Abstract
Background
Measuring temperature has always been a key observation in the diagnosis of infection. No studies have examined the usefulness of measuring temperature at the wrist to detect infection.
Aim
We sought to determine whether a watch measuring wrist temperature could accurately identify patients who are infected.
Methods
Prospective cross‐sectional pilot study of temperature monitoring in an unselected patients in a tertiary referral adult nephrology unit.
Results
One hundred and four data recording sessions revealed 88 useful data sets, with recording failures in the others. Patients were retrospectively classified as having no infection (Group A, n = 60), clinically diagnosed infection with less than 24 h of treatment with antibiotics (Group B, n = 5), and clinically diagnosed infection with greater than 24 h on antibiotics (Group C, n = 23). There was a significantly higher average maximum temperature in Group B (mean (SEM)) 38°C (0.6) compared with Groups A (36.1°C (0.1)) and C (36.3°C (0.3)). Based on receiver operating characteristics (ROC) a cut‐off temperature of ≥37.5°C gave sensitivity 80% and specificity 98%. Mean electrodermal activity was significantly higher in Groups B and C.
Conclusions
ROC of peripheral skin temperature measurements suggest that such a device may identify many patients requiring treatment for infection. This proof of principle study showed value in using a wearable device in the detection of infection and its potential as an early warning or monitoring device.
Keywords: temperature, fever, infection, monitoring, sepsis
Introduction
In many countries, infection is second only to cardiovascular disease as the cause of death in patients with kidney disease.1 Increased susceptibility to infection may be due to multiple comorbidities, defective phagocytic function, older age, high prevalence of diabetes mellitus as well as the repetitive exposure to infectious risks during dialysis or immunosuppression in renal transplantation and treatment of other immune‐based diseases.2, 3
Early identification and treatment of infection has been shown to reduce mortality,4, 5 and is now recognised as a time‐sensitive emergency, with early antibiotic therapy for bacterial sepsis saving lives. Yet diagnosis remains challenging, especially early in the illness. Physicians rely on relatively nonspecific symptoms, intermittently recorded temperatures and abnormal laboratory values to identify patients.
From the time temperature measurement was first introduced following the detailed reports of Carl Wündelich in the mid‐19th century,6 physicians have focussed on the presence or absence of fever based on a single time point measurement. Increasing evidence suggests that continuous measurements or variability in the patterns of physiological measures may be more specific for infection and an earlier indicator of sepsis.7
Temperature monitoring can now be performed using wearable technology and we set out to assess the feasibility of using a clinical grade device measuring peripheral temperature to detect infection in patients with kidney disease. The secondary objective was to evaluate whether peripheral temperature and other physiological variables could be used as an index of infection, potentially leading to an automated device that might alert an at risk non‐medically trained user to infection in order to seek help before it became clinically apparent.
Methods
This study was performed in accordance with the National Statement on Ethical Conduct in Human Research and the Note for Guidance on Good Clinical Practice. The Melbourne Health Human Research Ethics Committee approved the study (HREC 2017.047) and all participants provided written informed consent.
This was a single centre prospective cross‐sectional pilot study conducted at The Royal Melbourne Hospital on an unselected population of patients admitted to the adult nephrology ward and included patients with chronic kidney disease (CKD), on dialysis and with kidney transplants. Patients admitted between August 2017 and March 2018 were approached for inclusion selected by convenience between Monday and Friday irrespective of the time of admission. Participants with kidney disease aged over 18 years were eligible and the only exclusion criterion was pregnancy.
Participant characteristics were recorded at the time of study recruitment; age, gender, CKD stage and renal replacement therapy modality (haemodialysis, peritoneal dialysis or transplant). After obtaining informed consent the patient was requested to wear a fully charged wearable device (WD) on the wrist for the duration of the battery life, removing it only for showering or during surgical procedures.
Infection was retrospectively determined by examining the hospital record and supporting microbiological data, and antibiotic usage was noted from the drug chart and medical history. Participants were classified into one of three categories: No infection at the start of monitoring (Group A), infection but with less than 24 h of antibiotics treatment (Group B) or infection with more than 24–168 h on antibiotics (Group C). Any antibiotic taken more than 1 week prior to admission (as far as could be ascertained from the history) was disregarded.
Participants wore a clinical grade WD (Empatica E4 Watch, Empatica, Milan, Italy) at the wrist and we pre‐specified that this should be for a minimum of 4 h. An oral temperature was recorded up to three times within the first 20 min of wearing the WD with a hospital grade sublingual thermometer. The Empatica device sampled temperature (T) at 4 Hz through an infrared thermopile as well as recording heart rate (HR) and electrodermal activity (EDA) (electrical skin conduction measured at the wrist and related to the moisture content between two contacts on the WD). The watch was removed during showering and surgical procedures, and data from these periods were discarded. At the end of the monitoring period, data from each device were downloaded and stored as a comma separated values file (csv). The csv data files were loaded into a pandas data structure, to allow for the different sampling frequencies of the different variables. Data were cleaned using a programme written in Python 3.7.0 0 (Python Software Foundation, Wilmington, DE, USA) and run on a Jupyter notebook. The data were cleaned to exclude temperatures less than 31°C as these temperatures were likely recorded when the watch had been removed or was not making good skin contact and since patients were admitted, the hospital ambient temperature never exceeded this temperature. One‐way analysis of variance followed by Dunnett's multiple comparisons test was performed using GraphPad Prism version 8.0.0 for Mac (GraphPad Software, San Diego, CA, USA; www.graphpad.com). Tests were two‐tailed, and P‐values less than 0.05 were considered statistically significant.
Results
One hundred and four recording sessions were performed from 91 unique individuals and some patients had repeated admissions.
While the theoretical maximum battery life of the WD after one charge was approximately 3 days, in practice we rarely obtained more than 44 h worth of data before the battery was exhausted. Eighty‐eight temperature data sets were eventually available for analysis.
There were 16 data sets from 15 patients (8 HD, 4 Tx, 2 CKD, 1 PD) where data were excluded due to recording errors, these recording episodes were categorised as 10 Group A, 0 Group B, 6 Group C.
The reasons for these errors we believe were mainly device failure, but cannot exclude poor skin contact or inadvertent participant intervention to turn off the WD. There were some missing data in other variables.
Demographic data and results from the study are summarised in Table 1. The median age of study participants was 54 years (IQR 19–88) and 42.5% were female. There were 60 data sets with no infection (Group A), 5 data sets with infection less than 24 h on antibiotics (Group B) and 23 data sets with infection greater than 24 h on antibiotics (Group C). The median C‐reactive protein (CRP) was not normally distributed and has been log transformed and is significantly different between groups (P < 0.0001). All patients received standard hospital care which in many cases included the antipyretic, paracetamol. The median study recording time for all participants was 41.0 h (IQR 26.5–45.7 h). The maximum temperatures between groups were statistically significant (P < 0.001) (Fig 1) but with no statistically significant difference in the mean temperature. Post hoc Tukey's Honest Significant Difference analysis showed that the average maximum temperature of Group B (infection <24 h on antibiotics) was significantly higher when compared with Groups A and C. Using a cut off of 37°C we identified 4/5 (80%) of the infected patients and 3/60 (5%) of the uninfected patients, the receiver operating characteristic (ROC) analysis of temperature sensitivity and specificity is shown in Figure 2. One false positive was a day 2 post‐renal transplant, which reached a maximum of 39.9°C on WD. A fever was also noted on the observation chart, but resolved spontaneously and did not recur. This patient was treated only with paracetamol and the CRP was 3 mg/L. The other two false‐positives were in patients admitted with shortness of breath, their watch temperature maximums were 37.4 and 37.2°C on the watch with corresponding CRP values of 27 and 8 mg/L respectively. In both cases their fever defervesced with paracetamol or time and they had a clinical illness consistent with viral upper respiratory tract infection and were therefore not treated with antibiotics. Average EDA was also higher in Groups B and C compared with Group A.
Table 1.
| Group A | Group B | Group C | P | |
|---|---|---|---|---|
| Demographics | ||||
| Number of recordings | 60 | 5 | 23 | |
| Age, years (mean (SEM)) | 54 (4) | 50(9) | 59 (3) | |
| M:F | 30:30 | 5:00 | 13:10 | |
| Infection diagnosis | N/A | Urosepsis (2), biliary sepsis (1), line‐related bacteraemia (1), gastroenteritis (1) | Pneumonia (5), urosepsis (4), PD peritonitis (4), soft tissue infection (3), line‐related bacteraemia (2), osteomyelitis (1), pericarditis (1), diverticulitis (1), empirical (1) | |
| Haemodialysis | 20 | 1 | 9 | |
| Peritoneal dialysis | 8 | 0 | 5 | |
| Chronic kidney disease | 6 | 1 | 4 | |
| Renal transplant | 26 | 3 | 5 | |
| C‐reactive protein (CRP) | ||||
| CRP median (IQR) | 9 (5–39) | 150 (87–361) | 61 (22–113) | <0.0001† |
| CRP date ± days (SE) | 5 (1) | 1 (1) | 1 (1) | (A vs B and A vs C) |
| Missing values | 9 | 0 | 2 | |
| Temperature (°C) | ||||
| 25% temperature (mean (SEM)) | 33.2 (0.1) | 33.5 (0.6) | 33.1 (0.2) | ns |
| Median temperature | 34.0 (0.1) | 34.4 (0.4) | 33.9 (0.2) | ns |
| Mean temperature | 33.9 (0.1) | 34.5 (0.4) | 33.8 (0.2) | ns |
| 75% temperature | 34.7 (0.1) | 35.3 (0.2) | 34.7 (0.2) | ns |
| Maximum temperature | 36.1 (0.1) | 38.0 (0.6) | 36.3 (0.3) | <0.001 (A vs B) |
| Patients identified by T ≥ 37.0 | 3/60 | 4/5 | 7/23 | |
| % readings >37 (SEM) | <0.1 (0.0) | 4 (0.1) | 1.4 (0.1) | |
| Patients identified by T ≥ 37.5 | 1/60 | 4/5 | 4/23 | |
| % readings ≥37.5 | 0.0 (0.0) | 0.5 (0.2) | 0.3 (0.2) | |
| Electrodermal activity (EDA, microseimens) | ||||
| Average EDA (mean (SEM)) | 0.42 (0.05) | 0.91 (0.43) | 0.87 (0.31) | <0.001 (A vs B, A vs C) |
| Heart rate (HR) | ||||
| Average HR, bpm (SEM) | 84 (1) | 89 (5) | 81 (3) | ns |
| HR IQR, bpm (SEM) | 16 (1) | 17 (3) | 17 (3) | ns |
Wilcoxon rank test.
Figure 1.
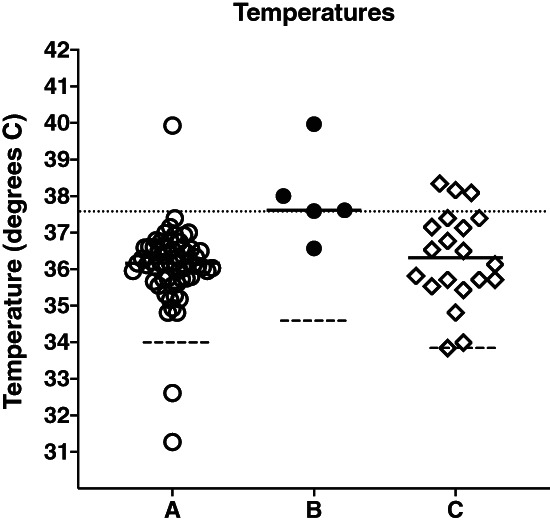
This graph plots the maximum temperatures recorded in each patient. Solid lines represent the mean of the maximum temperatures in each group. The dashed lines indicate the mean of all temperature values recorded in each group. A dotted line across the graph shows 37.5°C. Group B has significantly higher maximum temperatures (ANOVA, P < 0.002).
Figure 2.
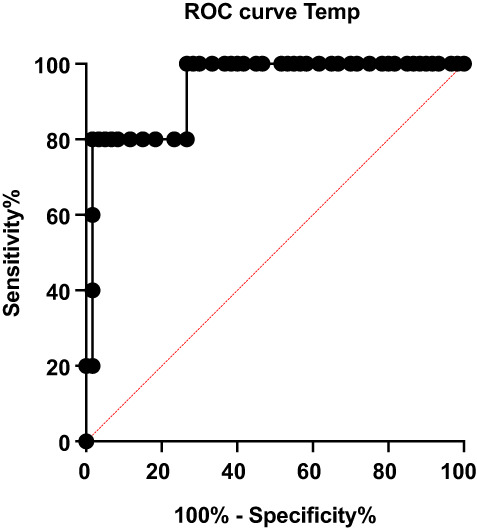
ROC analysis of maximum temperature showed an areas under the curve (AUC) of 0.9367 (95% CI 0.84–1.0), P < 0.01 (A vs B). Likelihood ratio of 48 at 37.5°C.
There was no statistical difference with respect to average, median or interquartile ranges of temperatures, or HR (Table 1). There was no relationship between oral temperature taken with a ward thermometer in the first 20 min of WD application and the average temperature recorded on the watch in the same timeframes (data not shown).
Discussion
Infection is an important cause of morbidity and mortality in patients with renal failure1, 3 and early aggressive treatment dramatically improves survival.5, 8 Strategies to enable earlier diagnosis of infection could have a significant impact on outcomes. The findings of this study provide an indication that continuous peripheral temperature monitoring might aid in the early detection and monitoring of infection. Measuring body temperature is one of the oldest clinical tools known to be helpful in diagnosing infection. Fever is often regarded as a dichotomous variable, where the presence or absence of fever may assist a diagnosis. However, body temperature patterns and variability may convey other meaningful clinical information regardless of the actual temperature. The American Infectious Diseases Society cites a threshold for a fever as a core temperature measurement of 38.3°C or higher,9 however measuring core temperature is often impractical. Varela et al.10 used approximate entropy and detrended fluctuation analysis methods to determine the complexity of temperature curves in different disease states. Papaioannou et al.11 studied temperature patterns using wavelet features for the differentiation of patients with systemic inflammatory response syndrome, sepsis and septic shock in an intensive care unit (ICU) setting. They compared results with the conventional Sequential Organ Failure Assessment score and found that the temperature curve complexity was inversely related to the severity of a patient's illness. More recently, Drewry et al.12 reported that abnormal body temperature patterns could act as a predictor of the diagnosis of sepsis in critically ill patients, again in an ICU setting.
In our current study, we sought to assess the feasibility of using a clinical grade watch‐like WD to measure peripheral body temperature to detect infection in unselected patients with kidney disease. We employed simple algorithms to see if WD measured peripheral body temperatures could be used to classify infection. We found that a larger percentage of temperature measurements over 37°C was associated with active infection. The single infected patient we failed to identify (false negative) was admitted 10 days after a renal transplant on high dose post‐transplant immunosuppression and was taking regular paracetamol. The difficulty in identifying patients with sepsis who are taking high dose steroids is well known, because while adrenal corticosteroids are probably required to generate a fever, large steroid doses inhibit temperature rise.13
Most of the key trials in continuous temperature monitoring have been conducted in the intensive care settings.10, 11, 12 Continuous temperature monitoring is potentially more useful in a ward setting or for patients at home as a tool for detecting infection before they develop symptomatic shock and end organ dysfunction requiring intensive and expensive support. This is the first study that the authors are aware of which investigates continuous temperature monitoring in patients with renal failure in an inpatient medical ward environment.
The WD measured peripheral temperature at the level of the wrist and so we had expected that temperature readings attained would be lower than that measured through the oral or tympanic route. We actually found little relation to such measurement. However, our purpose was not to measure or extrapolate the core temperature, but rather to analyse the thermoregulatory system by measuring the output pattern of one of its pathways.10, 14
Additionally, we sought to identify whether there was a significant correlation between infection status and variability in HR and EDA. Other studies have investigated the utility of measuring other physiological variables apart from temperature and how this correlated with the development of infection. Bravi and coworkers7 examined continuous HR monitoring to create a composite measure to track the HR variability during the development of sepsis in oncology patients with neutropenia. In our study, we found no statistically significant correlation between variability in HR and T in our participants with and without infection. Other studies utilised complex signal analysis to study physiological output patterns and such methodology has not been applied to our data.
This study has several limitations. The small sample and possible overrepresentation of transplant patients in our sample curtails our ability to make generalisations about our findings and validation with a more extended data set is a necessary future step. We were unable to include 20% of our participants' data in our final analysis due to several technical issues. This includes incomplete uploading of temperature or other data to the device software, non‐recording of the device, the device being worn incorrectly or being removed.
The watch once removed simply recorded ambient temperature and this was dealt with by simply ignoring values less than 31°C, this could be a problem where ambient temperatures are high. Detection of hypothermia was not tested in this study. Ambient room temperature did not seem to be an issue since all of our patients were hospitalised, and thus in a relatively temperature‐controlled environment, so this may not have been a major factor. In a home environment or other outpatient setting we recognise this could be more of a challenge. The fact that oral temperature bore no relationship with WD measured skin temperature at the wrist, could speak to the accuracy of measuring oral temperatures, the usefulness of single/few time point measurements or the validity of current observation charts. Given that we were able to detect patients who were infected with the WD we suggest that continuous wrist monitoring may be as good as, or perhaps better than, conventional recording of intermittent oral temperatures in a ward setting.
On average, EDA was higher in the infected Groups B and C. This finding has not been previously reported and is intriguing. However, EDA varied markedly within groups and we need to establish further the utility of this finding and its relation to sweating and infection, together with potential confounding factors such as washing.
Another issue we faced in this study was the battery life of the WD used, which was rarely able to record more than 44 h without requiring recharge. We are now working towards achieving a substantially longer recording cycle. Similarly, we expect reliability of the device to improve and real time telemetry could provide an early link to healthcare providers both in an in‐patient and an out‐patient setting. Simple pattern analysis may be more easily integrated into a practical, patient worn, continuous temperature monitoring device that might have a useful real‐world application.
We acknowledge a further limitation is possible misclassification of patients, the distinction being somewhat arbitrarily based upon clinical history and time on antibiotics. It may be more clinically relevant to conduct future studies monitoring patients out of hospital prospectively, and retrospectively review whether the monitoring was able to detect early signs of infection at presentation.
Conclusion
In this proof of principle study, we have concluded that peripheral temperature monitoring has the potential to identify patients with infection. We plan to develop a real time system for early detection of infection by continuously monitoring physiological variables that could provide an early marker of sepsis. This may not only give the user an early warning of impeding infection but may prevent much of the morbidity and mortality associated with delayed treatment of sepsis, and mitigate the significant costs associated with treatment of severe infection. We are working to improve the hardware and software aspects of this pilot study in order to conduct a prospective evaluation of this methodology and algorithm.
Acknowledgements
We are grateful for grant funding from The Victorian Department of Health and Human Services.
Funding: Funding for this study was obtained from the Victorian Department of Health and Human Services. The researchers were independent of the funding. The grant was used to fund the purchase of the wearable device and some study coordinator time.
Conflict of interest: None.
References
- 1. ANZDATA . ANZDATA Registry. 41st Report. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia; 2018.
- 2. Aslam N, Bernardini J, Fried L, Burr R, Piraino B. Comparison of infectious complications between incident hemodialysis and peritoneal dialysis patients. Clin J Am Soc Nephrol 2006; 1: 1226–33. [DOI] [PubMed] [Google Scholar]
- 3. Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end‐stage renal disease compared with the general population. Kidney Int 2000; 58: 1758–64. [DOI] [PubMed] [Google Scholar]
- 4. Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S et al Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34: 1589–96. [DOI] [PubMed] [Google Scholar]
- 5. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R et al Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017; 45: 486–552. [DOI] [PubMed] [Google Scholar]
- 6. MacKowiak PA, Morgan DJ, Huang E, Jordan SC. Feel the heat: a short history of body temperature. BMJ 2017; 14: j5697. [DOI] [PubMed] [Google Scholar]
- 7. Buchan CA, Bravi A, Seely AJEE. Variability analysis and the diagnosis, management, and treatment of sepsis. Curr Infect Dis Rep 2012; 14: 512–21. [DOI] [PubMed] [Google Scholar]
- 8. Nguyen HB, Corbett SW, Steele R, Banta J, Clark RT, Hayes SR et al Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med 2007; 35: 1105–12. [DOI] [PubMed] [Google Scholar]
- 9. O'Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC et al Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med 2008; 36: 1330–49. [DOI] [PubMed] [Google Scholar]
- 10. Varela M, Churruca J, Gonzalez A, Martin A, Ode J, Galdos P. Temperature curve complexity predicts survival in critically ill patients. Am J Respir Crit Care Med 2006; 174: 290–8. [DOI] [PubMed] [Google Scholar]
- 11. Papaioannou VE, Chouvarda IG, Maglaveras NK, Pneumatikos IA. Temperature variability analysis using wavelets and multiscale entropy in patients with systemic inflammatory response syndrome, sepsis and septic shock. Crit Care 2012; 16: R51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drewry AM, Fuller BM, Bailey TC, Hotchkiss RS. Body temperature patterns as a predictor of hospital‐acquired sepsis in afebrile adult intensive care unit patients: a case–control study. Crit Care 2013; 17: R200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schöbitz B, Holsboer F, Sutanto W, Gross G, Schönbaum ED, de Kloet ER. Corticosterone modulates interleukin‐evoked fever in the rat. Neuroendocrinology 1994; 59: 387–95. [DOI] [PubMed] [Google Scholar]
- 14. Varela M, Calvo M, Chana M, Gomez‐Mestre I, Asensio R, Galdos P. Clinical implications of temperature curve complexity in critically ill patients. Crit Care Med 2005; 33: 2764–71. [DOI] [PubMed] [Google Scholar]


