Abstract
Objectives
The human sense of smell constitutes the main part of flavor perception. Typically, patients with loss of olfactory function complain of diminished perception during eating and drinking. However, some patients with smell loss still report normal enjoyment of foods. The aim of the present study was to compare orthonasal and retronasal olfactory function in patients with non‐sinonasal smell loss and subjectively normal flavor perception.
Methods
Nineteen patients (mean age [range] 52.0 [8–83 years]) with self‐reported olfactory impairment but subjective normal flavor perception were included. Olfactory performance was assessed using the Sniffin’ Sticks (TDI) for orthonasal and the Candy Smell Test (CST) for retronasal function. Visual analogue scales were used for self‐assessment of odor (SOP), taste (STP), and flavor perception (SFP), ranging from 0 (no perception) to 10 (excellent perception).
Results
Mean (SD) SFP was 8.0 (1.8). Mean (SD) orthonasal TDI‐score of all patients was 14.4 (5.3, range 6–25.3) with 11 patients classified as anosmic and eight as hyposmic. Mean/SD retronasal CST‐score was 8.8 (2.7, range 3–13) within the range of anosmia/hyposmia. No correlation was found between SFP and the CST (P = .62).
Conclusion
The present results showed that despite claiming normal flavor perception, our patients were ortho‐ and retronasally dysosmic using standard tests for olfactory function. Although other explanations could be possible, we suggest that this subjective flavor perception might be due to unconscious memory recall from previously experienced cross‐modal sensory interactions.
Level of Evidence
4 Laryngoscope, 130:1629–1633, 2020
Keywords: Olfactory dysfunction, anosmia, flavor perception, retronasal olfactory function, flavor templates
INTRODUCTION
Flavor perception has a prominent role in our daily lives.1 Our olfactory system is based on orthonasal smell representing the pathway of odor molecules to the olfactory bulb via the nostrils, as well as retronasal smell (ie, molecules travelling via the pharynx). The latter smell plays a key part in flavor perception while interacting with all other sensory modalities (taste, trigeminal perception mediating texture and temperature, vision, and hearing). Besides the important contribution of the sense of smell to our daily food enjoyment, the olfactory system is believed to influence mate choice and modulate emotional states.2 Sudden loss of olfactory function is usually noticed (eg, post‐infectious or posttraumatic), whereas gradual deterioration of smell function may go unnoticed (eg, in relation to age or neurodegenerative diseases).3 Olfactory dysfunction (OD) affects up to a quarter of the general population with increasing prevalence in the older population.4, 5, 6 Correct diagnosis is often difficult and time‐consuming. In addition to the general and smell‐related medical history, physical examination, and adequate imaging, olfactory tests are core parts of patient management. Self‐assessment of olfactory function in healthy subjects is frequently unrelated to olfactory test results, whereas moderate but significant correlation between self‐assessment of smell function and olfactory test results have been shown in patients with olfactory loss.7
Although the majority of patients with loss of olfactory function complain of decreased flavor perception, olfactory function is normally only tested with established orthonasal testing procedures (eg, University of Pennsylvania Smell Identification Test [UPSIT]8 or the Sniffin’ Sticks Test [TDI]9, 10). Retronasal olfactory tests, such as the Candy Smell Test (CST) or the taste powders still need higher acceptance in clinical routine.11, 12, 13
Occasionally patients with smell loss report normal flavor perception.14, 15 Concerning this group of patients, this raises the following three issues: A) whether the subjectively normal flavor perception is due to normal or partially functional retronasal olfactory function, as seen in some patients with nasal polyps,15 B) whether retronasal smell perception might be diminished without being apparent to the patients, and C) whether there any correlations between measured and self‐assessed olfactory function.
The aim of the present investigation was to evaluate retronasal and orthonasal olfactory function in this subgroup of patients with smell loss but intact flavor perception.
MATERIALS AND METHODS
Ethical Considerations
This study was conducted at the Medical University of Vienna, Department of Otorhinolaryngology and at the Friedrich‐Alexander University Erlangen‐Nürnberg, Institute of Experimental and Clinical Pharmacology and Toxicology, according to the Declaration of Helsinki on biomedical research involving human subjects. The study protocol was approved by the local ethics committees (Approval No. EK‐Nr.: 1411/2017 and EK‐Nr.: 383_14 B).
Subjects
Outclinic patients with self‐reported OD, corresponding orthonasal olfactory test results (at least hyposmia according to normative data3) but normal flavor perception (self‐assessment of flavor perception using visual analogue scales) were recruited for retronasal olfactory and gustatory testing. A complete ear, nose and throat examination including the patient's history and nasal endoscopy as well as imaging procedures (whenever necessary) were used to determine possible reasons for olfactory loss. Patients with sinonasal diseases (eg, nasal polyps, chronic rhinosinusitis, obstructed olfactory cleft), neurodegenerative diseases, and fructose malabsorption or hypersensitivity (due to use of the Sorbitol‐based CST in this study, see below) were not eligible for the study. The study included 19 patients (10 female, nine male, mean age [range], 52.0 [8–83] years) with no history of prior olfactory testing. Reasons for OD were idiopathic cause (10 patients), followed by upper respiratory airway infection (four patients), congenital cause (three patients), and head trauma (two patients). Mean (SD) duration of impairment was 17.0 (18.8) months (except congenital cause). Orthonasal olfactory performance was tested using the TDI,10 retronasal olfactory performance using the CST11, 12 and gustatory function using the Taste Strips Test (TST).16 Feedback was given to the patients at the end of all tests and examinations only.
Self‐Assessment of Flavor Perception
Prior to olfactory tests, patients rated subjective chemosensory function, namely odor perception (SOP) (“How would you rate your sense of smell?”), flavor perception (SFP) (“How would you rate your fine taste, eg, during eating and drinking?), and taste perception (STP) (“How would you rate your basic taste: sweet, sour, salty, bitter?) by using visual analogue scales (VAS) of 10 cm length ranging from 0 (“no smell/taste/flavor perception”; left hand end) to 10 (“excellent smell/flavor/taste perception”; right hand end). A VAS of at least 4 for SFP was required to be eligible for this study.
Sniffin’ Sticks
Orthonasal olfactory function was tested using the TDI (Burghart Medical Technology, Wedel, Germany), which is based on reusable odor dispensing “pens” and has been validated for both children and adults.11, 17 The test consists of three parts: Odor Threshold (T), Odor Discrimination (D), and Odor Identification (I). A three‐alternative forced‐choice method is used for Odor Threshold (T) and Odor Discrimination (D) with 16 pen‐triplets, respectively. Odor Identification (I) is based on a four‐alternative forced‐choice method with 16 pens containing well‐known odors. Each subtest has a maximum score of 16 resulting in maximum total TDI score of 48. The individual TDI score was compared with normative data to distinguish between normosmia, hyposmia, and functional anosmia (further termed “anosmia”).3, 9, 18 The 10th percentile has been defined in previous studies to separate hyposmia from normosmia. TDI‐scores equal or higher than 30.75 were defined as normosmia and TDI‐scores less than 30.75 and higher than 16 as hyposmia. Functional anosmia was defined as TDI scores equal or less than 16.3
Candy Smell Test
Retronasal olfactory function was tested using the newly developed, extended CST (CST27), consisting of 27 (instead of 2311) white candies with a diameter of 9 mm, each containing 500 mg sorbitol and one targeted aroma. The CST was developed for children and has shown reliable and valid results from the age of 6 upwards.11, 12 The candies were manufactured in the Division of Pharmaceutics, University Erlangen‐Nürnberg. After placing the candy on the tongue, subjects were asked to suck or chew the candy and to choose one from four possible answers on a form (four alternative forced‐choice method), for example: banana, cinnamon, coffee, and orange. Subjects were asked to rinse the mouth with tap water after each candy. The result of the CST27 test was the sum of the results for individual candies with a maximum score of 27. Since normative data have not been published so far for the extended CST, the individual CST score was compared with normative collected data, available for the 23‐item CST (CST23).11 We used the cut‐off limit lower than 12 to distinguish anosmic patients from hyposmic/normosmic, since this limit has been proven to have the highest specificity for anosmia.11
Taste Strips
Gustatory function was tested using the clinically validated TST. The TST has been successfully applied in children and adults using paper strips with a length of 8 cm and an impregnated area of 2 cm2 (Burghart, Wedel, Germany).16, 19, 20 Four concentrations of sweet (sucrose), sour (citric acid), salty (sodium chloride) and bitter (quinine hydrochloride) taste were used for impregnation. Sixteen taste strips and two blanks were presented in increasing concentrations in a randomized order and placed on the middle of the tongue. Patients were asked to choose from one of five possible answers (sweet, sour, salty, bitter, and no taste) and to rinse their mouth after each taste strip. The result of the TST was the sum of the results for individual taste qualities with a maximal attainable test score of 16. The individual TST‐score was compared with normative collected data to distinguish between normogeusia and hypogeusia. As described in previous studies, the 10th percentile was used as the cut‐off limit, therefore TST‐scores lower than 9 were defined as hypogeusia.16, 17
Statistical Analysis
Statistical analysis was carried out using R Statistical Computing Software 3.4.4 (R Development Core Team, 2008; R Foundation for Statistical Computing, Vienna, Austria). R Statistical Computing Software 3.4.4 (R Development Core Team, 2008; R Foundation for Statistical Computing, Vienna, Austria) and GraphPrism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA) were used for graphical visualization. P‐value was set at <.05 and normality of data were tested using the Kolmogorov‐Smirnov test. Correlation analysis were performed using Spearman statistics.
RESULTS
Ortho‐ and Retronasal Olfactory Dysfunction with Concurrent Unimpaired Gustatory Function
Mean score of the orthonasal olfactory TDI was 14.4 ± 5.3, with 12 patients classified as anosmic and 7 as hyposmic. The retronasal olfactory test (CST27) revealed a mean score of 8.8 ± 2.7, with 16 patients classified as anosmic and three as hyposmic. Mean score of the TST was 9.6 ± 2.1 with 15 patients classified as normogeusic and four as hypogeusic (Table 1).
Table 1.
Results of Chemosensory Testing and Self‐Assessment Scores.
| Number | Mean | SD/Range | |
|---|---|---|---|
| Age | 52.0 | 21.7 | |
| Duration of OD in months (except congenital causes) | 17.0 | 18.8 | |
| Idiopathic OD | 10 | ||
| Postinfectious OD | 4 | ||
| Congenital OD | 3 | ||
| Traumatic OD | 2 | ||
| T (dilution steps) | 2.0 | 1.5 | |
| D (number of correct discriminations) | 6.8 | 2.8 | |
| I (number of correct identifications) | 5.9 | 2.2 | |
| TDI | 14.4 | 5.3 | |
| CST | 8.8 | 2.7 | |
| TST | 9.6 | 2.1 | |
| SOP | 1.6 | 0‐4.9 | |
| STP | 8.7 | 2.2‐10 | |
| SFP | 8.0 | 4‐10 |
CST = Candy smell test, OD = Olfactory dysfunction, TDI = threshold, discrimination, and identification (TDI), TDI = Sniffin’ Sticks test, TST = Taste strips test, SOP = self‐assessment of odor perception; STP = self‐assessment of taste perception, SFP = self‐assessment of flavor perception; SD = standard deviation.
Difference Between Self‐Assessment of Odor and Flavor Perception
Mean (range) SOP was 1.6 (0–4.9) with six patients stating no odor perception (patients stated the lowest possible VAS score of 0). Mean (range) SFP was 8.0 (4–10) with two patients stating excellent flavor perception (patients stated the highest possible VAS score of 10). Mean taste perception was 8.7 (2.2–10) with five patients stating excellent taste perception (Table 1, Fig. 1).
Figure 1.
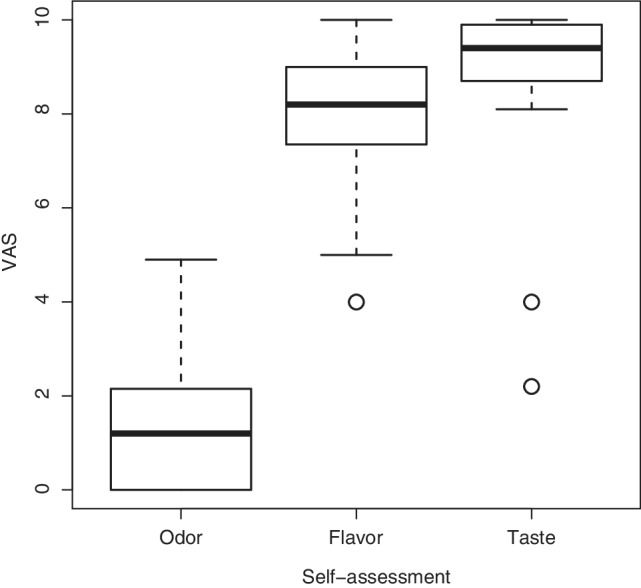
Boxplot of self‐assessment scores. VAS = visual analogue scale ranging from 0 (minimum) to 10 (maximum), Odor = self‐assessment of odor perception, Flavor = self‐assessment of flavor perception, Taste = self‐assessment of taste perception, boxes (1.quartile to 3.quartile) represent the middle 50% of data and the inside horizontal lines mark the median value, the two horizontal lines mark the whiskers (lower horizontal line = 1. Quartile – 1.5 interquartile range, upper horizontal line = 3. Quartile +1.5 interquartile range), outliers are shown as individual data points.
Nonsignificant Correlation Between SFP and Retronasal Olfactory Function
To assess whether there was an association between SFP and retronasal olfactory function, we performed a correlation analysis revealing no significant correlation (P = .60, r = −0.13, Spearman correlation; Fig. 2). To test whether there was an association between SOP and orthonasal olfactory function, we also performed a correlation analysis revealing significant correlations (for TDI, P < .01, r = 0.66, Fig. 3; for T, P = .32, r = 0.24; for D, P < .01, r = 0.76; for I, P = .02, r = 0.53, all Spearman correlation). Finally, to test whether there was an association between STP and gustatory function, we performed a correlation analysis revealing no significant correlation (P = .25. r = 0.28, Spearman correlation).
Figure 2.
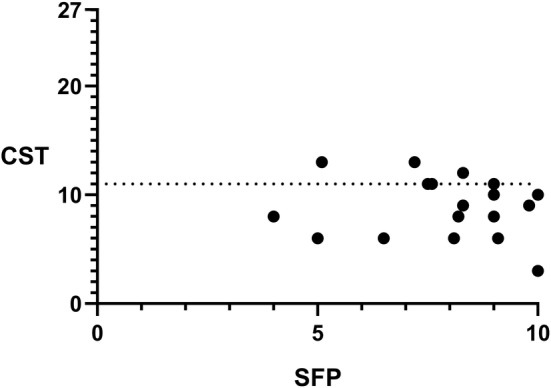
Nonsignificant correlation between self‐assessment of flavor perception and retronasal olfactory test (P = .60, r = −0.13). CST = Candy Smell Test, SFP = self‐assessment of flavor perception, dotted line showing the cut‐off point between hyposmic and normosmic patients (CST ≤11).
Figure 3.
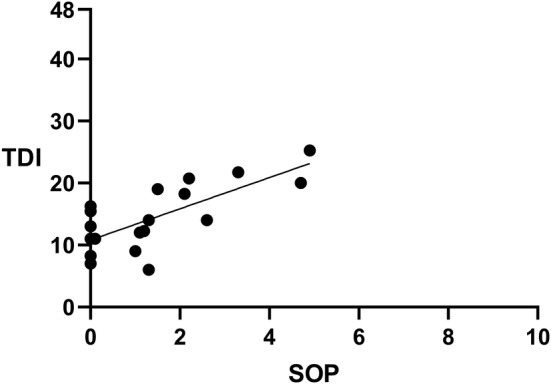
Correlation between self‐assessment of odor perception and orthonasal olfactory test (P < .01, r = 0.66). TDI = Sniffin’ Sticks Test, SOP = self‐assessment of odor perception, straight line showing regression line.
DISCUSSION
An estimated 25% of all people over 50 years of age experience olfactory impairment.5, 6, 21, 22 A survey from Vennemann et al. on the prevalence of olfactory dysfunctions in the general population showed impairments in almost 18% of the general population with 3.6% classified as functionally anosmic.6 The olfactory system plays the leading role in human multisensory flavor perception,23, 24 therefore it is expected that a loss of olfactory function leads to altered perception of flavors, which is also confirmed in larger series of patients.25 However, some patients report smell loss but simultaneously state normal to excellent flavor perception. Published26 and unpublished data of our group demonstrate a relatively low percentage of patients with severe olfactory dysfunction but normal subjective flavor perception at the same time (between 3.7% for VAS = 10 and 28% for VAS ≥4).
As the major finding of our study, retronasal olfactory performance as measured by an established retronasal smell test did not confirm normal flavor perception in the investigated subjects. All patients yielded scores within the range of hyposmia/anosmia with ortho‐ and retronasal tests, demonstrating striking discrepancies between subjective and measured flavor identification abilities. In contrast, orthonasal smell test results correlated significantly with self‐assessed olfactory abilities. Our findings are in accordance with current scientific publications stating a moderate but significant correlation between self‐assessment of smell perception and measured olfactory acuity in patients with olfactory dysfunction and confirm a trend that self‐assessment of olfactory function becomes more accurate with decreasing performance.7, 14, 27 However, it has to be kept in mind, that on an individual patient's level, olfactory performance can only be assessed by means of validated smell tests.28
Regarding gustatory function, the question could arise of whether gustatory function in patients with smell loss and subjectively normal flavor perception is increased, compared to patients with smell loss and concordant loss of flavor perception. This was not found to be the case in our patients, as the majority of achieved TST scores projected in the medium to lower percentile range of normogeusia compared with normative data,16 which is also in accordance with a previously published study showing no significant influence of smell loss on gustatory function.29 As previously described, normosmic patients tend to rely on their odor imagery abilities for self‐assessment of olfactory function7 although this ability seems to decrease with the duration of olfactory loss.30, 31 A tendency of these patients to rely more on gustatory, textural, auditory (during mastication), and visual information of foods could be a reason for the lack of correlation between self‐assessment and test results of retronasal olfactory function.32 Our findings show that relying exclusively on subjective reports on flavor perception in patients with olfactory dysfunction can be misleading and additional testing of retronasal olfactory function can provide more information for the management regarding hazardous events (eg, ingestion of spoiled food).33
Why does the loss of retronasal olfactory function go unnoticed in some patients? Although we cannot give answers to this question based on our results, some thoughts might be relevant for further research. In our patients subjectively normal flavor perception during food intake was not mediated by intact retronasal olfactory function. In another investigation retronasal olfactory event‐related potentials could be recorded from some patients with unimpaired flavor perception which were ortho‐ and retronasally tested to be dysosmic by means of psychophysical tests.14 However, this might not be clinically relevant, since olfactory event‐related potentials can also be present in patients with functional anosmia, for whom residual olfactory function is not useful in everyday life.17, 18, 34
Part of the contribution of retronasal smell stimuli to overall flavor perception seems to be mediated by memory recall. Therefore unconscious memory recall of “flavor templates” from previously experienced cross‐modal sensory interactions (eg, somatosensory–olfactory interactions) may be an explanation for normal flavor perception in orthonasally anosmic patients with noncongenital causes.30, 35, 36 All three patients in our study with congenital smell loss yielded scores within the range of anosmia in ortho‐ and retronasal tests presuming “flavor” is an individual concept, consisting of interaction of all other sensory modalities (for example vision, taste, sound, and somatosensory) independently from olfactory perception. Long‐term olfactory recognition memory, which plays a vital role in food preference and food habits, happens unconsciously and incidentally through repeated presentation of individual components together, as is the case with food and beverages.37, 38, 39 A further mentionable point is that the development of our multisensory flavor perception probably already starts in the mother's womb39 and continues into adulthood. The frequent presentation and co‐occurrence of olfactory stimuli with other sensory stimuli, eg, of gustatory and olfactory quality, consequently allow qualities of one sensory system to evoke qualities in another.40 Further studies using functional imaging methods, for example, are needed for more clarity regarding different brain activities with variability of self‐assessment of different sensory modalities.
Finally, as shown in a recent publication, olfactory changes are not as strongly perceived as visual changes. While olfactory changes were only detected with an accuracy of 61%, visual changes were detected with an accuracy of over 97%. Only 24% of the participants were able to detect olfactory changes reliably above chance. Notably, these subjects also rated their personal interest in olfaction and its use in daily life as most important.41 Regarding our subgroup of patients with smell loss and no subjective change in flavor perception, it might be speculated that these patients rely more on visual, gustatory, and trigeminal cues during eating and drinking leading to an unawareness of a decreased retronasal odor identification ability.
CONCLUSION
The present results show that normal subjective flavor perception in patients with non‐sinonasal smell loss is not confirmed by retronasal smell test results. In most of the investigated patients orthonasal and retronasal smell test results yielded scores within the range of anosmia. These findings suggest that part of the representation of retronasal smell within flavor perception may be mediated by unconscious memory recall from previous experienced cross‐modal sensory interactions. Possible anatomical, histological, and physiological differences between groups of anosmic patients with and without noticing the loss in flavor perception will be the subject of future investigations.
ACKNOWLEDGMENTS
Parts of this study were presented at the 90th Annual Meeting of the German Society of Oto‐Rhino‐Laryngology‐Head and Neck Surgery, Berlin, Germany, in June 2019.
Editor's Note: This Manuscript was accepted for publication on August 9, 2019.
The authors have no conflicts of interest or funding to declare.
BIBLIOGRAPHY
- 1. Clark JE. Taste and flavour: their importance in food choice and acceptance. Proc Nutr Soc 1998;57(04):639–643. [DOI] [PubMed] [Google Scholar]
- 2. Stevenson RJ. An initial evaluation of the functions of human olfaction. Chem Senses 2010;35:3–20. [DOI] [PubMed] [Google Scholar]
- 3. Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol 2019;276:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brämerson A, Johansson L, Ek L, et al. Prevalence of olfactory dysfunction: the skovde population‐based study. Laryngoscope 2004;114:733–737. [DOI] [PubMed] [Google Scholar]
- 5. Murphy C, Schubert CR, Cruickshanks KJ, Klein BEK, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. J Am Med Assoc 2002;288:2307–2312. [DOI] [PubMed] [Google Scholar]
- 6. Vennemann MM, Hummel T, Berger K. The association between smoking and smell and taste impairment in the general population. J Neurol 2008;255:1121–1126. [DOI] [PubMed] [Google Scholar]
- 7. Kollndorfer K, Kowalczyk K, Nell S, Krajnik J, Mueller CA, Schöpf V. The inability to self‐evaluate smell performance. How the vividness of mental images outweighs awareness of olfactory performance. Front Psychol 2015;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania smell identification test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984;32:489–502. [DOI] [PubMed] [Google Scholar]
- 9. Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S. “Sniffin’ Sticks”: screening of olfactory performance. Rhinology 1996;34:222–226. [PubMed] [Google Scholar]
- 10. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. “Sniffin' Sticks”. Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 1997;22:39–52. [DOI] [PubMed] [Google Scholar]
- 11. Renner B, Mueller CA, Dreier J, Faulhaber S, Rascher W, Kobal G. The Candy Smell Test: a new test for retronasal olfactory performance. Laryngoscope 2009;119:487–495. [DOI] [PubMed] [Google Scholar]
- 12. Haxel BR, Bertz‐Duffy S, Faldum A, et al. The Candy Smell Test in clinical routine. Am J Rhinol Allergy 2011;25:145–148. [DOI] [PubMed] [Google Scholar]
- 13. Heilmann S, Hummel T. A new method for comparing orthonasal and retronasal olfaction. Behav Neurosci 2004;118:412–419. [DOI] [PubMed] [Google Scholar]
- 14. Landis BN, Frasnelli J, Reden J, Lacroix JS, Hummel T. Differences between orthonasal and retronasal olfactory functions in patients with loss of the sense of smell. Arch Otolaryngol Head Neck Surg 2005;131:977–981. [DOI] [PubMed] [Google Scholar]
- 15. Landis BN, Giger R, Ricchetti A, et al. Retronasal olfactory function in nasal polyposis. Laryngoscope 2003;113:1993–1997. [DOI] [PubMed] [Google Scholar]
- 16. Mueller C, Kallert S, Renner B, et al. Quantitative assessment of gustatory function in a clinical context using impregnated “taste strips.” Rhinology 2003;41:2–6. [PubMed] [Google Scholar]
- 17. Kobal G, Klimek L, Wolfensberger M, et al. Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol 2000;257:205–211. [DOI] [PubMed] [Google Scholar]
- 18. Hummel T, Kobal G, Gudziol H, Mackay‐Sim A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol 2007;264:237–243. [DOI] [PubMed] [Google Scholar]
- 19. Welge‐Lüssen A, Dörig P, Wolfensberger M, Krone F, Hummel T. A study about the frequency of taste disorders. J Neurol 2011;258:386–392. [DOI] [PubMed] [Google Scholar]
- 20. Hill CA, Beach M, Smith MC, Chen EY. Incidence of and factors associated with hypogeusia in healthy children. JAMA Otolaryngol Head Neck Surg 2016;142:229–233. [DOI] [PubMed] [Google Scholar]
- 21. Landis BN, Konnerth CG, Hummel T. A study on the frequency of olfactory dysfunction. Laryngoscope 2004;114:1764–1769. [DOI] [PubMed] [Google Scholar]
- 22. Nordin S, Monsch AU, Murphy C. Unawareness of smell loss in normal aging and alzheimer's disease: discrepancy between self‐reported and diagnosed smell sensitivity. J Gerontol B Psychol Sci Soc Sci 1995;50:187–192. [DOI] [PubMed] [Google Scholar]
- 23. Stuckey B. Taste What You're Missing: The Passionate Eater's Guide to Why Good Food Tastes Good. Reiss L, transl. Hoboken, NJ: Wiley; 2012. [Google Scholar]
- 24. Chartier F. Taste Buds and Molecules: The Art and Science of Food, Wine, and Flavor. Boston, MA: Houghton Mifflin Harcourt; 2012. [Google Scholar]
- 25. Deems DA, Doty RL, Settle RG, et al. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg 1991;117:519–528. [DOI] [PubMed] [Google Scholar]
- 26. Besser G, Liu DT, Renner B, Hummel T, Mueller CA. Olfactory implant: demand for a future treatment option in patients with olfactory dysfunction. Laryngoscope 2019;129:312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Welge‐Luessen A, Hummel T, Stojan T, Wolfensberger M. What is the correlation between ratings and measures of olfactory function in patients with olfactory loss? Am J Rhinol 2005;19:567–571. [PubMed] [Google Scholar]
- 28. Lötsch J, Hummel T. Clinical usefulness of self‐rated olfactory performance—a data science‐based assessment of 6000 patients. Chem Senses 2019;44:357–364. [DOI] [PubMed] [Google Scholar]
- 29. Stinton N, Atif MA, Barkat N, Doty RL. Influence of smell loss on taste function. Behav Neurosci 2010;124:256–264. [DOI] [PubMed] [Google Scholar]
- 30. Flohr ELR, Arshamian A, Wieser MJ, et al. The fate of the inner nose: odor imagery in patients with olfactory loss. Neuroscience 2014;30:118–127. [DOI] [PubMed] [Google Scholar]
- 31. Han P, Hummel T, Raue C, Croy I. Olfactory loss is associated with reduced hippocampal activation in response to emotional pictures. Neuroimage 2019;188:84–91. [DOI] [PubMed] [Google Scholar]
- 32. Shepherd GM. New perspectives on olfactory processing and human smell In: Menini A, ed: The Neurobiology of Olfaction. Boca Raton, FL: CRC Press/Taylor & Francis; 2010. [PubMed] [Google Scholar]
- 33. Pence TS, Reiter ER, DiNardo LJ, Costanzo RM. Risk factors for hazardous events in olfactory‐impaired patients. JAMA Otolaryngol Head Neck Surg 2014;140:951–955. [DOI] [PubMed] [Google Scholar]
- 34. Lötsch J, Hummel T. The clinical significance of electrophysiological measures of olfactory function. Behav Brain Res 2006;170:78–83. [DOI] [PubMed] [Google Scholar]
- 35. Köster MA, Prescott J, Köster EP. Incidental learning and memory for three basic tastes in food. Chem Senses 2004;29:441–453. [DOI] [PubMed] [Google Scholar]
- 36. Mojet J, Köster EP. Texture and flavour memory in foods: an incidental learning experiment. Appetite 2002;38:110–117. [DOI] [PubMed] [Google Scholar]
- 37. Schaal B. Human foetuses learn odours from their pregnant mother's diet. Chem Senses 2000;25:729–737. [DOI] [PubMed] [Google Scholar]
- 38. Mennella JA, Beauchamp GK. Understanding the origin of flavor preferences. Chem Senses 2005;30(Suppl 1):i242–i243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mennella JA, Jagnow CP, Beauchamp GK. Prenatal and postnatal flavor learning by human infants. Pediatrics 2001;107(6):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stevenson RJ, Boakes RA, Prescott J. Changes in odor sweetness resulting from implicit learning of a simultaneous odor‐sweetness association: an example of learned synesthesia. Learn Motiv 1998;29:113–132. [Google Scholar]
- 41. Menzel S, Hummel T, Schäfer L, Hummel C, Croy I. Olfactory change detection. Biol Psychol 2019;140:75–80. [DOI] [PubMed] [Google Scholar]


