Abstract
Introduction
Cold allodynia is often seen in the acute phase of oxaliplatin treatment, but the underlying pathophysiology remains unclear.
Methods
Patients scheduled for adjuvant oxaliplatin for colorectal cancer were examined with quantitative sensory testing and nerve excitability tests at baseline and after the second or third oxaliplatin cycle at different skin temperatures.
Results
Seven patients were eligible for examination. All patients felt evoked pain and tingling when touching something cold after oxaliplatin infusion. Oxaliplatin decreased motor nerve superexcitability (P < .001), increased relative refractory period (P = .011), and caused neuromyotonia‐like after‐activity. Cooling exacerbated these changes and prolonged the accommodation half‐time.
Discussion
The findings suggest that a combined effect of oxaliplatin and cooling facilitates nerve excitability changes and neuromyotonia‐like after‐activity in peripheral nerve axons. A possible mechanism is the slowing in gating of voltage‐dependent fast sodium and slow potassium channels, which results in symptoms of cold allodynia.
Keywords: allodynia, nerve excitability testing, neuropathy, oxaliplatin toxicity, potassium channel dysfunction, sodium channel dysfunction
Abbreviations
- CAPOX
capecitabine and oxaliplatin
- CDT
cold detection threshold
- CMAP
compound muscle action potential
- CPT
cold pain threshold
- HPT
heat pain threshold
- NRS
numerical rating scale
- OXP
oxaliplatin
- RC
recovery cycle
- SDTC
strength‐duration time constant
- TE
threshold electrotonus
- VDT
vibration detection threshold
- WDT
warm detection threshold
1. INTRODUCTION
Oxaliplatin used in the treatment of several types of gastrointestinal cancer may cause an acute, partly reversible neuropathy characterized by distal and perioral tingling, muscle stiffness, and a striking cold allodynia characterized by pricking dysesthesia and pain upon touching something cold.1, 2, 3, 4 The pathophysiology underlying this cold hypersensitivity is not well understood.5, 6
Oxaliplatin has been shown to slow the inactivation of voltage‐dependent Na+ channels.5 This effect may be enhanced by cooling, which has been shown to prolong the refractoriness in peripheral axons.7, 8, 9 In addition, cooling slows the kinetics in the activation of axonal slow K+ (Kv7) channels that regulate accommodation in excitability.7, 8, 9, 10 Cooling also results in membrane depolarization of axons.8, 9
Electrophysiological effects of cooling on these parameters in oxaliplatin‐treated patients have not been described. The aim of this study was to use nerve excitability testing11 to examine changes in nerve excitability and repetitive activity in motor axons from baseline to after oxaliplatin treatment and the effect of cold on these changes.
2. METHODS
2.1. Ethics approval
This study was approved by the Central Denmark Region Committees on Health Research Ethics (No. 1‐10‐72‐154‐16) and the Danish Data Protection Agency (No. 1‐16‐02‐89‐16). The study was carried out in accordance with the Declaration of Helsinki. All subjects signed a written informed consent.
2.2. Study protocol
Patients with high‐risk colon cancer (stage II or stage III) scheduled to receive adjuvant combination chemotherapy with oxaliplatin and capecitabine (CAPOX) at the Department of Oncology, Aarhus University Hospital, Denmark, were invited to participate in this prospective clinical study in 2017‐2018. The patients were excluded if they had metastatic disease, previous systemic chemotherapy, alcohol or medicine abuse, a severe psychiatric disorder, known diabetes, spinal stenosis, peripheral vascular disease, polyneuropathy, or chronic pain with an intensity of at least 3 on the 0 to 10 numerical rating scale (NRS).
Patients were examined before chemotherapy (baseline) and second or third day after the second or third cycle of oxaliplatin (follow‐up). There was a 3‐week interval between cycles if hematology parameters allowed. They underwent interviews regarding symptoms and filled out questionnaires about oxaliplatin‐related symptoms.12
Quantitative sensory testing was performed according to the German Research Network of Neuropathic Pain protocol.13 Vibration (VDT), cold (CDT), and warm (WDT) detection thresholds and cold pain (CPT) and heat pain (HPT) thresholds were assessed at the cheek, the thenar eminence of the hand, and the dorsal lateral side of the foot. Thermal stimuli were applied using a thermal sensory analyzer (Medoc, Ramat Yishai, Israel) and VDT was assessed with a Rydel‐Seiffer graded tuning fork (conventional 68 Hz, 8/8 scale). Cold‐evoked pain and dysesthesia were also assessed by asking the patient to hold a cold (~6°C) custom‐made metal cylinder (Ventzel, Aarhus, Denmark) for 10 seconds and to rate the intensity of pain and unpleasantness on the NRS.14
Nerve excitability testing was recorded from the median nerve using the automated TRONDOLM (motor) and TRONDOLS (sensory) protocols (Institute of Neurology, London, UK).15 Multiple excitability parameters were assessed including the stimulus‐response curve, strength‐duration time constant (SDTC), threshold electrotonus (TE), and recovery cycle (RC). Electrodes were placed as described in previous studies.5, 16 Examinations were performed at a skin temperature above 32°C at baseline and a skin temperature above 32°C, as well as with the wrist cooled during the protocol with packed ice to skin temperature below 27°C (mean 25.6°C) at follow‐up.11 The ice was only removed when measuring the temperature, or if the patients felt that it was very unpleasant.
2.3. Statistical analysis
Nerve excitability testing data were analyzed with QtracP software and other analyses were performed with STATA version 14.2 (StataCorp, College Station, Texas) and Equista version 1.3.5. Data are presented as mean and standard deviation (SD). Normally distributed paired data were analyzed with t tests, and otherwise with Wilcoxon signed rank tests. The significance level was set at P < .05. We did not correct for multiple testing.
3. RESULTS
Ten patients were included at baseline, and 7 were eligible for follow‐up after CAPOX (Table S1). Three patients dropped out because the oxaliplatin treatment had been terminated early due to persistent neuropathy and hematological suppression.
At follow‐up, all 7 patients felt tingling and pain or discomfort in the hands when touching something cold (Table S2). One patient had involuntary twitches of the thumb. Six patients felt pain with a mean intensity of 7.5 when holding the cold Ventzel cylinder (Table S2). For the hands and cheek, CPT was significantly closer to baseline temperatures, indicating increased sensitivity and VDT was significantly further from baseline thresholds showing decreased sensitivity (Figure S1).
There were no significant differences in the sensory recordings from baseline to after oxaliplatin. After cooling, recordings were missing in five of the seven patients due to neuromyotonia‐like afterpotentials and discomfort, and therefore sensory results are not shown.
The results for motor nerve excitability testing are shown in Table 1 and Figure 1. Recordings at baseline, follow‐up, and follow‐up with cooling revealed statistically significant changes in several parameters (Table 1). There was a decrease from baseline to follow‐up in superexcitability (P < .001), an increase in subexcitability (P = .003), and slowing of the relative refractory period (RRP) (P = .011) (Figure 1C). There were no consistent changes in the stimulus‐response properties, SDTC, or rheobase. Also, TE parameters were not changed (Table 1).
Table 1.
Motor nerve excitability testing
| Baseline (n = 7) | Follow‐up (n = 7) | Baseline vs follow‐up (P value) | Cooling (n = 7) | Follow‐up vs cooling (P value) | |
|---|---|---|---|---|---|
| Skin temperature (°C) | 33.7 (1.0) | 34.3 (1.3) | .31a | 25.6 (2.7) | <.001 |
| Latency (ms) | 7.0 (1.2) | 6.8 (0.8) | .16a | 7.1 (0.9) | <.016 * |
| Stimulus‐response and strength‐duration properties | |||||
| Stimulus (mA): 50% max CMAP | 6.6 (2.2) | 7.8 (2.9) | .24 | 6.4 (1.9) | .015 |
| SDTC (ms) | 0.5 (0.1) | 0.4 (0.1) | .051 | 0.5 (0.2) | .50 |
| Rheobase (mA) | 4.3 (1.7) | 5.3 (2.2) | .16 | 4.7 (1.8) | .29 |
| Threshold electrotonus | |||||
| TEd (10‐20 ms) | 68.1 (4.1) | 68.1 (5.0) | .92 | 64.6 (4.6) | .021 |
| TEd (peak) | 66.4 (3.5) | 66.4 (4.6) | .89 | 64.5 (4.1) | .12 |
| TEd (40‐60 ms) | 47.8 (3.5) | 48.5 (3.4) | .45 | 54.8 (3.3) | .002 |
| TEd (90‐100 ms) | 43.4 (3.5) | 44.5 (4.1) | .099 | 42.7 (5.8) | .35 |
| S2 accommodation | 23.1 (3.2) | 21.8 (3.3) | .099 | 21.8 (5.5) | .94 |
| TEd20 (10‐20 ms) | 37.4 (2.8) | 37.0 (3.1) | .64 | 32.4 (3.3) | .003 * |
| TEd20 (peak) | 38.4 (2.7) | 38.6 (3.4) | .84 | 34.8 (3.7) | .019 |
| TEd40 (accomodation) | 23.5 (2.7) | 22.1 (3.1) | .066 | 20.7 (5.2) | .56 |
| TEh (10‐20 ms) | −80.1 (8.4) | −79.6 (7.0) | .70 | −74.8 (10.6) | .086 |
| TEh (20‐40 ms) | −101.5 (12.3) | −99.6 (11.9) | .40 | −88.4 (15.9) | .016 |
| TEh (90‐100 ms) | −138.9 (21.9) | −135.4 (21.5) | .31 | −110.1 (24.1) | .014 |
| TEh (peak, ‐70%) | −290.0 (40.9) | −279.8 (39.4) | .007 | −246.5 (47.1) | .006 |
| Accommodation half‐time (ms) | 36.5 (4.0) | 35.9 (2.8) | .36 | 54.2 (8.2) | .001 |
| Recovery cycle | |||||
| Relative refractory period (ms) | 2.9 (0.3) | 3.6 (0.3) | .011 | 5.8 (1.9) | ― |
| Superexcitability (%) | −19.2 (3.1) | −5.7 (7.0) | <.001 | −2.7 (5.0) | .044 |
| Subexcitability (%) | 17.2 (5.4) | 23.3 (6.9) | .003 | 20.8 (8.9) | .23 |
| Refractoriness at 2.5 ms (%) | 16.7 (12.6) | 37.5 (16.0) | .003 | ― | ― |
Note. Values are presented as mean (standard deviation). All significant P values are indicated in bold.
Abbreviations: CMAP, compound motor action potential; max, maximal; SDTC, strength‐duration time constant; TEd/TEh, threshold electrotonus depolarization/hyperpolarization.
Calculated using the Wilcoxon signed rank test, otherwise paired t test was used.
Figure 1.
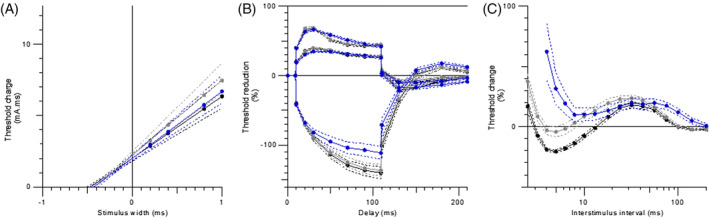
Nerve excitability testing for the motor nerve. A) Strength‐duration time constant, B) threshold electrotonus, and C) recovery cycle. Dark line: baseline; gray line: follow‐up without cooling; blue (triangle) line: follow‐up with cooling. Filled lines: means; dashed lines: standard errors [Color figure can be viewed at wileyonlinelibrary.com]
During cooling, there was a strong prolongation of the RRP and a complete loss of superexcitability. The amplitude of the late subexcitability remained constant, although it increased in duration. Cooling resulted in a significant change in the accommodation half‐time (P = .001), which is also revealed by the increase in excitability seen during long depolarizing current pulses (TEd 40 to 60 milliseconds) (P = .002) (Figure 1B). None of the participants felt any discomfort in the hands with cooling.
Oxaliplatin and cooling caused no consistent changes in SDTC and rheobase (Figure 1A).
There were no neuromyotonia‐like afterpotentials at baseline (Figure 2). At follow‐up, the number of afterpotentials with a voltage amplitude of more than 2% of maximal compound muscle action potential (CMAP) in the time interval from maximal CMAP until 35 to 40 milliseconds ranged from one to three (median of two) at normal skin temperature and from two to five (median of four) after cooling (P = .0083). Representative examples of such recordings are shown in Figure 2.
Figure 2.
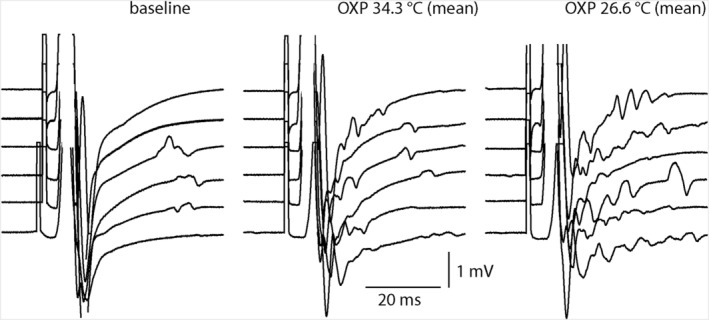
Afterpotentials after maximum CMAP at baseline, follow‐up, and follow‐up with cooling. The number of afterpotentials increased with cooling. Only six of the seven patients are shown due to excess voluntary activity in one patient. CMAP, compound muscle action potential; OXP, oxaliplatin
4. DISCUSSION
In this study we have confirmed previously shown nerve excitability changes in motor nerve fibers5 and further showed that cooling increases refractoriness and enhanced poststimulus repetitive muscle action potential activity. We did not see such an effect in sensory nerve fibers due to baseline noise when cooling the wrist, but cold aggravated oxaliplatin‐induced afterpotential has been demonstrated in an isolated mouse skin‐nerve preparation.17
One likely candidate, as suggested by animal models,18 for the nerve excitability changes is the voltage‐dependent sodium channel Nav1.6, which is present in both motor and sensory axons.19 A proposed mechanistic explanation for the neuromyotonia‐like afterpotentials is an increase in the Nav1.6‐induced resurgent and persistent current due to the slowing of the inactivation rate constant of the channel.17, 20 Cooling in the presence of oxaliplatin further increased the time constant of the inactivation of Nav1.6, which may produce more resurgent Na+ currents and neuromyotonia‐like after‐activity.17 In another study, Nav1.6‐related burst activity lasted up to 50 milliseconds after peak of sensory nerve action potential in mouse sural nerve,17 which is within the same time scale observed in the current study. Slowing of the inactivation of sodium channels has also been shown in oxaliplatin‐treated patients.5 This is the most likely reason for cold‐induced changes in the RC of oxaliplatin‐treated patients in the current study,7, 8 which have been shown to be reversible.5
Another candidate for abnormal excitability produced by cooling of oxaliplatin‐treated patients is slow nodal potassium (Kv7) channels.21, 22 The present data reveal slowing of accommodation seen in TE recordings, suggesting that cooling reduced the rate constant activation in Kv7 channels. Such an effect of cooling in recordings of TE in probands has been described.7, 8 Inhibition of Kv7 channels facilitates repetitive firing of axons and nerve terminals.10, 22 Conversely, activation of Kv7 channels reduces this type of neuromyotonia‐like after‐activity.23
A third possibility is that cooling results in depolarization of axons. Effects of cold on the TE in the form of “fanning‐in” have been described in healthy probands.8 We observed neither fanning‐in nor changes in SDTC that could suggest depolarization, but we also did not reach the same low tissue temperatures (less than 20°C).8
A limitation of this study is that we assessed motor fibers in the nerve trunk as a surrogate method and did not directly evaluate the small sensory afferents distally. Furthermore, cooling at the wrist did not cause symptoms. Because capecitabine was used concurrently, we cannot exclude its possible involvement. In addition, we could not examine the association between pharmacokinetic aspects and ion channel kinetics due to the limited number of subjects.
Taken together, our data indicate that oxaliplatin and cold additively affect ion channels present in the axonal membrane of axons and/or terminals in motor nerve fibers. The most likely candidates are voltage‐gated fast sodium and slow potassium channels.
5. CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
FIGURE S1 Quantitative sensory testing of the cheek, hand, and foot. A) CDT, cold detection threshold, B) CPT, cold pain threshold, C) WDT, warm detection threshold, D) HPT, heat pain threshold, and E) VDT, vibration detection threshold. The baseline values are indicated with a dashed line. For WDT and HPT high thresholds indicate decreased sensitivity, whereas for CDT, CPT and VDT decreased sensitivity is shown by low thresholds.”
Table S1 Patient characteristics
Table S2. Outcomes from the oxaliplatin‐specific questionnaire and Ventzel cold cylinder.
ACKNOWLEDGMENTS
The authors thank Helle O. Andersen for secretarial and language assistance. We also thank the patients who took the time to complete the questionnaires and participate in clinical examinations.
Bennedsgaard K, Ventzel L, Grafe P, et al. Cold aggravates abnormal excitability of motor axons in oxaliplatin‐treated patients. Muscle Nerve. 2020;61:796–800. 10.1002/mus.26852
Funding information Aarhus Universitet; Danish National Research Foundation, Grant/Award Number: DNRF121; EU Horizon 2020 research and innovation programme, Grant/Award Number: 633491, DOLORisk
REFERENCES
- 1. Pachman DR, Qin R, Seisler DK, et al. Clinical course of oxaliplatin‐induced neuropathy: results from the randomized phase III trial N08CB (Alliance). J Clin Oncol. 2015;33:3416‐3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grothey A. Oxaliplatin‐safety profile: neurotoxicity. Semin Oncol. 2003;30:5‐13. [DOI] [PubMed] [Google Scholar]
- 3. Attal N, Bouhassira D, Gautron M, et al. Thermal hyperalgesia as a marker of oxaliplatin neurotoxicity: a prospective quantified sensory assessment study. Pain. 2009;144:245‐252. [DOI] [PubMed] [Google Scholar]
- 4. Cersosimo RJ. Oxaliplatin‐associated neuropathy: a review. Ann Pharmacother. 2005;39:128‐135. [DOI] [PubMed] [Google Scholar]
- 5. Heide R, Bostock H, Ventzel L, et al. Axonal excitability changes and acute symptoms of oxaliplatin treatment: in vivo evidence for slowed sodium channel inactivation. Clin Neurophysiol. 2018;129:694‐706. [DOI] [PubMed] [Google Scholar]
- 6. Park SB, Lin CS, Krishnan AV, et al. Dose effects of oxaliplatin on persistent and transient Na+ conductances and the development of neurotoxicity. PLoS One. 2011;6:e18469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kiernan MC, Cikurel K, Bostock H. Effects of temperature on the excitability properties of human motor axons. Brain. 2001;124:816‐825. [DOI] [PubMed] [Google Scholar]
- 8. Franssen H, Gebbink TA, Wokke JH, et al. Is cold paresis related to axonal depolarization? J Peripher Nerv Syst. 2010;15:227‐237. [DOI] [PubMed] [Google Scholar]
- 9. Kovalchuk MO, Franssen H, Van Schelven LJ, et al. Comparing excitability at 37 degrees C versus at 20 degrees C: differences between motor and sensory axons. Muscle Nerve. 2018;57:574‐580. [DOI] [PubMed] [Google Scholar]
- 10. Passmore GM, Reilly JM, Thakur M, et al. Functional significance of M‐type potassium channels in nociceptive cutaneous sensory endings. Front Mol Neurosci. 2012;5:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kiernan MC, Bostock H, Park SB, et al. Measurement of axonal excitability: consensus guidelines. Clin Neurophysiol. 2020;131:308‐323. [DOI] [PubMed] [Google Scholar]
- 12. Leonard GD, Wright MA, Quinn MG, et al. Survey of oxaliplatin‐associated neurotoxicity using an interview‐based questionnaire in patients with metastatic colorectal cancer. BMC Cancer. 2005;5:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231‐243. [DOI] [PubMed] [Google Scholar]
- 14. Ventzel L, Madsen CS, Jensen AB, et al. Assessment of acute oxaliplatin‐induced cold allodynia: a pilot study. Acta Neurol Scand. 2016;133:152‐155. [DOI] [PubMed] [Google Scholar]
- 15. Bostock H, Cikurel K, Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve. 1998;21:137‐158. [DOI] [PubMed] [Google Scholar]
- 16. Park SB, Lin CS, Kiernan MC. Nerve excitability assessment in chemotherapy‐induced neurotoxicity. J Vis Exp. 2012; April;26(62). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sittl R, Lampert A, Huth T, et al. Anticancer drug oxaliplatin induces acute cooling‐aggravated neuropathy via sodium channel subtype Na(V)1.6‐resurgent and persistent current. Proc Natl Acad Sci USA. 2012;109:6704‐6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deuis JR, Zimmermann K, Romanovsky AA, et al. An animal model of oxaliplatin‐induced cold allodynia reveals a crucial role for Nav1.6 in peripheral pain pathways. Pain. 2013;154:1749‐1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caldwell JH, Schaller KL, Lasher RS, et al. Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc Natl Acad Sci USA. 2000;97:5616‐5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cannon SC, Bean BP. Sodium channels gone wild: resurgent current from neuronal and muscle channelopathies. J Clin Invest. 2010;120:80‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Devaux JJ, Kleopa KA, Cooper EC, et al. KCNQ2 is a nodal K+ channel. J Neurosci. 2004;24:1236‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwarz JR, Glassmeier G, Cooper EC, et al. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol. 2006;573:17‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sittl R, Carr RW, Fleckenstein J, et al. Enhancement of axonal potassium conductance reduces nerve hyperexcitability in an in vitro model of oxaliplatin‐induced acute neuropathy. Neurotoxicology. 2010;31:694‐700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Quantitative sensory testing of the cheek, hand, and foot. A) CDT, cold detection threshold, B) CPT, cold pain threshold, C) WDT, warm detection threshold, D) HPT, heat pain threshold, and E) VDT, vibration detection threshold. The baseline values are indicated with a dashed line. For WDT and HPT high thresholds indicate decreased sensitivity, whereas for CDT, CPT and VDT decreased sensitivity is shown by low thresholds.”
Table S1 Patient characteristics
Table S2. Outcomes from the oxaliplatin‐specific questionnaire and Ventzel cold cylinder.


