Abstract
Background
Although antiretroviral therapy (ART) has reduced the risk of Kaposi sarcoma (KS), KS cases still occur in HIV‐infected people.
Objective
To describe all KS cases observed between 2010 and 2015 in a country with high ART coverage.
Methods
Retrospective study using longitudinal data from 44 642 patients in the French Dat’AIDS multicenter cohort. Patients’ characteristics were described at KS diagnosis according to ART exposure and to HIV‐plasma viral load (HIV‐pVL) (≤50 or >50) copies/mL.
Results
Among the 209 KS cases diagnosed during the study period, 33.2% occurred in ART naïve patients, 17.3% in ART‐experienced patients and 49.5% in patients on ART, of whom 23% for more than 6 months. Among these patients, 24 (11.5%) had HIV‐pVL ≤50 cp/mL, and 16 (66%) were treated with a boosted‐PI‐based regimen. The distribution of KS localization did not differ by ART status nor by year of diagnosis.
Limitations
Data on human herpesvirus 8, treatment modalities for KS and response rate were not collected.
Conclusion
Half of KS cases observed in the study period occurred in patients not on ART, reflecting the persistence of late HIV diagnosis. Factors associated with KS in patients on ART with HIV‐pVL ≤50 cp/mL remain to be explored.
Introduction
Kaposi sarcoma (KS) has long remained the most common malignant condition in people living with HIV (PLWHIV). Although ART reduces the risk of KS and modifies its clinical features and prognosis,1, 2 KS continues to occur in ART‐treated patients, even in those with a sustained undetectable HIV‐plasma viral load (HIV‐pVL) and immune restoration.3 To date, however, the prevalence of such clinical presentation has not been clearly identified.
We aimed to describe all KS cases observed between 2010 and 2015 in a large multicenter French cohort to determine who experienced KS in a country with high ART coverage.
Methods
This multicenter retrospective analysis was performed using longitudinal data from the French Dat'AIDS cohort (NCT 02898987 ClinicalTrials.gov). In 2010, this cohort represented a collaboration of 17 major French HIV clinical centres using a common electronic medical record for the follow‐up of HIV, hepatitis B virus (HBV) and hepatitis C virus (HCV)‐infected adults (NADIS®).4 The data collection was approved by the French National Commission on Informatics and Liberty (CNIL 2001/762876; MR004 2210731v.0), and all patients signed an informed consent form before being included in this database. For this study, we selected patients followed in the cohort between 1 January 2010, and 31 December 2015, including those with a history of KS and/or malignant disease. ICD10 codes were used to identify KS cases (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD10), World Health Organization, Geneva), ICD code C46). The study period for patients included before to 1 January 2010, began on 1 January 2010; for patients included after 1 January 2010, the study period began at the date of cohort inclusion. The end of follow‐up was the date of KS occurrence.
First, KS cases were analysed according to ART exposure as follows: (i) ART‐naïve patients, (ii) patients who were ART experienced but not on ART at the time of KS diagnosis (iii) and patients on ART. Then, we selected patients on ART for more than 6 months and analysed their characteristics according to HIV‐pVL >50 copies/mL or ≤50 copies/mL at the time of KS diagnosis. Gender, age, duration of HIV infection follow‐up, HIV transmission group, CDC stage, history of KS and/or malignant disease, hepatitis C (HCV) positive serology, nadir CD4 T‐cell count (cells/mm3; <200, 200–500, >500), CD4 and CD8 T‐cell count (cells/mm3; CD4: <200, 200–500, >500; CD8 > 1000), CD4 : CD8 ratio (median, <1 and ≥1), HIV‐pVL and time with HIV‐pVL ≤50 copies/mL, antiretroviral exposure (duration of ART exposure; ART regimen: nucleoside/tide reverse transcriptase inhibitor (NRTI)‐, non‐nucleoside reverse transcriptase inhibitor (NNRTI)‐, boosted protease inhibitor (bPI)‐ or integrase strand transfer inhibitor (INSTI)‐based regimen and date of antiretroviral treatment initiation before: <1995 (pre ART era), between 1996 and ‐2006 (ART era with PI availability), ≥2007 (ART era with INSTI availability) were collected at the time of KS diagnosis.
Statistical analyses were performed with SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA) and Stata/SE software (version 14; StataCorp LP, College Station, TX, USA).
Results
During the study period, 44 642 PLWHIV were included in the Dat'AIDS cohort, of whom 13 543 were females (30.3%) and 31 099 were males (69.6%), representing 180 206 person‐years of follow‐up. Over the course of 180 2016 person‐years, 209 KS cases were diagnosed: 52 (24.9%) cases in 2010, 41 (19.6%) in 2011, 33 (15.8%) in 2012, 28 (13.4%) in 2013, 30 (14.4%) in 2014 and 25 (12%) in 2015.
Most cases occurred in males (91.9%) and men who have sex with men (MSM) (63.6%), with a median age of 43.0 years (IQR: 35.7; 49.9) and a median HIV time follow‐up of 2.1 years (IQR: 0.1; 9.4).
Description of 209 KS cases according to ART exposure at the time of KS diagnosis
Among the 209 KS cases, ART status at time of KS diagnosis was not available in one patient; 69 KS cases occurred in ART‐naïve patients, of whom 41 patients had a concomitant HIV diagnosis with a median CD4 of 41/mm3 (IQR: 25;160); 36 KS cases occurred in patients who were ART experienced but not on ART at the time of KS diagnosis; and 103 KS cases occurred in patients on ART of whom 54 for more than 6 months (CD4/mm3): 252 [53; 469]). The patients’ characteristics are reported in Table 1. The distribution of KS cases according to these three conditions did not differ according to the year of diagnosis. Likewise, the median CD4 T‐cell count in ART‐naïve, ART‐experienced and ART‐treated patients at the time of KS diagnosis was similar each year of the study period (Fig. 1).
Table 1.
Patient's characteristics of 209 KS cases according to ART exposure at time of KS diagnosis in the French Dat'AIDS cohort between 2010 and 2015
| Median [IQR]; N (%) | All | ART Naive | ART interrupted | On ART | P |
|---|---|---|---|---|---|
| N = 209b | N = 69 | N = 36 | N = 103 | ||
| Gender | |||||
| Male | 192 (91.9) | 64 (92.8) | 34 (94.4) | 93 (90.3) | 0.84a |
| Female | 17 (8.1) | 5 (7.2) | 2 (5.6) | 10 (9.7) | |
| Age (years) | 43.0 [35.7; 49.9] | 42.3 [34.6; 49.2] | 43.7 [35.2; 51.2] | 43.4 [36.1; 49.3] | 0.10 |
| ≤25 | 4 (1.9) | 2 (2.9) | 0 | 2 (1.9) | 0.82a |
| 25–50 | 153 (73.2) | 52 (75.4) | 25 (69.4) | 76 (73.8) | |
| >50 | 52 (24.9) | 15 (21.7) | 11 (30.6) | 25 (24.3) | |
| >65 | 7 (3.3) | 4 (5.8) | 0 | 2 (1.9) | |
| HIV Transmission group | |||||
| MSM | 133 (63.6) | 40 (58) | 28 (77.8) | 65 (63.1) | 0.45a |
| Heterosexual | 48 (23) | 20 (29) | 5 (13.9) | 22 (21.4) | |
| IVDU | 6 (2.9) | 1 (1.4) | 1 (2.8) | 4 (3.9) | |
| Others/unknown | 22 (10.6) | 8 (11.6) | 2 (5.6) | 12 (11.7) | |
| HIV time follow‐up (years) | 2.1 [0.1; 9.4] | 0.1 [0; 2.8] | 6 [1.9; 13.7] | 5 [0.3; 12.7] | <0.001 |
| ≤1 | 90 (43.1) | 45 (65.2) | 8 (22.2) | 37 (35.9) | <0.001 |
| [1–5] | 33 (15.8) | 12 (17.4) | 6 (16.7) | 15 (14.6) | |
| [5–10] | 37 (17.7) | 7 (10.1) | 9 (25) | 21 (20.4) | |
| [10–15] | 24 (11.5) | 2 (2.9) | 7 (19.4) | 15 (14.6) | |
| 15 | 25 (12.0) | 3 (4.3) | 6 (16.7) | 15 (14.6) | |
| Number of cases diagnosed/year | |||||
| 2010 | 52 (24.9) | 16 (23.2) | 7 (19.4) | 29 (28.2) | 0.51 |
| 2011 | 41 (19.6) | 16 (23.2) | 5 (13.9) | 20 (19.4) | |
| 2012 | 33 (15.8) | 8 (11.6) | 11 (30.6) | 14 (13.6) | |
| 2013 | 28 (13.4) | 11 (15.9) | 3 (8.3) | 14 (13.6) | |
| 2014 | 30 (14.4) | 10 (14.5) | 5 (13.9) | 14 (13.6) | |
| 2015 | 25 (12.0) | 8 (11.6) | 5 (13.9) | 12 (11.7) | |
| Stage C at KS (n = 206) | |||||
| Yes | 58 (27.8) | 8 (11.8) | 8 (22.2) | 41 (40.6) | <0.001 |
| No | 148 (70.8) | 60 (88.2) | 28 (77.8) | 60 (59.4) | |
| HCV coinfection | |||||
| No | 191 (91.4) | 63 (91.3) | 33 (91.7) | 94 (91.3) | 1a |
| Yes | 18 (8.6) | 6 (8.7) | 3 (8.3) | 9 (8.7) | |
| Nadir CD4 cell count/mm3 | 123 [30; 264] | 105 [35; 311] | 150 [53; 296] | 122 [15; 250] | 0.34 |
| <200/mm3 | 121 (57.9) | 36 (66.7) | 22 (61.1) | 62 (60.8) | 0.32a |
| 200–500/mm3 | 62 (29.7) | 15 (27.8) | 10 (27.8) | 37 (36.3) | |
| >500/mm3 | 10 (4.8) | 3 (5.6) | 4 (11.1) | 3 (2.9) | |
| CD4 T‐cell count/mm3 | 188 [49; 360] | 90 [33; 267] | 184 [93; 428] | 243 [61; 429] | 0.12 |
| <200/mm3 | 107 (51.2) | 45 (69.2) | 18 (52.9) | 44 (42.7) | 0.02a |
| 200‐500/mm3 | 69 (33) | 14 (21.5) | 10 (29.4) | 44 (42.7) | |
| >500/mm3 | 27 (12.9) | 6 (9.2) | 6 (17.6) | 15 (14.6) | |
| CD8 T‐cell count/mm3 | 761 [463; 1247] | 659 [383; 1154] | 863 [647; 1057] | 798 [512; 1296] | 0.25 |
| >1000/mm3 | 62 (29.7) | 18 (29.5) | 9 (28.1) | 35 (38.9) | 0.37 |
| CD4 : CD8 ratio | 0.2 [0.1; 0.4] | 0.11 [0.04; 0.29] | 0.18 [0.05; 0.38] | 0.22 [0.05; 0.42] | 0.10 |
| <1 | 175 (83.7) | 60 (98.4) | 31 (100) | 83 (92.2) | 0.12a |
| ≥1 | 8 (3.8) | 1 (1.6) | 0 | 7 (7.8) | |
| HIV‐pVL (Log/mL) | 4.6 [2.2‐5.4] | 5.2 [4.8; 5.7] | 5 [4; 5.6] | 2.6 [1.6; 4.6] | <0.001 |
| HIV‐pVL copies/mL (205) | |||||
| ≤50 | 38 (18.5) | 1 (1.5) | 4 (11.4) | 32 (31.4) | <0.001 |
| 51–1000 | 33 (16.1) | 4 (6) | 3 (8.6) | 26 (25.5) | |
| 1001–10 000 | 16 (7.8) | 1 (1.5) | 2 (5.7) | 13 (12.7) | |
| 10 001–100 000 | 36 (17.6) | 18 (26.9) | 9 (25.7) | 9 (8.8) | |
| >100 001 | 82 (40.0) | 43 (64.2) | 17 (48.6) | 22 (21.6) | |
| Time with HIV‐pVL ≤ 50 (years) | 0.4 [0.1; 2.6] | – | 2.4 [0.9; 3.7] | 0.4 [0.1; 2.6] | 0.16 |
| On first line of ART | 51 (24.4) | – | – | 51 (49.5) | |
| Time exposure to ART (years) | 0.7 [0.2; 6.8] | – | – | 0.7 [0.2; 6.6] | |
| ART initiation | – | – | |||
| Before 1995 | 4 (3.9) | NA | NA | ||
| 1996–2006 | 23 (22.3) | NA | NA | ||
| ≥2007 | 76 (73.8) | NA | NA | ||
| Time exposure to the last ART (m) | 2.7 [0.7; 10.4] | NA | NA | 2.7 [0.7; 10.4] | |
| Last ART regimen | – | – | |||
| 2NRTI + 1bPI | 60 (28.7) | NA | NA | 60 (58.3) | |
| 2NRTI + 1NNRTI | 13 (6.2) | NA | NA | 13 (12.6) | |
| 2NRTI + 1INSTI | 9 (4.3) | NA | NA | 9 (8.7) | |
| OtherscNA | 21 (10) | NA | NA | 21 (20.4) | |
| Previous malignant disease | 10 (4.8) | 5 (7.2) | 1 (2.8) | 3 (2.9) | 0.40a |
| Kaposi localization | |||||
| Cutaneous | 140 (67.0) | 47 (68.1) | 21 (58.3) | 72 (69.9) | 0.93 |
| Oral mucosa/palate | 15 (7.2) | 4 (5.8) | 4 (11.1) | 7 (6.8) | |
| Bronchopulmonary | 11 (5.3) | 4 (5.8) | 1 (2.8) | 6 (5.8) | |
| Intestinal | 9 (4.3) | 3 (4.3) | 3 (8.3) | 3 (2.9) | |
| Lymph node | 7 (3.3) | 2 (2.9) | 1 (2.8) | 3 (2.9) | |
| Disseminated | 6 (2.9) | 3 (4.3) | 1 (2.8) | 2 (1.9) | |
| Data not available | 21 (10.0) | 6 (8.7) | 5 (13.9) | 10 (9.7) | |
NA, not applicable.
Fisher exact test.
ART status was not available in one patient.
Others: 2NRTI + CCR5‐inhibitor: n = 1; bPI + INSTI: n = 2; bPI + CCR5‐inhibitor: n = 2; 1NRTI + 1bPI: n = 2; 1NRTI + 1bPI + 1INSTI: n = 1; 1NRTI + 1NNRTI + 1bPI: n = 1; 1NNRTI + 1bPI + CCR5‐inhibitor: n = 1; 2NRTI + 1bPI + 1INSTI: n = 3; 2NRTI + 1bPI + CCR5‐inhibitor: n = 2; 2NRTI + 1NNRTI + 1bPI + T20: n = 1; 2NRTI + 1NNRTI + 1bPI + INSTI: n = 2; 2NRTI + INSTI:1; 2NRTI + 2NNRTI:1; 3NRTI + bPI: n = 1.
Figure 1.
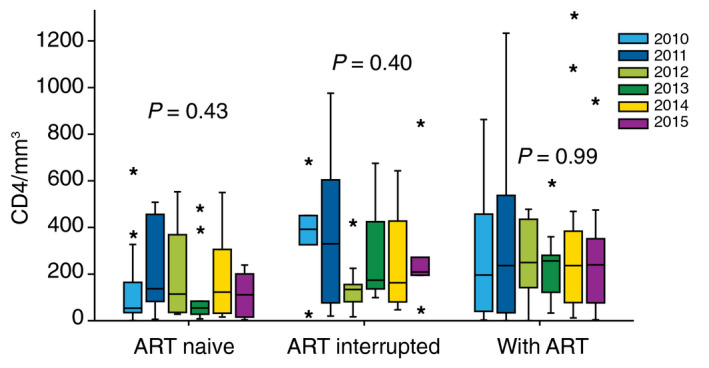
Median CD4 T‐cell count in 209 patients with Kaposi sarcoma according to ART exposure at time of KS diagnosis in the French DAT’AIDS cohort between 2010 and 2015.
The three groups did not differ in median age, sex ratio or HIV transmission groups, but the median duration of HIV follow‐up was significantly lower in ART‐naïve patients, most of whom were followed since 1 year or less.
The proportion of prior CDC stage C at the time of KS diagnosis was significantly higher in patients on ART (P < 0.001). The nadir CD4 and median CD4 T‐cell count as the CD4 : CD8 ratio did not differ between the three groups, but the proportion of patients with CD4 ≤200/mm3 at the time of KS diagnosis was significantly lower in patients on ART than in the other two groups.
HIV‐pVL was significantly higher among ART‐naïve patients. Although the number of patients with HIV‐pVL ≤50 copies/mL was significantly higher in patients on ART (n = 32 (31.4%), one ART‐naïve patient and four experienced ART patients had HIV‐pVL ≤50 copies/mL.
Data concerning KS localization were available for 188 patients (89.9%). Overall, the most frequent KS localization was cutaneous in 67% of cases, followed by oromucosal in 7.2%, bronchopulmonary in 5.3%, intestinal in 4.3% and nodal in 3.3%. In 2.9% of cases, KS was disseminated. The distribution of KS localization did not differ between the three groups or by year of diagnosis (Table 2). However, patients aged more than 50 years had more visceral (i.e. bronchopulmonary, intestinal, lymph nodes) and disseminated localization (P=0.04) than those aged <50 (Table 3).
Table 2.
Kaposi localization distribution by year of diagnosis of 209 KS cases diagnosed between 2010 and 2015 in the French Dat'AIDS Cohort
| Kaposi localization | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | P |
|---|---|---|---|---|---|---|---|
| N = 52 | N = 41 | N = 33 | N = 28 | N = 30 | N = 25 | ||
| Skin | 40 (76.9) | 30 (73.2) | 20 (60.6) | 20 (71.4) | 19 (63.3) | 11 (44) | 0.41 |
| Oral mucosa/palate | 1 (1.9) | 3 (7.3) | 3 (9.1) | 3 (10.7) | 1 (3.3) | 4 (16) | |
| Bronchopulmonary | 3 (5.8) | 1 (2.4) | 2 (6.1) | 1 (3.6) | 2 (6.7) | 2 (8) | |
| Intestinal | 1 (1.9) | 0 | 2 (6.1) | 2 (7.1) | 1 (3.3) | 3 (12) | |
| Lymph node | 1 (1.9) | 2 (4.9) | 0 | 1 (3.6) | 2 (6.7) | 1 (4) | |
| Disseminated | 2 (3.8) | 3 (7.3) | 1 (3) | 0 | 0 | 0 | |
| Data not available | 4 (7.7) | 2 (4.9) | 5 (15.2) | 1 (3.6) | 5 (16.7) | 4 (16) |
Table 3.
Kaposi localization distribution according to age ≥ or <50 years of 209 KS cases diagnosed between 2010 and 2015 in the French Dat'AIDS Cohort
| Kaposi localizationa | Age < 50 years | Age ≥ 50 years | P |
|---|---|---|---|
| N = 138 | N = 50 | ||
| Skin | 103 (74.6) | 37 (74) | 0.04 |
| Oral mucosa/palate | 14 (10.1) | 1 (2.0) | |
| Visceral | 18 (13.0) | 9 (18.0) | |
| Bronchopulmonary | 6 (33.0) | 5 (55.5) | |
| Intestinal | 8 (44.4) | 1 (11.1) | |
| Lymph node | 4 (22.2) | 3 (33.3) | |
| Disseminated | 3 (1.9) | 3 (6.0) |
Data not available for 19 (12.1) of patients aged <50 and for 2 (3.8) of patients aged ≥50.
Description of 54 KS cases in patients on ART for more than 6 months
Among the 103 patients on ART, 54 patients were on ART for more than 6 months, of whom 24 patients had HIV‐pVL ≤50 copies/mL, with a median time of 0.58 years [IQR: 0.26; 3], and 30 patients had HIV‐pVL >50 copies/mL [median HIV‐pVL: 3.4 (IQR: 2.3–4.9)] at the time of KS diagnosis. The patients’ characteristics are reported in Table 4.
Table 4.
Patient's characteristics at time of KS diagnosis of KS cases occurred in patients on ART for more than 6 months between 2010 and 2015 in the French Dat'AIDS cohort according to HIV‐pVL > or ≤50 copies/mL
| Median [IQR] or N (%) | KS on ART† with HIV VL > 50 cp/mL n = 30 | KS on ART† with HIV VL ≤ 50 cp/mL n = 24 | P |
|---|---|---|---|
| Age (years) | 43.5 [36; 51.2] | 48.2 [39; 54.7] | 0.14 |
| Gender | |||
| Male | 26 (86.7) | 21 (87.5) | 1 |
| Female | 4 (13.3) | 3 (12.5) | |
| HIV Transmission group | |||
| MSM | 16 (53.3) | 14 (58.3) | 0.46 |
| Heterosexual | 7 (23.3) | 8 (33.3) | |
| IVDU | 2 (6.7) | 1 (4.2) | |
| Others/unknown | 5 (16.7) | 1 (4.2) | |
| HIV time follow‐up (y) | 12.8 [6.4; 15.8] | 7.4 [2.8; 20.7] | 0.21 |
| ≤1 | 0 | 3 (12.5) | 0.14 |
| [1–5] | 2 (6.7) | 5 (20.8) | |
| [5–10] | 11 (36.7) | 6 (25) | |
| [10–15] | 9 (30) | 4 (16.7) | |
| 15 | 8 (26.7) | 6 (25) | |
| Year of diagnosis | |||
| 2010 | 10 (33.3) | 1 (4.2) | |
| 2011 | 5 (16.7) | 5 (20.8) | 0.05a |
| 2012 | 5 (16.7) | 5 (20.8) | |
| 2013 | 1 (3.3) | 5 (20.8) | |
| 2014 | 6 (20) | 3 (12.5) | |
| 2015 | 3 (10) | 5 (20.8) | |
| Stage C at KS | |||
| Yes | 12 (42.9) | 7 (29.2) | 0.31 |
| No | 16 (57.1) | 17 (70.8) | |
| Nadir CD4/mm3 | 13 [5; 154] | 242 [120; 363] | <0.001 |
| <200/mm3 | 22 (75.9) | 9 (37.5) | 0.005 |
| CD4/mm3 | 69 [12; 301] | 467 [255; 819] | <0.001 |
| CD4 < 200/mm3 | 19 (63.3) | 4 (16.7) | 0.001 |
| CD4 > 500/mm3 | 1 (3.3) | 9 (37.5) | 0.003a |
| CD8/mm3 | 665 [507; 1321] | 960 [662; 1360] | 0.30 |
| CD8 ≥ 1000/mm3 | 9 (37.5) | 10 (41.7) | 0.77 |
| Ratio CD4 : CD8 | 0.2 [0; 0.3] | 0.5 [0.2; 0.9] | <0.001 |
| Ratio < 1 | 24 (100) | 18 (75) | 0.022a |
| HIV‐pVL (log/mL) | 3.4 [2.3; 4.9] | NA | |
| ART‐Time exposure (years) | 8 [3.9; 13.1] | 4.1 [1; 10.3] | 0.16 |
| ART initiation | |||
| Before 1995 | 2 (6.7) | 2 (8.3) | 0.30a |
| 1996–2006 | 15 (50) | 7 (29.2) | |
| ≥2007 | 13 (43.3) | 15 (62.5) | |
| Time exposure to the last ART (m) | 3.9 [0.9; 12.4] | 13.8 [7.6; 28.9] | 0.001 |
| Last ART | |||
| 2NRTI + 1bPI | 17 (56.7) | 8 (33.3) | 0.25a |
| 2NRTI + 1NNRTI | 2 (6.7) | 5 (20.8) | |
| 2NRTI + 1INSTI | 3 (10) | 2 (8.3) | |
| Others‡ | 8 (26.7) | 9 (37.5) | |
| KS Localizationb | |||
| Cutaneous | 20 (66.7) | 18 (75.0) | 0.57 |
| Oral mucosa/palate | 1 (3.3) | 2 (8.3) | |
| Bronchopulmonary | 4 (13.3) | 1 (4.2) | |
| Intestinal | 0 | 0 | |
| Lymph node | 2 (6.7) | 0 | |
| Disseminated | 0 | 0 | |
b, boosted; INSTI, integrase strand transfer inhibitor; NA, not applicable; NNRTI, non‐nucleoside reverse transcriptase inhibitor; NRTI, nucleotide reverse transcriptase inhibitor; PI, boosted protease inhibitor.
Fisher exact test.
For more than 6 months.
Others: 2NRTI + CCR5‐inhibitor: n = 1; bPI + INSTI: n = 2; bPI + CCR5‐inhibitor: n = 1; 1NRTI + 1bPI: n = 1; 2NRTI + 1bPI + 1INSTI: n = 2; 2NRTI + 1bPI + CCR5‐inhibitor: n = 1; 3NRTI + bPI: n = 1.
Data not available for 3 (10.0) patients with HIV‐pVL >50 cp/mL and for 3 (12.5) patients with HIV VL ≤50 cp/mL.
The two groups did not differ in median age, sex ratio and HIV transmission groups or time of HIV follow‐up. The distribution of KS cases that occurred in patients on ART according to HIV‐pVL differed by the year of diagnosis. Indeed, 33% of KS cases in patients with detectable HIV‐pVL occurred in 2010, while in patients with undetectable HIV‐pVL, most KS cases occurred after 2010. Data concerning KS localization were available for 48 patients (88.8%), and the distribution of KS localization did not differ between the two groups. Patients on ART with HIV‐pVL ≤50 copies/mL presented significantly better immune parameters (median CD4 count and %, %CD4 >500/mm3, nadir CD4, ratio CD4 : CD8) than those with detectable HIV‐pVL. Moreover, the proportion of patients with nadir <200/mm3 was significantly higher in patients with detectable HIV‐pVL, as was the proportion of patients with CD4 : CD8 ratio <1, but the two groups did not differ in CD8 T‐cell count (median and proportion with CD8 ≥1000/mm3).
Antiretroviral therapy‐time exposure and period of ART initiation were similar regardless of the detectability of HIV‐pVL, but time exposure to the last treatment was significantly higher in patients with undetectable HIV‐pVL. Additionally, the last ART regimen did not differ between those two groups. Among the 24 patients on ART with HIV‐pVL ≤50 copies/mL, sixteen patients (66%) were treated with an ART regimen including a bPI (darunavir/ritonavir in 12 patients, atazanavir/ritonavir in 3 and lopinavir/ritonavir in 1). There were five patients (21%) receiving an ART regimen including one NNRTI (efavirenz: n = 4; nevirapine: n = 1), six patients (25%), one INSTI (raltegravir) and three patients, a CCR5‐inhibitor (maraviroc). Nine patients were on their first line of ART, including five patients treated with 2NRTI + 1bPI, two patients treated with 2NRTI + 1NNRTI, one patient treated with 2NRTI + 1bPI + CCR5‐inhibitor and one patient treated with 2NRTI + 1 CCR5‐inhibitor. For these patients, the median delay of KS occurrence was 13.8 months (IQR: 10.7; 21.3). Six patients were on a second line of ART with a median delay of KS occurrence after the switch of 6.6 months (IQR: 5;7.5). Among them, 3 were treated with a bPI‐based regimen, one with an NNRTI‐based regimen and one with an INSTI‐based regimen. Eight patients were previously exposed to 3 or more lines of ART (3 to >10).
Discussion
In this retrospective study performed on a large cohort of PLWHIV, the prevalence of KS cases was 0.47%. This low prevalence rate is in line with the trend of KS incidence, which has declined since 1996 in France and in other resource‐rich countries, as we recently reported.5
However, more relevantly, this analysis highlights the need to differentiate KS occurrence circumstances in the post‐ART period, half of them occurring in patients not on ART, of whom 41 had concomitant HIV diagnosis. These patients fulfilled the criteria defining late presenters among HIV‐infected people, a situation that has remained common around the world,6, 7 including in France as we reported recently,8 and which increases patient morbidity and mortality and limits the effectiveness of all subsequent steps in the cascade of HIV care.9, 10
In our study, KS cases that occurred in late presenters were observed indifferently throughout the study period. This persistent delay in HIV testing in France stresses the need to reinforce HIV screening strategies in our country. Although KS may have different clinical presentations11 (sometimes atypical12) and should occur in conditions other than HIV infection,13, 14 nevertheless, cutaneous and mucosal involvement are the most frequent,15 as we have reported herein, highlighting the important role of dermatologists and general practitioners in HIV screening. Two recent studies reported, however, that much progress remains to be made in this area.16, 17
The 36 KS cases occurring in patients who stopped ART confirms the importance of improving retention in care with a specific strategy, as recently reported.18
This study also highlights that not being on ART or being on ART with a persistence of HIV replication remains a risk factor for KS;19 apart from worsening immune deficiency, the lack of control of HIV viral replication allows the promoting effect of HIV‐1 Tat protein on HHV8‐induced angiogenesis and tumorigenesis.20, 21
However, more worryingly, this study highlights, as observed in studies performed over previous calendar periods,3, 22, 23 that a sustained undetectable HIV viral load and a high CD4 cell count on ART is not enough to completely prevent KS occurrence. In our cohort, 11.4% of KS cases occurred in patients on ART for more than 6 months with an undetectable HIV‐pVL. This rate is higher than that reported in a recent retrospective study conducted between 1987 and 2011 in a German cohort of 16 500 HIV‐infected patients in which 7.4% of KS cases had HIV‐pVL ≤50 copies/mL.3 Some clinicians wondered about the possible impact of new ART guidelines,24, 25 which recommend an INSTI‐based regimen as the first line and advocate optimizing ART with less risk of drug‐drug interactions between antiretroviral drugs and concomitant medications, especially for older patients.26, 27 In our study, most patients with undetectable HIV‐pVL were treated with a bPI‐based regimen at the time of KS diagnosis, including those in first line of ART, and when we considered KS cases that occurred after ART switching, most of them switched for a bPI‐based regimen. This result is not consistent with that of a previous study showing that a lower KS incidence was associated with bPI exposure for at least 3 years.28 However, in our cohort, the median time of exposure to the last treatment in patients with undetectable HIV‐pVL was shorter than 3 years. The objective of this study was not to investigate factors associated with KS in patients on ART with undetectable HIV‐pVL, such as premature immune senescence or the loss of specific HHV8 immunity.29, 30 However, regardless of the cause, such KS cases, although few, remained steady throughout the study period, stressing the need to maintain a cautious examination and follow‐up of PLWHIV.
In this cohort, the distribution of KS localization did not differ according to the year of diagnosis, but patients aged over 50 had a higher percentage of visceral/disseminated forms. Specific clinical features in paediatric KS with more extensive and severe forms and a predilection for primary lymph node involvement are well identified.31 We did not find any previous report on specific features of KS according to age in adult HIV‐infected people. Thus, our results should be confirmed by further studies.
This study has several limitations. First, virological data on human herpesvirus 8 (HHV8)32 were not available. Thus, we could not determine whether KS cases in patients on ART with undetectable HIV‐pVL were related to HHV8 reactivation or even to HHV8 primary infection. In our study, 58% of affected individuals were men who have sex with men (MSM). High‐risk sexual behaviours with unprotected sex and concomitant sexually transmitted diseases are two factors associated with HHV8 infection in MSM.33 Unfortunately, sexual behaviour data, condom use and previous or concomitant sexually transmitted disease at the time of KS diagnosis were not collected. Second, the study design did not allow us to clearly identify KS cases related to an immune reconstitution syndrome, as CD4 T‐cell count data were not collected at the time of first ART initiation. Third, we could not determine the median delay of KS occurrence in experienced ART patients, as no information concerning the time of treatment withdrawal was available. Fourth, among patients on ART with undetectable HIV‐pVL who switched ART, the reasons for switching were not reported, nor was the previous ART regimen before the switch. Finally, treatment modalities for KS and response rate data were not collected, which did not allow us to evaluate the percentage of patients requiring new therapeutic options.34
Conclusion
Although the KS prevalence among PLWHIV remained low between 2010 and 2015 in France, this retrospective study highlights the persistent delay in HIV testing in France and the need to reinforce HIV screening strategies in our country. KS occurrence in patients who have discontinued ART confirms the importance of improving retention in care, and KS cases in patients on ART with control of viremia reinforces the need to maintain cautious examination and follow‐up, including for patients on a bPI‐based regimen. Further studies are needed to better understand KS pathogenesis mechanisms.
Acknowledgements
Dat'AIDS Study Group: C. Drobacheff‐Thiébaut, A. Foltzer, K. Bouiller, L. Hustache‐Mathieu, C. Chirouze, Q. Lepiller, F. Bozon, O. Babre, A.S. Brunel, P. Muret (Besançon); H. Laurichesse, O. Lesens, M. Vidal, N. Mrozek, C. Aumeran, O. Baud, V. Corbin, P. Letertre‐Gibert, S. Casanova, J. Prouteau, C. Jacomet (Clermont Ferrand); I. Lamaury, I. Fabre, E. Curlier, R. Ouissa, C. Herrmann‐Storck, B. Tressieres, T. Bonijoly, M.C. Receveur, F. Boulard, C. Daniel, C. Clavel (Guadeloupe); D. Merrien, P. Perré, T. Guimard, O. Bollangier, S. Leautez, M. Morrier, L. Laine (La Roche sur Yon); F. Ader, A. Becker, F. Biron, A. Boibieux, L. Cotte, T. Ferry, P. Miailhes, T. Perpoint, S. Roux, C. Triffault‐Fillit, S. Degroodt, C. Brochier, F. Valour, C. Chidiac (Lyon); A. Ménard, A.Y. Belkhir, P. Colson, C. Dhiver, A. Madrid, M. Martin‐Degioanni, L. Meddeb, M. Mokhtari, A. Motte, A. Raoux, I. Ravaux, C. Tamalet, C. Toméi, H. Tissot Dupont (Marseille IHU Méditerranée); S. Brégigeon, O. Zaegel‐Faucher, V. Obry‐Roguet, H. Laroche, M. Orticoni, M.J. Soavi, P. Geneau de Lamarlière, E. Ressiot, M.J. Ducassou, I. Jaquet, S. Galie, A. Galinier, P. Martinet, M. Landon, A.S. Ritleng, A. Ivanova, C. Debreux, C. Lions, I. Poizot‐Martin (Marseille Ste Marguerite); S. Abel, O. Cabras, L. Cuzin, K. Guitteaud, M. Illiaquer, S. Pierre‐François, L. Osei, J. Pasquier, K. Rome, E. Sidani, J.M. Turmel, C. Varache, A. Cabié (Martinique); N. Atoui, M. Bistoquet, E. Delaporte, V. Le Moing, A. Makinson, N. Meftah, C. Merle de Boever, B. Montes, A. Montoya Ferrer, E. Tuaillon, J. Reynes (Montpellier); M. André, L. Boyer, M.P. Bouillon, M. Delestan, C. Rabaud, T. May, B. Hoen (Nancy); C. Allavena, C. Bernaud, E. Billaud, C. Biron, B. Bonnet, S. Bouchez, D. Boutoille, C. Brunet‐Cartier, C. Deschanvres, N. Hall, T. Jovelin, P. Morineau, V. Reliquet, S. Sécher, M. Cavellec, A. Soria, E. Paredes, V. Ferré, E. André‐Garnier, A. Rodallec, M. Lefebvre, O. Grossi, O. Aubry, F. Raffi (Nantes); P. Pugliese, S. Breaud, C. Ceppi, D. Chirio, E. Cua, P. Dellamonica, E. Demonchy, A. De Monte, J. Durant, C. Etienne, S. Ferrando, R. Garraffo, C. Michelangeli, V. Mondain, A. Naqvi, N. Oran, I. Perbost, S. Pillet, C. Pradier, B. Prouvost‐Keller, K. Risso, V. Rio, P.M. Roger, E. Rosenthal, S. Sausse, I. Touitou, S. Wehrlen‐Pugliese, G. Zouzou (Nice); L. Hocqueloux, T. Prazuck, C. Gubavu, A. Sève, A. Maka, C. Boulard, G. Thomas (Orleans); A. Cheret, C. Goujard, Y. Quertainmont, E. Teicher, N. Lerolle, O. Deradji, A. Barrail‐Tran (Paris Hop. Bicètre); R. Landman, V. Joly, J. Ghosn, C. Rioux, S. Lariven, A. Gervais, F.X. Lescure, S. Matheron, F. Louni, Z. Julia, C. Mackoumbou‐Nkouka, S. Le Gac, C. Charpentier, D. Descamps, G. Peytavin, Y. Yazdanpanah (Paris Hop. Bichat); K. Amazzough, G. Benabdelmoumen, P. Bossi, G. Cessot, C. Charlier, P.H. Consigny, F. Danion, A. Dureault, C. Duvivier, J. Goesch, R. Guery, B. Henry, K. Jidar, F. Lanternier, P. Loubet, O. Lortholary, C. Louisin, J. Lourenco, P. Parize, B. Pilmis, F. Touam (Paris Hop. Necker Pasteur); M.A. Valantin, R. Tubiana, R. Agher, S. Seang, L. Schneider, R. PaLich, C. Blanc, C. Katlama (Paris Hop. Pitié Salpétrière); J.L. Berger, Y. N'Guyen, D. Lambert, I. Kmiec, M. Hentzien, A. Brunet, V. Brodard, F. Bani‐Sadr (Reims) P. Tattevin, M. Revest, F. Souala, M. Baldeyrou, S. Patrat‐Delon, J.M. Chapplain, F. Benezit, M. Dupont, M. Poinot, A. Maillard, C. Pronier, F. Lemaitre, C. Guennoun, M. Poisson‐Vanier, T. Jovelin, J.P. Sinteff, C. Arvieux (Rennes); E. Botelho‐Nevers, A. Gagneux‐Brunon, A. Frésard, V. Ronat, F. Lucht (St Etienne); P. Fischer, M. Partisani, C. Cheneau, M. Priester, M.L. Batard, C. Bernard‐Henry, E. de Mautort, S. Fafi‐Kremer, D. Rey (Strasbourg); M. Alvarez, N. Biezunski, A. Debard, C. Delpierre, P. Lansalot, L. Lelièvre, G. Martin‐Blondel, M. Piffaut, L. Porte, K. Saune, P. Delobel (Toulouse); F. Ajana, E. Aïssi, I. Alcaraz, V. Baclet, L. Bocket, A. Boucher, P. Choisy, T. Huleux, B. Lafon‐Desmurs, A. Meybeck, M. Pradier, O. Robineau, N. Viget, M. Valette (Tourcoing).
Conflicts of interest
Tristan Ferry: Travel grant (MSD, Pfizer, Astellas); Consultancies (MaaT Pharma, Pfizer, Debiopharm, Atlangram); Research grant (HERAEUS), outside the submitted work. Alain Makinson: Travel grants (MSD, Mylan; Donations (MSD), outside the submitted work. André CABIE: Travel grants (Gilead Science, ViiV HealthCare), outside the submitted work. Fresard Anne: Travel and congress grants: (Gilead, merck, viiv, Janssen), outside the submitted work. David Rey: travel grants for conferences (Gilead, ViiV, BMS, Mylan), honoraria (Gilead), outside the submitted work. I Poizot‐Martin: consultancies (Gilead Sciences, MSD), travel/accommodation/meeting expenses (Gilead sciences, ViiVhealthcare), outside the submitted work. Claudine Duvivier: consultancies, honoraria, paid expert testimony and travel grants (Bristol Myers Squibb, Gilead Sciences Janssen‐ Cilag, Merck Sharp & Dohme and ViiV Healthcare), outside the submitted work. Christine Jacomet: honoraria (MSD, Janssen); travel grants (MSD, Gilead, Janssen), outside the submitted work. Thomas Huleux: congress and travel grants (GILEAD, ViiV, MSD, JANSSEN); expert/speaker (GILEAD, JANSSEN). Clotilde Allavena: honoraria and/or travel grants (Gilead, ViiV Healthcare, Teva, Mylan, MSD and Janssen). Romain Palich: travel grants for conferences (ViiV Healthcare, Janssen, Gilead et MSD). Antoine Cheret: Grant (Viiv, Janssens) and personnal fies (Viiv). Firouze Banisadr, Caroline Lions, Véronique Obry‐Roguet, Pascal Pugliese, Pierre Delobel, Catherine Chirouze, Isabelle Lamaury: No conflict of interest.
Funding sources
None.
Meetings: A part of this work was presented partially at the Conference on Retrovirus and Opportunistic Infections (CROI), March 4–7 2019, Seattle (Poster number 0275).
Contributor Information
I. Poizot‐Martin, Email: isabelle.poizot@ap-hm.fr.
The Dat'AIDS study group:
C. Drobacheff‐Thiébaut, A. Foltzer, K. Bouiller, C. Chirouze, Q. Lepiller, F. Bozon, O. Babre, A.S. Brunel, P. Muret, H. Laurichesse, O. Lesens, M. Vidal, N. Mrozek, C. Aumeran, O. Baud, V. Corbin, P. Letertre‐Gibert, S. Casanova, J. Prouteau, I. Fabre, E. Curlier, R. Ouissa, C. Herrmann‐Storck, B. Tressieres, T. Bonijoly, M.C. Receveur, F. Boulard, C. Daniel, C. Clavel, D. Merrien, P. Perré, T. Guimard, O. Bollangier, S. Leautez, M. Morrier, L. Laine, F. Ader, A. Becker, F. Biron, A. Boibieux, L. Cotte, P. Miailhes, T. Perpoint, S. Roux, C. Triffault‐Fillit, S. Degroodt, C. Brochier, F. Valour, C. Chidiac, A. Ménard, A.Y. Belkhir, P. Colson, C. Dhiver, A. Madrid, M. Martin‐Degioanni, L. Meddeb, M. Mokhtari, A. Motte, A. Raoux, I. Ravaux, C. Tamalet, C. Toméi, H. Tissot Dupont, O. Zaegel‐Faucher, H. Laroche, M. Orticoni, M.J. Soavi, P. Geneau de Lamarlière, E. Ressiot, M.J. Ducassou, I. Jaquet, S. Galie, A. Galinier, P. Martinet, M. Landon, A.S. Ritleng, A. Ivanova, C. Debreux, S. Abel, O. Cabras, L. Cuzin, K. Guitteaud, M. Illiaquer, S. Pierre‐François, L. Osei, J. Pasquier, K. Rome, E. Sidani, J.M. Turmel, C. Varache, N. Atoui, M. Bistoquet, E. Delaporte, V. Le Moing, N. Meftah, C. Merle de Boever, B. Montes, A. Montoya Ferrer, E. Tuaillon, J. Reynes, M. André, L. Boyer, M.P. Bouillon, M. Delestan, C. Rabaud, T. May, B. Hoen, C. Bernaud, E. Billaud, C. Biron, B. Bonnet, S. Bouchez, D. Boutoille, C. Brunet‐Cartier, C. Deschanvres, N. Hall, T. Jovelin, P. Morineau, V. Reliquet, S. Sécher, M. Cavellec, A. Soria, E. Paredes, V. Ferré, E. André‐Garnier, A. Rodallec, M. Lefebvre, O. Grossi, O. Aubry, F. Raffi, S. Breaud, C. Ceppi, D. Chirio, E. Cua, P. Dellamonica, E. Demonchy, A. De Monte, J. Durant, C. Etienne, S. Ferrando, R. Garraffo, C. Michelangeli, V. Mondain, A. Naqvi, N. Oran, I. Perbost, S. Pillet, C. Pradier, B. Prouvost‐Keller, K. Risso, V. Rio, P.M. Roger, E. Rosenthal, S. Sausse, I. Touitou, S. Wehrlen‐Pugliese, G. Zouzou, L. Hocqueloux, T. Prazuck, C. Gubavu, A. Sève, A. Maka, C. Boulard, G. Thomas, C. Goujard, Y. Quertainmont, E. Teicher, N. Lerolle, O. Deradji, A. Barrail‐Tran, R. Landman, V. Joly, J. Ghosn, C. Rioux, S. Lariven, A. Gervais, F.X. Lescure, S. Matheron, F. Louni, Z. Julia, C. Mackoumbou‐Nkouka, S. Le Gac, C. Charpentier, D. Descamps, G. Peytavin, Y. Yazdanpanah, K. Amazzough, G. Benabdelmoumen, P. Bossi, G. Cessot, C. Charlier, P.H. Consigny, F. Danion, A. Dureault, J. Goesch, R. Guery, B. Henry, K. Jidar, F. Lanternier, P. Loubet, O. Lortholary, C. Louisin, J. Lourenco, P. Parize, B. Pilmis, F. Touam, M.A. Valantin, R. Tubiana, R. Agher, S. Seang, L. Schneider, C. Blanc, C. Katlama, J.L. Berger, Y. N'Guyen, D. Lambert, I. Kmiec, M. Hentzien, A. Brunet, V. Brodard, P. Tattevin, M. Revest, F. Souala, M. Baldeyrou, S. Patrat‐Delon, J.M. Chapplain, F. Benezit, M. Dupont, M. Poinot, A. Maillard, C. Pronier, F. Lemaitre, C. Guennoun, M. Poisson‐Vanier, T. Jovelin, J.P. Sinteff, C. Arvieux, E. Botelho‐Nevers, A. Gagneux‐Brunon, A. Frésard, V. Ronat, F. Lucht, P. Fischer, M. Partisani, C. Cheneau, M. Priester, M.L. Batard, C. Bernard‐Henry, E. de Mautort, S. Fafi‐Kremer, M. Alvarez, N. Biezunski, A. Debard, C. Delpierre, P. Lansalot, L. Lelièvre, G. Martin‐Blondel, M. Piffaut, L. Porte, K. Saune, F. Ajana, E. Aïssi, I. Alcaraz, V. Baclet, L. Bocket, A. Boucher, P. Choisy, B. Lafon‐Desmurs, A. Meybeck, M. Pradier, O. Robineau, N. Viget, and M. Valette
References
- 1. Yanik EL, Achenbach CJ, Gopal S et al Changes in clinical context for Kaposi's sarcoma and Non‐Hodgkin Lymphoma among people with HIV infection in the United States. J Clin Oncol 2016; 34: 3276–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yarchoan R, Uldrick TS. HIV‐associated cancers and related diseases. N Engl J Med 2018; 378: 1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klingenberg R‐E, Esser S, Brockmeyer NH et al Profile of Kaposi sarcoma patients in the competence network HIV/AIDS. Hautarzt 2018; 69: 143–148. [DOI] [PubMed] [Google Scholar]
- 4. Pugliese P, Cuzin L, Cabié A et al A large French prospective cohort of HIV‐infected patients: the Nadis Cohort. HIV Med 2009; 10: 504–511. [DOI] [PubMed] [Google Scholar]
- 5. Poizot‐Martin I, Makinson A, Protopopescu C et al Kaposi sarcoma incidence between 2010‐2015 in the French Dat'AIDS cohort. In: Vol 0275. Seattle: Conference on Retroviruses and Opportunistic Infections; 2019:0275.
- 6. Wilton J, Light L, Gardner S et al Late diagnosis, delayed presentation and late presentation among persons enrolled in a clinical HIV cohort in Ontario, Canada (1999‐2013). HIV Med 2019; 20: 110–120. [DOI] [PubMed] [Google Scholar]
- 7. Komninakis SV, Mota ML, Hunter JR, Diaz RS. Late presentation HIV/AIDS is still a challenge in Brazil and worldwide. AIDS Res Hum Retroviruses 2018; 34: 129–131. [DOI] [PubMed] [Google Scholar]
- 8. Lions C, Cabras O, Cotte L et al Missed opportunities of HIV pre‐exposure prophylaxis in France: a retrospective analysis in the French DAT'AIDS cohort. BMC Infect Dis 2019; 19: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Darling KE, Hachfeld A, Cavassini M, Kirk O, Furrer H, Wandeler G. Late presentation to HIV care despite good access to health services: current epidemiological trends and how to do better. Swiss Med Wkly 2016; 146: w14348. [DOI] [PubMed] [Google Scholar]
- 10. O'Connell S, Enkelmann J, Sadlier C, Bergin C. Late HIV presentation – missed opportunities and factors associated with a changing pattern over time. Int J STD AIDS 2017; 28: 814–821. [DOI] [PubMed] [Google Scholar]
- 11. Curtiss P, Strazzulla LC, Friedman‐Kien AE. An update on Kaposi's Sarcoma: epidemiology, pathogenesis and treatment. Dermatol Ther (Heidelb) 2016; 6: 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lukoviek V, Markthaler M, Arteaga‐Henríquez M et al Kaposi's varicelliform eruption in a patient with bullous pemphigoid. J Am Acad Dermatol 2017; 76: AB421. [Google Scholar]
- 13. Saxena A, Netchiporouk E, Al‐Rajaibi R, Billick R, Roshdy O. Iatrogenic Kaposi's sarcoma after immunosuppressive treatment for granulomatosis with polyangiitis (Wegener's). JAAD Case Rep 2015; 1: 71–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tremblay C, Friedmann D. Kaposi sarcoma–associated with immunosuppressive therapy. J Am Acad Dermatol 2017; 76: AB421. [Google Scholar]
- 15. Lebbe C, Garbe C, Stratigos AJ et al Diagnosis and treatment of Kaposi's sarcoma: European consensus‐based interdisciplinary guideline (EDF/EADO/EORTC). Eur J Cancer 2019; 114: 117–127. [DOI] [PubMed] [Google Scholar]
- 16. Esson GA, Holme SA. HIV testing in dermatology – a national audit. Int J STD AIDS 2018; 29: 611–613. [DOI] [PubMed] [Google Scholar]
- 17. Deblonde J, Van Beckhoven D, Loos J et al HIV testing within general practices in Europe: a mixed‐methods systematic review. BMC Public Health 2018; 18: 1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li M‐C, Ko N‐Y, Wang L‐Y. The moderator effect of retention in care on late presentation in HIV‐infected patients. AIDS Care 2019; 32: 93–97. [DOI] [PubMed] [Google Scholar]
- 19. Guiguet M, Boué F, Cadranel J et al Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH‐ANRS CO4): a prospective cohort study. Lancet Oncol 2009; 10: 1152–1159. [DOI] [PubMed] [Google Scholar]
- 20. Zhou F, Xue M, Qin D et al HIV‐1 Tat promotes Kaposi's sarcoma‐associated herpesvirus (KSHV) vIL‐6‐induced angiogenesis and tumorigenesis by regulating PI3K/PTEN/AKT/GSK‐3β signaling pathway. PLoS ONE 2013; 8: e53145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen X, Cheng L, Jia X et al Human immunodeficiency virus type 1 Tat accelerates Kaposi sarcoma‐associated herpesvirus Kaposin A‐mediated tumorigenesis of transformed fibroblasts in vitro as well as in nude and immunocompetent mice. Neoplasia 2009; 11: 1272–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krown SE, Lee JY, Dittmer DP, AIDS Malignancy Consortium . More on HIV‐associated Kaposi's sarcoma. N Engl J Med 2008; 358: 535–536. author reply 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mani D, Neil N, Israel R, Aboulafia DM. A retrospective analysis of AIDS‐associated Kaposi's sarcoma in patients with undetectable HIV viral loads and CD4 counts greater than 300 cells/mm(3). J Int Assoc Physicians AIDS Care (Chic) 2009; 8: 279–285. [DOI] [PubMed] [Google Scholar]
- 24. Simonetti FR, Ricaboni D, Cattaneo D, Micheli V, Rusconi S, Gervasoni C. Relapse of Kaposi's Sarcoma and HHV‐8 viremia in an HIV‐infected patient switching from protease inhibitor to integrase inhibitor‐based antiretroviral therapy. J Clin Virol 2016; 74: 75–77. [DOI] [PubMed] [Google Scholar]
- 25. Philibert P, Chiche L, Caillères S et al HHV8 and Kaposi's sarcoma: should we really give up protease inhibitors in all HIV‐infected patients? AIDS 2017; 31: 2167–2169. [DOI] [PubMed] [Google Scholar]
- 26. EACS guidelines version9.1 [WWW document]. URL www.eacsociety.org/files/guidelines_changes_from_v9.0_to_v9.1.pdf (last accessed: 13 November 2018).
- 27. Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV [WWW document]. URL https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf (last accessed: 30 July 2019).
- 28. Kowalkowski MA, Kramer JR, Richardson PR, Suteria I, Chiao EY. Use of boosted protease inhibitors reduces Kaposi sarcoma incidence among male veterans with HIV infection. Clin Infect Dis 2015; 60: 1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cloarec N, Faucher O, Bregigeon S et al Kaposi's sarcoma in a treated and well‐controlled HIV infected patient: discussion on the role of immunosenescence. HIV & AIDS Rev 2014; 13: 131–134. [Google Scholar]
- 30. Guihot A, Dupin N, Marcelin A‐G et al Low T cell responses to human herpesvirus 8 in patients with AIDS‐related and classic Kaposi sarcoma. J Infect Dis 2006; 194: 1078–1088. [DOI] [PubMed] [Google Scholar]
- 31. El‐Mallawany NK, McAtee CL, Campbell LR, Kazembe PN. Pediatric Kaposi sarcoma in context of the HIV epidemic in sub‐Saharan Africa: current perspectives. Pediatric Health Med Ther 2018; 9: 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moore PS, Chang Y. Detection of herpesvirus‐like DNA sequences in Kaposi's sarcoma in patients with and those without HIV infection. N Engl J Med 1995; 332: 1181–1185. [DOI] [PubMed] [Google Scholar]
- 33. Liu Z, Fang Q, Zuo J et al Global epidemiology of human herpesvirus 8 in men who have sex with men: A systematic review and meta‐analysis. J Med Virol 2018; 90: 582–591. [DOI] [PubMed] [Google Scholar]
- 34. Palich R, Veyri M, Valantin M‐A et al Recurrence and occurrence of Kaposi's sarcoma in HIV‐infected patients on antiretroviral therapy despite suppressed HIV viremia. Clin Infect Dis 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


