Abstract
Western flower thrip, Frankliniella occidentalis (Pergande), is among the most economically important agricultural pests globally, attacking a wide range of vegetable and horticultural crops. In addition to causing extensive crop damage, the species is notorious for vectoring destructive plant viruses, mainly belonging to the genera Orthotospovirus, Ilarvirus, Alphacarmovirus and Machlomovirus. Once infected by orthotospoviruses, thrips can remain virulent throughout their lifespan and continue transmitting viruses to host plants when and wherever they feed. These irruptive viral outbreaks in crops will permanently disrupt functional integrated pest management systems, and typically require a remedial treatment involving insecticides, contributing to further development of insecticide resistance. To mitigate against this continuing cycle, the most effective management is early and comprehensive surveillance of the pest species and recognition of plant viruses in the field. This review provides information on the pest status of F. occidentalis, discusses the current global status of the viruses vectored by this thrip species, examines the mechanisms involved in transmitting virus‐induced diseases by thrips, and reviews different management strategies, highlighting the potential management tactics developed for various cropping systems. The early surveillance and the utilization of potential methods for control of both F. occidentalis and viruses are proposed.
Keywords: global distribution, integrated pest management, invasion, thrips, viruses transmission
Introduction
Thrips (order Thysanoptera) are minute insects only a few millimeters or less in length. Of the approximately 5500 described species of thrips in the world, scarcely 1% are considered to be serious pests of commercial crops (Morse & Hoddle, 2006; Healey et al., 2017). Among these pests, several species stand out as being among the most important global agricultural pests. These include four of the major thrip pests, the western flower thrip, Frankliniella occidentalis Pergande, the onion thrip, Thrips tabaci Lindeman, the melon thrip, T. palmi Karny and the yellow tea thrip (chili thrip), Scirtothrips dorsalis Hood (Mound, 2002; Riley et al., 2018). F. occidentalis is a polyphagous and ubiquitous invader of key agri‐ and horticultural crops in diverse field and greenhouse environments. This is due to the damage caused directly by its feeding and oviposition, and indirectly through transmission of plant viruses, of which tomato spotted wilt orthotospovirus (TSWV) is the most economically important (Schneweis et al., 2017). F. occidentalis was first described in 1895 in California, USA, and beginning in the late 1970s has since become a major global pest (Kirk & Terry, 2003). This species has been the most intensively studied member of the order Thysanoptera since 1980, accounting for over one‐third of the publications based on this order (Reitz et al., 2011). F. occidentalis has continued its spread around the world and is now distributing in at least 57 countries (Fig. 1, Table S1). The spread of F. occidentalis and various vectored orthotospoviruses have frequently caused failure of established integrated pest management (IPM) systems for agricultural crops (Morse & Hoddle, 2006).
Figure 1.
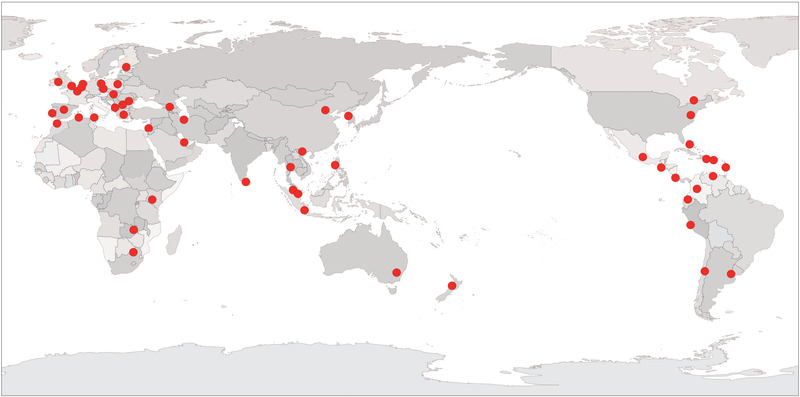
The worldwide distribution of Frankliniella occidentalis. GS (2019) 4551.
Phytophagous thrips have many traits that predispose them to be successful invaders, such as minute size, cryptic habits, high reproductive potential and high dispersal capability. F. occidentalis has superior or additional features, promoting their worldwide spread and sustained damage. The possible reasons for the start of the spread is intensive insecticide use in the 1970s and 1980s, which was reviewed by Kirk and Terry (2003). Biological factors facilitating invasion by thrips was reviewed by Morse and Hoddle (2006). Biological processes and molecular interactions involved in the virus acquisition and transmission by thrips was reviewed by Whitfield et al. (2005a). Over a decade after these reports, we aim to provide a summary of the extraordinary attributes that make for a successful invader with major economical damage potential. We have reconstructed in a chronological order the current global distribution of F. occidentalis, as well as several viruses transmitted. We have also summarized the control strategies based on IPM of F. occidentalis, stressing the recent progress in biological control.
Biology and ecology
F. occidentalis possesses several biological and ecological characteristics that enable it to become a dominant thrip species in many of the areas it has invaded. Its short generation time and high reproductive potential, often with a predisposition to parthenogenesis, enhances the likelihood of establishment (Kirk & Terry, 2003; Zhang et al., 2010); its cryptic behavior and high level of vagility enable it to disperse to a wide variety of crops (Cloyd, 2009; Reitz, 2009); its polyphagous nature likely supplements its predisposition to evolve resistance to many classes of insecticides through metabolic detoxification pathways (Demirozer et al., 2012); its widespread resistance to most major insecticides, in turn, makes it difficult to control (Bielza, 2010; Gao et al., 2012a); its highly efficient exploitation of food sources provides it with a competitive advantage over indigenous species and enables it to become successfully established in new regions (Morse & Hoddle, 2006; Demirozer et al., 2012). However, its propensity to transmit viruses often results in serious losses in a wide range of crops (Wijkamp et al., 2010; Ogada & Poehling, 2015). In most cases, combinations of these attributes contribute to its high invasion success, ultimately resulting in severe economic damage to crops throughout the world.
Viruses transmitted by F. occidentalis
Thrips are the only known vectors of orthotospoviruses, but only 0.16% of the known species have been implicated in their transmission (Mound, 2004). Thrips transmit viruses belonging to at least four virus groups, including ilarviruses, machlomoviruses, alphacarmoviruses and orthotospoviruses (Fig. 2, Table S2). (Jones, 2005; Morse & Hoddle, 2006). Using TSWV as an example, this particular virus, which is one of the most economically important members of the genus Orthotospovirus (Tospoviridae) (Mumford et al., 1996), has long been associated with F. occidentalis – one of the most important and efficient vector thrips (Wijkamp, 1995a; Arthurs et al., 2018a). TSWV acquisition by F. occidentalis is a developmental‐stage dependent process, with the 1st instar larval stage considered as the most susceptible phase (Rotenberg et al., 2015). The interactions between TSWV and F. occidentalis and its dissemination route in thrips has been thoroughly reviewed by Whitfield et al. (2015a), Rotenberg et al. (2015) and Dietzgen et al. (2016). TSWV is acquired by the thrips’ stylets and travels across the alimentary canal to the anterior region of the midgut (MG), where the surface glycoproteins, Gn and Gc, facilitate its entrance into the thrips’ MG (Whitfield et al., 2004, 2005b). Subsequently, TSWV replicates and accumulates in the visceral muscles of the gut, later spreading back into the salivary glands through the connected ligaments, and is then transmitted to the plant by the stylets (Fig. 3). Abe et al. (2011) suggested that TSWV infection facilitates the production of thrips of the next generation, which will contribute to further spread of TSWV. Stafford et al. (2011) reported that TSWV infection directly influences the feeding behavior of thrips, and enhances the transmission efficiency of the virus, whereas, viruses such as ilarviruses are also thought to be transmitted very transiently by F. occidentalis (Aramburu et al., 2010), where the transmission starts when the thrips feed on virus‐laden pollen and ends once the virus‐laden pollen is gone.
Figure 2.
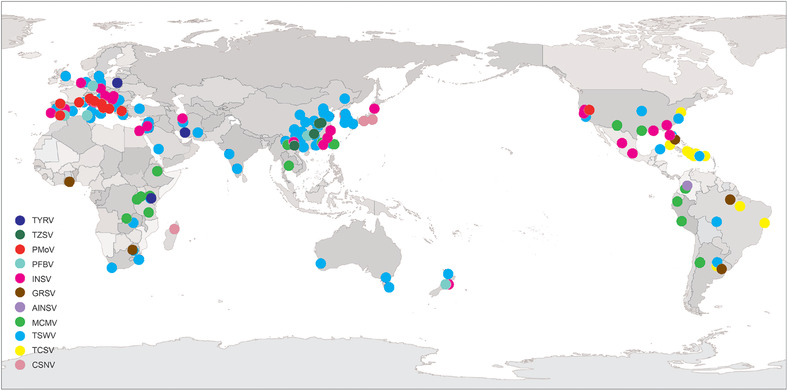
The worldwide distribution of viruses transmitted by Frankliniella occidentalis. The viruses shown in the map are: AlNSV, alstroemeria necrotic streak orthotospovirus; CSNV, chrysanthemum stem necrosis orthotospovirus; GRSV, groundnut ringspot orthotospovirus; INSV, impatiens necrotic spot orthotospovirus; TCSV, tomato chlorotic spot orthotospovirus; TSWV, tomato spotted wilt orthotospovirus; TYRV, tomato yellow ring virus; TZSV, tomato zonate spot orthotospovirus; PMoV, parietaria mottle virus; PFBV, pelargonium flower break virus; MCMV, maize chlorotic mottle virus. Arcgis 10.0 software was used to create the map. GS (2019) 4551.
Figure 3.
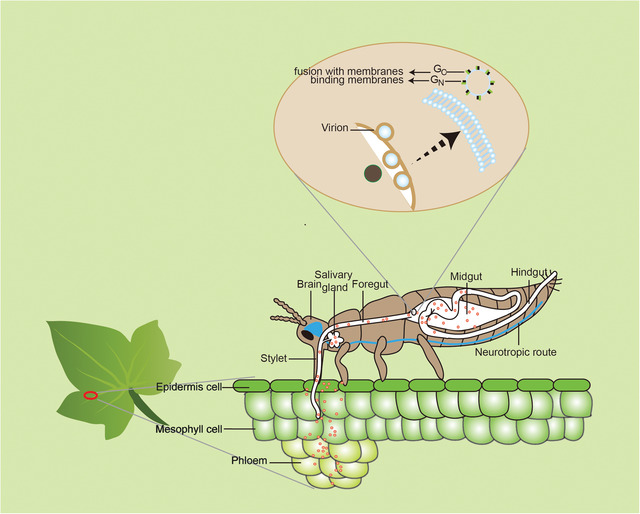
Virus localization sites in Frankliniella occidentalis. Viruses are initially acquired through the stylets. They then travel across the alimentary canal to the anterior region of the midgut (MG), where the surface glycoproteins, Gn and Gc, facilitate their entrance into the thrips’ MG. Subsequently, the viruses replicate, accumulate in the visceral muscles of the gut, and then spread to the salivary glands through the connective ligaments, where they are then transmitted back to the plants through the stylets.
Current global status of the viruses transmitted by F. occidentalis
Presently, a total of 11 viruses have been reported vectored by F. occidentalis. These include eight species in the genus Orthotospovirus (Tospoviridae): alstroemeria necrotic streak orthotospovirus (AlNSV), chrysanthemum stem necrosis orthotospovirus (CSNV), groundnut ringspot orthotospovirus (GRSV), impatiens necrotic spot orthotospovirus (INSV), tomato chlorotic spot orthotospovirus (TCSV), TSWV, tomato yellow ring virus (TYRV), and tomato zonate spot orthotospovirus (TZSV); parietaria mottle virus (PMoV) in the genus Ilarvirus (Bromoviridae); and pelargonium flower break virus (PFBV) in the genus Alphacarmovirus, and maize chlorotic mottle virus (MCMV) in the genus Machlomovirus (both in Tombusviridae) (Fig. 2, Table S2). The global distributions, hosts, emergence and dissemination of these viruses are discussed below.
AlNSV
AlNSV was first described in Colombia, when it was found to cause necrotic streaks on the leaves of Peruvian lilies (Alstroemeria sp.) (Hassani‐Mehraban et al., 2010). According to the nucleocapsid (N) protein gene sequence, phylogenetic analysis revealed that AlNSV clustered with those orthotospoviruses from the American continent into a single lineage with a significantly close serological relationship (Hassani‐Mehraban et al., 2010; Liu et al., 2017). Similar to other reference orthotospoviruses, AlNSV is capable of infecting ornamentals as well as vegetables locally or systemically, and is transmitted by F. occidentalis under experimental conditions (Hassani‐Mehraban et al., 2010).
CSNV
CSNV was first identified from chrysanthemums in Brazil in 1996 (Resende et al., 1996), followed by the Netherlands, Slovenia, UK, Japan and South Korea (Resende et al., 1996; Verhoeven et al., 1996; Mumford et al., 2003; Ravnikar et al., 2003; Okuda et al., 2013; Yoon et al., 2017a). In Brazil, CSNV also infects tomatoes with necrosis and necrotic spots on the stem and leaves, showing symptoms similar to those seen in chrysanthemums (Bezerra et al., 1999; Nagata et al., 2004). F. schultzei and F. intonsa have also been identified as vectors of CSNV although their efficiencies as vectors are much lower than F. occidentalis (Nagata et al., 2004; Okuda et al., 2013).
GRSV
GRSV was first described from South Africa and Brazil from peanuts and tomatoes, respectively (Wijkamp, 1995b; Pappu et al., 2009). It was subsequently reported from Argentina, the USA, the Caribbean basin and Ghana from a relatively narrow host range compared to TSWV (Webster et al., 2010; Webster et al., 2011; Camelo‐Garca et al., 2014; Spadotti et al., 2014; Leão et al., 2015; Webster et al., 2015; Appiah et al., 2016). In Brazil, GRSV was identified from several hosts including sweet peppers, coriander, cocona, cucumbers, cubiu, peanuts and watermelons (Lima et al., 1999; Boari et al., 2002; Camelo‐Garca et al., 2014; Spadotti et al., 2014; Leão et al., 2015). In North America, GRSV was initially reported from tomatoes in south Florida in 2009, subsequently from peppers, tomatilloes and eggplants in peninsular Florida, and later in South Carolina and New York (Webster et al., 2010, 2011, 2015). Interestingly, a reassortant isolate GRSV‐LGMTSG, composed of the L and S RNAs from GRSV and the M RNA from TCSV, was reported from tomatoes in Florida in 2010 (Webster et al., 2011). The recent outbreaks of GRSV in Brazil and North America were probably driven by its major thrip vectors, F. occidentalis, F. schultzei, and F. gemina (Pappu et al., 2009; Gilbertson et al., 2015; Webster et al., 2015). Among the three species involved, F. schultzei, the local species from Brazil and North America, has a more efficient transmission than the other two thrip species (Nagata et al., 2004; Gilbertson et al., 2015; Webster et al., 2015).
INSV
INSV, which is considered to be an important pathogen of ornamental crops, was initially characterized and distinguished from New Guinea impatiens in the Netherlands in the late 1980s (Ávila et al., 1992). It is now widespread throughout much of the world (Vaira et al., 1993; Peters et al., 1996; Lebas & Ochoa‐Corona, 2007; Pappu et al., 2009). In northern Africa, the Middle East, Southeast Asia, southern New Zealand, the Caribbean and Central America, INSV has been reported from numerous field and greenhouse‐grown ornamentals, including freesia, impatiens, lobelia, primula, ranunculus, begonia, chrysanthemum, and so on, (Lebas et al., 2004; Jones, 2005; Lebas & Ochoa‐Corona, 2007; Pappu et al., 2009), as well as a number of weed species (Okuda et al., 2010). Traditionally, INSV was also believed to be a pathogen on some vegetable crops, although it is only capable of causing limited local symptoms or is symptomless on sweet peppers, pepinos, spinach, tomatoes and cucumbers (Verhoeven & Roenhorst, 1998; Vicchi et al., 1999; Sialer & Gallitelli, 2000; Mavric & Ravnikar, 2001). However, INSV has recently emerged as an important pathogen of lettuce caused by F. occidentalis transferring from ornamental hosts in coastal regions of California (Pappu et al., 2009; Kuo et al., 2014; Gilbertson et al., 2015). In addition to F. occidentalis, INSV can also be transmitted by F. intonsa and F. fusca, but with a lower efficiency (Naidu et al., 2001; Sakurai et al., 2004).
TCSV
In 1990, TCSV was first characterized as a distinct serotype of TSWV from tomatoes in Brazil (De Avila et al., 1990, 1993). Subsequently, TCSV was isolated from sweet peppers, potatoes, endives, celery, lisianthus and various weeds with mosaic, necrosis, chlorotic or stunting symptoms in Argentina and Brazil (Boiteux et al., 1993; Gracia et al., 1999; Colariccio et al., 2001a; Dal Bio et al., 2001; Eiras et al., 2002), and from outbreaks on lettuce and gilo in Brazil (Colariccio et al., 2001b; Rabelo et al., 2002). In the USA, TCSV was first detected from tomatoes in south Florida in 2012 (Londoño et al., 2012), and then from lettuce, impatiens and peppers (Webster et al., 2015). In Puerto Rico, TCSV was also found from tomatoes, peppers, jimsonweed (Datura stramonium), and lettuce in 2013 (Estévez de Jensen et al., 2013; Estévez de Jensen & Adkins, 2014). More recently, TCSV was identified from tomatoes in the Dominican Republic (Batuman et al., 2014).
TSWV
At the beginning of the 20th century, spotted wilt disease of tomato was first described in Australia in 1915 (Brittlebank, 1919). Afterwards, it was considered as a viral disease caused by TSWV and transmitted by T. tabaci and F. schultzei (Pittman, 1927; Samue et al., 1930). Normally, TSWV‐infecting tomatoes shows bronzing, curling necrotic streaks and spots on the leaves, and a paler red or yellow skin color on the fruits. In addition to tomatoes, TSWV was subsequently isolated from many other plants such as aubergine, artichoke, bell peppers, cabbage, chrysanthemums, cowpeas, cucumbers, butternut squash, hot peppers, common beans, lettuce, petunias, papaya, peas, peanut, eggplants, pineapples, potatoes, strawberries, mangos, soybeans, celery, spinach, sweet peppers, broad beans, tobacco, cauliflower and assorted weeds (Chatzivassiliou et al., 2000a; Jones, 2005; Whitfield et al., 2005a; Reitz et al., 2011).
In Europe, TSWV was first described in England in 1929 from ornamental winter cherries (Solanum capsicastrum) with concentric ring symptoms on leaves (Smith, 1932). In the early 20th century, TSWV was transmitted mainly by T. tabaci. An obvious decline of TSWV‐infecting crops was evident when effective controls successfully managed this vector. When F. occidentalis extended its range to Europe, TSWV began to be a major threat to European horticultural crops (Jones, 2005). Today, TSWV is well established in almost all European countries, including Albania (Cota & Merkuri, 2004), Bulgaria (Dikova et al., 2013), Bosnia and Herzegovina (Trkulja et al., 2013), France (Marchoux et al., 1991), Greece (Chatzivassilou et al., 1996; Chatzivassiliou et al., 2000b), Hungary (Salamon et al., 2012), Montenegro (Zindović et al., 2011), Spain (Jorda, 1993; Aramburu et al., 1997), Portugal (Louro, 1996), and Slovenia (Mavric & Ravnikar, 2001). In Serbia, TSWV was isolated from Gerbera hybrida in 2009 (Stanković et al., 2011), onions, garlic and chrysanthemums in 2011 (Stanković et al., 2012, 2013), and Brugmansia sp. in 2012 (Nikolić et al., 2013).
In Africa, TSWV was first described from a wilt disease of tobacco in South Africa as early as 1905 (Moore, 1933), and later found in several provinces of the country infecting tobacco, tomatoes, peppers and potato crops (Moore & Andessen, 1939). After F. occidentalis was introduced into Africa, TSWV became widespread in other African countries (Moussa et al., 2000; Ben Moussa et al., 2005). Recently, TSWV has been found from Amaranthus thunbergii in South Africa (Kisten et al., 2016), butternut squash (Cucurbita moschata) and peppers in Zimbabwe in 2015 (Karavina et al., 2016a,b).
In Asia, TSWV was first recorded in the Middle Eastern countries. In July 1998, TSWV was identified from Pittosporum tobira shrubs with foliar ring spots, mild mosaic, and tip necrosis symptoms in a nursery in the Sharon Valley of Israel (Gera et al., 2000a), and later from several vegetables (Gera et al., 2000b). Similarly, the virus was also detected from potatoes in 1998 in Iran (Pourrahim et al., 2001), and subsequently from soybeans, tomatoes, and cucurbits (Golnaraghi et al., 2001; Massumi et al., 2007, 2009). TSWV has also been isolated from many important vegetable crops in Jordan and Lebanon (Anfoka et al., 2006; Abou‐Jawdah et al., 2006). More recently, TSWV was reported from lettuce showing necrotic lesions, necrosis of the lamina of the younger leaves, and leaf curling symptoms in March 2014 from the Al‐Uyaynah area, in the central region of Saudi Arabia. In eastern Asia, TSWV is now widely distributed in China, Korea, and Japan in a number of vegetable and horticultural crops such as celery, peppers, cowpeas, lettuce, Bidens pilosa, tomatoes, potatoes, Brugmansia suaveolens, Eustoma grandiflorum, and miscellaneous other wild plant species (Choi et al., 2004; Zheng et al., 2010; Reitz et al., 2011; Okazaki et al., 2007, 2011; Choi & Choi, 2015; Li et al., 2015; Xiao et al., 2016; Yoon et al., 2017b). In India, TSWV was found on sunflowers exhibiting severe mosaic, systemic necrosis, leaf distortion, and ringspots symptoms in Tirupati in January 1998 (Subbaiah et al., 2000), and more recently from chrysanthemums grown in the Nilgiris district of Tamil Nadu State in August 2013 (Renukadevi et al., 2015).
In America and the Caribbean, TSWV was first discovered from pineapples causing yellow spot disease as early as 1926 in Hawaii (Kucharek et al., 2000). In the 1970s, the virus was reported from peanuts in Texas (Haliwell & Philley, 1974), and then from peppers, tobacco and tomatoes in Georgia and other areas of the southeastern USA in the mid‐1990s (Culbreath et al., 1991). In the latest 20 years, TSWV has become widespread throughout most of the states in the USA (Groves et al., 1998; Holcomb et al., 1999; Díaz‐Pérez & Pappu, 2000; Holcomb & Valverde, 2000; Momol et al., 2000; Adkins et al., 2003; Whitfield et al., 2003; Mullis et al., 2004; Yang et al., 2004; Adkins & Baker, 2005; Mullis et al., 2006; Nischwitz et al., 2006a,b; Baker et al., 2007; Baker et al., 2009; Barkley et al., 2009; Crosslin et al., 2009) causing significant economic losses (Pearce, 2005). More recently, TSWV was isolated from Stevia rebaudiana and tomatoes with the Sw‐5 orthotospovirus‐resistance gene in Carolina (Koehler et al., 2016; Batuman et al., 2017). TSWV was found in a commercial chrysanthemum field of Mexico infesting several weeds including Taraxacum officinale, Bidens sp., Reseda luteola, Mirabilis jalapa being transmitted by F. occidentalis (Martinez et al., 1999). In 2005 to 2006, tomatoes showing chlorosis, malformation of apical leaves, stunting, and ringspot lesions caused by TSWV were first noticed in the Baja California peninsula of Mexico (Holguín‐Peña & Rueda‐Puente, 2007). In the Dominican Republic, TSWV transmitted by F. occidentalis was found to be widely distributed in commercial peppers and tomatoes growing under protected greenhouse conditions (Martínez et al., 2014). In South America, tomato, pepper, and lettuce crops infected by TSWV were reported from Argentina, Brazil, Chile, and Venezuela causing a significant threat to the vegetable industry (Maluf et al., 1991; Gracia et al., 1999; EPPO, 2004; Lebas & Ochoa‐Corona, 2007; Rosales et al., 2007; Marys et al., 2014; Pérez‐Colmenares et al., 2015).
The first recognition that tomato spotted wilt disease was caused by TSWV occurred in Australia as early as 1915, although it was considered as an introduction from elsewhere following European colonization (Brittlebank, 1919; Pittman, 1927; Samuel et al., 1930; Smith, 1932). In Australia, TSWV was mainly transmitted by T. tabaci and F. shultzei on several vegetables with limited spread occurring over a span of many decades. When F. occidentalis was detected in southwestern Australia in 1993, an outbreak of TSWV was being reported in eastern and southeastern Australia (Latham & Jones, 1997; Wilson et al., 2000; Pappu et al., 2009). Similarly, TSWV was detected from tomatoes and other vegetables in New Zealand soon after its discovery on the Australian continent, and, at the time, was also transmitted by T. tabaci (Chamberlain & Taylor, 1936, 1938). More recently, a serious epidemic of TSWV on ornamental plants grown in greenhouses on the North Island was caused by F. occidentalis rather than T. tabaci (Fletcher et al., 2005; Pappu et al., 2009).
TYRV
TYRV, a tentative orthotospovirus species, is closely related to iris yellow spot virus. The virus was first identified from tomatoes in Teheran Province, Iran (Hassani‐Mehraban et al., 2005). Subsequently, TYRV was isolated from chrysanthemums, gazanias, potatoes, soybeans, and cineraria with high diversity in the N gene in Iran (Hassani‐Mehraban et al., 2005, 2007; Rasoulpour & Izadpanah, 2007). In 2012, TYRV was isolated from tomatoes with chlorotic ring spots on fruits and necrosis of stems and leaves in Kenya (Birithia et al., 2012). TYRV has now been found in Europe in tomato plants having symptoms of necrosis on leaves and stalks, and chlorotic and necrotic ringspots on fruits in Kujawsko‐Pomorskie Province, Poland (Zarzyńska‐Nowak et al., 2016).
TZSV
TZSV was first reported to naturally infect tomatoes, causing zoned ring spots on fruits in Yunnan Province, China (Dong et al., 2008). In Yunnan Province, TZSV was subsequently isolated from chili peppers (Capsicum annuum), peppers, tobacco, Iris tectorum, potatoes and several weeds, including Bidens pilosa and Rumex dentatus (Dong et al., 2010; Zheng et al., 2014; Huang et al., 2015; Liu et al., 2015; Wu et al., 2016a). During a survey from 2008 to 2010 in Guangxi Province, China, TZSV was also detected from tobacco. Infection symptoms included dwarfing, midrib browning, distorted apical buds, and concentric ringspot (Cai et al., 2011).
PMoV
PMoV, a member of the genus Ilarvirus, was originally isolated from the weed Parietaria officinalis in 1989 (Caciagli et al., 1989), and afterwards from tomato, Mirabilis jalapa, Capsicum annuum, Diplotaxis tenuifolia in Italy (Roggero et al., 2000; Parrella, 2002; Parrella et al., 2016, 2017). Besides Italy, PMoV has also been detected from tomatoes in France, Spain and Greece, and from Capsicum annuum in Spain (Ramasso et al., 1997; Roggero et al., 2000; Aramburu, 2001; Galipienso et al., 2005; Janssen et al., 2005).
PFBV
PFBV is a member of the genus Alphacarmovirus, affecting Pelargonium spp. which causes white flower streaking, chlorotic spotting of leaves and stunting on some cultivars. It was originally identified in Europe and has now spread throughout much of the world (Stone & Hollings, 1973; Bouwen & Maat, 1992; Blystad et al., 1995; Ivars et al., 2004; Rico et al., 2004; Rico & Hernández, 2006; Rico et al., 2006; Wei et al., 2015). It is primarily known for its detrimental effects on the production and quality of some Pelargonium spp. cultivars (Bouwen & Maat, 1992; Blystad et al., 1995; Krczal et al., 1995; Ivars et al., 2004; Wei et al., 2015). PFBV is frequently transmitted and dispersed by vegetative propagation and irrigation systems as well as by the western flower thrip, F. occidentalis (Krczal et al., 1995).
MCMV
MCMV, a member the genus Machlomovirus in the family Tombusviridae, was first identified from maize in the Americas including plants from Peru and the USA (Castillo‐Loayza, 1977; Niblett & Clafin, 1978; Jiang et al., 1990). In maize, MCMV is among the important pathogens that characteristically induce typical symptoms in the plants such as mosaicism, stunting and necrosis (Niblett & Clafin, 1978; Mahuku et al., 2015; Chen et al., 2017). MCMV, together with other maize‐infecting potyviruses, are responsible for inducing corn lethal necrosis disease, which was first described in Peru in 1974 and has since spread worldwide (Castillo‐Loayza, 1977; Niblett & Clafin, 1978; Morales et al., 1999; Adams et al., 2014; Deng et al., 2014; Lukanda et al., 2014; Gowda et al., 2015; Mahuku et al., 2015; Quito‐Avila et al., 2016; Chen et al., 2017). In addition to maize, MCMV also can infect sorghum, Coix seed and finger millet in several Asia and Africa regions, probably due to its diverse transmission methods including by seeds, mechanical inoculation, and insects including thrips and beetles (Jiang et al., 1990; Cabanas et al., 2013; Deng et al., 2014; Kusia et al., 2015; Achon et al., 2017; Chen et al., 2017).
In conclusion, from the emergence and dissemination of F. occidentalis and its transmitting viruses, we speculate that the western flower thrip has spread from its original distribution in western North America to tropical, subtropical, temperate and cold temperate zones of the world by the movement of horticultural material, such as cuttings, seedlings and potted plants, while the spread of F. occidentalis‐transmitting viruses is shared along with the migration pattern (or trends) of its vector, especially TSWV and INSV. F. occidentalis was rarely reported in cold zones especially in areas with temperature dropping to −10 °C in winter which is 100% lethal to F. occidentalis attempting to overwinter outdoors.
Management of F. occidentalis
Because of their small size and the difficulty involved in detection and identification, successful invasions of thrips often occur unnoticed. As a result, F. occidentalis has become a major global pest with immense damage potential in only 30 years. In addition, adults are capable of migrating long distances to new host plants and are able to quickly transmit their viruses (Kliot et al., 2016). With these risks in mind, the most effective means of dealing with this potentially invasive and pestiferous thrip species is to prevent its entry and establishment into nonendemic regions. For example, methyl bromide was used for post‐harvest fumigation of a number of commodities either by the exporting or importing country (provinces) after a thrip infestation is noticed (Morse & Hoddle, 2006). However, because of its ozone depletion effect, methyl bromide is being phased out worldwide (Deewatthanawong et al., 2016). The alternatives to methyl bromide include irradiation, sulfuryl fluoride, phosphine, ethanedinitrile, low oxygen treatments, heat and cold treatments (Cox, 2017). A number of sustainable tactics have been developed in IPM programs for managing F. occidentalis and to inhibit its persistent spread worldwide with ever increasing damage to its many host crops.
Chemical control
Management of F. occidentalis has been a difficult task. Use of insecticides has traditionally been the primary strategy for control of F. occidentalis, especially in virus‐sensitive crops (Bielza, 2008). The insecticides that are normally applied can be separated into two major groups: broad‐spectrum insecticides, which include pyrethroids, neonicitinoids, organophosphates and carbamates, and narrow‐spectrum insecticides, which include pyridalyl and lufenuron (Mouden et al., 2017). However, frequent applications of insecticides, especially those containing pyrethroids, organophosphates, neonicitinoids and carbamates have also decimated large percentages of natural enemies and led to the rapid development of insecticide resistance in F. occidentalis (Mouden et al., 2017). This propensity of F. occidentalis for developing insecticide resistance has been a primary factor in promoting its pest status.
Spinosad and the related spinetoram, which tend to be compatible with natural enemies, are now being extensively used and currently provide the most effective chemical control of F. occidentalis (Gao et al., 2012a; Li et al., 2016). However, the applications of any insecticide will eventually contribute to resistance development in a given pest species. Evidence has shown that Spinosad resistance is now present in some populations of F. occidentalis in the USA (Weiss et al., 2009), Australia (Herron et al., 2014) and China (Li et al., 2016). If deemed necessary, insecticide use should be accurate, precise and complement other compatible control approaches.
Agricultural practices
Thrips often overwinter in patches of uncultivated plants and migrate into cropping systems in the spring (Pearsall & Myers, 2000), with cropping systems often serving as a sink, with sources of insect populations occurring in field margins and fencerows. F. occidentalis, which is a highly polyphagous pest of many cultivated as well as wild plants, has been shown to feed on more than 240 host plants (Tommasini & Maini, 1995), including many weed species. In France, the weed, gallant soldier (Galinsoga parviflora Cav.), has been reported by Nyasani et al. (2013) as an excellent host of F. occidentalis for both feeding and reproduction under field conditions, and serves as a potential source of thrip outbreaks in French bean fields. In instances such as this, where alternative hosts are identified and known to be present, sound agricultural practices such as seasonal mowing of these weeds will likely decrease the number of thrips migrating into the cropping systems (Northfield et al., 2008).
Additional practices for managing F. occidentalis include creating a less favorable environment by irrigation to reduce numbers of F. occidentalis adults (Schuch et al., 1998), by decreasing the levels of nitrogen fertilization to reduce populations of F. occidentalis in ornamentals (Brodbeck et al., 2001; Chow et al., 2012), and by growing trap plants to draw F. occidentalis away from susceptible crops, thereby reducing the number of thrips on the target crop (Cook et al., 2006).
Physical control
Because of their small size, fine mesh screens have been widely used to cover greenhouse openings such as vents to help physically prevent thrips from immigrating onto protected crops (Arthurs et al., 2018b). It was reported by Tinoco et al. (2014) that using appropriate mesh size screens on greenhouse windows would reduce the incidence of F. occidentalis by 20% in protected tomatoes. Because thrips find suitable host plants by utilizing different cues, including visual cues in the ultraviolet (UV) spectrum (Terry, 1997), using materials that reflect UV radiation can obscure their host‐locating cues. Several researchers have found that using UV‐reflective mulch significantly reduced early season abundance of adult thrips and disease incidence (Stavisky et al., 2002; Kigathi & Poehling, 2012).
Another conventional measure, sticky cards, are widely used by growers for monitoring thrip populations in greenhouses (Ren et al., 2008). It was reported that blue cards are highly attractive to F. occidentalis (Otieno et al., 2018). Because adult thrips explore their host range in part through volatiles, the commercially available F. occidentalis semiochemicals are frequently used as lures in conjunction with sticky card traps (Broughton et al., 2015) to attract and monitor or eliminate thrips.
Biological control
There has been considerable interest in the use of biological control agents to reduce thrip populations, especially in protected crops. An effective use of agents has been shown to improve thrip management. Inoculative release of agents, beginning at crop initiation before the resident thrips approach economically damaging levels, is recommended (Reitz et al., 2011). The large number of biological control agents that have been reported to attack F. occidentalis can be separated into two groups: macrobials (predators and parasitoids) and microbials (fungal pathogens and entomopathogenic nematodes) (Mouden et al., 2017). The macrobials currently being widely and effectively used are anthocorid bugs (Orius spp.) (Mo et al., 2013; Aragón‐Sánchez et al., 2018), green lacewing species (Sarkar et al., 2019) and predatory phytoseiid mites (Messelink et al., 2006; Ahmed & Lou, 2018), which predominantly attack 1st instar thrips on foliage, and soil‐dwelling predaceous laelapid mites (Berndt et al., 2004; Wu et al., 2016b), which consume thrip pupae in soil.
Fungal pathogens used as biocontrol agents of F. occidentalis, are Beauveria bassiana (Gao et al., 2012b; Lee et al., 2017), Metarhizium anisopliae (Maniania et al., 2003; Toledo‐Hernández et al., 2017) and Lecanicillium lecanii (Gouli et al., 2009; Wang et al., 2013). The various nematode species used against soil‐inhabiting pupae of F. occidentalis are in the genera Steinernema and Heterorhabditis (Ebssa et al., 2004; Buitenhuis & Shipp, 2005). There is currently interest in combinations of different biological control agents, which may result in an additive suppression of F. occidentalis populations (Messelink & Janssen, 2014; Saito & Brownbridge, 2016; Wu et al., 2017). Furthermore, fungal‐based granular formulations of entomopathogenic fungi are regarded as an effective strategy for thrip management by controlling the soil‐dwelling developmental stages of thrips (Lee et al., 2017). The latest research demonstrates that the application of B. bassiana granules to the soil surface can successfully suppress F. occidentalis under greenhouse conditions (Zhang et al., 2019).
Concluding remarks
With the continuing increase in global trade in ornamental greenhouse plants, it is likely that F. occidentalis will continue its rapid spread into, as yet, uninfested areas around the world, causing substantial amounts of damage from feeding and virus transmission. Another consideration is that F. occidentalis may also be capable of expanding its range to new areas as a consequence of global climate warming (Wu et al., 2018). Considering the economic importance of F. occidentalis both as a pest and a vector of several notorious plant viruses, it is essential to establish early surveillance systems of the species and to encourage the rapid recognition of plant virus symptoms while keeping a constant vigil on further spread of the species, especially in cold zones where F. occidentalis has not been reported.
Aggregation pheromones of F. occidentalis have been identified and shown to be cost‐effective for monitoring detection of this thrip species in the field (Kirk, 2017). Huang et al. (2010) and Zhang et al. (2012) provided a diagnostic polymerase chain reaction detection system, which can quickly and accurately identify F. occidentalis from thrip larvae to complement the traditional morphological identification. This method can also be used for on‐site testing of samples at ports‐of‐entry in the future. Standard area diagrams (SADs) have been used as a tool to improve the accuracy and reliability of visual estimates of leaf spotting diseases. More than 100 diseases with a range of plant organs were validated by SAD (Del Ponte et al., 2017). Presently, enhanced accessibility of cameras and image analysis software has accelerated the development of more realistic, stylized color representations or diagrams based on photographs of diseased plant organs (Del Ponte et al., 2019). Hence, we consider that it is a potential method for rapid recognition of F. occidentalis‐transmitting virus symptoms in the near future.
Most of the vector thrip species have high fecundity, short reproductive cycles and extensive plant host ranges (Whitfield et al., 2005a). F. occidentalis populations tend to be efficient vectors of multiple orthotospovirus species. Although the research on biological processes involved in the transmission of orthotospoviruses and their thrip vectors has made progress during the last decade (Hogenhout et al., 2008; Rotenberg et al., 2015), there is still a lack of effective measures for management of F. occidentalis and its transmitted viruses. At present, there is still a heavy reliance on insecticides, which will continue to play an important role in thrip management in the foreseeable future. The increased incidence of F. occidentalis throughout the world that we are currently witnessing, could be a consequence of increased insecticide applications over the past 30 years. There is mounting evidence that synthetic pyrethroids can stimulate reproduction of F. occidentalis (Funderburk, 2009) and promote insecticide resistance (Gao et al., 2012a). Further studies, including the virus‐vector relationship of F. occidentalis with insecticide resistance are needed to improve our understanding of basic biological concepts and develop alternative measures for thrip control.
Although significant research progress in control of F. occidentalis has been made after using alternative measures, this thrip species continues to threaten the production of many crops worldwide because of the severity of viruses and difficulty in preventing thrip transmission. Therefore, both the thrips and plant virus diseases transmitted should be taken into account in control tactics. First, because immature F. occidentalis can be found consistently in tomato blossoms (Beaudoin, 2011) and have been shown to acquire TSWV from infected tomato and then transmit to susceptible plants (Szostek et al., 2017), it was suggested that management of F. occidentalis infestations during the blooming season may be important for effective control of TSWV in susceptible tomato cultivars (Houle & Kennedy, 2017). Second, because adult thrips oviposit in plant tissue and prefer tight spaces, contact insecticides are often not effective against thrips. Induced systemic resistance (ISR) has recently gained more interest and might be the important option for management of thrips and transmitted virus (Mouden et al., 2017). It was reported that Pseudomonas strains induced resistance against virus diseases (Vasanthi et al., 2010); the combination of ISR by Pseudomonas and Neem oil, would be a best alternative in the future (Vasanthi et al., 2017). In addition, integration of new biotechnology‐based strategies, as well as advances in computational systems will provide a powerful tool to drive innovation in reducing virus transmission and vector populations.
Disclosure
The authors declare no competing interests.
Supporting information
Table S1. The worldwide distribution of Frankliniella occidentalis.
Table S2. The worldwide distribution and host of viruses transmitted by Frankliniella occidentalis.
Acknowledgments
We wish to thank Dr. Cecil L. Smith (University of Georgia, USA) for help with the language editing of the manuscript. This work was supported by National Key Research & Developments (R&D) plan (Grant No. 2016YFD0201002), Key project at central government level: The ability establishment of sustainable use for valuable Chinese medicine resources (2060302) and the National Natural Science Foundation of China (No. 31601604).
The copyright for this article was changed on May 21 after original online publication.
References
- Abe, H. , Tomitaka, Y. , Shimoda, T. , Seo, S. , Sakurai, T. , Kugimiya, S . et al (2011) Antagonistic plant defense system regulated by phytohormones assists interactions among vector insect, thrips and a tospovirus. Plant Cell Physiology, 53(1), 204–212. [DOI] [PubMed] [Google Scholar]
- Abou‐Jawdah, Y. , El Mohtar, C. , Sobh, H. and Nakhla, M.K. (2006) First report of Tomato spotted wilt virus on tomatoes in Lebanon. Plant Disease, 90, 376. [DOI] [PubMed] [Google Scholar]
- Achon, M.A. , Serrano, L. , Clemente‐Orta, G. and Sossai, S. (2017) First report of Maize chlorotic mottle virus on a perennial host, Sorghum halepense, and maize in Spain. Plant Disease, 101, 393. [Google Scholar]
- Adams, I.P. , Harju, V. , Hodges, T. , Hany, U. , Skelton, A. , Rai, S . et al (2014) First report of maize lethal necrosis disease in Rwanda. New Disease Reports, 29, 22. [Google Scholar]
- Adkins, S. and Baker, C.A. (2005) Tomato spotted wilt virus identified in desert rose in Florida. Plant Disease, 89, 526. [DOI] [PubMed] [Google Scholar]
- Adkins, S. , Breman, L. , Baker, C.A. and Wilson, S. (2003) First report of Tomato spotted wilt virus in blackberry lily in North America. Plant Disease, 87, 102. [DOI] [PubMed] [Google Scholar]
- Ahmed, N. and Lou, M. (2018) Efficacy of two predatory phytoseiid mites in controlling the western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) on cherry tomato grown in a hydroponic system. Egyptian Journal Biological Pest Control, 28, 15. [Google Scholar]
- Anfoka, G.H. , Abhary, M. and Stevens, M.R. (2006) Occurrence of tomato spotted wilt virus (TSWV) in Jordan. EPPO Bulletin, 36, 517–522. [Google Scholar]
- Appiah, A.S. , Offei, S.K. , Tegg, R.S. and Wilson, C.R. (2016) Varietal response to groundnut rosette disease and thefirst report of groundnut ringspot virus in Ghana. Plant Disease, 100, 946–952. [DOI] [PubMed] [Google Scholar]
- Aragón‐Sánchez, M. , Román‐Fernández, L.R. , Martínez‐García, H. , Aragón‐García, A. , Pérez‐Moreno, I. and Marco‐Mancebón, V.S. (2018) Rate of consumption, biological parameters, and population growth capacity of Orius laevigatus fed on Spodoptera exigua . BioControl, 63, 785–794. [Google Scholar]
- Aramburu, J. (2001) First report of Parietaria mottle virus on tomato in Spain. Plant Disease, 85, 1210. [DOI] [PubMed] [Google Scholar]
- Aramburu, J. , Galipienso, L. , Aparicio, F. , Soler, S. and López, C. (2010) Mode of transmission of Parietaria mottle virus . Journal of Plant Pathology, 92, 679–684. [Google Scholar]
- Aramburu, J. , Laviña, A. , Moriones, E. , Riudavets, J. and Arnó, J. (1997) The proportion of viruliferous individuals in field populations of Frankliniella occidentalis: Implications for tomato spotted wilt virus epidemics in tomato. European Journal of Plant Pathology, 103, 623–629. [Google Scholar]
- Arthurs, S.P. , Heinz, K.M. and Mitchell, F.L. (2018a) Comparison of Frankliniella fusca and Frankliniella occidentalis (Thysanoptera: Thripidae) as vectors for a peanut strain of tomato spotted wilt Orthotospovirus. Environmental Entomology, 47, 623–628. [DOI] [PubMed] [Google Scholar]
- Arthurs, S.P. , Krauter, P.C. , Gilder, K. and Heinz, K.M. (2018b) Evaluation of deltamethrin‐impregnated nets as a protective barrier against Western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae) under laboratory and greenhouse conditions. Crop Protection, 112, 227–231. [Google Scholar]
- Ávila, A.C. , Haan, P. De , Kitajima, E.W. , Kormelink, R. , Resende, R.D.O. , Goldbach, R.W . et al (1992) Characterization of a distinct isolate of Tomato spotted wilt virus (TSWV) from Impatiens sp. in the Netherlands. Journal of Phytopathology, 134, 133–151. [Google Scholar]
- Baker, C.A. , Davison, D. and Jones, L. (2007) Impatiens necrotic spot virus and Tomato spotted wilt virus diagnosed in Phalaenopsis orchids from two Florida nurseries. Plant Disease, 91, 1515. [DOI] [PubMed] [Google Scholar]
- Baker, C.A. , Jones, L. , Leahy, R.M. and Soltis, D.E. (2009) Tomato spotted wilt virus found in five species of the genus Tragopogon in a Florida greenhouse. Plant Disease, 93, 546. [DOI] [PubMed] [Google Scholar]
- Barkley, N.A. , Pinnow, D.L. , Wang, M.L. and Pederson, G.A. (2009) First report of Tomato spotted wilt virus infecting African clover (Trifolium tembense) in Georgia. Plant Disease, 93, 202. [DOI] [PubMed] [Google Scholar]
- Batuman, O. , Rojas, M. , Almanzar, A. and Gilbertson, R. (2014) First report of Tomato chlorotic spot virus in processing tomatoes in the Dominican Republic. Plant Disease, 98, 286. [DOI] [PubMed] [Google Scholar]
- Batuman, O. , Turini, T.A. , Oliveira, P.V. , Rojas, M.R. , Macedo, M. , Mellinger, H.C . et al (2017) First report of a resistance‐breaking strain of Tomato spotted wilt virus infecting tomatoes with the Sw‐5 tospovirus‐resistance gene in California. Plant Disease, 101, 637. [Google Scholar]
- Beaudoin, A.P.L. (2011) Temporal and spatial patterns of thrips dispersal in relation to the epidemiology of tomato spotted wilt virus. PhD Dissertation, Order No. 3497228, North Carolina State University.
- Ben Moussa, A. , Marrakchi, M. and Makni, M. (2005) Characterisation of tospovirus in vegetable crops in Tunisia. Infection Genetics and Evolution, 5, 312–322. [Google Scholar]
- Berndt, O. , Meyhofer, R. and Poehling, H.M. (2004) The edaphic phase in the ontogenesis of Frankliniella occidentalis and comparison of Hypoaspis miles and Hypoaspis aculeifer as predators of soil‐dwelling thrips stages. Biological Control, 30, 17–24. [Google Scholar]
- Bezerra, I.C. , Resende, R. , Pozzer, L. , Nagata, T. , Kormelink, R. and De Ávila, A.C. (1999) Increase of tospoviral diversity in Brazil with the identification of two new tospovirus species, one from chrysanthemum and one from zucchini. Phytopathology, 89, 823–830. [DOI] [PubMed] [Google Scholar]
- Bielza, P. (2008) Insecticide resistance management strategies against the western flower thrips, Frankliniella occidentalis . Pest Management Science, 64, 1131–1138. [DOI] [PubMed] [Google Scholar]
- Bielza, P. (2010) Insecticide resistance management strategies against the western flower thrips, Frankliniella occidentalis . Pest Management Science, 64, 1131–1138. [DOI] [PubMed] [Google Scholar]
- Birithia, R. , Subramanian, S. , Villinger, J. , Muthomi, J.W. , Narla, R.D. and Pappu, H.R. (2012) First report of Tomato yellow ring virus (Tospovirus, Bunyaviridae) infecting tomato in Kenya. Plant Disease, 96, 1384. [DOI] [PubMed] [Google Scholar]
- Blystad, D.R. , Naess, V. and Haugslien, S. (1995) Optimizing immunosorbent electron microscopy for detection of pelargonium flower‐break carmovirus in pelargonium. EPPO Bulletin, 25, 239–245. [Google Scholar]
- Boari, A.J. , Maciel‐zambolim, E. , Lau, D.D. , Lima, G.S.A. , Kitajima, E.W. , Brommonschenkel, S.S.H . et al (2002) Detection and partial characterization of an isolate of Groundnut ringspot virus in Solanum sessiliflorum . Fitopatologia Brasileira, 27, 249–253. [Google Scholar]
- Boiteux, L. , Nagata, T. , Dusi, A. and de Avila, A. (1993) Natural occurrence of the two tospovirus species infecting Capsicum spp. in Brazil. Capsicum Eggplant Newsleter, 12, 75. [Google Scholar]
- Bouwen, I. and Maat, D.Z. (1992) Pelargonium flower‐break and pelargonium line pattern viruses in the Netherlands; purification, antiserum preparation, serological identification, and detection in pelargonium by ELISA. Netherlands Journal ofPlant Pathology, 98, 141–156. [Google Scholar]
- Brittlebank, C. (1919) Tomato diseases. Journal of Agricultural Victoria, 27, 231–235. [Google Scholar]
- Brodbeck, B.V. , Stavisky, J. , Funderburk, J.E. , Andersen, P.C. and Olson, S.M. (2001) Flower nitrogen status and populations of Frankliniella occidentalis feeding on Lycopersicon esculentum . Entomologia Experimentalis et Applicata, 99, 165–172. [Google Scholar]
- Broughton, S. , Cousins, D.A. and Rahman, T. (2015) Evaluation of semiochemicals for their potential application in mass trapping of Frankliniella occidentalis (Pergande) in roses. Crop Protection, 67,130–135. [Google Scholar]
- Buitenhuis, R. and Shipp, J.L. (2005) Efficacy of entomopathogenic nematode Steinernema feltiae (Rhabditida: Steinernematidae) as influenced by Frankliniella occidentalis (Thysanoptera: Thripidae) developmental stage and host plant stage. Journal of Economic Entomology, 98, 1480–1485. [DOI] [PubMed] [Google Scholar]
- Cabanas, D. , Watanabe, S. , Higashi, C.H.V. and Bressan, A. (2013) Dissecting the mode of maize chlorotic mottle virus transmission (Tombusviridae: Machlomovirus) by Frankliniella williamsi (Thysanoptera: Thripidae). Journal of Economic Entomology, 106, 16–24. [DOI] [PubMed] [Google Scholar]
- Caciagli, P. , Boccardo, G. and Lovisolo, O. (1989) Parietaria mottle virus, a possible new ilarvirus from Parietaria officinalis (Urticaceae). Plant Pathology, 38, 577–584. [Google Scholar]
- Cai, J.H. , Qin, B.X. , Wei, X.P. , Huang, J. , Zhou, W.L. , Lin, B.S . et al (2011) Molecular identification and characterization of Tomato zonate spot virus in tobacco in Guangxi, China. Plant Disease, 95, 1483. [DOI] [PubMed] [Google Scholar]
- Camelo‐Garca, V.M. , Lima, E.F.B , Mansilla‐Crdova, P.J. , Rezende, J.A.M. , Kitajima, E.W. and Barreto, M. (2014) Occurrence of Groundnut ringspot virus on Brazilian peanut crops. Journal of General Plant Pathology, 80, 282–286. [Google Scholar]
- Castillo‐Loayza, J. (1977) Maize virus and virus‐like diseases in Peru. Proceedings of International Maize Virus Disease Colloquim Workshop (eds. L.E. Williams, D.T. Gordon & L.R. Nault) pp. 40–44.
- Chamberlain, E. and Taylor, G. (1936) The occurrence of spotted‐wilt on tomatoes in New Zealand. New Zealand Journal of Agriculture, 52, 9–17. [Google Scholar]
- Chamberlain, E. and Taylor, G. (1938) Spotted wilt. Host range and transmission by thrips. New Zealand Journal of Science and Technology, Section A., 20, 133–142. [Google Scholar]
- Chatzivassiliou, E.K. , Weekes, R. , Morris, J. , Wood, K.R. , Barker, I. and Katis, N.I. (2000a) Tomato spotted wilt virus (TSWV) in Greece: its incidence following the expansion of Frankliniella occidentalis, and characterisation of isolates collected from various hosts. Annual of Appliled Biology, 137, 127–134. [Google Scholar]
- Chatzivassiliou, E.K. , Livieratos, I. , Jenser, G. and Katis, N. (2000b) Ornamental plants and thrips populations associated with tomato spotted wilt virus in Greece. Phytoparasitica, 28, 257–264. [Google Scholar]
- Chatzivassilou, E.K. , Livieratos, I. , Katis, N. , Avegelis, A. and Lykouressis, D. (1996) Occurrence of tomato spotted wilt virus in vegetables and ornamentals in Greece. Acta Horticulturae, 431, 44–50. [Google Scholar]
- Chen, L. , Jiao, Z. , Liu, D. , Liu, X. , Xia, Z. , Deng, C . et al (2017) One‐step reverse transcription loop‐mediated isothermal amplification for the detection of Maize chlorotic mottle virus in maize. Journal of Virological Methods, 240, 49–53. [DOI] [PubMed] [Google Scholar]
- Choi, G.S. , Kim, J.S. , Choi, J.K. and Kim, J.H. (2004) Characterization of Tomato spotted wilt virus from paprika in Korea. Plant Pathology Journal, 20, 297–301. [Google Scholar]
- Choi, S.K. and Choi, G.S. (2015) First report of Tomato spotted wilt virus in Solanum tuberosum in Korea. Plant Disease, 99, 1657. [DOI] [PubMed] [Google Scholar]
- Chow, A. , Chau, A. and Heinz, K.M. (2012) Reducing fertilization: a management tactic against western flower thrips on roses. Journal of Applied Entomology, 136, 520–529. [Google Scholar]
- Cloyd, R.A. (2009) Western flower thrips (Frankliniella occidentalis) management on ornamental crops grown in greenhouses: have we reached an impasse? Pest Technology, 3, 1–9. [Google Scholar]
- Colariccio, A. , Chaves, A. , Eiras, M. and Chagas, C. (2001a) Identification of the tomato chlorotic spot virus in endive (Cichorium endiva L.). Summa Phytopathology, 27, 325–327. [Google Scholar]
- Colariccio, A. , Eiras, M. , Alexandre, L. , Chaves, R. and Chagas, C. (2001b) Characterization of tomato chlorotic spot virus from hydroponic grown lettuce in Brazil. Thrips and Tospoviruses: Proceedings of the 7th International Symposium on Thysanoptera (eds. R. Marullo & L. Mound), pp. 99–104. Reggio Calabria, Italy: ANIC.
- Cook, S.M. , Khan, Z.R. and Pickett, J.A. (2006) The use of push‐pull strategies in integrated pest management. Annual Review of Entomology, 52, 375–400. [DOI] [PubMed] [Google Scholar]
- Cota, E. and Merkuri, J. (2004) Introduction of Frankliniella occidentalis and occurrence of Tomato spotted wilt tospovirus in Albania. EPPO Bulletin, 34, 421–422. [Google Scholar]
- Cox, D. (2017) Quarantine and pre‐shipment uses of methyl bromide 2013–2016 and the potential for its replacement. Report to the Australian Government Department of the Environment and Energy.
- Crosslin, J.M. , Mallik, I. and Gudmestad, N.C. (2009) First report of Tomato spotted wilt virus causing potato tuber necrosis in Texas. Plant Disease, 93, 845. [DOI] [PubMed] [Google Scholar]
- Culbreath, A.K. , Csinos, A.S. , Bertrand, P.F. and Demski, J.W. (1991) Tomato spotted wilt virus epidemic in flue‐cured tobacco in Georgia. Plant Disease, 75, 483–485. [Google Scholar]
- Dal Bio, E. , Chiarrone, G. , Rolleri, J. and Ronc, L. (2001) New tospoviruses found in La Plata. Revista de la Facultad Agronnomia (La Plata), 104, 35–40. [Google Scholar]
- De Avila, A.C. , De Haan, P. , Kormelink, R. , De Resende, O.R. , Goldbach, R.W. and Peters, D. (1993) Classification of tospoviruses based on phylogeny of nucleoprotein gene sequences. Journal of General Virology, 74, 153–159. [DOI] [PubMed] [Google Scholar]
- Díaz‐Pérez, J.C. and Pappu, H.R. (2000) First report of Tomato spotted wilt virus infection of tomatillo in Georgia. Plant Disease, 84, 1155. [DOI] [PubMed] [Google Scholar]
- Dietzgen, R. , Mann, K. and Johnson, K. (2016) Plant virus–insect vector interactions: current and potential future research directions. Viruses, 8, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Avila, A.C. , Huguenot, C. , De Resende, O.R. , Kitajima, E.W. , Goldbach, R.W. and Peters, D. (1990) Serological differentiation of 20 isolates of tomato spotted wilt virus. Journal of General Virology, 71, 2801–2807. [DOI] [PubMed] [Google Scholar]
- Deewatthanawong, R. , Chanapan, S. and Suwanagul, A. (2016) Evaluation of methyl bromide alternatives to control thrips in orchid cut‐flowers. I International Symposium on Tropical and Subtropical Ornamentals 1167, pp. 393–398.
- Del Ponte, E.M. , Pethybridge, S.J. , Bock, C.H. , Michereff, S.J. , Machado, F.J. and Spolti, P. (2017) Standard area diagram aids for aiding severity estimation: Scientometrics, pathosystems and methodological trends in the last 25 years. Phytopathology, 107, 1161–1174. [DOI] [PubMed] [Google Scholar]
- Del Ponte, E.M. , Nelson, S.C. and Pethybridge, S.J. (2019) Evaluation of app‐embedded disease scales for aiding visual severity estimation of cercospora leaf spot of Table Beet. Plant Disease, 103, 1347–1356. [DOI] [PubMed] [Google Scholar]
- Demirozer, O. , Tyler‐Julian, K. , Funderburk, J. , Norm, L. and Stuart, R. (2012) Frankliniella occidentalis (Pergande) integrated pest management programs for fruiting vegetables in Florida. Pest Management Science, 68, 1537–1545. [DOI] [PubMed] [Google Scholar]
- Deng, T.C. , Chou, C.M. , Chen, C.T. , Tsai, C.H. and Lin, F.C. (2014) First report of Maize chlorotic mottle virus on sweet corn in Taiwan. Plant Disease, 98, 1748. [DOI] [PubMed] [Google Scholar]
- Dikova, B. , Petrov, N. , Djourmanski, A. and Lambev, H. (2013) First report of Tomato spotted wilt virus on a new host Leuzea carthamoides in Bulgaria and the World. Plant Disease, 97, 1258. [DOI] [PubMed] [Google Scholar]
- Dong, J. , Zhang, Z. , Yin, Y. , Cheng, X. , Ding, M. and Fang, Q. (2010) Natural host ranges of Tomato zonate spot virus in Yunnan. Journal of Insect Science, 10, 12–13.20575743 [Google Scholar]
- Dong, J.H. , Cheng, X.F. , Yin, Y.Y. , Fang, Q. , Ding, M. , Li, T.T . et al (2008) Characterization of tomato zonate spot virus, a new tospovirus in China. Archives of Virology, 153, 855–864. [DOI] [PubMed] [Google Scholar]
- Ebssa, L. , Borgemeister, C. and Poehling, H.M. (2004) Effectiveness of different species/strains of entomopathogenic nematodes for control of western flower thrips (Frankliniella occidentalis) at various concentrations, host densities and temperatures. Biological Control, 29, 145–154. [Google Scholar]
- Eiras, M. , Chaves, A. , Colariccio, A. , Harakava, R. , de Araujo, J. and Chagas, C. (2002) Characterization of a Tomato chlorotic spot virus isolated from gilo in Peraiba Valley, Sao Paulo, Brazil. Fitopatologia Brasileira, 27, 285–291. [Google Scholar]
- EPPO (2004) Data sheets on quarantine pests: Tomato spotted wilt tospovirus . Data Sheets Quar. Pests (revision Orig. 1997c data sheet). [Google Scholar]
- Estévez de Jensen, C. and Adkins, S. (2014) First report of Tomato chlorotic spot virus in lettuce in Puerto Rico. Plant Disease, 98, 1015. [DOI] [PubMed] [Google Scholar]
- Estévez de Jensen, C. , Rivera‐Vargas, L.I. , Rodrigues, J.C.V. , Mercado, W. , Frantz, G. , Mellinger, H.C . et al (2013) First report of Tomato chlorotic spot virus (TCSV) in tomato, pepper, and jimsonweed in Puerto Rico. Plant Health Progress, 10.1094/PHP-2013-0812-01-BR [DOI] [Google Scholar]
- Fletcher, J. , France, C. and Butler, R. (2005) Virus surveys of lettuce crops and management of lettuce big‐vein disease in New Zealand. New Zealand Plant Protection, 58, 239–244. [Google Scholar]
- Funderburk, J. (2009) Management of the western flower thrips (Thysanoptera: Thripidae) in fruiting vegetables. Florida Entomologist, 92, 1–6. [Google Scholar]
- Galipienso, L. , Herranz, M.C. , Pallás, V. and Aramburu, J. (2005) Detection of a tomato strain of Parietaria mottle virus (PMoV‐T) by molecular hybridization RT‐PCR in field samples from north‐eastern Spain. Plant Pathology, 54, 29–35. [Google Scholar]
- Gao, Y. , Lei, Z. and Reitz, S.R. (2012a) Western flower thrips resistance to insecticides: detection, mechanisms and management strategies. Pest Management Science, 68, 1111–1121. [DOI] [PubMed] [Google Scholar]
- Gao, Y.L. , Reitz, S.R. , Wang, J. , Tamez‐Guerra, P. , Wang, E.D. , Xu, X.N . et al (2012b) Potential use of the fungus Beauveria bassiana against the western flower thrips Frankliniella occidentalis without reducing the effectiveness of its natural predator Orius sauteri (Hemiptera: Anthocoridae). Biocontrol Science and Technology, 22, 803–812. [Google Scholar]
- Gera, A. , Kritzman, A. and Cohen, J. (2000a) Pittosporum tobira: a new host for Tomato spotted wilt virus . Plant Disease, 84, 491. [DOI] [PubMed] [Google Scholar]
- Gera, A. , Kritzman, A. , Cohen, J. , Raccah, B. and Antignus, Y. (2000b) Tospoviruses infecting vegetable crops in Israel. EPPO Bulletin, 30, 289–292. [Google Scholar]
- Gilbertson, R.L. , Batuman, O. , Webster, C.G. and Adkins, S. (2015) Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Annual Review of Virology, 2, 67–93. [DOI] [PubMed] [Google Scholar]
- Golnaraghi, A.R. , Shahraeen, N. , Pourrahim, R. , Ghorbani, S. and Farzadfar, S. (2001) First report of Tomato spotted wilt virus on soybean in Iran. Plant Disease, 85, 1290. [DOI] [PubMed] [Google Scholar]
- Gouli, V.V. , Gouli, S.Y. , Skinner, M. and Shternshis, M.V. (2009) Effect of the entomopathogenic fungi on mortality and injury level of western flower thrips, Frankliniella occidentalis . Archives of Phytopathology and Plant Protection, 42(2), 118–123. [Google Scholar]
- Gowda, M. , Das, B. , Makumbi, D. , Babu, R. , Semagn, K. , Mahuku, G . et al (2015) Genome‐wide association and genomic prediction of resistance to maize lethal necrosis disease in tropical maize germplasm. Theoretical and Applied Genetics, 128, 1957–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia, O. , De Borbon, C.M. , Granval De Millan, N. and Cuesta, G.V. (1999) Occurrence of different tospoviruses in vegetable crops in Argentina. Journal of Phytopathology, 147, 223–227. [Google Scholar]
- Groves, R.L. , Kennedy, G.G. , Walgenbach, J.F. and Moyer, J.W. (1998) Inoculation of Tomato spotted wilt virus into Cotton. Plant Disease, 82, 959. [DOI] [PubMed] [Google Scholar]
- Haliwell, R. and Philley, G. (1974) Spotted wilt of peanut in Texas. Plant Disease Report, 58, 23–25. [Google Scholar]
- Hassani‐Mehraban, A. , Botermans, M. , Verhoeven, J.T.J. , Meekes, E. , Saaijer, J. , Peters, D . et al (2010) A distinct tospovirus causing necrotic streak on Alstroemeria sp. in Colombia. Archives of Virology, 155, 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani‐Mehraban, A. , Saaijer, J. , Peters, D. , Goldbach, R. and Kormelink, R. (2005) A new tomato‐infecting Tospovirus from Iran. Phytopathology, 95, 852–858. [DOI] [PubMed] [Google Scholar]
- Hassani‐Mehraban, A. , Saaijer, J. , Peters, D. , Goldbach, R. and Kormelink, R. (2007) Molecular and biological comparison of two tomato yellow ring virus (TYRV) isolates: challenging the tospovirus species concept. Archievs of Virology, 152, 85–96. [DOI] [PubMed] [Google Scholar]
- Healey, M.A. , Senior, L.J. , Brown, P.H. and Duff, J. (2017) Relative abundance and temporal distribution of adult Frankliniella occidentalis (Pergande) and Frankliniella schultzei (Trybom) on French bean, lettuce, tomato and zucchini crops in relation to crop age. Journal of Asia‐Pacific Entomology, 20, 859–865. [Google Scholar]
- Herron, G.A. , Gunning, R.V. , Cottage, E.L. , Borzatta, V. and Gobbi, C. (2014) Spinosad resistance, esterase isoenzymes and temporal synergism in Frankliniella occidentalis (Pergande) in Australia. Pesticide Biochemistry and Physiology, 114, 32–37. [DOI] [PubMed] [Google Scholar]
- Hogenhout, S.A. , Ammar, E.D. , Whitfield, A.E. and Redinbaugh, M.G. (2008) Insect vector interactions with persistently transmitted viruses. Annual Review of Phytopathology, 46, 327–359. [DOI] [PubMed] [Google Scholar]
- Holcomb, G.E. and Valverde, R.A. (2000) First report of Oidium sp. powdery mildew and Tomato spotted wilt virus on Melampodium divaricatum . Plant Disease, 84, 1152–1152. [DOI] [PubMed] [Google Scholar]
- Holcomb, G.E. , Valverde, R.A. , Sim, J. and Nuss, J. (1999) First report on natural occurrence of Tomato spotted wilt tospovirus in Basil (Ocimum basilicum). Plant Disease, 83, 966. [DOI] [PubMed] [Google Scholar]
- Holguín‐Peña, R.J. and Rueda‐Puente, E.O. (2007) Detection of Tomato spotted wilt virus in tomato in the Baja California Peninsula of Mexico. Plant Disease, 91, 1682. [DOI] [PubMed] [Google Scholar]
- Houle, J.L. and Kennedy, G.G. (2017) Tomato spotted wilt virus can infect resistant tomato when western flower thrips inoculate blossoms. Plant Diseae, 101, 1666–1670. [DOI] [PubMed] [Google Scholar]
- Huang, C.J. , Liu, Y. , Yu, H.Q. and Li, B.L. (2015) Occurrence of Tomato zonate spot virus on potato in China. Plant Disease, 99, 733. [DOI] [PubMed] [Google Scholar]
- Huang, K.S. , Lee, S.E. , Yeh, Y. , Shen, G.S. , Mei, E. and Chang, C.M. (2010) Taqman real‐time quantitative PCR for identification of western flower thrip (Frankliniella occidentalis) for plant quarantine. Biology letters, 6(4), 555–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivars, P. , Alonso, M. , Borja, M. and Hernández, C. (2004) Development of a non‐radioactive dot‐blot hybridisation assay for the detection of Pelargonium flower break virus and Pelargonium line pattern virus . Euroupean Journal of Plant Pathology, 110, 275–283. [Google Scholar]
- Janssen, D. , Saez, E. , Segundo, E. , Martín, G. , Gil, F. and Cuadrado, I.M. (2005) Capsicum annuum – a new host of Parietaria mottle virus in Spain. Plant Pathology, 54, 567. [Google Scholar]
- Jiang, X. , Wilkinson, D. and Berry, J. (1990) An outbreak of maize chlorotic mottle virus in Hawaii and possible association with thrips. Phytopathology, 80, 1060. [Google Scholar]
- Jones, D.R. (2005) Plant viruses transmitted by thrips. Euroupean Journal Plant Pathology, 113, 119–157. [Google Scholar]
- Jorda, C. (1993) Nuevas virosis de mayor incidencia en cultivos horticolas. Phytoma Espana, 50, 7–13. [Google Scholar]
- Karavina, C. , Ibaba, J.D. and Gubba, A. (2016a) First report of Tomato spotted wilt virus infecting butternut squash (Cucurbita moschata) in Zimbabwe. Plant Disease, 100, 870. [Google Scholar]
- Karavina, C. , Ximba, S. , Ibaba, J.D. and Gubba, A. (2016b) First report of a mixed infection of Potato virus Y and Tomato spotted wilt virus on pepper (Capsicum annuum) in Zimbabwe. Plant Disease, 100, 1513. [Google Scholar]
- Kigathi, R. and Poehling, H.M. (2012) UV‐absorbing films and nets affected the dispersal of western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). Journal of Applied Entomology, 136, 761–771. [Google Scholar]
- Kirk, W.D.J. and Terry, L.I. (2003) The spread of the western flower thrips Frankliniella occidentalis (Pergande). Agricultural and Forest Entomology, 5, 301–310. [Google Scholar]
- Kirk, W.D. (2017) The aggregation pheromones of thrips (Thysanoptera) and their potential for pest management. International Journal of Tropical Insect Science, 37, 41–49. [Google Scholar]
- Kisten, L. , Moodley, V. , Gubba, A. and Mafongoya, P.L. (2016) First detection of Tomato spotted wilt virus (TSWV) on Amaranthus thunbergii in South Africa. Plant Disease, 100, 2176. [Google Scholar]
- Kliot, A. , Kontsedalov, S. , Lebedev, G. and Ghanim, M. (2016) Advances in whiteflies and thrips management Advances in Insect Control and Resistance Management. pp. 205–218. Springer, Cham. [Google Scholar]
- Koehler, A.M. , Brown, J.A. , Huber, B. , Wehner, T.C. and Shew, H.D. (2016) First report of Tomato spotted wilt virus in Stevia rebaudiana in North Carolina. Plant Disease, 100, 1251. [Google Scholar]
- Krczal, G. , Albouy, J. , Damy, I. , Kusiak, C. , Deogratias, J.M. , Moreau, J.P . et al (1995) Transmission of pelargonium flower break virus (PFBV) in irrigation systems and by thrips. Plant Disease, 79, 163–166. [Google Scholar]
- Kucharek, T. , Brown, L. , Johnson, F. and Funderburk, J. (2000) Tomato spotted wilt virus of agronomic, vegetable, and ornamental crops Plant Pathology Fact Sheet Circ‐914., pp. 13 Florida Cooperative Extension Service/Institute of Food and Agricultural Sciences/University of Florida. [Google Scholar]
- Kuo, Y.W. , Gilbertson, R.L. , Turini, T. , Brennan, E.B. , Smith, R.F. and Koike, S.T. (2014) Characterization and epidemiology of outbreaks of Impatiens necrotic spot virus on lettuce in coastal California. Plant Disease, 98, 1050–1059. [DOI] [PubMed] [Google Scholar]
- Kusia, E.S. , Subramanian, S. , Nyasani, J.O. , Khamis, F. , Villinger, J. , Ateka, E.M . et al (2015) First report of lethal necrosis disease associated with co‐infection of finger millet with Maize chlorotic mottle virus and Sugarcane mosaic virus in Kenya. Plant Disease, 99, 899. [Google Scholar]
- Latham, L.J. and Jones, R.A.C. (1997) Occurrence of tomato spotted wilt tospovirus in native flora, weeds, and horticultural crops. Australian Journal of Agricultural Research, 48, 359. [Google Scholar]
- Leão, E.U. , de Almeida Spadotti, D.M. , Rocha, K.C.G. , de Fátima da CunhaPantoja, K. , Rezende, J.A.M. , Pavan, M.A . et al (2015) Citrullus lanatus is a new natural host of Groundnut ringspot virus in Brazil. Journal of Phytopathology, 163, 1014–1018. [Google Scholar]
- Lebas, B.S. and Ochoa‐Corona, F. (2007) Impatiens necrotic spot virus. Characterization, Diagnosis and Management of Plant Viruses. Vol. 4: Grain Crops and Ornamentals (eds. Rao G.P., Bragard C. & Lebas B.S.M.), pp. 221–243. Texas: Stadium Press LLC. [Google Scholar]
- Lebas, B.S. , Ochoa‐Corona, F. , Elliott, D. , Tang, Z. , Alexander, B.J. and Froud, K.J. (2004) An investigation of an outbreak of Impatiens necrotic spot virus in New Zealand. Phytopathology, 94, S57–S58. [Google Scholar]
- Lee, S.J. , Kim, S. , Kim, J.C. , Lee, M.R. , Hossain, M. , Shin, T.S . et al (2017) Entomopathogenic Beauveria bassiana granules to control soil‐dwelling stage of western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). BioControl, 62, 639–648. [Google Scholar]
- Li, D.G. , Shang, X.Y. , Reitz, S.R. , Nauen, R. , Lei, Z.R. , Lee, S.H . et al (2016) Field resistance to spinosad in western flower thrips Frankliniella occidentalis (Thysanoptera: Thripidae). Journal of Integrative Agriculture, 15, 2803–2808. [Google Scholar]
- Li, Y.Y. , Xiao, L. , Tan, G.L. , Fu, X.P. , Li, R.H. and Li, F. (2015) First report of Tomato spotted wilt virus on celery in China. Plant Disease, 99, 734. [Google Scholar]
- Lima, M.F. , de Ávila, A.C. , da G Wanderley, L.J. , Nagata, T. and da Gama, L.J.W. (1999) Coriander: a new natural host of Groundnut ring spot virus in Brazil. Plant Disease, 83, 878. [DOI] [PubMed] [Google Scholar]
- Liu, L.Y. , Ye, H.Y. , Chen, T.H. and Chen, T.C. (2017) Development of a microarray for simultaneous detection and differentiation of different tospoviruses that are serologically related to Tomato spotted wilt virus . Journal of Virology, 14, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Huang, C.J. , Tao, X.R. and Yu, H.Q. (2015) First report of Tomato zonate spot virus in Iris tectorum in China. Plant Disease, 99, 164. [DOI] [PubMed] [Google Scholar]
- Londoño, A. , Capobianco, H. , Zhang, S. and Polston, J.E. (2012) First record of Tomato chlorotic spot virus in the USA. Tropical Plant of Pathology, 37, 333–338. [Google Scholar]
- Louro, D. (1996) Detection and identification of tomato spotted wilt virus and impatiens necrotic spot virus in Portugal. Acta Horticulturae, 431, 99–105. [Google Scholar]
- Lukanda, M. , Owati, A. , Ogunsanya, P. , Valimunzigha, K. , Katsongo, K. , Ndemere, H . et al (2014) First report of Maize chlorotic mottle virus infecting maize in the Democratic Republic of the Congo. Plant Disease, 98, 1448. [DOI] [PubMed] [Google Scholar]
- Mahuku, G. , Lockhart, B.E. , Wanjala, B. , Jones, M.W. , Kimunye, J.N. , Stewart, L.R . et al (2015) Maize lethal necrosis (MLN), an emerging threat to maize‐based food security in Sub‐Saharan Africa. Phytopathology, 105, 956–965. [DOI] [PubMed] [Google Scholar]
- Maluf, W. , Toma‐Brahini, M. and Corte, R.D. (1991) Progress in breeding tomatoes for resistance to tomato spotted wilt. Brazilian Journal of Genetics, 14, 509–525. [Google Scholar]
- Maniania, N.K. , Ekesi, S. , Löhr, B. and Mwangi, F. (2003) Prospects for biological control of the western flower thrips, Frankliniella occidentalis, with the entomopathogenic fungus, Metarhizium anisopliae, on chrysanthemum. Mycopathologia, 155, 229–235. [DOI] [PubMed] [Google Scholar]
- Marchoux, G. , Gebre‐selassie, K. and Villevieille, M. (1991) Detection of tomato spotted wilt virus and transmission by Frankliniella occidentalis in France. Plant Pathology, 40, 347–351. [Google Scholar]
- Martínez, R.T. , Poojari, S. , Tolin, S.A. , Cayetano, X. and Naidu, R.A. (2014) First report of Tomato spotted wilt virus in peppers and tomato in the Dominican Republic. Plant Disease, 98, 163. [DOI] [PubMed] [Google Scholar]
- Martinez, D.L.O. , Zavaleta‐Mejía, E. , Mora‐Aguilera, G. and Johansen N.R.M. (1999) Implications of weed composition and thrips species for the epidemiology of tomato spotted wilt in chrysanthemum (Dendranthema grandiflora). Plant Pathology, 48, 707–717. [Google Scholar]
- Marys, E. , Mejías, A. , Rodríguez‐Román, E. , Avilán, D. , Hurtado, T. , Fernández, A. et al (2014) The first report of Tomato spotted wilt virus on gerbera and chrysanthemun in Venezuela. Plant Disease, 98, 1161. [DOI] [PubMed] [Google Scholar]
- Massumi, H. , Samei, A. , Pour, A.H. , Shaabanian, M. and Rahimian, H. (2007) Occurrence, distribution, and relative incidence of seven viruses infecting greenhouse‐grown cucurbits in Iran. Plant Disease, 91, 159–163. [DOI] [PubMed] [Google Scholar]
- Massumi, H. , Shaabanian, M. , Pour, A.H. , Heydarnejad, J. and Rahimian, H. (2009) Incidence of viruses infecting tomato and their natural hosts in the southeast and central regions of Iran. Plant Disease, 93, 67–72. [DOI] [PubMed] [Google Scholar]
- Mavric, I. and Ravnikar, M. (2001) First report of Tomato spotted wilt virus and Impatiens necrotic spot virus in Slovenia. Plant Disease, 85, 1288. [DOI] [PubMed] [Google Scholar]
- Messelink, G.J. and Janssen, A. (2014) Increased control of thrips and aphids in greenhouses with two species of generalist predatory bugs involved in intraguild predation. Biological Control, 79, 1–7. [Google Scholar]
- Messelink, G.J. , van Steenpaal, S.E.F. and Ramakers, P.M.J. (2006) Evaluation of phytoseiid predators for control of western flower thrips on greenhouse cucumber. BioControl, 51, 753–768. [Google Scholar]
- Mo, L.F. , Zhi, J.R. and Tian, T. (2013) Biological control efficiency of Orius similis Zheng (Hemiptera: Anthocoridae) on Frankliniella occidentalis (Pergande) under different spatial and caged conditions. Acta Ecologica Sinica, 33, 7132–7139. [Google Scholar]
- Momol, M.T. , Pappu, H.R. , Dankers, W. , Rich, J.R. and Olson, S.M. (2000) First report of Tomato spotted wilt virus in habanero and tabasco peppers in Florida. Plant Disease, 84, 1154. [DOI] [PubMed] [Google Scholar]
- Moore, E. (1933) The kromnek or kat river diease of tobacco and tomato in the east province (South Africa). Deptment of Agriculture, Union of South Africa. Science Bulletin, 123, 5–28. [Google Scholar]
- Moore, E. and Andessen, E.E. (1939) Notes on plant virus diseases in South Africa. the kromnek disease of tobacco and tomato. Department of Agriculture, Union of South Africa. Science Bulletin, 182, 1–36. [Google Scholar]
- Morales, F. , Arroyave, J.A. , Castillo, J. and Leon, C.D. (1999) Cytopathology of Mazie chlorotic mottle virus in Zea mays L. Maydica, 44, 231–235. [Google Scholar]
- Morse, J.G. and Hoddle, M.S. (2006) Invasion biology of thrips. Annual Review of Entomology, 51, 67–89. [DOI] [PubMed] [Google Scholar]
- Mouden, S. , Sarmiento, K.F. , Klinkhamer, P.G. and Leiss, K.A. (2017) Integrated pest management in western flower thrips: past, present and future. Pest Management Science, 73, 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mound, L.A. (2002) So many thrips–so few tospoviruses//Thrips and tospoviruses. Proceedings of the 7th International Symposium on Thysanoptera. Australian National Insect Collection, Canberra, 15–18.
- Mound, L.A. (2004) Thysanoptera: diversity and interactions. Annual Review of Entomology, 50, 247–269. [DOI] [PubMed] [Google Scholar]
- Moussa, A. Ben , Makni, M. and Marrakchi, M. (2000) Identification of the principal viruses infecting tomato crops in Tunisia. EPPO Bulletin, 30, 293–296. [Google Scholar]
- Mullis, S.W. , Csinos, A.S. , Gitaitis, R.D. and Martinez‐Ochoa, N. (2006) First report of pinaceae in Georgia naturally infected with Tomato spotted wilt virus . Plant Disease, 90, 376. [DOI] [PubMed] [Google Scholar]
- Mullis, S.W. , Langston, D.B. , Gitaitis, R.D. , Sherwood, J.L. , Csinos, A.C. , Riley, D.G . et al (2004) First report of vidalia onion (Allium cepa) naturally infected with Tomato spotted wilt virus and Iris yellow spot virus (family Bunyaviridae, genus Tospovirus) in Georgia. Plant Disease, 88, 1285. [DOI] [PubMed] [Google Scholar]
- Mumford, R.A. , Barker, I. and Wood, K.R. (1996) The biology of the tospoviruses. Annals of Applied Biology, 128, 159–183. [Google Scholar]
- Mumford, R.A. , Jarvis, B. , Morris, J. and Blockley, A. (2003) First report of Chrysanthemum stem necrosis virus (CSNV) in the UK. Plant Pathology, 52, 779. [Google Scholar]
- Nagata, T. , Almeida, A.C.L. , Resende, R.O. and de Avila, A.C. (2004) The competence of four thrips species to transmit and replicate four tospoviruses. Plant Pathology, 53, 136–140. [Google Scholar]
- Naidu, R.A. , Deom, C.M. and Sherwood, J.L. (2001) First report of Frankliniella fusca as a vector of Impatiens necrotic spot tospovirus . Plant Disease, 85, 1211. [DOI] [PubMed] [Google Scholar]
- Niblett, C. and Clafin, L. (1978) Maize lethal necrosis–a new virus disease of maize in Kansas. Plant Disease Report, 62, 15–19. [Google Scholar]
- Nikolić, D. , Stanković, I. , Vučurović, A. , Ristić, D. , Milojević, K. , Bulajić, A . et al (2013) First report of Tomato spotted wilt virus on Brugmansia sp. in Serbia. Plant Disease, 97, 850. [DOI] [PubMed] [Google Scholar]
- Nischwitz, C. , Mullis, S.W. , Gitaitis, R.D. and Csinos, A.S. (2006a) First report of Tomato spotted wilt virus in Soybean (Glycine max) in Georgia. Plant Disease, 90, 524. [DOI] [PubMed] [Google Scholar]
- Nischwitz, C. , Mullis, S.W. , Gitaitis, R.D. , Csinos, A.S. and Olson, S.M. (2006b) First report of Tomato spotted wilt virus in leek (Allium porrum) in the United States. Plant Disease, 90, 525. [DOI] [PubMed] [Google Scholar]
- Northfield, T.D. , Paini, D.R. , Funderburk, J.E. and Reitz, S.R. (2008) Annual cycles of Frankliniella spp. (Thysanoptera: Thripidae) thrips abundance on North Florida uncultivated reproductive hosts: predicting possible sources of pest outbreaks. Annals of the Entomological Society of America, 101, 769–778. [Google Scholar]
- Nyasani, J.O. , Meyhöfer, R. , Subramanian, S. and Poehling, H.M. (2013) Feeding and oviposition preference of Frankliniella occidentalis for crops and weeds in Kenyan French bean fields. Journal of Applied Entomology, 137, 204–213. [Google Scholar]
- Ogada, P.A. and Poehling, H.M. (2015) Sex‐specific influences of Frankliniella occidentalis (western flower thrips) in the transmission of Tomato spotted wilt virus (Tospovirus). Journal of Plant Disease Protection, 122, 264–274. [Google Scholar]
- Okazaki, S. , Okuda, M. , Komi, K. , Yamasaki, S. , Okuda, S. , Sakurai, T . et al (2011) The effect of virus titre on acquisition efficiency of Tomato spotted wilt virus by Frankliniella occidentalis and the effect of temperature on detectable period of the virus in dead bodies. Australas Plant Pathology, 40, 120–125. [Google Scholar]
- Okazaki, S. , Okuda, M. , Komi, K. , Yoshimatsu, H. and Iwanami, T. (2007) Overwintering viruliferous Frankliniella occidentalis (Thysanoptera: Thripidae) as an infection source of Tomato spotted wilt virus in green pepper Fields. Plant Disease, 91, 842–846. [DOI] [PubMed] [Google Scholar]
- Okuda, M. , Fuji, S. , Okuda, S. , Sako, K. and Iwanami, T. (2010) Evaluation of the potential of thirty two weed species as infection sources of Impatiens necrotic spot virus . Journal of Plant Pathology, 92, 357–361. [Google Scholar]
- Okuda, S. , Okuda, M. , Matsuura, S. , Okazaki, S. and Iwai, H. (2013) Competence of Frankliniella occidentalis and Frankliniella intonsa strains as vectors for Chrysanthemum stem necrosis virus . European Journal of Plant Pathology, 136, 355–362. [Google Scholar]
- Otieno, J.A. , Stukenberg, N. , Weller, J. and Poehling, H.M. (2018) Efficacy of LED‐enhanced blue sticky traps combined with the synthetic lure Lurem‐TR for trapping of western flower thrips (Frankliniella occidentalis). Journal of Pest Science, 91, 1301–1314. [Google Scholar]
- Pérez‐Colmenares, Y. , Mejías, A. , Rodríguez‐Román, E. , Avilán, D. , Gómez, J.C. , Marys, E . et al (2015) Identification of Tomato spotted wilt virus associated with fruit damage during a recent virus outbreak in pepper in Venezuela. Plant Disease, 99, 896. [Google Scholar]
- Pappu, H.R. , Jones, R.A.C. and Jain, R.K. (2009) Global status of tospovirus epidemics in diverse cropping systems: successes achieved and challenges ahead. Virus Reseach, 141, 219–236. [DOI] [PubMed] [Google Scholar]
- Parrella, G. (2002) First report of Parietaria mottle virus in Mirabilis jalapa . Plant Pathology, 51, 401. [Google Scholar]
- Parrella, G. , Greco, B. and Troiano, E. (2016) Severe symptoms of mosaic and necrosis in bell pepper associated with Parietaria mottle virus in Italy. Plant Disease, 100, 1514. [Google Scholar]
- Parrella, G. , Greco, B. and Troiano, E. (2017) First report of Parietaria mottle virus associated with yellowing disease in Diplotaxis tenuifolia in Italy. Plant Disease, 101, 850. [Google Scholar]
- Pearce, M. (2005) 2004 Georgia plant disease loss estimates. University of Georgia, Cooperative Extension Service, 24.
- Pearsall, I.A. , and Myers, J.H. (2000) Population dynamics of western Bower thrips (Thysanoptera: Thripidae) in nectarine orchards in British Columbia. Journal of Economic Entomology, 93, 264–275. [DOI] [PubMed] [Google Scholar]
- Peters, D. , Wijkamp, I. , van De Wetering, F. and Goldbach, R. (1996) Vector relations in the transmission and epidemiology of tospoviruses. Acta Horticulturae, 431, 29–43. [Google Scholar]
- Pittman, H. (1927) Spotted wilt of tomatoes. preliminary note concerning the transmission of the spotted wilt of tomatoes by an insect vector (Thrips tabaci Lind.). Australian Council for Scientific and Industrial Research Bulliten, 1, 74–77. [Google Scholar]
- Pourrahim, R. , Farzadfar, S. , Moini, A.A. , Shahraeen, N. and Ahoonmanesh, A. (2001) First report of tomato spotted wilt virus on potatoes in Iran. Plant Disease, 85, 442. [DOI] [PubMed] [Google Scholar]
- Quito‐Avila, D.F. , Alvarez, R.A. and Mendoza, A.A. (2016) Occurrence of maize lethal necrosis in Ecuador: a disease without boundaries. Eurpean Journal of Plant Pathology, 146, 705–710. [Google Scholar]
- Rabelo, L. , Pedrazzoli, D. , Novaes, Q. , Nagata, T. , Rezende, E. and Kitajima, J. (2002) High incidence of tomato chlorotic spot virus in the state of Sao Paulo, Brazil. Fitopatologia Brasileira, 27, 105–112. [Google Scholar]
- Ramasso, E. , Roggero, P. , Dellavalle, G. and Lisa, V. (1997) Necrosi apicale del pomodoro causata da un Ilarvirus. Mikologiya I Fitopatologiya, 1, 71–77. [Google Scholar]
- Rasoulpour, R. and Izadpanah, K. (2007) Characterisation of cineraria strain of Tomato yellow ring virus from Iran. Australas. Plant Pathology, 36, 286–294. [Google Scholar]
- Ravnikar, M. , Vozelj, N. , Mavriè, I. , Svigelj, S. , Zupanèiè, M. and Petroviè, N. (2003) Detection of Chrysanthemum stem necrosis virus and Tomato spotted wilt virus in chrysanthemum. Abstracts 8th International Congress of Plant Pathology ICPP, Christchurch (NZ).
- Reitz S.R. (2009) Biology and ecology of the western flower thrips (Thysanoptera: Thripidae): the making of a pest. Florida Entomology, 92, 7–13. [Google Scholar]
- Reitz, S.R. , Gao, Y.L. and Lei, Z.R. (2011) Thrips: Pests of concern to China and the United States. Journal of Integrative Agriculture, 10, 867–892. [Google Scholar]
- Ren, J. , Lei, Z.R. and Hua, L. (2008) The study on trapping effects of colorful card on Frankliniella occidentalis (Pergande). Chinese Plant Protection, 28, 34–35. [Google Scholar]
- Renukadevi, P. , Nagendran, K. , Nakkeeran, S. , Karthikeyan, G. , Jawaharlal, M. , Alice, D . et al (2015) First report of Tomato spotted wilt virus infection of chrysanthemum in India. Plant Disease, 99, 1190. [Google Scholar]
- Resende, R. , Pozzer, L. , Nagata, T. , Bezerra, I. , Lima, M. , Giordano, L . et al (1996) New tospoviruses found in Brazil. Acta Horticulture, 431, 78–89. [Google Scholar]
- Rico, P. and Hernández, C. (2006) Infectivity of in vitro transcripts from a full‐length cDNA clone of perlargonium flower break virus in an experimental and a natural host. Journal of Plant Pathology, 88, 103–106. [Google Scholar]
- Rico, P. , Hernndez, C. and Hernández, C. (2004) Complete nucleotide sequence and genome organization of Pelargonium flower break virus . Archievs Virology, 149, 641–651. [DOI] [PubMed] [Google Scholar]
- Rico, P. , Ivars, P. , Elena, S.F. and Hernandez, C. (2006) Insights into the selective pressures restricting Pelargonium flower break virus genome variability: evidence for host adaptation. Journal of Virology, 80, 8124–8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley, D. , Sparks Jr, A. , Srinivasan, R. , Kennedy, G. , Fonsah, G. , Scott, J. et al (2018) Thrips: Biology, ecology, and management Sustainable Management of Arthropod Pests of Tomato, pp. 49–71. Academic Press. [Google Scholar]
- Roggero, P. , Ciuffo, M. , Katis, N. , Alioto, D. , Crescenzi, A. , Parrella, G . et al (2000) Necrotic disease in tomatoes in greece and southern Italy caused by the tomato strain of Parietaria mottle virus . Journal of Plant Pathology, 82, 159. [Google Scholar]
- Rosales, M. , Pappu, H. , Arayam, C. and Aljaro, A. (2007) Characterization of Tomato spotted wilt virus (Tospovirus, Bunyaviridae) from lettuce (Lactuca sativa) in Chile. Phytopathology, 97, S101. [Google Scholar]
- Rotenberg, D. , Jacobson, A.L. , Schneweis, D.J. and Whitfield, A.E. (2015) Thrips transmission of tospoviruses. Current Opinion of Virology, 15, 80–89. [DOI] [PubMed] [Google Scholar]
- Saito, T. and Brownbridge, M. (2016) Compatibility of soil‐dwelling predators andmicrobial agents and their efficacy in controlling soil‐dwelling stages of western flower thrips Frankliniella occidentalis . Biological Control, 92, 92–100. [Google Scholar]
- Sakurai, T. , Inoue, T. and Tsuda, S. (2004) Distinct efficiencies of Impatiens necrotic spot virus transmission by five thrips vector species (Thysanoptera: Thripidae) of tospoviruses in Japan. Applied Entomology and Zoology, 39, 71–78. [Google Scholar]
- Salamon, P. , Nemes, K. , Salánki, K. and Palkovics, L. (2012) First report of natural infection of pea (Pisum sativum) by Tomato spotted wilt virus in Hungary. Plant Disease, 96, 295. [DOI] [PubMed] [Google Scholar]
- Samuel, G. , Bald, J. and Pittman, H. (1930) Investigations on “spotted wilt” of tomatoes. Australian Council for Scientific and Industrial Research, 44, 1–64. [Google Scholar]
- Sarkar, S.C. , Wang, E.D. , Zhang, Z.K. , Wu, S.Y. and Lei, Z.R. (2019) Laboratory and glasshouse evaluation of the green lacewing, Chrysopa pallens (Neuroptera: Chrysopidae) against the western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). Applied Entomology and Zoology, 10.1007/s13355-018-0601-9 [DOI] [Google Scholar]
- Schneweis, D.J. , Whitfield, A.E. and Rotenberg, D. (2017) Thrips developmental stage‐specific transcriptome response to tomato spotted wilt virus during the virus infection cycle in Frankliniella occidentalis, the primary vector. Virology, 500, 226–237. [DOI] [PubMed] [Google Scholar]
- Schuch, U.K. , Rdak, R.A. and Behtke, J.A. (1998) Cultivar, fertilizer and irrigation effect vegetative growth and susceptibility of chrysanthemum to western flower thrips. Journal of America Society Horticulture Science, 123, 727–733. [Google Scholar]
- Sialer, M.M.F. and Gallitelli, D. (2000) The occurrence of Impatiens necrotic spot virus and Tomato spotted wilt virus in mixed infection in tomato. Journal of Plant Pathology, 82, 244. [Google Scholar]
- Smith, K. (1932) Studies on plant virus diseases XI. Further experiments with a ringspot virus: its identification with spotted wilt of tomato. Annals of Applied Biology, 19, 305–320. [Google Scholar]
- Spadotti, D. , Leão, E. and Rocha, K. (2014) First report of Groundnut ringspot virus in cucumber fruits in Brazil. New Dosease Reports, 5197.
- Stafford, C.A. , Walker, G.P. and Ullman, D.E. (2011) Infection with a plant virus modified vector feeding behavior. Proceedings of the National Academy of Sciences USA, 108, 9350–9355. [DOI] [PMC free article] [PubMed]
- Stanković, I. , Bulajić, A. , Vučurović, A. , Ristić, D. , Jović, J. and Krstić, B. (2011) First report of Tomato spotted wilt virus on Gerbera hybrida in Serbia. Plant Disease, 95, 226. [DOI] [PubMed] [Google Scholar]
- Stanković, I. , Bulajić, A. , Vučurović, A. , Ristić, D. , Milojević, K. , Nikolić, D . et al (2012) First report of Tomato spotted wilt virus infecting onion and garlic in Serbia. Plant Disease, 96, 918. [DOI] [PubMed] [Google Scholar]
- Stanković, I. , Bulajić, A. , Vučurović, A. , Ristić, D. , Milojević, K. , Nikolić, D . et al (2013) First report of Tomato spotted wilt virus on chrysanthemum in Serbia. Plant Disease, 97, 150. [DOI] [PubMed] [Google Scholar]
- Stavisky, J. , Funderburk, J.E. , Brodbeck, B.V. , Olson, S.M. and Andersen, P.C. (2002) Population dynamics of Frankliniella spp. and tomato spotted wilt incidence as influenced by cultural management tactics in tomato. Journal of Economic Entomology, 95, 1216–1221. [DOI] [PubMed] [Google Scholar]
- Stone, B.O.M. and Hollings, M. (1973) Some properties of pelargonium flower‐break virus. Annual of Applied Biology, 75, 15–23. [Google Scholar]
- Subbaiah, K.V. , Sai Gopal, D.V.R. and Krishna Reddy, M. (2000) First report of a tospovirus on sunflower (Helianthus annus L.) from India. Plant Disease, 84, 1343. [DOI] [PubMed] [Google Scholar]
- Szostek, S.A. , Rodriguez, P. , Sanchez, J. , Adkins, S. and Naidu, R.A. (2017) Western flower thrips can transmit Tomato spotted wilt virus from virus‐infected tomato fruits. Plant Health Prog, 18(1), 1–6. [Google Scholar]
- Terry, L.I. (1997) Host selection, communication and reproductive behavior Thrips as Crop Pests (ed. Lewis T.), pp. 65–118. CAB International, New York. [Google Scholar]
- Tinoco, C.E. , Gutiérrez, G.A.M. , Bolaños, T.A. and Sánchez, D.M. (2014) Screen porosity and exclusion of pest in greenhouse tomatoes (Solanum lycopersicum L.). Southwest Entomology, 39, 625–634. [Google Scholar]
- Toledo‐Hernández, R.A. , Ortiz‐Girón, J.A. and Sánchez, D. (2017) Preliminary observations on pathogenicity of commercial formulations of Metarhizium anisopliae and Beauveria bassiana entomopathogenic fungi for control of Frankliniella invasor under laboratory conditions. Southwest Entomology, 42, 1035–1040. [Google Scholar]
- Tommasini, M.G. and Maini, S. (1995) Frankliniella occidentalis and other thrips harmful to vegetable and ornamental crops in Europe Biological Control of Thrips (eds. Loomans A.J.L., van Lenteren J.C., Tommasini M.G., Maini S. & Riudavets J.), Papers 95.1, pp. 1–42. Wageningen Agricultural University, the Netherlands. [Google Scholar]
- Trkulja, V. , Salapura, J.M. , Ćurković, B. , Stanković, I. , Bulajić, A. , Vučurović, A . et al (2013) First report of Impatiens necrotic spot virus on begonia in Bosnia and Herzegovina. Plant Disease, 97, 1004. [DOI] [PubMed] [Google Scholar]
- Vaira, A.M. , Roggero, P. , Luisoni, E. , Masenga, V. , Milne, R.G. and Lisa, V. (1993) Characterization of two tospoviruses in Italy: tomato spotted wilt and impatiens necrotic spot. Plant Pathology, 52, 530–542. [Google Scholar]
- Vasanthi, V.J. , Kandan, A. , Raguchander, T. , Ramanathan, A. , Balasubramanian, P. and Samiyappan, R. (2010) Pseudomonas fluorescens‐based formulations for management of tomato leaf curl gemini virus (TLCV) and enhanced yield in tomato. Archives of Phytopathology, 17, 553–569. [Google Scholar]
- Vasanthi, V.J. , Samiyappan, R. and Vetrivel, T. (2017) Management of tomato spotted wilt virus (TSWV) and its thrips vector in tomato using a new commercial formulation of Pseudomonas fluorescens strain and neem oil. Journal of Entomol Zoology Studies, 5, 1441–1445. [Google Scholar]
- Verhoeven, J. , Roenhorst, J. , Cortes, I. and Peters, D. (1996) Detection of a novel tospovirus in chrysanthemum. Acta horticulture, 432, 44–51. [Google Scholar]
- Verhoeven, T.J. and Roenhorst, J.W. (1998) Occurrence of tospoviruses in the Netherlands. Fourth International Symposium on Tospoviruses and Thrips in Floral and Vegetable Crops pp. 77. Wageningen, The Netherlands: Graduate Schools of Experimental Plant Sciences and Production Ecology.
- Vicchi, V. , Fini, P. and Cardoni, M. (1999) Presence of impatiens necrotic spot tospovirus (INSV) on vegetable crops in Emilia‐Romagna region. Mikologiya I Fitopatologiya, 49, 52–55. [Google Scholar]
- Wang, H. , Lei, Z. , Reitz, S. , Li, Y. and Xu, X. (2013) Production of microsclerotia of the fungal entomopathogen Lecanicillium lecanii (Hypocreales: Cordycipitaceae) as a biological control agent against soil‐dwelling stages of Frankliniella occidentalis (Thysanoptera: Thripidae). Biocontrol Science and Technology, 23, 234–238. [Google Scholar]
- Webster, C.G. , Frantz, G. , Reitz, S.R. , Funderburk, J.E. , Mellinger, H.C. , McAvoy, E . et al (2015) Emergence of Groundnut ringspot virus and Tomato chlorotic spot virus in vegetables in Florida and the southeastern United States. Phytopathology, 105, 388–398. [DOI] [PubMed] [Google Scholar]
- Webster, C.G. , Perry, K.L. , Lu, X. , Horsman, L. , Frantz, G. , Mellinger, H.C . et al (2010) First report of Groundnut ringspot virus infecting tomato in South Florida. Plant Health Progress. [Google Scholar]
- Webster, C.G. , Reitz, S.R. , Perry, K.L. and Adkins, S. (2011) A natural M RNA reassortant arising from two species of plant‐ and insect‐infecting bunyaviruses and comparison of its sequence and biological properties to parental species. Virology, 413, 216–225. [DOI] [PubMed] [Google Scholar]
- Webster, C.G. , Turechek, W.W. , Mellinger, H.C. , Frantz, G , Roe, N. , Yonce, H . et al (2011) Expansion of Groundnut ringspot virus host and geographic ranges in solanaceous vegetables in Peninsular Florida. Plant Health Progress. [Google Scholar]
- Wei, M.S. , Li, G.F. , Ma, J. and Kong, J. (2015) First report of Pelargonium flower break virus infecting Pelargonium plants in China. Plant Disease, 99, 735. [Google Scholar]
- Weiss, A. , Dripps, J. and Funderburk, J. (2009) Assessment of implementation and sustainability of integrated pest management programs. Florida Entomologist, 92, 24–28. [Google Scholar]
- Whitfield, A.E. , Campbell, L.R. , Sherwood, J.L. and Ullman, D.E. (2003) Tissue blot immunoassay for detection of Tomato spotted wilt virus in Ranunculus asiaticus and other ornamentals. Plant Disease, 87, 618–622. [DOI] [PubMed] [Google Scholar]
- Whitfield, A.E. , Ullman, D.E. , and German, T.L. (2004) Expression and characterization of a soluble form of tomato spotted wilt virus glycoprotein GN. Journal of Virology, 78(23), 13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield, A.E. , Ullman, D.E. and German, T.L. (2005a) Tospovirus–thrips interactions. Annual Review of Phytopathology, 43, 459–489. [DOI] [PubMed] [Google Scholar]
- Whitfield, A.E. , Ullman, D.E. and German, T.L. (2005b) Tomato spotted wilt virus glycoprotein GC is cleaved at acidic pH. Virus Research, 110, 183–186. [DOI] [PubMed] [Google Scholar]
- Whitfield, A.E. , Falk, B.W. and Rotenberg, D. (2015) Insect vector‐mediated transmission of plant viruses. Virology, 479, 278–289. [DOI] [PubMed] [Google Scholar]
- Wijkamp, I. (1995a) Distinct levels of specificity in thrips transmission of tospoviruses. Phytopathology, 85, 1069–1074. [Google Scholar]
- Wijkamp, I. , Fvande, W. , Goldbach, R. and Peters, D. (2010) Transmission of tomato spotted wilt virus by Frankliniella occidentalis; median acquisition and inoculation access period. Annual of Applied Biology, 129, 303–313. [Google Scholar]
- Wijkamp, I. (1995b) Virus‐Vector Relationships in the Transmission of Tospoviruses. Ph.D. thesis, Wageningen, The Netherlands.
- Wilson, C.R. , Wilson, A.J. and Pethybridge, S.J. (2000) First report of Tomato spotted wilt virus in common agapanthus. Plant Disease, 84, 491. [DOI] [PubMed] [Google Scholar]
- Wu, K. , Zheng, K.Y. , Zhang, Z.K. , McBeath, J.H. and Dong, J.H. (2016a) First report of Crinum asiaticum as a natural host of tomato zonate spot virus in China. Journal of Plant Pathology, 97, 76. [Google Scholar]
- Wu, S.Y. , Zhang, Z.K. , Gao, Y.L. , Xu, X.N. and Lei, Z.R. (2016b) Interactions between foliage‐ and soil‐dwelling predatory mites and consequences for biological control of Frankliniella occidentalis . BioControl, 61, 717–727. [Google Scholar]
- Wu, S.Y. , Tang, L.D. , Zhang, X.R. , Xing, Z.L. , Lei, Z.R. and Gao, Y.L. (2018) A decade of a thrips invasion in China: lessons learned. Ecotoxicology, 27, 1032–1038. [DOI] [PubMed] [Google Scholar]
- Wu, S.Y. , He, Z. , Wang, E.D. , Xu, X.N. and Lei, Z.R. (2017) Application of Beauveria bassiana and Neoseiulus barkeri for improved control of Frankliniella occidentalis in greenhouse cucumber. Crop Protection, 96, 83–87. [Google Scholar]
- Xiao, L. , Li, Y.Y. , Lan, P.X. , Tan, G.L. , Ding, M. , Li, R.H . et al (2016) First report of Tomato spotted wilt virus infecting cowpea in China. Plant Disease, 100, 233. [Google Scholar]
- Yang, H. , Ozias‐Akins, P. , Culbreath, A.K. , Gorbet, D.W. , Weeks, J.R. , Mandal, B . et al (2004) Field evaluation of Tomato spotted wilt virus resistance in transgenic peanut (Arachis hypogaea). Plant Disease, 88, 259–264. [DOI] [PubMed] [Google Scholar]
- Yoon, J.Y. , Choi, G.S. and Choi, S.K. (2017a) First report of Chrysanthemum stem necrosis virus on Chrysanthemum morifolium in Korea. Plant Disease, 101, 264. [Google Scholar]
- Yoon, J.Y. , Choi, G.S. and Choi, S.K. (2017b) First report of Tomato spotted wilt virus in Eustoma grandiflorum in Korea. Plant Disease, 101, 515. [DOI] [PubMed] [Google Scholar]
- Zarzyńska‐Nowak, A. , Rymelska, N. , Borodynko, N. and Hasiów‐Jaroszewska, B. (2016) The occurrence of Tomato yellow ring virus on tomato in Poland. Plant Disease, 100, 234. [Google Scholar]
- Zhang, X.R. , Lei, Z.R. , Reitz, S.R. , Wu, S.Y. and Gao, Y.L. (2019) Laboratory and greenhouse evaluation of a granular formulation of Beauveria bassiana for control of western flower thrips, Frankliniella occidentalis . Insects, 10(2), 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z.J. , Wu, Q.J. , Li, X.F. , Zhang, Y.J. , Xu, B.Y. and Zhu, G.R. (2010) Life history of western flower thrips, Frankliniella occidentalis (Thysan., Thripae), on five different vegetable leaves. Journal of Applied Entomology, 131, 347–354. [Google Scholar]
- Zhang, G.F. , Meng, X.Q. , Min, L. , Qiao, W.N. and Wan, F.H. (2012) Rapid diagnosis of the invasive species, Frankliniella occidentalis (Pergande): a species‐specific COI marker. Journal of Applied Entomology, 136, 410–420. [Google Scholar]
- Zheng, X. , Zhang, J. , Chen, Y. , Dong, J. and Zhang, Z. (2014) Effects of Tomato zonate spot virus infection on the development and reproduction of its vector Frankliniella occidentalis (Thysanoptera: Thripidae). Florida Entomology, 97, 549–554. [Google Scholar]
- Zheng, Y.X. , Huang, C.H. , Cheng, Y.H. , Kuo, F.Y. and Jan, F.J. (2010) First report of Tomato spotted wilt virus in sweet pepper in Taiwan. Plant Disease, 94, 920. [DOI] [PubMed] [Google Scholar]
- Zindović, J. , Bulajić, A. , Krstić, B. , Ciuffo, M. , Margaria, P. and Turina, M. (2011) First report of Tomato spotted wilt virus on pepper in Montenegro. Plant Disease, 95, 882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The worldwide distribution of Frankliniella occidentalis.
Table S2. The worldwide distribution and host of viruses transmitted by Frankliniella occidentalis.


