Abstract
Objectives
The aim of this study was to compare the diagnostic accuracy of salivary pepsin with oropharyngeal pH monitoring using the Restech measurement system (Dx‐pH) for the diagnosis of laryngopharyngeal reflux (LPR).
Study Design
Prospective cohort study.
Methods
Seventy patients with primary symptoms related to LPR underwent gastroscopy, high‐resolution manometry, pH throughout 24‐hour monitoring (MII‐pH), and barium esophagography between October 2015 and May 2018. In addition, an ear, nose, and throat examination was performed, including assessment of Belafsky Reflux Finding Score (RFS). Clinical symptoms were evaluated with the Belafsky Reflux Symptom Index (RSI) and the Gastrointestinal Quality of Life Index (GIQLI). Simultaneous to MII‐pH, pepsin determination and Dx‐pH were performed.
Results
Of 70 patients, 41 (58.6%) subjects with a pathological DeMeester score showed higher mean values of pepsin (mean value: 216 ng/mL, 95% confidence interval [CI]: 172 to 260), compared to patients with a normal DeMeester score (mean value: 161 ng/mL, 95% CI: 115 to 207). Salivary pepsin showed a specificity of 86.2% and sensitivity of 41.5% for diagnosing LPR using the optimal cutoff value of 216 ng/mL. Furthermore, a significant correlation between the values of salivary pepsin and the RSI score was seen in patients with pathological results in MII‐pH (r = 0.344; P = 0.046).
However, elevated Dx‐pH measurements showed no significant correlation with either MII‐pH, RSI score, RFS score, or GIQLI score, or with the results of pepsin measurement.
Conclusion
Pepsin measurement in saliva could be an alternative tool to assist office‐based diagnosis of LPR, whereas Dx‐pH does not seem to be an adequate test.
Level of Evidence
2B Laryngoscope, 130:1780–1786, 2020
Keywords: GERD, salivary pepsin, oropharyngeal pH monitoring, laryngopharyngeal reflux
INTRODUCTION
Laryngopharyngeal reflux (LPR) results in 10% of otolaryngology consultations.1, 2 The clinical challenge is to determine if primarily present symptoms such as hoarseness, frequent throat clearing, cough, or asthma‐like symptoms are related to an exacerbated gastroesophageal reflux disease (GERD) or are caused by other potential etiologies such as allergies, sinusitis, chronic bronchitis, or a postnasal drip syndrom.3 The main problems are that the current standard methods are not sufficient and accurate enough to definitively diagnose LPR.4, 5 The lack of LPR‐specific tests often leads to evaluation of an empirical treatment response to GERD or multichannel intraluminal impedance pH throughout 24‐h monitoring (MII‐pH), which is still the gold standard measurement tool to assess if present extraesophageal symptoms are caused by GERD.5 However, MII‐pH is an invasive and costly method and cannot be performed on all patients with suspicion to suffer from LPR.6, 7 There is a need for noninvasive and inexpensive methods that allow for a more sensitive and accurate diagnosis of LPR to optimize the treatment strategies for this patient cohort.
Recently, a few studies reported that oropharyngeal pH monitoring using the Restech measurement system (Dx‐pH) appears to be more sensitive than MII‐pH in the evaluation of patients with LPR, although the authors state that Dx‐pH is still limited due to a lack of consensus on normal and abnormal cutoff values, as well as missing well‐controlled prospective studies.8, 9
Controversial data is provided by Willhelm et al., who reported that 60% of asymptomatic gastrectomy patients showed positive results in Dx‐pH. Therefore, the authors state that Dx‐pH is not useful to guide any diagnostic or therapeutic decisions.10
As another diagnostic method, pepsin determination in saliva has been proposed as a tool to improve the diagnosis of GERD as well as LPR.
Pepsin is a proteolytic enzyme, which is activated by its precursor pepsinogen in the stomach and can be detected in saliva as well as secretion samples from the lung, sinus, middle ear, trachea, and exhaled breath condensate.11, 12, 13, 14, 15
Hayat et al. showed the value of salivary pepsin to discriminate patients with GERD. The authors speculate that this noninvasive Peptest (Peptest, RDBiomed, Hull, U.K.) could lead to an improvement in the diagnosis of patients with GERD as well as patients with LPR.7 A recently published state‐of‐the‐art review on the evaluation and management of LPR disease by Lechien et al. underlines these speculations, although the authors state that a multiparameter diagnostic approach should be established.5 Nevertheless, the role of pepsin determination in saliva and Dx‐pH for the diagnosis of LPR remains controversial.
The aim of this prospective study was to compare the value of salivary pepsin determination and Dx‐pH measurement as tools in a multiparameter diagnostic pathway for patients with LPR.
MATERIALS AND METHODS
Study Population
From October 2015 to May 2018, 635 patients with typical and atypical clinical symptoms related to GERD were assessed for eligibility in the Department of Surgery at Ordensklinikum Linz Sisters of Charity Hospital in Linz, Austria (Fig. 1). Seventy patients with primarily atypical GERD symptoms and suspicion to suffer from LPR were included in the study. A multiparameter diagnostic approach, including gastroscopy, barium esophagography, high‐resolution esophageal manometry (HRM), MII‐pH, Dx‐pH, and measurement of salivary pepsin concentration was performed. Only patients with primary laryngopharyngeal/atypical reflux symptoms despite treatment with a proton‐pump inhibitor (PPI) for at least 6 months were considered.
Figure 1.
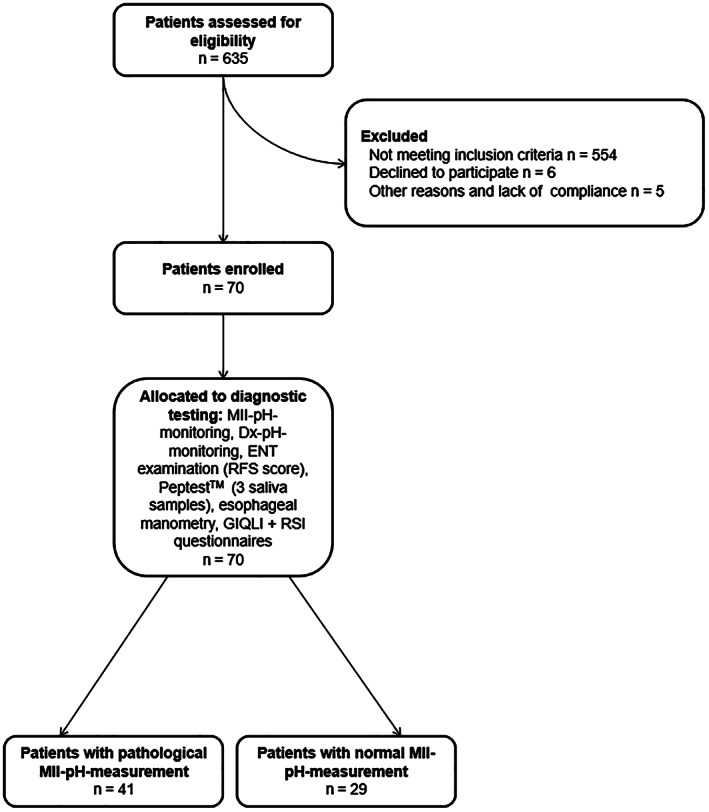
Flowchart showing patient recruitment process and classification. ENT = ear, nose and throat; GIQLI (mean normal 122.6) = Gastrointestinal Quality of Life Index; RFS = Belafsky Reflux Finding Score; RSI = Belafsky Reflux Symptom Index; MII‐pH = multichannel intraluminal impedance pH throughout 24‐h monitoring; Dx‐pH = oropharyngeal pH monitoring using the Restech measurement system.
Patient exclusion criteria were the following: age younger than 18 years, American Society of Anesthesiologists physical status classification >II, previous esophageal or gastric surgery, pregnancy, presence of higher grade esophageal dysmotility (e.g., achalasia), and other potential causes of laryngopharyngeal/atypical reflux symptoms (e.g., heterotopic gastric mucosa of the cervical esophagus).
Written informed consent for participation in the study was obtained from all patients, and study approval was obtained by the institution's ethical committee.
Study Design and Study Run
The study design is a prospective single‐center trial on the value of pepsin dtermination in saliva and Dx‐pH as sufficient tools to diagnose LPR. All measurements took place in an inpatient setting, with patients off PPIs for at least 10 days. On day 1, patients had to undergo gastroscopy and HRM. On day 2, MII‐pH simultaneously to Dx‐pH were performed, as well as the collection of three saliva samples to determine the pepsin concentration. On day 3, patients had to undergo an ENT examination including assessment of the Belafskys Reflux Finding Score (RFS) score. Furthermore, during the study period from day 1 through day 3, quality of life and clinical symptoms were assessed in all patients by the GIQLI and Belafsky Reflux Symptom Index (RSI) score, respectively.
High‐Resolution Esophageal Manometry
All patients had to undergo the measurement after an overnight fast in the supine position. HRM using the Sierra system ManoScan Z, Model A200 (Given Imaging, Duluth, GA) was performed in order to evaluate patients for esophageal motility disorders. A structurally defective lower esophageal sphincter (LES) was defined as an overall length below 2.4 cm, an intraabdominal length below 0.9 cm, and/or the presence of a hiatal hernia. Pressure levels beyond <29.8 or > 180.2 mmHg were rated as abnormal, and detected motility disorders were classified according to the Chicago Classification, version 3.0.16
Quality‐of‐Life Evaluation
Quality‐of life was assessed by means of the German Gastrointestinal Quality of Life Index (GIQLI).17 This questionnaire has been validated in German language and it is recommended by the European Study Group for Antireflux Surgery.18 The GIQLI includes 36 items, which are divided into five subdimensions: gastrointestinal symptoms, emotional status, social functions, physical functions, and a single item for stress of medical treatment, for a minimum of 0 and a maximum of 144 points. A better QoL is indicated by higher points. The mean normal in the healthy population is set at 122.6 points.17, 18
Multichannel Intraluminal Impedance pH Throughout 24‐Hour Monitoring
All patients had to be off antisecretory therapy for at least 10 days before examination. Furthermore, all patients were encouraged to maintain their normal activities and to remain upright during the day, except for one short nap allowed. Patients were asked to have three main meals a day, without eating snacks in between. The Digitrapper‐multichannel intraluminal impedance‐pH monitoring system (Medtronic, Minneapolis, MN) was used for assessment. A 2.1 mm nasogastric probe was inserted with two antimony pH electrodes located 5 cm above the manometrically located LES and 15 cm more distal the LES and eight impedance electrodes, allowing measurement of intraluminal impedance in six segments at 3, 5, 7, 9, 15, and 17 cm above LES.19
GERD was diagnosed if the reflux‐related composite pH score according to DeMeester exceeded 14.7, in combination with a total number of reflux events in 24 hours of more than 73.19, 20, 21, 22
Belafsky Reflux Symptom Index
Laryngopharyngeal/extra‐esopahgeal reflux symptoms were evaluated using the standardized RSI questionnaire. The RSI includes nine items and it is self‐administered. The score range for each item is between 0 (no problem) and 5 (severe problem) points, with a maximum total score of 45. An RSI of >13 is considered to indicate the presence of reflux.23, 24
Belafskys Reflux Finding Score
An ear, nose, and throat (ENT) examination was performed by an otolaryngologist, including a fiberoptic laryngoscopy and photographic documentation. Furthermore, the RFS was determined. RFS ranges were set from the lowest possible score of 0 (normal larynx) to the highest possible score of 26. A score of >7 was defined as pathological.23, 25
Oropharyngeal pH Monitoring
Simultaneous to MII‐pH, patients had to undergo Dx‐pH as well. The Restech Dx‐pH measurement system, version 1.0 (Restech Dx‐pH, Restech, San Diego, CA) was used. Therefore, a probe was placed in a standardized way, as recommended by the provider in the oropharynx above the upper esophageal sphincter. The measurements are evaluated by the Ryan score, which was considered to be pathological when higher 9.4 in the upright position (pH < 5.5) or higher 6.8 in the supine position (pH < 5.0).26
Pepsin Determination in Saliva
Subjects collected the saliva samples on waking, 1 hour after finishing lunch, and 1 hour after finishing dinner during the MII‐pH and simultaneous Dx‐pH monitoring period. The early morning sample was collected before eating, drinking, smoking, or brushing the teeth of the patients. Saliva was collected into tubes containing 0.5 mL of 0.01 M citric acid. Subjects returned the samples immediately after collection, and the samples were sent to the laboratory.
Samples were refrigerated at 4°C and analyzed within 2 days after collection.
Pepsin values were determined in a standardized procedure using Peptest (RDBiomed), as previously described.7
The value of 16 ng/mL was used as cutoff for a positive sample (as determined by the manufacturer). Samples with a pepsin concentration above the upper limit of 500 ng/mL had 501 ng/mL in the results. The mean value out of three samples was used to perform correlation analysis.
Statistical Analysis
Statistical analysis was performed using SPSS statistical analysis software, version 25.0 (SPSS Inc., Chicago, IL). Data were compared using a paired t test or the Wilcoxon signed rank test. If normally distributed, datasets were additionally presented as means and standard deviation. Multiple group comparisons were performed using a one‐way analysis of variance, followed by Kolmogorov–Smirnov Test for normal distributed data and the Kruskall–Wallis Test with Dunns comparison for non‐normal data. Receiver operating characteristic (ROC) curves were constructed to determine and compare the sensitivity and specificity of different pepsin cutoff concentrations and their predictive value to diagnose or refute the diagnosis of GERD and extra‐esophagel/laryngophyrangeal reflux‐related symptoms. Likelihood ratios were calculated, and P < 0.05 was regarded as statistically significant.
RESULTS
Patient Characteristics and Classification
Seventy patients were enrolled in the study. There were 30 (42.9%) male and 40 (57.1%) female patients with a mean age of 54.44 (±13.23) years. The mean body mass index (BMI) was 23.21 (±3.2) kg/m2. Patients were objective classified according to a pathological DeMeester score (>14.72), and having more than 73 reflux events in 24 hours to suffer from laryngopharyngeal/extra‐esophageal reflux symptoms related to GERD.
Finally, 41 patients (58.6%) were included in the group showing a pathological DeMeester score and were classified having “true” LPR (LPR group). Twenty‐nine patients (41.4%) built the group with clinical symptoms of LPR and normal results in MII‐pH (non‐LPR group) (Fig. 1).
In each group, none of the patients showed high‐grade esophageal motility disorders, a paraesophageal hiatal hernia, or upside‐down stomach in barium esophagography. In gastroscopy, 24 of 41 patients (58.5%) in the LPR group showed signs of esophagitis (grade I or II) compared to four of 29 patients (13.8%) in the non‐LPR group (P = 0.0001). Differences in ages, BMI, and sex distribution of subjects among the two groups were not significant (P > 0.05 for all).
Except for RFS score measurements, reflux episodes detected in MII‐pH, and DeMeester scores, no significant differences in the mean values of RSI score, as well as Ryan score and GIQLI, were seen (Table 1). However, significantly more patients in the LPR group (32 of 41; 78.0%) showed a pathological result in the RSI score compared to the non‐LPR group (14 of 29; 48.3%) (P = 0.045).
Table 1.
Demographic Data and Mean Values of MII‐pH, Reflux Episodes, Dx‐pH, RSI Score, RFS Score, and GIQLI Score For Each Group of Patients.
| Mean Values When DeMeester Score Pathological (n = 41) | Mean Values When DeMeester Score Normal (n = 29) | Significance | |
|---|---|---|---|
| Sex (male) | 21 (51.2%) | 12 (41.4%) | P = 0.724 |
| Age (years) | 55.2 (SD ± 12.4) | 53.2 (SD ± 14.1) | P = 0.758 |
| Body mass index (kg/m2) | 24.3 (SD ± 3.5) | 23.2 (SD ± 3.2) | P = 0.841 |
| DeMeester score | 40.8 (SD ± 36.2) | 8.8 (SD ± 3.7) | P = 0.000 |
| Total acid exposure time (%) | 12.3 (SD ± 6.1) | 2.3 (SD ± 1.1) | P = 0.000 |
| Reflux episodes MII‐pH (total) | 141.7 (SD ± 111.2) | 41.8 (SD ± 22.0) | P = 0.000 |
| Reflux episodes MII‐pH (proximal; total) | 38.7 (SD ± .27.8) | 8.2 (SD ± 4.4) | P = 0.000 |
| Reflux episodes MII‐pH (proximal; acidic) | 25.7 (SD ± .20.1) | 4.4 (SD ± 2.3) | P = 0.000 |
| Reflux episodes MII‐pH (proximal; weakly acidic) | 12.9 (SD ± .7.7) | 3.8 (SD ± 2.1) | P = 0.000 |
| Reflux episodes MII‐pH (proximal; nonacidic) | 2.1 (SD ± .2.0) | 0.5 (SD ± 0.5) | P = 0.887 |
| GIQLI score (points) | 96.5 (SD ± 22.6) | 101.6 (SD ± 21.9) | P = 0.327 |
| RSI score (points) | 18.1 (SD ± 8.4) | 15.7 (SD ± 10.2) | P = 0.276 |
| RFS score (points) | 5.7 (SD ± 2.1) | 4.5 (SD ± 2.6) | P = 0.048 |
| Ryan Score upright position (< 9.41) | 43.3 (SD ± 102.4) | 29.3 (SD ± 49.3) | P = 0.472 |
| Ryan Score supine position (< 6.79) | 4.7 (SD ± 9.8) | 3.3 (SD ± 5.0) | P = 0.467 |
GIQLI (mean normal 122.6) = Gastrointestinal Quality of Life Index; RFS = Belafsky Reflux Finding Score; Dx‐pH = oropharyngeal pH monitoring using the Restech measurement system; MII‐pH = multichannel intraluminal impedance pH throughout 24‐h monitoring; RSI = Belafsky Reflux Symptom Index; Ryan Score (upright and supine position) = results of oropharyngeal pH monitoring with Restech Dx‐pH Measurement System (Restech, San Diego, CA); SD = standard deviation.
There were no significant differences considering the number of patients with a pathological RFS score between both groups (P > 0.05).
Prevalence of Positive Pepsin Detection/Concentration in Saliva
In total, 35 of 41 (85.4%) patients with a pathological DeMeester score (LPR group) had one or more saliva samples positive for pepsin. In comparison, 21 of 29 (72.4%) patients in the non‐LPR group showed at least one positive sample (P > 0.05). The prevalence in the LPR group of having a positive sample was 61.0% on waking, 68.3% on lunch, and 56.1% on dinner. In the non‐LPR group, the prevalence of having a positive sample was 65.5% prevalence on waking, 89.7% prevalence on lunch, and 58.6% prevalence on dinner.
The mean salivary pepsin concentration out of three samples in the LPR group was 216 (±127) ng/mL, whereas patients in the non‐LPR group had a mean concentration of 161 (±114) ng/mL (Table 2).
Table 2.
Concentrations of Pepsin in Saliva for Each Group of Patients.
| n = 70 | Mean Concentration of Positive Samples (± SEM) | Median Concentration (25‐75th centiles), 95th Centile | Highest Pepsin Concentration (median (25–75th centile), 95th Centile) |
|---|---|---|---|
| DeMeester score pathological (n = 41) | 216 (± 127) | 171 (103–271), 378 | 313 (139–501), 501 |
| DeMeester score normal (n = 29) | 161 (± 114) | 92.5 (30–319), 501 | 233 (78–501), 501 |
Unit of concentrations: ng/mL.
SEM = standard error of the mean.
Neither mean concentrations of pepsin on waking (LPR vs. non‐LPR:100 [±150] ng/mL vs. 115 [±158] ng/mL) or after lunch (LPR vs. non‐LPR:192 [±189] ng/mL vs. 208 [±180] ng/mL) or dinner (LPR vs. non‐LPR:204 [±209] ng/mL vs. 202 [±202] ng/mL) showed significant differences when comparing both groups.
Values of Salivary Pepsin Concentration to Differentiate Patients With LPR From Patients With Non‐LPR
Using the ROC curve, we identified the optimal cutoff value of salivary pepsin concentration to differentiate patients with LPR from non‐LPR patients. The area under the ROC curve was 0.658 ±0.084 (95% CI, 0.387 to 0.720, P < 0.05). The best cutoff value was determined to be 216 ng/mL, and the value of the Youden index was largest. The specificity of the Peptest (RDBiomed, Hull, U.K.) was 86.2%, and the sensitivity was 41.5% at the measured optimal cutoff value. If at least one sample was positive (>16 ng/mL), the test showed a specificity of 85.4% and a sensitivity of 27.6%, with a negative predictive value of 57.1%. With one sample positive, the usefulness of the test depends on the pepsin concentration, which is shown beside predictive values and likelihood ratios in Table 3.
Table 3.
Patients With at Least One Positive Sample, Sensitivities, Specificities, Positive and Negative Predictive Values, and Likelihood Ratios for a Range of Pepsin Concentrations and Their Ability to Identify Patients with LPR.
| n = 70 | DeMeester Score Pathological (%) | DeMeester Score Nomal (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | +ve Likelihood Ratio | − ve Likelihood Ratio |
|---|---|---|---|---|---|---|---|---|
| At least 1 sample > 16 ng/mL | 35/41 (85.4) | 21/29 (72.4) | 85.4 | 27.6 | 62.5 | 57.1 | 1.18 | 0.53 |
| At least 1 sample > 50 ng/mL | 32/41 (78.1) | 17/29 (58.6) | 78.1 | 41.4 | 65.3 | 57.1 | 1.33 | 0.53 |
| At least 1 sample > 100 ng/mL | 28/41 (68.3) | 12/29 (41.4) | 68.3 | 58.6 | 70.0 | 56.7 | 1.65 | 0.54 |
| At least 1 sample > 150 ng/mL | 22/41 (53.7) | 9/29 (31.0) | 53.7 | 69.0 | 71.0 | 51.3 | 1.73 | 0.67 |
| At least 1 sample > 216 ng/mL | 17/41 (41.5) | 4/29 (13.8) | 41.5 | 86.2 | 81.0 | 51.0 | 3.00 | 0.69 |
LPR = laryngopharyngeal reflux; NPV = negative predictive value; PPV = positive predictive value.
Correlation Between Pepsin in Saliva/Dx‐pH and RFS, RSI, and GIQLI
The mean values of RSI score, RFS score, and GIQLI are presented in Table 1.
Significant correlations between GIQLI score and RSI score (r = −0.419; P = 0.000), as well as between GIQLI score and RFS score (r = −0.262; P = 0.026), were recognized in all patients.
Patients with a pathological result in MII‐pH showed a significant correlation between the values of salivary pepsin and the measurements of RSI score (r = 0.344; P = 0.046).
Furthermore, the pepsin test with the highest level out of three samples in each patient of both groups (LPR + non‐LPR) showed a significant correlation with the RFS score (r = 0.246; P = 0.043).
Correlation Between Pepsin in Saliva/Dx‐pH and HRM As Well As MII‐pH
Lower esophageal sphincter resting pressure (LESP) was significantly lower in the LPR group with a mean of 17.69 (±9.01) mmHg compared to non‐LPR group with a mean of 27.49 (±13.3) mmHg (P = 0.0001). All patients showed normal values of the upper esophageal sphincter pressure and integrated relaxation pressure as well as distal contractile integral. None of the patients presented higher‐grade esophageal motility disorders. There were no significant correlations between LESP measurements and the results of pepsin determination in saliva, as well as results of Dx‐pH (Ryan score in upright and supine position) and LESP measurements in both groups (P > 0.05). In addition, correlation analysis between the DeMeester score and salivary pepsin values as well as between pepsin values and the results of Ryan score (supine + upright) showed no significant correlations either (P > 0.05). Furthermore, there were no significant correlations between the mean pepsin values or the highest pepsin test out of three samples in the LPR‐ and non‐LPR group and the number of proximal reflux episodes (total count and separated acidic, weakly acidic, nonacidic events) measured by MII‐pH.
Oropharyngeal pH Monitoring
In summary, elevated Dx‐pH measurements showed no significant correlations with either the DeMeester score, RSI score, RFS score, GIQLI score, outcomes of HRM, or the results of pepsin measurement in saliva.
DISCUSSION
The accurate value of salivary pepsin and DX‐pH in the diagnosis of LPR remains controversial because of heterogeneous data and the lack of multiparameter prospective studies and adequate cutoff values.5, 27 For patients, who seem to suffer from LPR, no optimal cutoff values for a pepsin measurement with the Peptest (RDBiomed) exist thus far. The aim of our prospective trial was to close that gap. Thus far, this is the largest‐scale prospective study in which the diagnostic value of the Peptest (RDBiomed) and Restech Dx‐pH measurement system (Restech) for the objective diagnosis of LPR confirmed by MII‐pH is assessed.
To date, two studies in which the value of pepsin in saliva for the diagnosis of GERD has been assessed have provided cutoff values.7, 28
Hayat et al. recently reported that the optimal cutoff was at >210 ng/mL, showing a sensitivity of 44.0% and a specificity of 98.2% to diagnose patients with GERD.7
Du et al. stated that the Pepstest (RDBiomed) had a sensitivity of 73% and a specificity of 88.3 for diagnosing GERD using the optimal cutoff value of 76 ng/mL. The authors explained that the differences in their results from those in the study of Hayat et al. are due to use of a different study protocol and MII‐pH plus endoscopy to lower the rate of false negative results.28
A metanalysis performed by Wang et al. encompassing 11 studies described a moderate value of pepsin determination in saliva for the diagnosis of LPR, with a pooled sensitivity of 64% (95% CI 43 to 80%) and specificity of 68% (95% CI 55 to 78) due to heterogeneous study designs, lack of confirmation by reliable parameters such as results of MII‐pH, and different time points of pepsin collection.29
In addition, several studies showed promising results using Dx‐pH to determine whether extraesophageal symptoms can be attributed to GERD, whereas other studies have already reported the lack of correlation between Dx‐pH and catheter‐based MII‐pH during simultaneous measurements.30, 31, 32 Furthermore, Willhelm et al. already stated that, based on their measurements, Dx‐pH is not useful to guide any diagnostic or therapeutic decisions.10
The optimal cutoff value for pepsin in saliva of 216 ng/mL that we used to differentiate between patients with LPR and non‐LPR is quite similar to 210 ng/mL used by Hayet et al. to differentiate between patients with GERD and healthy subjects. Nevertheless, specificity (86.2% vs. 98.2%) of the Pepstest (RDBiomed) was lower in our study group compared to the results reported by Hayet et al., whereas sensitivity was quite similar (41.5% vs. 44.0%).7
This could be explained by the different study design and patient cohorts. It should be noted that both Hayat et al. and Du et al. have assessed the value of pepsin in saliva to diagnose GERD, whereas we are focusing hereto on patients with symptoms of LPR. We also hypothesized that atypical symptoms are a result of laryngeal or pharyngeal alterations due to increased stress with higher pepsin levels, although the patients had a normal DeMeester score. This can be underlined by the fact that the mean value of the RSI score in both study groups proved to be pathological. In addition, there were signs of reflux esophagitis seen in the LPR and non‐LPR group, which can be due to the fact that catheter‐based pH monitoring may fail to diagnose patients with GERD and patients may show a day‐to‐day variability during measurement.20, 33
There also might be some kind of silent reflux present that cannot be detected adequately by MII‐pH. Those combined reasons could explain the low sensitivity of the Peptest (RDBiomed) of 41.5% in our patient cohort.
The results of our trial also showed that patients with LPR had a higher mean pepsin concentration out of three samples than patients with primarily LPR‐related symptoms and a normal DeMeester score. Furthermore, a significant correlation between the values of salivary pepsin and the measurements of RSI score in patients with a pathological result in MII‐pH was recognized, and significantly more patients in the LPR group showed a pathological result in the RSI score compared to the non‐LPR group. Based on these findings, a combined application of the cutoff value for pepsin in saliva of 216 ng/mL and the use of the RSI score could increase the specificity and sensitivity of those diagnostic tests to discriminate patients with LPR in clinical practice. This would be a useful, noninvasive, and inexpensive option to diagnose LPR. In addition, the fact that the pepsin test with the highest level in each patient showed that a significant correlation with the RFS score could also allow a combined multiparameter approach of the Peptest (RDBiomed), RSI score, and RFS score to diagnose LPR.
Further specific research is necessary to prove that hypothesis.
Na et al. reported the best moment to determine the presence of pepsin in saliva, showing the highest values was upon waking.34 The results of our study cannot confirm this observation. Our patients showed the highest values of pepsin after lunch and dinner, which can be explained by the fact that heartburn likewise generally occurs 1 or 2 hours after a meal.35 Furthermore, based on the evidence above, it seems that postprandial salivary samples may have a more powerful ability to differentiate GERD patients from non‐GERD patients as well as patients with LPR from non‐LPR. For that reason, the mean value of three samples was used to perform correlation analysis in our trial.
Moreover, the pepsin level had no influence on the esophageal motility, which our results share with those of previous studies.28
All in all, the results of our study underline the role of pepsin in the pathophysiology of laryngopharyngeal/extraesophageal reflux symptoms and encourage performing further research.
Nevertheless, correlation analysis between results of Dx‐pH and measurements of objective parameters such as MII‐pH, pepsin in saliva, and RFS score, as well as subjective parameters such as RSI and GIQLI, was not conclusive in our patient cohort.
These findings underline the results of previous published trials10, 30, 31, 32; therefore, Dx‐pH may have no value in the diagnosis of patients with LPR.
Limitations of our study are the lack of follow‐up data to assess treatment outcomes after diagnostic decision based on salivary pepsin testing and that standardization of meals was not included. The patients were only asked to have three main meals so we could best simulate the patient's real life.
CONCLUSION
The results of this study show that salivary pepsin could be an alternative, cost‐effective, noninvasive measurement tool to assist office‐based diagnosis of LPR, whereas Dx‐pH appears not to be an adequate test. However, larger controlled trials are required to reach more definite conclusions.
ACKNOWLEDGMENT
We want to thank Astrid Reizner and Friedrich Radlmair from the laboratory of the Department of Nuclear Medicine, Ordensklinikum Linz Sisters of Charity Hospital for analyzing the saliva samples. We also want to thank Mag. Christian Steinlechner for statistical support.
Editor's Note: This Manuscript was accepted for publication on September 5, 2019.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1. Hammer HF. Reflux associated laryngitis and laryngopharyngeal reflux: a gastroenterologist's point of view. Dig Dis 2009;27:14–17. [DOI] [PubMed] [Google Scholar]
- 2. Campagnolo AM, Priston J, Heidrich Thoen R, et al. Laryngopharyngeal reflux: diagnosis, treatment and latest research. Int Arch Otorhinolaryngol 2014;18:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hom C, Vaezi MF. Extraesophageal manifestations of gastroesophageal reflux disease. Gastroenterol Clin North Am 2013;42:71–91. [DOI] [PubMed] [Google Scholar]
- 4. Koufman JA, Aviv JE, Casiano RR, Shaw GY. Laryngopharyngeal reflux: position statement of the committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology‐Head and Neck Surgery. Otolaryngol Head Neck Surg 2002;127:32–35. [DOI] [PubMed] [Google Scholar]
- 5. Lechien JR, Akst LM, Hamdan AL, et al. Evaluation and management of laryngopharyngeal reflux disease: state of the art review. Otolaryngol Head Neck Surg 2019;160:762–782. [DOI] [PubMed] [Google Scholar]
- 6. Postma G. Ambulatory pH monitoring methodology. Ann Otol Rhinol Laryngol 2000;109:10–14. [DOI] [PubMed] [Google Scholar]
- 7. Hayat JO, Gabieta‐Somnez S, Yazaki E, et al. Pepsin in saliva for the diagnosis of gastro‐oesophageal reflux disease. Gut 2015;64:373–380. [DOI] [PubMed] [Google Scholar]
- 8. Yuksel E, Slaughter JC, Mukhtar N, et al. An oropharyngeal pH monitoring device to evaluate patients with chronic laryngitis. Neurogastroenterol Motil 2013;25:315–323. [DOI] [PubMed] [Google Scholar]
- 9. Patel DA, Harb AH, Vaezi MF. Oropharyngeal reflux monitoring and atypical gastroesophageal reflux disease. Curr Gastroenterol Rep 2016;18:12. [DOI] [PubMed] [Google Scholar]
- 10. Willhelm D, Jell A, Feussner H, Schmid RM, Bajoubi M, Becker V. Pharyngeal pH monitoring in gastrectomy patients–what do we really measure? United European Gastroenterol J 2015;4:541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farhath S, He Z, Nakhla T, et al. Pepsin, a marker of gastric contents, is increased in tracheal aspirates from preterm infants who develop bronchopulmonary dysplasia. Pediatrics 2008;121:253–259. [DOI] [PubMed] [Google Scholar]
- 12. Stovold R, Forrest IA, Corris PA, et al. Pepsin, a biomarker of gastric aspiration in lung allografts: a putative association with rejection. Am J Respir Crit Care Med 2007;175:1298–1303. [DOI] [PubMed] [Google Scholar]
- 13. Crapko M, Kerschner JE, Syring M, Johnston N. Role of extra‐esophageal reflux in chronic otitis media with effusion. Laryngoscope 2007;117:1419–1423. [DOI] [PubMed] [Google Scholar]
- 14. Knight J, Lively MO, Johnston N, Dettmar PW, Koufman JA. Sensitive pepsin immunoassay for detection of laryngopharyngeal reflux. Laryngoscope 2005;115:1473–1478. [DOI] [PubMed] [Google Scholar]
- 15. Yates DH, Krishnan A, Chow S, Thomas PS. Non‐invasive assessment of exhaled biomarkers in lung transplantation. J Breath Res 2011;5:024001. [DOI] [PubMed] [Google Scholar]
- 16. Kahrilas PJ, Bredenoord AJ, Fox M; International High Resolution Manometry Working Group, et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eypasch E, Williams JI, Wood‐Dauphinee S, Ure BM, Schmulling C, Neugebauer E, Troidl H. Gastrointetinal quality of life index: development, validation and application of a new instrument. Br J Surg 1995;82:216–222. [DOI] [PubMed] [Google Scholar]
- 18. Fuchs KH, Feussner H, Bonavina L, Collard JM, Coosemans W. Current status and trends in laparoscopic antireflux surgery: results of a consensus meeting. The European Study Group for Antireflux Surgery (ESGARS). Endoscopy 1997;29:298–308. [DOI] [PubMed] [Google Scholar]
- 19. Koch OO, Kaindlstorfer A, Antoniou SA, Spaun G, Pointner R, Swanstrom LL. Subjective and objective data on esophageal manometry and impedance pH monitoring 1 year after endoscopic full‐thickness plication for the treatment of GERD by using multiple plication implants. Gastrointest Endosc 2013;77:7–14. [DOI] [PubMed] [Google Scholar]
- 20. Zerbib F, Roman S, Ropert A, et al. Esophageal pH‐Impedance monitoring and symptom analysis in GERD: a study in patients off and on therapy. Am J Gastroenterol 2006;101:1956–1963. [DOI] [PubMed] [Google Scholar]
- 21. Shay S, Tutuian R, Sifrim D, et al. Twenty‐four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol 2004;99:1037–1043. [DOI] [PubMed] [Google Scholar]
- 22. Zerbib F, Bruley des Varannes S, Roman S, et al. Normal values and day to day variability of 24‐h ambulatory oesophageal impedance‐pH monitoring in a Belgian‐French cohort of healthy subjects. Aliment Pharmacol Ther 2005;22:1011–1021. [DOI] [PubMed] [Google Scholar]
- 23. Belafsky PC, Postma GN, Koufman JA. Laryngopharyngeal reflux symptoms improve before changes in physical findings. Laryngoscope 2001;111:979–981. [DOI] [PubMed] [Google Scholar]
- 24. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice 2002;16:274–277. [DOI] [PubMed] [Google Scholar]
- 25. Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope 2001;111:1313–1317. [DOI] [PubMed] [Google Scholar]
- 26. Ayazi S, Lipham JC, Hagen JA, et al. A new technique for measurement of pharyngeal pH: normal values and discriminating pH threshold. J Gastrointest Surg 2009;13:1422–1429. [DOI] [PubMed] [Google Scholar]
- 27. Patel DA, Harb AH, Vaezi MF. Oropharyngeal reflux monitoring and atypical gastroesophageal reflux disease. Curr Gastroenterol Rep. 2016;18:12. [DOI] [PubMed] [Google Scholar]
- 28. Du X, Wang F, Hu Z, et al. The diagnostic value of pepsin detection in saliva for gastro‐esophageal reflux disease: a preliminary study from China. BMC Gastroenterol. 2017;17:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang J, Zhao Y, Ren J, Xu Y. Pepsin in saliva as a diagnostic biomarker in laryngopharyngeal reflux: a meta‐analysis. Eur Arch Otorhinolaryngol 2018;275:671–678. [DOI] [PubMed] [Google Scholar]
- 30. Becker V, Graf S, Schlag C, et al. First agreement analysis and day‐to‐day comparison of pharyngeal pH monitoring with pH/impedance monitoring in patients with suspected laryngopharyngeal reflux. J Gastrointest Surg 2012;16:1096–1101. [DOI] [PubMed] [Google Scholar]
- 31. Mazzoleni G, Vailati C, Lisma DG, Testoni PA, Passaretti S. Correlation between oropharyngeal pH‐monitoring and esophageal pH‐impedance monitoring in patients with suspected GERD‐ related extra‐esophageal symptoms. Neurogastroenterol Motil 2014;26:1557–1564. [DOI] [PubMed] [Google Scholar]
- 32. Desjardin M, Roman S, des Varannes SB, et al. Pharyngeal pH alone is not reliable for the detection of pharyngeal reflux events: a study with oesophageal and pharyngeal pH‐impedance monitoring. United European Gastroenterol J 2013;1:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wiener GJ, Morgan TM, Copper JB, et al. Ambulatory 24‐hour esophageal pH monitoring. Reproducibility and variability of pH parameters. Dig Dis Sci 1988;3:1127–1133. [DOI] [PubMed] [Google Scholar]
- 34. Na SY, Kwon OE, Lee YC, Eun YG. Optimal timing of saliva collection to detect pepsin in patients with laryngopharyngeal reflux. Laryngoscope 2016;126:2770–2773. [DOI] [PubMed] [Google Scholar]
- 35. Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24‐hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101:(suppl 53):1–78. [DOI] [PubMed] [Google Scholar]


