Abstract
Adeno‐associated virus serotype 8 (AAV8) gene therapy has shown efficacy in several clinical trials and is considered a highly promising technology to treat monogenic diseases such as hemophilia A and B. However, a major drawback of AAV8 gene therapy is that it can be applied only once because anti‐AAV8 immunity develops after the first treatment. Readministration may be required in patients who are expected to need redosing, eg, due to organ growth, or to boost suboptimal expression levels, but no redosing protocol has been established. We have developed a preventive immune‐suppressive protocol for a human factor IX (FIX) vector with an intended dose of ~5 × 1011 vg/kg that inhibits the development of anti‐AAV8 neutralizing‐antibody (NAb) responses and anti‐AAV8 T‐cell responses using CTLA4‐IgG (abatacept). In a preclinical model, transient treatment with abatacept during initial human FIX gene therapy efficiently inhibited the generation of AAV8‐specific cellular and humoral responses, and thus permitted redosing of FIX. Furthermore, our data suggest that by suppression of anti‐AAV8 NAb responses after the second higher dose (4 × 1012 vg/kg) this protocol can be used to enable redosing up to such high doses. An additional advantage of CTLA4‐IgG blocking CD28‐mediated signals is its potential suppression of AAV8‐specific cytotoxic CD8 T‐cell responses, which are believed to kill transduced hepatocytes and might interfere with a successful readministration. Redosing protocols using approved drugs would be beneficial for patients because they could effortlessly be applied in clinical trials and enable safe and efficient treatment options for patients undergoing AAV8 gene therapy.
Keywords: CTLA‐4‐Ig, gene therapy, hemophilia B, immunosuppression, neutralizing antibodies
Essentials.
AAV gene therapy can be only applied once due to anti‐AAV neutralizing antibody formation.
Abatacept blocks anti‐AAV8 T cell‐ and neutralizing antibody‐response in a preclinical model.
CTLA4‐IgG enables redosing with AAV8 vectors by blocking neutralizing antibodies.
CTLA4‐IgG could be used in patients to enable re‐dosing and control anti‐AAV8 T cell responses.
1. INTRODUCTION
Several clinical trials have shown that adeno‐associated virus serotype 8 (AAV8) gene therapy is a promising treatment for patients with monogenic diseases such as hemophilia.1, 2, 3 However, overcoming anti‐AAV8 immunity is considered a major challenge. AAV8 immunity results from anti‐AAV8 neutralizing antibodies, which can efficiently block transgene transfer even at low titers,4, 5 and anti‐AAV8 T‐cell responses, which may kill transduced hepatocytes.6 Treatment with AAV8 vectors also induces novel anti‐AAV8 neutralizing‐antibody (NAb) responses, and potentially anti‐AAV8 T‐cell responses, thereby precluding readministration to patients.1, 7
Repeated AAV8 vector administrations may be required to achieve sufficient transgene expression levels in patient populations which are negative for AAV8 NAbs and are expected to have low transgene expression after the first treatment. This could include patients treated with low‐vector doses for safety reasons1 or young patients with declining transgene expression because of body or organ growth.8 To enable readministration of the same vector, we here evaluated transient and preventive immune suppression using abatacept. This approach focused on enabling readministration in AAV8‐factor IX (FIX) gene therapy or similar gene therapies with low vector doses of about 5 × 1011 vg/kg. Abatacept was chosen because its immunosuppressive efficacy has been described in transplantation and rheumatoid arthritis.9, 10, 11 Abatacept blocks the costimulatory interaction between CD28 and CD80/CD86, thereby inhibiting CD4 and CD8 T‐cell differentiation and, consequently, the development of anti‐AAV8 NAb‐secreting plasma cells.11
2. RESULTS AND DISCUSSION
To characterize the anti‐AAV8 immune response, mice were injected intravenously with an AAV8‐FIX vector bearing the FIXR388L transgene. At day 28, the treatment resulted in high FIX plasma levels in all mice receiving the AAV8‐FIX vector, demonstrating the effectiveness of AAV8‐based vectors to deliver transgenes (Figure 1A). Treatment with AAV8‐FIX elicited AAV8‐specific binding antibodies (BAbs), including anti‐AAV8 NAbs that would block a subsequent gene therapy treatment (Figure 1B). At the same time, a weak but statistically significant AAV8‐specific T‐cell response was detected by an IFN‐γ secretion ELISpot (Figure 1C) and by flow cytometric assessment of the activation marker CD154, defining antigen‐specific CD4+ T cells (Figure 1D).12 The cytometric data showed that the cellular response elicited against AAV8 is mediated mostly by CD4+ T cells as the frequencies of IFN‐γ, CD107a, and TNF‐α elicited by CD8+ T cells were in the same range as the controls (data not shown). Further analysis of the cytokine expression revealed that AAV8 promotes a typical antiviral Th1‐cell polarization because the CD154+CD4+ T cells coexpressed predominantly IFN‐γ and TNF‐α, but no IL‐4 (Figure 1E).
Figure 1.
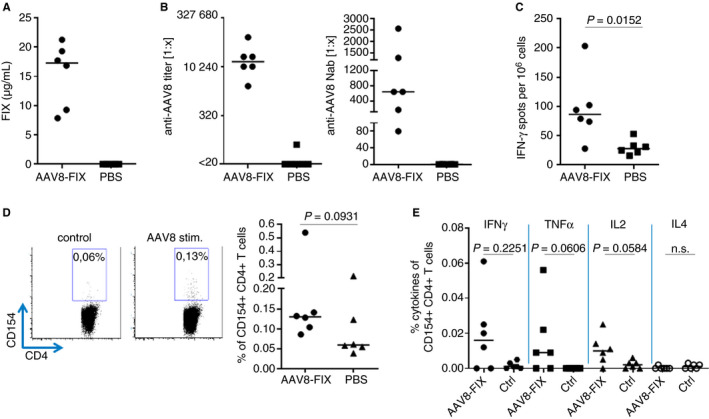
Adeno‐associated virus serotype 8 (AAV8)‐factor IX (FIX) gene therapy elicits robust anti‐AAV8 T‐cell and antibody responses, preventing redosing. Mice were immunized with AAV8‐FIX (FIXR338L) or buffer and killed on day 28 for analysis of FIX expression and anti‐AAV8 immunity. A, FIX expression on day 28. B, Anti‐AAV8 binding and neutralizing antibody titer at day 28 after AAV8 challenge. C, T‐cell response was characterized after in vitro restimulation with AAV8 peptide pools by IFN‐γ ELISpot. AAV8‐specific CD4+ T‐cell responses were analyzed by fluorescence‐activated cell sorting (D, E). Ctrl, Control; PBS, phosphate‐buffered saline (significant P > .005; n.s. = not significant)
After setting up the model and establishing that abatacept can suppress anti‐AAV8 immune responses sufficiently, we performed two independent experiments to show that prevention of anti‐AAV8 immunity enables redosing: A redosing scenario was mimicked to show the impact of AAV8‐FIX–induced immunity on subsequent treatment with AAV8‐FIX gene therapy. Mice immunized with low‐dose AAV8‐FIX without immunosuppression (Figure 2A) showed that FIX expression on day 28 could not be further boosted to reach the expression levels of the controls (about 20 µg/mL on day 77; Figure 2C). The boosting of FIX expression was blocked by the adaptive immune responses, the major barrier to redosing (Figure 2D‐E). Confirming previous studies, low anti‐AAV8 NAbs measured after a first low‐dose application appeared to inhibit the boost in FIX transgene expression (Figure 2E).4 The boosting of anti‐AAV8 antibody responses in double‐treated mice (day 77; Figure 2D‐E) compared with the mice receiving AAV8 only once (day 28; Figure 2D‐E) demonstrated that the mice receiving both applications developed a classic prime‐boost immune reaction against AAV8.
Figure 2.
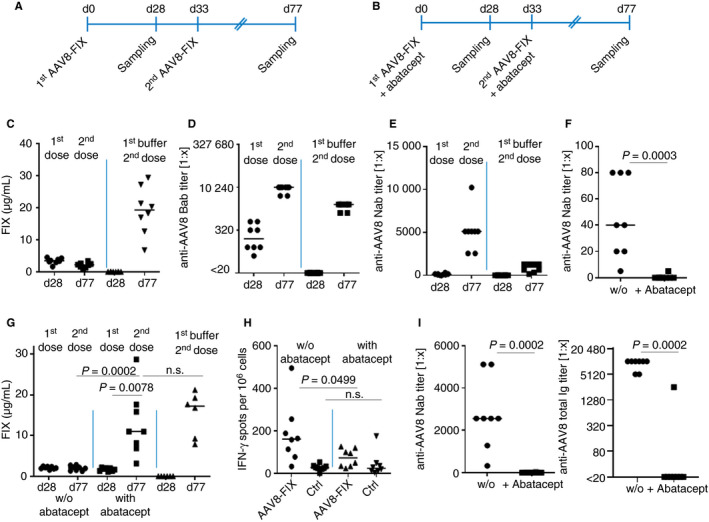
Abatacept efficiently suppresses anti‐adeno‐associated virus serotype 8 (AAV8) T‐cell and antibody responses, enabling redosing with AAV8‐factor IX (FIX). Mice were treated according to the indicated scheme once (A) or twice (B) with AAV8‐FIX or buffer. For repeated treatment, 5 × 1011 vg/kg AAV8‐FIX and a subsequent dose of 4 × 1012 vg/kg were used with and without immunosuppression. Treatment in the absence of abatacept showed that AAV8‐induced immune responses inhibit the application of the second treatment, thus blocking the boosting of FIX expression on day 77 after the second dose (C). Lower titers of AAV8 antibodies were induced after the first dose and boosted after the second (D, E). Abatacept suppresses anti‐AAV8 NAb responses (F), enabling redosing and boosting of FIX expression (G). Concomitant abatacept treatment during the second vector application suppresses the adaptive anti‐AAV8 immune response of day 77 (H, I) (significant P > .005; n.s. = not significant)
Generation of anti‐AAV8 immunity was prevented in two independent experiments by applying abatacept before and after the AAV8‐FIX injection (Figure 2B). Concomitant application of abatacept efficiently inhibited the development of AAV8‐specific NAb responses on day 28 after treatment (Figure 2F). Repeated application of AAV8 vectors in the presence of abatacept finally boosted FIX plasma levels on day 77 to within the range shown by the controls, which were treated only once with AAV8‐FIX (Figure 2G shows a representative experiment). A second application of abatacept was applied to show that anti‐AAV8 antibodies and T cells could be suppressed efficiently, even after the second higher dose, potentially facilitating a third AAV8 vector treatment (Figure 2H‐I).
Repeated administration of AAV vectors is required in patients who are expected to have low expression levels due to low vector doses or a decline in transgene expression over time.8 Hence, various redosing strategies have been suggested such as using different serotypes of vectors with13 and without14 preventive immunomodulation. These approaches have the disadvantage that new AAV vectors need to be developed and redosing efficacy might be impacted by cross‐reactivity of anti‐AAV immune responses. Accordingly, an approach using rapamycin‐filled nanoparticles, avoiding the development of new gene therapy vectors, was suggested.15 Our data indicate that a preventive approach based on CTLA‐4IgG would enable redosing without having to develop a new drug. The present protocol focused on AAV8‐FIX gene therapy using vector doses up to 5 × 1011 vg/mL. Based on our data, vector doses up to 5 × 1011 vg/mL could be used to enable redosing. However, a second treatment to boost FIX expression using a dose of 4 × 1012 vg/kg in the presence of abatacept showed that abatacept was capable of suppressing anti‐AAV8 NAb responses efficiently even at higher vector doses (Figure 2I). As the presence of neutralizing antibodies is the limiting factor for readministration, we conclude that with our immune suppressive regimen, vector doses up to 4 × 1012 vg/kg could be suppressed to enable redosing. Further studies are needed to enable readministration of doses higher than 4 × 1012 vg/kg because higher vector doses are likely to induce stronger anti‐AAV8 immunity.
Abatacept and the second‐generation product belatacept are approved drugs with known safety profiles that suppress T‐cell activation and accordingly primary antibody responses.9, 10, 11 CTLA‐4‐IgG targets T cells and, hence, antibody‐producing plasma cells that do not require T‐cell help would not be affected by CTLA4‐IgG.16 Accordingly, preexisting AAV antibody levels would not be reduced by using CTLA4‐IgG. Other technologies such as immune adsorption columns could be used to remove preexisting AAV8 antibodies.17 However, CTLA‐4 IgG could play an important role in suppressing preexisting memory T‐cell responses that are considered to kill transduced hepatocytes.18
In general, in both cases, anti‐AAV T‐cell immunity could be inhibited more specifically by CTLA‐4IgG, reducing the potential side effects of prednisone.19, 20 Accordingly, mice treated with abatacept did not show statistically significant different transgene expressions compared with the control group (Figure 2G), and no treatment‐related effects were observed (data not shown). Additionally, a preventive transient immunosuppression with a drug that has a known safety profile is intended, thus decreasing any potential side effects of the immune suppressant. Moreover, if required, abatacept could be safely combined with other immunosuppressants.21, 22 Our data suggest that AAV8 immune responses are T‐cell dependent and transient and preventive immune suppression targeting T cells can enable redosing in AAV8 gene therapy.
3. METHODS
3.1. Mice and gene vectors
C57BL/6J mice, purchased from Charles River Laboratories Inc, were challenged at age 8 to 10 weeks and killed at indicated time points. Purified full AAV8‐FIX capsids (90% full capsids) were used at the indicated doses.
3.2. Experimental gene therapy
Mice were treated with two doses (5 × 1011 vector genomes [vg] per kg of body weight and 4 × 1012 vg/kg) of AAV8‐FIX or buffer. The groups received 500 μg abatacept (Bristol‐Myers Squibb) intraperitoneally 3 days and 1 day before injection of AAV8 vectors, and at days 1, 3, and 6 after injection. AAV8‐FIX was further injected with and without abatacept in the same way. Blood was collected from the tail at the indicated time points. Spleens were harvested on day 77.
3.3. Cell preparation
Single‐cell suspensions were obtained from spleens by passing through a 40‐μm filter (BD Biosciences). After lysing erythrocytes with ACK lysis buffer (Thermo Fisher), cells were cultured in RPMI medium supplemented with 100 U/mL penicillin, 0.1 mg/mL streptomycin, 0.3 mg/mL glutamine, 10% inactivated fetal bovine serum (FBS; all PAA Laboratories Inc), and 50 μM β‐mercaptoethanol (Sigma‐Aldrich).
3.4. ELISpot
The IFN‐γ ELISpot was performed according to the manufacturer's protocol (Cellular Technology Limited) and as described using AAV8 peptide pools (15mer, offset 5, pool 1 = peptide 1‐50, pool 2 = peptide 51‐100, pool 3 = the remaining peptides; Anita Kruzik, Damir Fetahagic, Bettina Hartlieb, Sebastian Dorn, Frank M. Horling, FS, BMR, and M.d.l.R., manuscript submitted January 2019).
3.5. In vitro NAb assay
The in vitro NAb assay was performed using AAV8‐luciferase reporter constructs and Huh7 cells and a 1:5 cutoff.4
3.6. Detection of FIX protein concentration
FIX protein concentration was measured with an enzyme‐linked immunosorbent assay (ELISA) using commercially available polyclonal‐paired anti–human FIX antibodies as described.4
3.7. ELISA
Anti‐AAV8 IgG1 binding antibodies were detected by ELISA as described.4 In brief, AAV8 capsids were coated on 96‐well plates overnight at 4°C. For antibody detection, horseradish peroxidase‐conjugated secondary antibodies specific to murine IgG1 were incubated for 1 hour, and then tetramethylbenzidine used as a substrate. Anti‐AAV8 Ig binding antibody ELISA was performed as described above by using a peroxidase‐conjugated pan‐Ig specific secondary antibody.
3.8. Fluorescence‐activated cell sorting
Single‐cell suspensions were stimulated with AAV8 peptide pools for 6 hours and subsequently stained using CytoFix/CytoPerm (BD) and CD4‐BV605 (RM4‐5), CD8‐PerCpCy5.5 (53‐6.7), IFN‐γ‐ PeCy7 (XMG1.2), IL‐2‐APC (JES6‐5H4), IL‐4‐Pe (11B11), TNF‐α–A700 (MP6‐XT22 all from Biolegend), CD154‐FITC (MR1) from Invitrogen, and CD107a‐eFluor 450 (eBio1D4B) from eBioscience. Dead cells were discriminated by the Aqua Dead Cell Stain Kit (Thermo Fisher).
3.9. Statistical analysis
Between‐treatment group differences were assessed for endpoints using the non‐parametric unpaired Mann‐Whitney test and between time points in the same animals a non‐parametric, paired Wilcoxon test. The analyses were performed with Graphpad Prism 8.2.
CONFLICTS OF INTEREST
FS, BMR, and M.d.l.R. are employees of Baxalta Innovations GmbH, a member of the Takeda group of companies and are Takeda stock owners.
AUTHOR CONTRIBUTIONS
MF designed experiments, analyzed and interpreted the data, and wrote the manuscript; ASJ designed and performed experiments; MD performed experiments and established the assays; NM obtained approvals for animal research; AT provided intellectual input and editorial assistance; FS and BMR interpreted the data, facilitated the study, and reviewed the manuscript; M.d.l.R. designed the study, analyzed and interpreted the data, and wrote the manuscript; and all authors reviewed the manuscript critically for intellectual content, participated in drafting and/or revising the manuscript, and approved the final version for submission.
ACKNOWLEDGMENTS
The authors would like to thank Hanspeter Rottensteiner, Maria Schuster, Markus Weiller, Alfred Weber for their support of the studies, and Elise Langdon‐Neuner for English editing support. The authors thank the BCRT/Charité Flow Cytometry Laboratory for their highly competent support. This research was funded by Baxalta Innovations GmBH, a member of the Takeda group of companies, Vienna, Austria. Editing support for this manuscript was provided by Isobel Lever employee of Excel Medical Affairs (Southport, CT, USA), and was funded by Baxalta US Inc, a member of the Takeda group of companies, Lexington, MA, USA.
Frentsch M, Japp AS, Dingeldey M, et al. Blockade of the costimulatory CD28‐B7 family signal axis enables repeated application of AAV8 gene vectors. J Thromb Haemost. 2020;18:1075–1080. 10.1111/jth.14757
Manuscript handled by: David Lillicra
Final decision: 27 January 2020
REFERENCES
- 1. Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus‐associated virus vector‐mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357‐2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nathwani AC, Reiss UM, Tuddenham EG, et al. Long‐term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371(21):1994‐2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. George LA, Sullivan SK, Giermasz A, et al. Hemophilia B gene therapy with a high‐specific‐activity factor IX variant. N Engl J Med. 2017;377(23):2215‐2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kruzik A, Koppensteiner H, Fetahagic D, et al. Detection of biologically relevant low‐titer neutralizing antibodies against adeno‐associated virus require sensitive in vitro assays. Hum Gene Ther Methods. 2019;30(2):35‐43. [DOI] [PubMed] [Google Scholar]
- 5. Wang L, Calcedo R, Bell P, et al. Impact of pre‐existing immunity on gene transfer to nonhuman primate liver with adeno‐associated virus 8 vectors. Hum Gene Ther. 2011;22(11):1389‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV‐Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342‐347. [DOI] [PubMed] [Google Scholar]
- 7. Zaiss AK, Muruve DA. Immune responses to adeno‐associated virus vectors. Curr Gene Ther. 2005;5(3):323‐331. [DOI] [PubMed] [Google Scholar]
- 8. Corti M, Elder M, Falk D, et al. B‐cell depletion is protective against anti‐AAV capsid immune response: a human subject case study. Mol Ther Methods Clin Dev. 2014;1:14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Korhonen R, Moilanen E. Abatacept, a novel CD80/86‐CD28 T cell co‐stimulation modulator, in the treatment of rheumatoid arthritis. Basic Clin Pharmacol Toxicol. 2009;104(4):276‐284. [DOI] [PubMed] [Google Scholar]
- 10. Vincenti F, Larsen C, Durrbach A, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353(8):770‐781. [DOI] [PubMed] [Google Scholar]
- 11. Young JS, Chen J, Miller ML, et al. Delayed cytotoxic T lymphocyte‐associated protein 4‐immunoglobulin treatment reverses ongoing alloantibody responses and rescues allografts from acute rejection. Am J Transplant. 2016;16(8):2312‐2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frentsch M, Stark R, Matzmohr N, et al. CD40L expression permits CD8+ T cells to execute immunologic helper functions. Blood. 2013;122(3):405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McIntosh JH, Cochrane M, Cobbold S, et al. Successful attenuation of humoral immunity to viral capsid and transgenic protein following AAV‐mediated gene transfer with a non‐depleting CD4 antibody and cyclosporine. Gene Ther. 2012;19(1):78‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halbert CL, Rutledge EA, Allen JM, Russell DW, Miller AD. Repeat transduction in the mouse lung by using adeno‐associated virus vectors with different serotypes. J Virol. 2000;74(3):1524‐1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meliani A, Boisgerault F, Hardet R, et al. Antigen‐selective modulation of AAV immunogenicity with tolerogenic rapamycin nanoparticles enables successful vector re‐administration. Nat Commun. 2018;9(1):4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol. 2015;15(3):149‐159. [DOI] [PubMed] [Google Scholar]
- 17. Salas D, Kwikkers KL, Zabaleta N, et al. Immunoadsorption enables successful rAAV5‐mediated repeated hepatic gene delivery in nonhuman primates. Blood Adv. 2019;3(17):2632‐2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122(1):23‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lozada F, Silverman S Jr, Migliorati C. Adverse side effects associated with prednisone in the treatment of patients with oral inflammatory ulcerative diseases. J Am Dent Assoc. 1984;109(2):269‐270. [DOI] [PubMed] [Google Scholar]
- 20. Richards RN. Side effects of short‐term oral corticosteroids. J Cutan Med Surg. 2008;12(2):77‐81. [DOI] [PubMed] [Google Scholar]
- 21. Langford CA, Cuthbertson D, Ytterberg SR, et al. A randomized, double‐blind trial of abatacept (CTLA‐4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol. 2017;69(4):837‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. ACCESS Trial Group . Treatment of lupus nephritis with abatacept: the abatacept and cyclophosphamide combination efficacy and safety study. Arthritis Rheumatol. 2014;66(11):3096‐3104. [DOI] [PMC free article] [PubMed] [Google Scholar]


