error. *P = 0.009; **P < 0.001.
Period A
During period A, the proportion of patients experiencing at least one occurrence of flare was significantly lower in the ADAew group compared with the PBO group at each visit and overall (12.3% [n = 39] vs. 35.3% [n = 112]; P < 0.001; Fig. 3). In addition, mean time to flare was significantly longer for patients taking ADAew vs. those taking PBO (101 days vs. 57 days; P < 0.001; Fig. 4a), and duration of flare was significantly lower in ADAew‐treated patients compared with PBO‐treated patients (mean of 18.9 days vs. 32.0 days, respectively; P = 0.001; Fig. 4b). The proportion of patients experiencing multiple flares was also lower in the ADAew group vs. PBO group (5.4% vs. 17.7%, respectively; P < 0.001).
Figure 3.
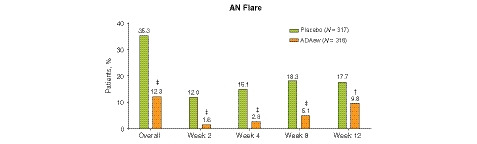
Proportion of patients experiencing (A) AN flare. †P < 0.01; ‡P < 0.001. ADAew, weekly adalimumab; AN, total abscess and inflammatory nodule count; PBO, placebo.
Figure 4.
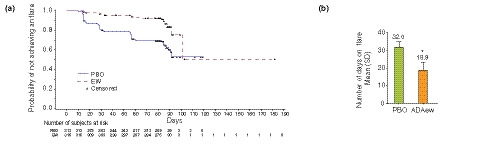
(a) Median time to flare (**P < 0.001) and (b) mean (SD) number of days on flare during period A (*P = 0.001). ADAew, weekly adalimumab; PBO, placebo; SD, standard deviation.
Period B
Among all patients who received continuous ADAew treatment during periods A and B (n = 99), 20.2% experienced flare between weeks 12 and 36. Among these patients, for those who had at least a partial initial response to ADAew at week 12 (defined as ≥ 25% improvement in total AN count relative to baseline; n = 70), only 5.7% experienced flare at any time between week 12 and week 36 (Fig. 5).
Figure 5.
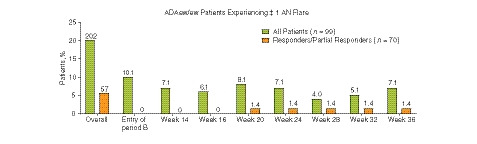
Proportion of ADAew/ew patients experiencing ≥ 1 AN flare during period B (including those who escaped to the OLE) by week 12 HiSCR response rate. ADAew, weekly adalimumab; AN, total abscess and inflammatory nodule count; HiSCR, hidradenitis suppurativa clinical response; OLE, open‐label extension.
Discussion
The impact of HS on quality of life is higher than other dermatological conditions.18, 19 For general inflammatory skin conditions, a change in DLQI score of ≥ 4 points is considered clinically important.20 In this analysis at week 12, the average improvement in DLQI from baseline was 4.8 points for patients who did not experience flare compared with 0.9 points for those who did, suggesting that prevention of flare is associated with a clinically relevant impact on HRQOL. Additionally, the proportion of patients who reported that HS had no effect at all on their life (DLQI score of 0–1) was also significantly higher for patients who did not experience flare (P < 0.001), further supporting the finding that flare plays an important role in the quality of life of patients with HS.
The results of this analysis demonstrate that ADAew is effective at preventing flares in the short and longer‐term treatment of patients with moderate‐to‐severe HS. During the 12‐week PBO‐controlled portion of the study, the proportion of ADAew patients experiencing flare was only about one‐third that of the proportion of PBO patients experiencing flare (12.3% vs. 35.3%), and few ADAew patients experienced multiple flares (5.4%). Furthermore, between weeks 12 and 36, most patients receiving continuous ADAew (80%) did not experience disease flare. Importantly, flare prevention over the longer‐term was most marked in those with at least a partial response to ADAew at week 12, as 94.3% of these patients experienced no flares between weeks 12 and 36. In addition to significantly reducing the proportion of patients who flare, ADA demonstrated improvement in flare‐related parameters vs. PBO, including longer time to flare (over 100 days to first flare vs. 57 days in terms of the 25% quantile; median time not estimable due to the fact that too few patients experienced flare) and noticeably shorter flare duration (> 13 days shorter flare on average).
Previous reports from the PIONEER I and II studies also demonstrate the efficacy of ADAew treatment in HS, with significantly higher clinical response rates vs. placebo at week 12.13, 14 Long‐term efficacy with continuous weekly ADA was assessed through the 3‐year OLE, demonstrating that the percentage of patients achieving clinical response remained consistent through 168 weeks.
Reinforced by the flare data presented here, continuous weekly ADA appears to be the optimal strategy for maintenance of disease control, especially in those patients who achieve at least a partial response by week 12. This finding was also supported across multiple efficacy parameters in the integrated analysis of the PIONEER I and II trials.21 It should be noted that, given the waxing and waning nature of disease activity in HS, patients can expect some degree of disease variation even with ADAew. However, patients appear to experience considerably less variation with continued weekly ADA treatment: in the analysis by Jemec et al. median worsening in AN count from week 12 at the worst point throughout period B was 1 (interquartile range 0–4) for patients continuing ADAew, while patients who withdrew from ADAew in period B had a median worsening of 3 (interquartile range 1–6).21 A multimodal approach can be considered to manage those patients who do experience occasional flare with intralesional corticosteroids, analgesics, antibiotics, deroofing, and incision and drainage.
Limitations of the current analysis include the lack of a reliable control group past 12 weeks of the PIONEER programme; as such, it is difficult to determine how flare incidence on ADAew treatment compares with that of the natural course of disease over the long term. Additionally, the definition of flare used in this analysis was developed as an objective and measurable outcome for reporting purposes. We also acknowledge that the study population was limited to patients with moderate‐to‐severe HS, and therefore may not represent all patients with HS.
Additional research is needed to examine factors associated with HS flare and the impact of longer‐term ADAew treatment on flare. A well‐established and validated definition of flare is also needed for clinical research purposes, along with additional treatment targets in HS.
Conclusions
This analysis provides valuable insight into the impact of disease flare on patients with HS, as there is a clinically insignificant change in quality of life in patients who experience flare; and a 5 × higher improvement in DLQI in patients who did not flare. As such, incidence of flare and associated measures should continue to be evaluated in future clinical trials for HS. These data demonstrate the positive effect of weekly adalimumab treatment on incidence of HS disease flare, time to flare and duration of flare. Importantly, this analysis also further supports the use of continued weekly adalimumab treatment in patients experiencing at least a partial response at week 12, which resulted in complete flare prevention in about 95% of patients through 36 weeks of treatment.
Data sharing statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial‐level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Author contributions
HHVDZ contributed to the original research project conception and design, facilitated data acquisition, interpreted data, reviewed and critiqued the manuscript throughout the editorial process, and approved the final manuscript draft submitted for publication. ML contributed to research project conception and design, interpreted data, reviewed and critiqued the manuscript throughout the editorial process, and approved the final manuscript draft submitted for publication. ZG contributed to the original research project conception and design, conducted statistical analysis, interpreted data, reviewed and critiqued the manuscript throughout the editorial process, and approved the final manuscript draft submitted for publication. AG contributed to the original research project conception and design, facilitated data acquisition, interpreted data, reviewed and critiqued the manuscript throughout the editorial process, and approved the final manuscript draft submitted for publication. All authors agree to be accountable for all aspects of the work, ensuring the accuracy and integrity of the publication.
Acknowledgements
This study was supported by AbbVie. AbbVie participated in the analysis of data, interpretation of the data, writing, review and approval of the publication. The authors would like to thank AbbVie for support of this manuscript. Medical writing support, funded by AbbVie, was provided by Caroline Walsh Cazares, PhD, of JB Ashtin, who developed the first draft based on an author‐approved outline and assisted in implementing author revisions. The authors thank Colleen M Wegzyn, PharmD, US Medical Affairs, Dermatology Therapeutic Area Lead of AbbVie, Inc. (North Chicago, Illinois) for her input and guidance on the content of this manuscript.
Conflicts of interest
Hessel H van der Zee has received grant/research support from AbbVie and InflaRX; and received honoraria or consulting fees from AbbVie, InflaRX, Novartis and Galderma. Michelle Longcore and Ziqian Geng are full‐time employee of AbbVie Inc. and may hold stock or stock options. Amit Garg has received grant/research support from AbbVie; has served as an investigator for UCB; and received honoraria or consulting fees from AbbVie, Amgen, Asana Biosciences, Janssen, Pfizer and UCB.
Funding sources
This study was funded by AbbVie, Inc.
References
- 1. Garg A, Kirby JS, Lavian J et al Sex‐ and age‐adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol 2017; 153: 760–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ingram JR, Jenkins‐Jones S, Knipe DW et al Population‐based clinical practice research datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol 2018; 178: 917–924. [DOI] [PubMed] [Google Scholar]
- 3. Vinding GR, Miller IM, Zarchi K et al The prevalence of inverse recurrent suppuration: a population‐based study of possible hidradenitis suppurativa. Br J Dermatol 2014; 170: 884–889. [DOI] [PubMed] [Google Scholar]
- 4. Ingram JR. The genetics of hidradenitis suppurativa. Dermatol Clin 2016; 34: 23–28. [DOI] [PubMed] [Google Scholar]
- 5. van der Zee HH, Laman JD, Boer J et al Hidradenitis suppurativa: viewpoint on clinical phenotyping, pathogenesis and novel treatments. Exp Dermatol 2012; 21: 735–739. [DOI] [PubMed] [Google Scholar]
- 6. Poli F et al Clinical presentation In: Jemec GBR, et al, eds. Hidradenitis Suppurativa. Springer, Berlin, Germany, 2006: 11–23. [Google Scholar]
- 7. Garg A, Papagermanos V, Midura M et al Incidence of hidradenitis suppurativa among tobacco smokers: a population‐based retrospective analysis in the U.S.A. Br J Dermatol 2018; 178: 709–714. [DOI] [PubMed] [Google Scholar]
- 8. Wiseman MC. Hidradenitis suppurativa: a review. Dermatol Ther 2004; 17: 50–54. [DOI] [PubMed] [Google Scholar]
- 9. Vanlaerhoven A, Ardon CB, van Straalen KR et al Hurley III hidradenitis suppurativa has an aggressive disease course. Dermatology 2018; 234: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin 2010; 28: 779–793. [DOI] [PubMed] [Google Scholar]
- 11. Ring HC, Theut Riis P, Zarchi K et al Prodromal symptoms in hidradenitis suppurativa. Clin Exp Dermatol 2017; 42: 261–265. [DOI] [PubMed] [Google Scholar]
- 12. Thorlacius L, Ingram JR, Villumsen B et al A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol 2018; 179: 642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kimball AB, Okun MM, Williams DA et al Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med 2016; 375: 422–434. [DOI] [PubMed] [Google Scholar]
- 14. Zouboulis CC, Okun MM, Prens EP et al Long‐term adalimumab efficacy in patients with moderate‐to‐severe hidradenitis suppurativa/acne inversa: 3‐year results of a phase 3 open‐label extension study. J Am Acad Dermatol 2018; 80: 60–69. [DOI] [PubMed] [Google Scholar]
- 15. Kimball AB, Jemec GB, Yang M et al Assessing the validity, responsiveness and meaningfulness of the hidradenitis suppurativa clinical response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol 2014; 171: 1434–1442. [DOI] [PubMed] [Google Scholar]
- 16. Kimball AB, Sobell JM, Zouboulis CC et al HiSCR (hidradenitis suppurativa clinical response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo‐controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol 2016; 30: 989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Finlay AY, Khan GK. Dermatology life quality index (DLQI). In: Series Dermatology Life Quality Index (DLQI). 1992. URL https://www.cardiff.ac.uk/medicine/resources/quality-of-life-questionnaires/dermatology-life-quality-index [DOI] [PubMed]
- 18. von der Werth JM, Jemec GB. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol 2001; 144: 809–813. [DOI] [PubMed] [Google Scholar]
- 19. Jemec GB, Heidenheim M, Nielsen NH. Hidradenitis suppurativa–characteristics and consequences. Clin Exp Dermatol 1996; 21: 419–423. [DOI] [PubMed] [Google Scholar]
- 20. Basra MK, Salek MS, Camilleri L et al Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology 2015; 230: 27–33. [DOI] [PubMed] [Google Scholar]
- 21. Jemec GBE, Okun MM, Forman SB et al Adalimumab medium‐term dosing strategy in moderate‐to‐severe hidradenitis suppurativa: integrated results from the phase 3, randomized, placebo‐controlled, PIONEER trials. Br J Dermatol 2019; 181: 967–975. 10.1111/bjd.17919 [DOI] [PMC free article] [PubMed] [Google Scholar]


