Abstract
Objective
The obesity epidemic is a public health concern, warranting further research into pharmacological treatments for weight management (WM) as an adjunct to lifestyle interventions. The Semaglutide Treatment Effect in People with obesity (STEP) program aims to investigate the effect of semaglutide versus placebo on weight loss, safety, and tolerability in adults with obesity or overweight.
Methods
Across five phase 3 trials (NCT03548935, WM; NCT03552757, WM in type 2 diabetes; NCT03611582, WM with intensive behavioral therapy; NCT03548987, sustained WM; and NCT03693430, long‐term WM), ~5,000 participants are being randomly assigned to receive semaglutide 2.4 mg once weekly subcutaneously versus placebo. Results will be available in 2020/2021. For all trials, the primary end point is change from baseline to end of treatment in body weight.
Results
Participants have a mean age of 46.2 to 55.3 years, are mostly female (mean: 74.1%‐81.0%), and have a mean BMI of 35.7 to 38.5 kg/m2 and a mean waist circumference of 113.0 to 115.7 cm.
Conclusions
The STEP program evaluates the efficacy and safety of semaglutide 2.4 mg subcutaneously once weekly in a broad population. The trials will provide insights on WM in people with obesity with and without type 2 diabetes and on long‐term follow‐up.

Study Importance.
What is already known?
-
►
Lifestyle intervention can often be insufficient in treating obesity; however, when combined with pharmacological treatments, clinically relevant weight loss and amelioration of obesity complications can be achieved.
-
►
The GLP‐1 receptor agonist liraglutide is approved for the treatment of people with obesity; a phase 2 trial with semaglutide, a GLP‐1 analogue, suggested greater efficacy.
What does this study add?
-
►
The Semaglutide Treatment Effect in People with obesity (STEP trials 1‐5) clinical development program is one of the largest clinical trial programs for the management of obesity and assessed the efficacy and safety of semaglutide 2.4 mg subcutaneously once weekly.
-
►
The STEP program is designed to elucidate key aspects of the medical management of obesity across various races and ethnicities, including whether semaglutide 2.4‐mg dosing once weekly is reliably effective (STEP trials 1‐5) for patients with and without diabetes, as an adjunct to intensive behavioral therapy plus low‐calorie diet, and with longer term administration for weight loss maintenance.
How might these results change the focus of clinical practice?
-
►
These pivotal trials will provide data on the efficacy and safety of a new treatment, semaglutide, which is anticipated to provide clinically meaningful and durable weight loss beyond what is currently achievable with the available agents for obesity.
Introduction
Burden of obesity
Obesity is a chronic, relapsing, progressive disease (1) with a multifactorial origin, including genetic, metabolic, behavioral, sociocultural, and environmental factors (2, 3). The clinical complications of obesity include cardiovascular diseases (CVD; e.g., ischemic heart disease, heart failure), metabolic diseases (type 2 diabetes [T2D]), mechanical dysfunction (musculoskeletal disorders [e.g., osteoarthritis]), sleep apnea, and malignancy (4, 5, 6, 7). Around 13% to 19.5% of adults globally have obesity, and the prevalence of obesity is predicted to continue to rise (5, 8). There is a recognition that much of the pathophysiology of obesity involves abnormal satiety and feeding signaling within the brain (9). The hypothalamus, mesolimbic system, and executive functioning are all implicated in the physiology of obesity (9). Thus, there is a necessity for developing more effective novel treatment approaches that address these central nervous system processes (2, 9, 10).
Treatment of obesity
Lifestyle interventions are the cornerstone of weight management (WM) (11), but alone they are generally associated with moderate weight loss (WL) that is gradually regained (9, 12, 13). Maintaining WL is inherently difficult because of counter‐regulatory neuroendocrine pathways that promote weight regain by influencing hunger and satiety, which are a component of appetite, and potentially by decreasing energy expenditure (14, 15). Antiobesity medications (AOMs) may provide a valuable adjunct to lifestyle interventions, which typically have a limited effect on WL, to help people achieve and maintain healthy behaviors that are consistent with sustaining WL.
The US Food and Drug Administration and European Medicines Agency have approved AOMs that have been shown to achieve clinically significant WL when used as adjuncts to lifestyle interventions (2, 16). However, most approved AOMs have moderate efficacy, quantified as a < 10% reduction in mean WL over that achieved with lifestyle intervention alone, with significant limitations related to adverse effects, cost, or restrictions on use (2). There is a need for additional AOMs that can induce and sustain greater clinically meaningful WL and that have a convenient form of administration that improves associated complications, such as T2D and CVD. One potential new AOM is the glucagon‐like peptide 1 (GLP‐1) analogue semaglutide, which has been developed with these characteristic features in mind (11, 17).
Semaglutide pharmacology
Semaglutide is a long‐acting GLP‐1 analogue that mimics the effects of native GLP‐1, which promotes WL by reducing energy intake, increasing satiety and satiation, and reducing hunger, as well as enhancing glycemic control (17). Many GLP‐1s have been approved for the treatment of T2D, but only liraglutide 3.0 mg daily has been approved for WM. Semaglutide is approved for treatment of diabetes at the dosage of ≤ 1.0 mg once weekly subcutaneously or in oral tablet form at a dosage of up to 14 mg (2, 17, 18, 19, 20).
Current phase 3 trials are investigating semaglutide as a new GLP‐1 analogue for the treatment of obesity because greater WL was observed with semaglutide than liraglutide (21). In the phase 2 trial of semaglutide in adults with obesity, a 0.4‐mg dose daily was well tolerated, and patients experienced a mean WL at week 52 from baseline of −13.8% compared with −7.8% for liraglutide 3.0 mg and −2.3% for placebo (21).
Semaglutide Treatment Effect in People with obesity program
The Semaglutide Treatment Effect in People with obesity (STEP) clinical trial development program is evaluating semaglutide 2.4 mg, administered subcutaneously once weekly, for WM in people with obesity or overweight. The purpose of the program is to demonstrate the effect, safety, and tolerability profile of semaglutide 2.4 mg on WL, to enable further clinical development, and to support regulatory approval of semaglutide for WM. The trial design, objectives, end points, and baseline characteristics of five of the STEP trials are presented in this article.
Methods
Trial designs
This article covers five of the ongoing phase 3, double‐blinded, randomized, multicenter, and multinational trials that assess semaglutide (2.4 mg subcutaneously once weekly) versus placebo for WM in adults with obesity or overweight and with and without T2D (Table 1). All participants receive periodic counseling, and support for all trials is provided by a multidisciplinary team, including a dietitian or a similarly qualified health care professional. Nonmonetary incentives are provided throughout the program, such as kettle balls and jump ropes, to encourage exercise and a healthy lifestyle.
TABLE 1.
Enrollment, objectives, and end points for STEP trials
| Trial | WM | WM in T2D | WM with IBT (US only) | Sustained WM | Long‐term WM |
|---|---|---|---|---|---|
| Other trial names | STEP 1, NCT03548935 | STEP 2, NCT03552757 | STEP 3, NCT03611582 | STEP 4, NCT03548987 | STEP 5, NCT03693430 |
| Participants enrolled, n | 1,961 | 1,210 | 611 | 902 | 304 |
| EOT, wk | 68 | 68 | 68 | 68 | 104 |
| Trial objectives | To show superiority of semaglutide 2.4 mg versus placebo a on WL and to compare safety and tolerability in adults with obesity or overweight, without T2D | To show superiority of semaglutide 2.4 mg versus placebo and semaglutide 1.0 mg OW a on WL and to compare safety and tolerability in adults with T2D and either obesity or overweight | To maximize the effect of semaglutide 2.4 mg versus placebo b on WL in adults with obesity or overweight, without T2D | To maintain the effect of semaglutide 2.4 mg versus placebo a on WL from randomization to EOT and baseline to EOT and to compare safety in adults with obesity or overweight who reached the target dose of semaglutide during run‐in | To show superiority of semaglutide 2.4 mg versus placebo a on WL and to compare safety and tolerability in adults with obesity or overweight after 2 years of treatment |
| Primary end points | |||||
| Change from baseline to EOT in body weight, % | X | X | X | X c | X |
| ≥ 5% WL from baseline after EOT, yes/no | X | X | X | NA | X |
| Confirmatory secondary end points | |||||
| ≥ 10% WL from baseline after EOT, yes/no | X | X | X | NA | X |
| ≥ 15% WL from baseline after EOT, yes/no | X | X | X | NA | X |
| Change from baseline to EOT | |||||
| Waist circumference, cm | X | X | X | X c | X |
| Systolic blood pressure, mmHg | X | X | X | X c | X |
| SF‐36 physical functioning | X | X | X | X c | NA |
| IWQOL‐Lite‐CT physical function | X | X | NA | NA | NA |
| Other | NA | Body weight of semaglutide 2.4 mg OW versus semaglutide 1 mg, %; HbA1c, % (mmol/mol) | NA | NA | NA |
As adjunct to lifestyle intervention (−500‐kcal/d diet + 150 min/wk of physical activity).
As adjunct to intensive behavioral therapy including initial 8‐week low‐calorie diet.
Primary and confirmatory secondary efficacy end points in STEP 4 are changes between randomization (week 20) and EOT (68 weeks).
EOT, end of treatment; HbA1c, hemoglobin A1c; IBT, intensive behavioral therapy; IWQOL‐Lite‐CT, Impact of Weight on Quality of Life, Lite Clinical Trials Version; NA, not applicable; OW, once weekly; SF‐36, Short Form36v2 Health Survey, Acute Version; STEP, Semaglutide Treatment Effect in People with obesity; T2D, type 2 diabetes; WL, weight loss; WM, weight management; X, included in trial.
In the WM trial (STEP 1, NCT03548935), 1,961 adults with obesity or overweight, without T2D, are being randomly assigned in a 2:1 manner to receive semaglutide 2.4 mg or placebo to assess WL (Figure 1, Table 1). A subpopulation of participants will have their body composition assessed by dual‐energy x‐ray absorptiometry (DXA) to test the hypothesis that WL is primarily caused by reduction in fat mass, resulting from treatment, in accordance with the European Medicines Agency and US Food and Drug Administration guidelines (22, 23).
Figure 1.
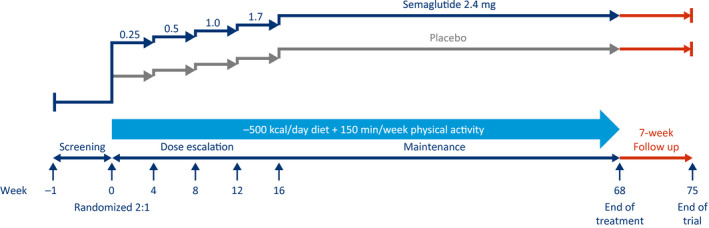
Weight management trial design (Semaglutide Treatment Effect in People with obesity 1). This is a 68‐week, randomized, double‐blind, placebo‐controlled, two‐armed, parallel‐group, multicenter, multinational clinical trial, with 7 weeks of follow‐up without treatment for safety assessments, comparing semaglutide 2.4 mg (subcutaneously, once weekly) with placebo, as an adjunct to lifestyle intervention, in people with obesity or overweight.
In the WM trial in T2D (STEP 2, NCT03552757), 1,210 adults with obesity or overweight, and with T2D, are being randomly assigned 1:1:1 to receive either semaglutide 2.4 mg, semaglutide 1.0 mg once weekly, or placebo to assess WL (Figure 2, Table 1).
Figure 2.
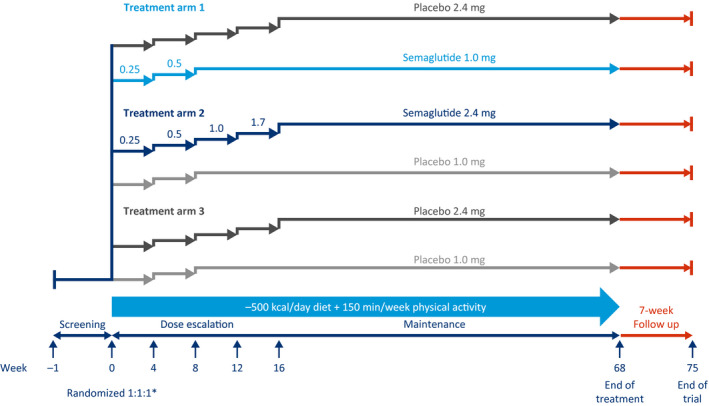
Weight management in type 2 diabetes (T2D) trial design (Semaglutide Treatment Effect in People with obesity 2). This is a 68‐week, randomized, double‐blind, double‐dummy, placebo‐controlled, three‐armed, multicenter, multinational clinical trial, with 7 weeks of follow‐up without treatment for safety assessments, comparing semaglutide 2.4 mg (subcutaneously, once weekly) with placebo, as an adjunct to lifestyle intervention, in people with obesity or overweight and T2D. *Randomization was stratified according to background diabetes treatment; diet and physical activity only or treatment with single‐compound metformin or sodium‐glucose cotransporter 2 inhibitor (SGLT2i) and single‐compound agents for diabetes (sulphonylurea [SU] or glitazone) or combination treatment with up to three agents for diabetes (metformin, SU, SGLT2i, or glitazone).
In the WM with intensive behavioral therapy (IBT) trial only conducted in the United States (STEP 3, NCT03611582), 611 adults with obesity or overweight, without T2D, are being randomly assigned in a 2:1 manner to receive semaglutide 2.4 mg or placebo to assess WL (Figure 3, Table 1). Treatment is administered as an adjunct to IBT, in addition to an initial 8‐week, low‐calorie diet, followed by 60 weeks of a hypocaloric diet and increased physical activity.
Figure 3.
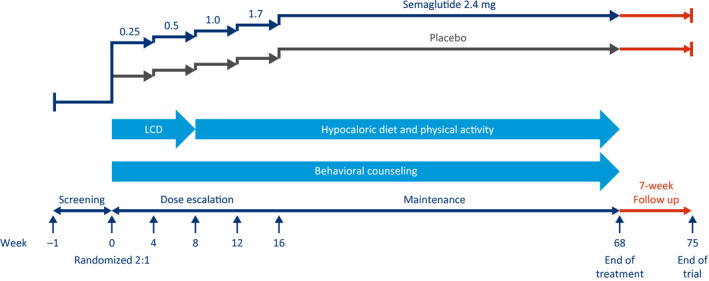
Weight management with intensive behavioral therapy trial design, only conducted in the United States (Semaglutide Treatment Effect in People with obesity 3). This is a 68‐week, randomized, double‐blind, placebo‐controlled, two‐armed, parallel‐group, multicenter clinical trial, with 7 weeks of follow‐up without treatment for safety assessments, comparing semaglutide 2.4 mg (subcutaneously, once weekly) with placebo, as an adjunct to intensive behavioral therapy and low‐calorie diet (LCD), in people with obesity or overweight.
In the sustained WM trial (STEP 4, NCT03548987), 902 participants with obesity or overweight, without T2D, are being treated with semaglutide 2.4 mg once weekly. Those completing a 20‐week run‐in period are being randomly assigned in a 2:1 manner to receive continued semaglutide 2.4 mg or placebo for an additional 48 weeks to assess WL (Figure 4, Table 1). Approximately 750 eligible participants will be randomly assigned. In addition, it has a withdrawal trial design to assess the change in weight after switching from semaglutide to placebo.
Figure 4.
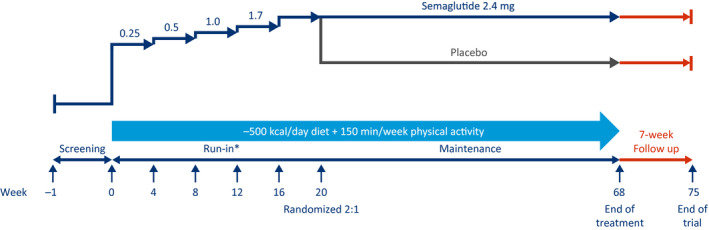
Sustained weight management trial design (Semaglutide Treatment Effect in People with obesity 4). This is a 68‐week, randomized, double‐blind, placebo‐controlled, two‐armed, multicenter, multinational withdrawal clinical trial, with 7 weeks of follow‐up without treatment for safety assessments, comparing semaglutide 2.4 mg (subcutaneously, once weekly) with placebo, as an adjunct to lifestyle intervention, in people with obesity or overweight. *During the 20‐week run‐in period, participants start a dose escalation (visit 2 [week 0]) with semaglutide 2.4 mg (subcutaneously, once weekly) as an adjunct to a reduced‐calorie diet and increased physical activity for 20 weeks. The run‐in period includes 4 weeks at the target dose (semaglutide subcutaneously, 2.4 mg once weekly).
In the long‐term WM trial (STEP 5, NCT03693430), 304 participants with obesity or overweight, without T2D, are being randomly assigned in a 1:1 manner to receive semaglutide 2.4 mg or placebo to assess WL (Figure 5, Table 1) over a 2‐year period.
Figure 5.
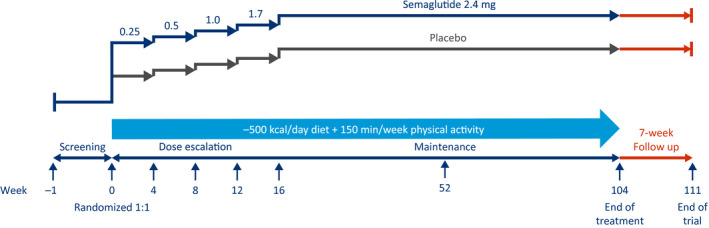
Long‐term weight management trial design (Semaglutide Treatment Effect in People with obesity 5). This is a 104‐week, randomized, double‐blind, placebo‐controlled, two‐armed, parallel‐group, multicenter, multinational clinical trial, with 7 weeks of follow‐up without treatment for safety assessments, comparing semaglutide 2.4 mg (subcutaneously, once weekly) with placebo, as an adjunct to lifestyle intervention in people with obesity or overweight.
Participants in all treatment groups, including placebo, are receiving the trial product as an adjunct to lifestyle intervention. In all trials except for the WM with IBT trial (STEP 3), this is defined as a 500‐kcal/d deficit relative to the estimated total energy expenditure calculated at randomization together with a recommended 150 min/wk of physical activity.
Randomization and treatment
Randomization for all participants is being conducted by an interactive Web‐based response system. In the WM in T2D trial (STEP 2), randomization is being stratified according to background diabetes treatment: diet and physical activity only or treatment with single‐compound metformin or sodium‐glucose cotransporter 2 inhibitors and single‐compound oral agents for diabetes (sulphonylurea or glitazone) versus combination treatment with up to three agents for diabetes (metformin, sulphonylurea, sodium‐glucose cotransporter 2 inhibitor, or glitazone). Participants are being further stratified by screening value of hemoglobin A1c (HbA1c): < 8.5% versus ≥ 8.5%.
Trial populations
The key eligibility criteria are shown in Table 2. Male or female participants qualifying for eligibility are ≥ 18 years of age and have a history of at least one self‐reported unsuccessful dietary effort to lose body weight. Adults are considered eligible for the DXA subtrial in STEP 1 if they have a BMI ≤ 40 kg/m2 at screening and if the quality of the baseline DXA is found to be acceptable by the imaging laboratory before randomization into the subtrial. For the WM trials without T2D (STEP trials 1, 3, 4, and 5), eligible adults have a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 with the presence of weight‐related complications (treated or untreated): dyslipidemia, obstructive sleep apnea, hypertension, or CVD. For the WM in T2D trial (STEP 2), eligible participants are required to have a BMI ≥ 27 kg/m2 and a diagnosis of T2D (HbA1c: 7%‐10% [53‐86 mmol/mol]) ≥ 180 days prior to the day of screening.
TABLE 2.
Key eligibility criteria for STEP trials
| Criteria | WM, STEP 1 | WM in T2D, STEP 2 | WM with IBT, STEP 3 | Sustained WM, STEP 4 | Long‐term WM, STEP 5 |
|---|---|---|---|---|---|
| Key inclusion | |||||
| Man or woman aged ≥ 18 years | X | X | X | X | X |
| BMI ≥ 27 kg/m2 | NA | X | NA | NA | NA |
| BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 with ≥ 1 weight‐related comorbidity (treated or untreated): hypertension, dyslipidemia, obstructive sleep apnea, or CVD | X | NA | X | X | X |
| History of at least 1 self‐reported unsuccessful dietary effort to lose weight | X | X | X | X | X |
| Diagnosed with T2D ≥ 180 days prior to screening | NA | X | NA | NA | NA |
| Treated with diet and exercise alone or stable treatment with metformin, SU, SGLT2i, glitazone as single‐agent therapy, or ≤ 3 agents for diabetes (metformin, SU, SGLT2i, or glitazone) according to local label | NA | X | NA | NA | NA |
| HbA1c 7%‐10% (53‐86 mmol/mol) | NA | X | NA | NA | NA |
| Key exclusion | |||||
| HbA1c ≥ 48 mmol/mol (6.5%) at screening a | X | NA | X | X | X |
| History of T1D or T2D | X | NA | X | X | X |
| Treatment with glucose‐lowering agent(s) < 90 days before screening | X | NA | X | X | X |
| Treatment with GLP‐1 RA < 180 days before screening | X | X | NA | NA | NA |
| Treatment with any medication for diabetes or obesity not stated in inclusion criteria < 90 days before screening | NA | X | NA | NA | NA |
| Treatment with any other investigational drugs for diabetes < 90 days before screening or any investigational drugs not affecting diabetes < 30 days before screening | NA | X | NA | NA | NA |
| Self‐reported change in body weight > 5 kg (11 lb) < 90 days before screening | X | X | X | X | X |
| Uncontrolled thyroid disease: TSH > 6.0 mIU/L or < 0.4 mIU/L at screening a | X | X | X | X | X |
| Participants unable to adhere to low‐calorie diet and physical activity | NA | NA | X | NA | NA |
| Acute pancreatitis < 180 days before screening | X | X | X | X | X |
| History or presence of chronic pancreatitis | X | X | X | X | X |
| Calcitonin ≥ 100 ng/L at screening a | X | X | X | X | X |
| Renal impairment eGFR 40 at screening a | |||||
| eGFR < 15 mL/min/1.73 m2 | X | NA | X | X | X |
| eGFR < 30 mL/min/1.73 m2 | NA | X | NA | NA | NA |
| eGFR < 60 mL/min/1.73 m2 | NA | X b | NA | NA | NA |
| MI, stroke, hospitalization for unstable angina, or TIA < 60 days before screening | X | X | X | X | X |
| Classified as New York Heart Association class 4 | X | X | X | X | X |
Central laboratory measured.
In participants treated with SGLT2i.
CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; GLP‐1 RA, glucagon‐like peptide 1 receptor agonist; HbA1c, hemoglobin A1c; IBT, intensive behavioral therapy; MI, myocardial infarction; NA, not applicable; SGLT2i, sodium‐glucose cotransporter 2 inhibitor; STEP, Semaglutide Treatment Effect in People with obesity; SU, sulphonylurea; T1D, type 1 diabetes; T2D, type 2 diabetes; TIA, transient ischemic attack; TSH, thyroid‐stimulating hormone; WM, weight management; X, included in trial.
Adults are excluded from the trials if there is a self‐reported change in body weight of > 5 kg within 90 days before screening. For trials that excluded patients with T2D (STEP trails 1, 3, 4, and 5), adults are excluded if they have a history of type 1 diabetes mellitus or T2D mellitus, HbA1c ≥ 6.5% (48 mmol/mol), or previous treatment with glucose‐lowering agents or any AOM within the past 90 days before screening.
For all trials, treatment discontinuation can be decided by the investigator or participant. After discontinuation, participants are encouraged to continue to attend visits per the schedule and may be given the option to restart trial medication. Protocol‐specified discontinuation criteria include safety concerns from the investigator, calcitonin level ≥ 100 ng/L, suspicion of pancreatitis, pregnancy or intention to become pregnant, and participation in another clinical trial. Participants can withdraw consent at any point and are considered lost to follow‐up if they repeatedly fail to attend scheduled visits and cannot be contacted.
The protocols allow for dose reductions in case a participant does not tolerate the recommended target dose of 2.4 mg and may stay at the lower dose level of 1.7 mg once weekly, if needed. This is only allowed if the participant would otherwise discontinue trial treatment completely and if it is considered safe to continue trial treatment, per the investigator’s discretion. It is recommended that the participant make at least one attempt to re‐escalate to the recommended target dose of 2.4 mg once weekly. Dose is recorded at selected visits throughout the trials.
Outcome measures
The primary and confirmatory secondary end points are described in Table 1. For all trials, the primary endpoints are percentage change from baseline at randomization (note that this was from week 20 in STEP 4) to end of treatment (EOT) in body weight and ≥ 5% WL from baseline after EOT (not applicable for the sustained WM trial [STEP 4]). Confirmatory secondary trial endpoints include the proportion of participants achieving a body weight reduction ≥ 10% or ≥ 15% from baseline to EOT (not applicable for the sustained WM trial [STEP 4]). Other confirmatory secondary endpoints for all the trials are change from baseline to EOT (or change from randomization [week 20] to EOT for the sustained WM trial [STEP 4]), in waist circumference (centimeters), systolic blood pressure (millimeters of mercury), and clinical outcome assessments.
Assessments
Serial assessments of randomly assigned participants are conducted throughout all of the trials and include height, body weight, waist circumference, glucose metabolism (fasting plasma glucose, HbA1c, fasting serum insulin), lipids (total cholesterol, free fatty acids, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, triglycerides, very‐low‐density lipoprotein cholesterol), biomarkers (high‐sensitivity C‐reactive protein, except in the sustained WM trial [STEP 4]), and vital signs (diastolic and systolic blood pressure [millimeters of mercury]). The WM in T2D trial (STEP 2) assessments also include self‐measured fasting plasma glucose. If a BMI ≤ 22.5 kg/m2 is reached, the recommended energy intake is recalculated, with no calorie deficit (maintenance diet) for the remainder of the trial.
Clinical outcome assessments are carried out throughout the duration of the STEP trials 1‐4 and include the following measures for physical functioning: Short Form36v2 Health Survey, Acute Version (24); Impact of Weight on Quality of Life, Lite Clinical Trials Version (25, 26); and Stanford Presenteeism Scale, version 2001. In addition, other measures included the Patient Global Impression of Status; Patient Global Impression of Change; International Consultation on Incontinence Questionnaire Urinary Incontinence Short Form; Work Productivity Activity Impairment–Specific Health Problem, version 2.0; Six‐Minute Walk Test; and Weight‐Related Sign and Symptom Measure.
For the WM with IBT trial (STEP 3), each IBT session consists of dietitian counseling and a participant handout based on the IBT protocol (27). Participants receive weekly intensive behavioral support in which they discuss progress, review their food diary/Web application (app), and address any adherence issues. For all other trials, participants receive diet and physical activity counseling provided by a dietitian or a similarly qualified health care professional. Counseling is provided every fourth week via visits/telephone contacts, and participants are instructed to record their food intake and physical activity daily via a paper diary, an app, or a similar tool.
For all trials, excluding the WM with IBT trial (STEP 3), the total energy expenditure is calculated by multiplying the estimated basal metabolic rate (as defined in the trial protocol) with a physical activity level value of 1.3.
Safety assessments of the randomly assigned participants for the trials include physical examinations, electrocardiograms, hematology and biochemistry assessments, detection of antibodies against semaglutide, and vital signs. Information on adverse events is collected throughout the trial periods, including the follow‐up period off treatment.
Statistical analysis
Effect end points will be analyzed using the full analysis set, which includes all randomly assigned participants according to the intention‐to‐treat principle. Safety endpoints will be analyzed using the safety analysis set, which includes all randomly assigned participants exposed to at least one dose of randomized treatment. Results from statistical analyses will generally be accompanied by two‐sided 95% CIs and corresponding P values. Superiority will be claimed if P values are less than 5% (P < 0.05) and the estimated treatment contrasts favor semaglutide 2.4 mg. The sample size for each trial gives an effective power (marginal powers multiplied) of 99% for STEP 1, 94% for STEP 2, 86% for STEP 3, 95% for STEP 4, and 43% for STEP 5. The sample sizes for the STEP trials 1 to 4 are primarily defined to support safety. The sample size for STEP 5 is primarily defined to support the co‐primary end points. As there are two primary end points included in the statistical testing hierarchy for STEP trials 1 to 3 and STEP 5, significant superiority of semaglutide 2.4 mg versus placebo must be demonstrated for each primary end point.
The use of two estimands in the STEP program will address different scientific questions of interest, and both contribute to the full clinical picture. Including more than one estimand allows evaluation of the treatment effect from different perspectives. The treatment‐policy estimand assesses the trial‐population average treatment effect of semaglutide or placebo. All randomly assigned participants contribute to data analysis regardless of adherence to treatment or participants starting unplanned interventions such as other AOMs or bariatric surgery. For all trials, all analyses in the statistical testing hierarchy are addressing the treatment‐policy estimand. The trial‐product estimand will evaluate the treatment effect of semaglutide 2.4 mg versus placebo under the assumption that all participants remain on their randomized treatment for the entire planned trial duration. Trial‐product estimand assessments include only participants who are taking the randomized treatment and have not initiated other AOMs or undergone bariatric surgery.
The treatment‐policy and trial‐product estimands correspond to the updated International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use regulatory guidelines on quantifying treatment effects of medications (28). For the treatment‐policy estimand, continuous end points are analyzed using ANCOVA with randomized treatment as a factor and baseline end point value as a covariate. Missing data are imputed using a multiple imputation approach similar to that described by McEvoy (29). Estimates and standard deviations will be pooled across imputed data sets using the Rubin formula. All categorical end points will be assessed at EOT and analyzed by logistic regression using randomized treatment (and stratification groups for the WM in T2D trial [STEP 2]) as a factor and baseline end point value as a covariate. For analyses of end points, the estimated treatment difference and odds ratio (apart from the sustained WM trial [STEP 4]) between semaglutide 2.4 mg and placebo will be reported with the associated two‐sided 95% CI and corresponding P value. For the trial‐product estimand, continuous end points are analyzed using a mixed model for repeated measurements.
Ethics
The trials are being conducted in accordance with good clinical practice guidelines (30) and the principles of the Declaration of Helsinki (31). All 750 sites in the five studies received independent ethics‐committee or institutional‐review‐board approval. The trials are designed and overseen by a steering group of clinical professionals, including representatives from the trial sponsor (Novo Nordisk, Søborg, Denmark).
Results
All the trials are ongoing, and results will be available in 2020, except for those from the long‐term WM trial (STEP 5), which finishes in 2021. A total of 4,988 participants were enrolled across all five trials to receive either semaglutide or placebo (Supporting Information Table S1). The key baseline demographics and characteristics for the participants in each trial are shown in Table 3. The participants had a mean age of 46.2 to 55.3 years; were mostly female (74.1%‐81.0%), excluding the WM in T2D trial (STEP 2); and had a mean BMI of 35.7 to 38.5 kg/m2. Waist circumference, blood pressure, cholesterol levels, overall estimated glomerular filtration rate, high‐sensitivity C‐reactive protein, and glycemic status were generally well balanced across the trials.
TABLE 3.
Key baseline characteristics and demographics of participants
| WM, STEP 1, N = 1,961 | WM in T2D, STEP 2, N = 1,210 | WM with IBT, STEP 3, N = 611 | Sustained WM, STEP 4, N = 902 | Long‐term WM, STEP 5, N = 304 | |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Female | 1,453 (74.1) | 616 (50.9) | 495 (81.0) | 717 (79.5) | 236 (77.6) |
| Male | 508 (25.9) | 594 (49.1) | 116 (19.0) | 185 (20.5) | 68 (22.3) |
| Age, y | 46.5 ± 12.7 | 55.3 ± 10.6 | 46.2 ± 12.7 | 46.4 ± 11.9 | 47.3 ± 11.0 |
| Race, n (%) a | |||||
| White | 1,472 (77.2) | 751 (62.1) | 465 (76.1) | 751 (83.3) | 283 (93.1) |
| Black or African American | 111 (5.8) | 100 (8.3) | 116 (19.0) | 123 (13.6) | 12 (3.9) |
| Asian | 261 (13.7) | 317 (26.2) | 11 (1.8) | 19 (2.1) | 2 (0.7) |
| American Indian or Alaskan Native | 27 (1.4) | 5 (0.4) | 1 (0.2) | 0 | 3 (1.0) |
| Native Hawaiian or other Pacific Islander | 2 (0.1) | 0 | 3 (0.5) | 1 (0.1) | 0 |
| Other | 33 (1.7) | 37 (3.1) | 15 (2.5) | 8 (0.9) | 4 (1.3) |
| Ethnic group, n (%) a | |||||
| Hispanic or Latino | 236 (12.0) | 155 (12.8) | 121 (19.8) | 70 (7.8) | 39 (12.8) |
| Not reported | 55 (2.8) | 0 | 0 | 0 | 0 |
| BMI, kg/m2 | 37.9 ± 6.7 | 35.7 ± 6.3 | 38.0 ± 6.7 | 38.3 ± 7.0 | 38.5 ± 6.9 |
| Waist circumference, cm | 114.7 ± 14.7 | 114.6 ± 14.1 | 113.0 ± 15.5 | 115.1 ± 15.6 | 115.7 ± 14.8 |
| FPG, mmol/L | 5.3 ± 0.6 | 8.6 ± 2.2 | 5.2 ± 0.5 | 5.4 ± 0.6 | 5.3 ± 0.6 |
| Blood pressure, mm Hg | |||||
| Systolic | 126.5 ± 14.3 | 130.0 ± 13.5 | 124.4 ± 14.8 | 126.4 ± 14.3 | 125.5 ± 14.5 |
| Diastolic | 80.3 ± 9.6 | 79.8 ± 9.0 | 80.5 ± 9.7 | 80.9 ± 9.9 | 80.1 ± 9.4 |
| HbA1c, % | 5.7 ± 0.32 | 8.1 ± 0.8 | 5.7 ± 0.3 | 5.7 ± 0.3 | 5.7 ± 0.3 |
| Cholesterol, mmol/L | |||||
| Total | 4.9 ± 20.0 | 4.4 ± 23.2 | 4.8 ± 19.7 | 5.0 ± 19.5 | 4.8 ± 19.1 |
| HDL | 1.3 ± 25.5 | 1.1 ± 24.5 | 1.3 ± 23.6 | 1.29 ± 24.6 | 1.23 ± 23.9 |
| LDL | 2.9 ± 28.7 | 2.3 ± 35.6 | 2.8 ± 28.5 | 3.0 ± 27.3 | 2.9 ± 26.1 |
| VLDL | 0.6 ± 51.1 | 0.8 ± 51.6 | 0.6 ± 48.1 | 0.6 ± 53.6 | 0.6 ± 48.1 |
| Triglycerides, mg/dL | 1.4 ± 70.0 | 1.8 ± 64.2 | 1.2 ± 49.8 | 1.4 ± 54.9 | 1.3 ± 48.6 |
| Free fatty acids, mmol/L | 0.4 ± 48.3 | 0.6 ± 44.6 | 0.4 ± 49.0 | 0.4 ± 51.5 | 0.4 ± 48.3 |
| Overall eGFR, mL/min/1.73 m2 b | 96.6 ± 17.2 | 93.7 ± 19.5 | 96.8 ± 19.5 | 97.6 ± 17.8 | 94.8 ± 16.6 |
| hsCRP, mg/L | 3.9 ± 117.5 | 3.4 ± 129.2 | 4.4 ± 99.3 | NA | 4.4 ± 125.2 |
| Diabetes duration, y | NA | 8.6 ± 6.2 | NA | NA | NA |
| Glycemic status, n (%) | |||||
| Normoglycemia | 1,106 (56.4) | NA | 306 (50.1) | 493 (54.7) | 163 (53.6) |
| Prediabetes | 855 (43.6) | NA | 305 (49.9) | 408 (45.3) | 141 (46.4) |
Baseline was defined as randomization for WM, WM in T2D, WM with IBT, and long‐term WM trials and as start of run‐in period for sustained WM trial. Plus‐minus values are reported as means ± SD. For cholesterol, triglycerides, free fatty acids, overall eGFR, and hsCRP, plus‐minus values are geometric means and coefficients of variations. For WM with or without T2D and maximizing and long‐term WM trials (STEP trials 1‐3 and 5), data are for all randomly assigned participants. For sustained WM trial (STEP 4), data are for all participants entering run‐in period.
Race and ethnic group were self‐reported.
eGFR is calculated according to chronic kidney disease–epidemiology collaboration (CKD‐EPI) creatinine equation as defined by Kidney Disease: Improving Global Outcomes (KDIGO) (32).
eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; hsCRP, high‐sensitivity C‐reactive protein; IBT, intensive behavioral therapy; LDL, low‐density lipoprotein; NA, not applicable; STEP, Semaglutide Treatment Effect in People with obesity; T2D, type 2 diabetes; VLDL, very‐low‐density lipoprotein; WM, weight management.
The racial composition of each trial is primarily white (62.1%‐93.1%), but overall there is broad variation in races/ethnicities across the trials. Both the WM with diabetes and the WM without diabetes trials (STEP 1 and STEP 2) have a higher proportion of Asians (13.7% and 26.2%, respectively) than the other trials. Compared with the WM with and without diabetes and long‐term WM trials (STEP trials 1, 2, and 5), there are slightly more Hispanic or Latino participants in the WM with IBT trial (STEP 3, 19.8%) and slightly fewer in the sustained WM trial (STEP 4, 7.8%). As expected, the WM in T2D trial (STEP 2), versus the other trials, has numerically higher levels of fasting plasma glucose, HbA1c, very‐low‐density lipoprotein, triglycerides, and free fatty acids.
Discussion
The STEP program represents the latest investigation to date of an AOM for chronic WM. These phase 3 trials aim to evaluate the effect of semaglutide 2.4 mg (administered subcutaneously once weekly) on WM in adults with obesity or overweight and provide a comprehensive overview of the efficacy, safety, and tolerability profile of semaglutide 2.4 mg.
Baseline characteristics are well balanced among randomized groups for many of the parameters. The baseline results presented from the sustained WM trial (STEP 4) are only available for the pre‐randomized participants in the run‐in phase. The variations in the race and ethnicity of participants across the trials are expected because of recruiting participants from various countries. The WM with or without diabetes trials (STEP 1 and STEP 2) are designed to have Asians as at least 10% of the population, which is the reason they had a higher proportion of Asians than the other trials. The WM in T2D trial (STEP 2) has a greater proportion of men (49.1%) than the other trials (19.0%‐25.9%). There are slightly more Hispanic or Latino participants in the WM with IBT trial (STEP 3), and there are slightly fewer in the sustained WM trial (STEP 4) than in the other trials.
Baseline characteristics were generally comparable between the STEP trials presented in this article and the phase 2 trial of semaglutide (21). In comparison with the WM in T2D trial (STEP 2), the phase 2 trial had a numerically higher BMI (39.3 kg/m2) and waist circumference (117.8 cm). This was due to STEP 2 having a lower threshold‐for‐BMI inclusion criterion ( ≥ 27 kg/m2) than the phase 2 trial (≥ 30 kg/m2). Overall, the phase 2 trial has a numerically lower HbA1c level (5.5%) than the STEP trials, particularly for STEP 2 (21). However, cross‐trial comparisons should be interpreted cautiously.
At the time of initiation of the phase 2 trial, it was hypothesized that daily dosing of semaglutide would result in a more favorable tolerability profile compared with weekly administration, and, therefore, the once‐daily administration of semaglutide was investigated. The phase 2 WM trial of semaglutide daily resulted in dose‐dependent, clinically relevant WL over 52 weeks and associated with an acceptable tolerability profile with respect to gastrointestinal symptoms (21). However, based on comparisons with studies with weekly administration of semaglutide, it was reported that there was no difference in gastrointestinal adverse events with the daily versus weekly dosing regimen of semaglutide (33). Furthermore, using population pharmacokinetic modeling, it was estimated that a once‐weekly maintenance dose of semaglutide 2.4 mg subcutaneously would not exceed the maximum concentration at steady state as obtained by the once‐daily semaglutide 0.4‐mg subcutaneous dose. Hence, a decision was made to change dosing for semaglutide from daily to weekly to improve adherence and convenience for participants.
There are several trials that demonstrate the promising effects of GLP‐1s in general and semaglutide in particular on WM in subpopulations of people with obesity. In the Satiety and Clinical Adiposity – Liraglutide Evidence in individuals with and without diabetes (SCALE) trial of 3,731 people without diabetes, WL was maintained at 56 weeks with liraglutide 3.0 mg versus placebo (−8.0% versus −2.6%, respectively; P < 0.001) (34). The SCALE trial of 846 people with T2D and obesity or overweight demonstrated a 6.0% (6.4‐kg) reduction of initial body weight with liraglutide 3.0 mg versus 1.8 mg and placebo (4.7% [5.0 kg] and 2.0% [2.2 kg], respectively) (35). The Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) clinical development program, which included > 8,000 people with T2D, demonstrated that semaglutide at doses of 0.5 mg and 1.0 mg weekly offered WM benefits of 2.5 to 5.7 kg and 2.0 to 7.9 kg, respectively (36). Liraglutide and semaglutide are administered at different frequencies and doses, and over a 24‐hour period, they have distinct pharmacodynamic effects (37, 38). However, they are equally associated with transient, mild, or moderate gastrointestinal symptoms, including nausea, vomiting, and diarrhea (17, 39). Clinical experience shows that slowing the initial escalation of liraglutide and semaglutide helps mitigate these side effects; hence, we have designed the trials in the STEP program to have a slow titration of semaglutide over a 16‐week period.
The primary goal of pharmacotherapies like semaglutide for chronic WM is to achieve a clinically meaningful WL when combined with lifestyle intervention and to provide long‐term WM and to minimize weight regain (2). More importantly, semaglutide, through both direct and indirect actions, is hoped to meaningfully impact obesity‐related comorbidities. Average WL of 10% to 15% has been shown to significantly alleviate many complications associated with obesity, including diabetes, hypertension, osteoarthritis, and gastroesophageal reflex disease (40, 41, 42). The benefits of WL have also been demonstrated in dyslipidemia, nonalcoholic fatty liver disease, sleep apnea, and stress incontinence (40, 41, 43, 44).
Obesity can adversely affect physical and mental health and reduces health‐related quality of life (45). The physical impairments appear to be more closely associated with severity of obesity than mental impairments (45). Despite a wide range of randomized controlled trials investigating these associations, a systematic review by Kolotkin et al. (45) found that these studies are inconclusive. Therefore, further investigations are necessary to explore the relationship between obesity and health‐related quality of life besides the physical and health advantages of WL. The STEP trials have been designed to monitor patient‐reported outcomes, including the Impact of Weight on Quality of Life, Lite Clinical Trials Version, and Short Form36v2 Health Survey, Acute Version.
One key difference among the STEP trials presented here is that, despite them all having the same primary end point of change in body weight, results from the primary end point in the sustained WM trial (STEP 4) will not reflect the full WL potential that is expected to be observed with semaglutide in the other STEP trials. This is due to the fact that in STEP 4, the randomization to semaglutide or placebo takes place after all participants have received a 20‐week run‐in treatment with semaglutide, and the primary end point for STEP 4 is the change in the percentage of body weight from randomization at week 20 to the EOT at week 68. There are currently four additional planned and ongoing trials in the STEP program. STEP 6 (NCT03811574) is investigating the efficacy and safety of semaglutide once weekly in East Asian adults with obesity or overweight. STEP TEENS (NCT04102189) is evaluating the efficacy and safety of semaglutide in adolescents. STEP 7 (NCT04251156) is planned to investigate the efficacy and safety of semaglutide also in the Chinese population. STEP 8 (NCT04074161) is assessing the effect and safety of semaglutide 2.4 mg once weekly compared with liraglutide 3.0 mg once daily on WM in people with obesity or overweight. Obesity is a strong risk factor for the development of diabetes, and both diabetes and obesity are associated with CVD; therefore, it is important to consider the effect of semaglutide on cardiovascular risk factors. Further research is ongoing in the Semaglutide Effects on Cardiovascular Outcomes in People With Overweight or Obesity (SELECT) trial (NCT03574597) to explore the impact of semaglutide 2.4 mg subcutaneously on reducing the risk of cardiovascular events in people with prior CVD and either obesity or overweight.
Conclusion
AOMs are an important treatment option for people living with obesity who are unable to lose weight and maintain WL or for those who do not meet the eligibility criteria for bariatric surgery or who failed to maintain WL following bariatric surgery. The STEP clinical development program with the GLP‐1 analogue semaglutide provides rigorous assessment regarding the use of semaglutide 2.4 mg once weekly to treat people with obesity, with an effort to gain a greater understanding of WL, WL maintenance, safety, and tolerability in adults with obesity as an adjunct to lifestyle intervention. We anticipate that these trials will demonstrate that semaglutide represents a new and effective medication that can be used to improve the health and quality of life for patients with obesity.
Funding agencies
These trials are funded by Novo Nordisk A/S, which also provided financial support for medical editorial assistance.
Disclosures
RFK has served on advisory boards for Novo Nordisk, received grant support during the conduct of these trials, and received fees for participating in educational films from Novo Nordisk. SC is an employee of and holds stock in Novo Nordisk. MD has received grant support from Novo Nordisk, Sanofi, Eli Lilly, Boehringer Ingelheim, and Janssen; has served on advisory boards and received consulting and speaker fees from AstraZeneca and Janssen; and has served on advisory boards for Servier and received speaker fees from Mitsubishi Tanabe Pharma Corporation and Takeda. DD has received grant support from Novo Nordisk and Boehringer Ingelheim and has served on advisory boards for and received consulting and speaker fees from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, Teva, and Sanofi. WTG has served on advisory boards for Novo Nordisk, Sanofi, BOYDSense, Amgen, Gilead, and Boehringer Ingelheim and has received support for institutionally sponsored research from Sanofi, Pfizer, and Novo Nordisk. BG is an employee of and holds stock in Novo Nordisk. IL has received grant support from Novo Nordisk, Novartis, Pfizer, Merck, Mylan, and GI Dynamics; grant support for institutionally sponsored research from Novo Nordisk; and consulting fees from Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Sanofi, MannKind, Valeritas, Intarcia, and Janssen. MT is an employee of and holds stock in Novo Nordisk. TAW has served on advisory boards for Novo Nordisk and Weight Watchers and has received grant support, on behalf of the University of Pennsylvania, from Novo Nordisk. SW has received grant support and honoraria from and served on advisory boards for Novo Nordisk, Bausch Health, Eli Lilly, and Janssen and received nonfinancial support during the conduct of these trials. JPHW has received grant support from AstraZeneca, Novo Nordisk, and Takeda; has received personal fees from Boehringer Ingelheim, Napp, Novo Nordisk, Eli Lilly, Mundipharma, Sanofi, Janssen, and Takeda; and has served as a consultant for Astellas, AstraZeneca, Boehringer Ingelheim, Napp, Novo Nordisk, Eli Lilly, Mundipharma, Rhythm Pharmaceuticals, Sanofi, Janssen, and Wilmington Healthcare. DR has served on advisory boards for Arena, Eisai, Zafgen, and Novo Nordisk; has served as a clinical trials investigator for Bristol‐Myers Squibb, Merck, Nutrisource, Arena, Eisai, Vivus, Orexigen, Sanofi, AstraZenca, Novo Nordisk, and Weight Watchers; and has participated on a speaker’s bureau for Novo Nordisk and received grant support from Obeseinov SARL.
Author contributions
RFK, DR, SC, and MT contributed to the design of the trials. RFK, MD, DD, WTG, IL, TAW, SW, JPHW, and DR contributed to the recruitment of trial participants and collection of data. BG provided support with statistical analyses. All authors participated in drafting and revision of the manuscript and reviewed and approved the final, submitted version.
Clinical trial registration
ClinicalTrials.gov identifiers NCT03548935 (STEP 1), NCT03552757 (STEP 2), NCT03611582 (STEP 3), NCT03548987 (STEP 4), and NCT03693430 (STEP 5).
Supporting information
Table S1
Acknowledgments
We acknowledge the trial participants and all personnel involved in these trials. We thank Lisa von Huth Smith for her contribution to the clinical outcome assessment section. This research was supported by the National Institute for Health Research Leicester Biomedical Research Centre. Medical editorial assistance was provided by Anna Bacon and Sara Shaw, PhD, of Articulate Science Ltd. (London, UK).
Deidentified participant data are available for this article on a specialized SAS data platform (SAS Institute, Cary, NC). Data sets from Novo Nordisk will be available permanently after research completion and approval of product and product use in both the European Union and United States. The study protocol and redacted clinical study report will be available according to Novo Nordisk data‐sharing commitments. Access to data can be granted through a request proposal form, and the access criteria can be found online. Data will be shared with bona fide researchers submitting a research proposal requesting access to data. Data use is subject to approval by the independent review board according to the Institutional Review Board Charter (see novonordisk‐trials.com).
References
- 1. Frühbeck G, Busetto L, Dicker D, et al. The ABCD of obesity: an EASO position statement on a diagnostic term with clinical and scientific implications. Obes Facts 2019;12:131‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bray GA, Frühbeck G, Ryan DH, Wilding JPH. Management of obesity. Lancet 2016;387:1947‐1956. [DOI] [PubMed] [Google Scholar]
- 3. Ralston J, Brinsden H, Buse K, et al. Time for a new obesity narrative. Lancet 2018;392:1384‐1386. [DOI] [PubMed] [Google Scholar]
- 4. Ritten A, LaManna J. Unmet needs in obesity management: from guidelines to clinic. J Am Assoc Nurse Pract 2017;29:S30‐S42. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . Obesity and overweight. Updated February 16, 2018. Accessed October 25, 2019. https://www.who.int/news‐room/fact‐sheets/detail/obesity‐and‐overweight
- 6. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health 2009;9:88. doi: 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. GBD 2015 Obesity Collaborators . Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Organisation for Economic Co‐operation and Development . Obesity update. Published 2017. Accessed October 25, 2019. https://www.oecd.org/els/health‐systems/Obesity‐Update‐2017.pdf
- 9. Acosta A, Abu Dayyeh BK, Port JD, Camilleri M. Recent advances in clinical practice challenges and opportunities in the management of obesity. Gut 2014;63:687‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coulter AA, Rebello CJ, Greenway FL. Centrally acting agents for obesity: past, present, and future. Drugs 2018;78:1113‐1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hope DCD, Tan TMM, Bloom SR. No guts, no loss: toward the ideal treatment for obesity in the twenty‐first century. Front Endocrinol (Lausanne) 2018;9:442. doi: 10.3389/fendo.2018.00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krentz AJ, Fujioka K, Hompesch M. Evolution of pharmacological obesity treatments: focus on adverse side‐effect profiles. Diabetes Obes Metab 2016;18:558‐570. [DOI] [PubMed] [Google Scholar]
- 13. Wadden TA, West DS, Delahanty L, et al.; Look AHEAD Research Group . The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saltiel AR. New therapeutic approaches for the treatment of obesity. Sci Transl Med 2016;8:323rv2. doi: 10.1126/scitranslmed.aad1811 [DOI] [PubMed] [Google Scholar]
- 15. Sweeting AN, Hocking SL, Markovic TP. Pharmacotherapy for the treatment of obesity. Mol Cell Endocrinol 2015;418(pt 2):173‐183. [DOI] [PubMed] [Google Scholar]
- 16. Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events a systematic review and meta‐analysis. JAMA 2016;315:2424‐2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide . Front Endocrinol (Lausanne) 2019;10:155. doi: 10.3389/fendo.2019.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Novo Nordisk . OZEMPIC (semaglutide) injection, for subcutaneous use Package insert. Novo Nordisk; 2017. Updated December 2017. Accessed October 8, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf [Google Scholar]
- 19. Novo Nordisk . RYBELSUS (semaglutide) tablets, for oral use Package insert. Novo Nordisk; 2019. Updated September 2019. Accessed October 8, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213051s000lbl.pdf [Google Scholar]
- 20. Novo Nordisk . VICTOZA (liraglutide 1.2/1.8 mg) injection, for subcutaneous use Package insert. Novo Nordisk; 2017. Updated August 2017. Accessed October 8, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022341s027lbl.pdf [Google Scholar]
- 21. O'Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double‐blind, placebo and active controlled, dose‐ranging, phase 2 trial. Lancet 2018;392:637‐649. [DOI] [PubMed] [Google Scholar]
- 22. European Medicines Agency . EMA/CHMP/311805/2014 Guideline on clinical evaluation of medicinal products used in weight management. Published June 23, 2016. Accessed October 25, 2019. https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐clinical‐evaluation‐medicinal‐products‐used‐weight‐management‐revision‐1_en.pdf
- 23. Food and Drug Administration . FDA guidance for industry: developing products for weight management. Published February 2007. Accessed October 25, 2019. https://www.fda.gov/media/71252/download
- 24. Maruish ME. User's Manual for the SF‐36v2 Health Survey. Lincoln: QualityMetric; 2011. [Google Scholar]
- 25. Kolotkin RL, Ervin CM, Meincke HH, Højbjerre L, Fehnel SE. Development of a clinical trials version of the Impact of Weight on Quality of Life‐Lite Questionnaire (IWQOL‐Lite Clinical Trials Version): results from two qualitative studies. Clin Obes 2017;7:290‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kolotkin RL, Williams VSL, Ervin CM, et al. Validation of a new measure of quality of life in obesity trials: impact of weight on quality of life‐lite clinical trials version. Clin Obes 2019;9:e12310. doi: 10.1111/cob.12310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wadden TA, Tsai AG, Tronieri JS. A protocol to deliver intensive behavioral therapy (IBT) for obesity in primary care settings: the MODEL‐IBT program. Obesity (Silver Spring) 2019;27:1562‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. International Council for Harmonisation . ICH Harmonised Guideline E9 (R1): Estimands and Sensitivity Analysis in Clinical Trials. International Council for Harmonization; 2017. [DOI] [PubMed] [Google Scholar]
- 29. McEvoy BW. Missing data in clinical trials for weight management. J Biopharm Stat 2016;26:30‐36. [DOI] [PubMed] [Google Scholar]
- 30. International Conference on Harmonisation Expert Working Group . ICH harmonised tripartite guideline: guideline for good clinical practice. Published June 10, 1996. Accessed October 25, 2019. https://apps.who.int/medicinedocs/documents/s22154en/s22154en.pdf
- 31. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191‐2194. [DOI] [PubMed] [Google Scholar]
- 32. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1‐150. Accessed October 25, 2019. https://kdigo.org/wp‐content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf [Google Scholar]
- 33. Lingvay I, Desouza CV, Lalic KS, et al. A 26‐week randomized controlled trial of semaglutide once daily versus liraglutide and placebo in patients with type 2 diabetes suboptimally controlled on diet and exercise with or without metformin. Diabetes Care 2018;41:1926‐1937. [DOI] [PubMed] [Google Scholar]
- 34. Pi‐Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11‐22. [DOI] [PubMed] [Google Scholar]
- 35. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015;314:687‐699. [DOI] [PubMed] [Google Scholar]
- 36. Ahrén B, Atkin SL, Charpentier G, et al. Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. Diabetes Obes Metab 2018;20:2210‐2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Degn KB, Juhl CB, Sturis J, et al. One week's treatment with the long‐acting glucagon‐like peptide 1 derivative liraglutide (NN2211) markedly improves 24‐h glycemia and alpha‐ and beta‐cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes 2004;53:1187‐1194. [DOI] [PubMed] [Google Scholar]
- 38. Jensen L, Helleberg H, Roffel A, et al. Absorption, metabolism and excretion of the GLP‐1 analogue semaglutide in humans and nonclinical species. Eur J Pharm Sci 2017;104:31‐41. [DOI] [PubMed] [Google Scholar]
- 39. Drucker DJ. Mechanisms of action and therapeutic application of glucagon‐like peptide‐1. Cell Metab 2018;27:740‐756. [DOI] [PubMed] [Google Scholar]
- 40. Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013;310:2416‐2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garvey WT, Mechanick JI, Brett EM, et al.; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines . American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract 2016;22(suppl 3):1‐203. [DOI] [PubMed] [Google Scholar]
- 42. Singh M, Lee J, Gupta N, et al. Weight loss can lead to resolution of gastroesophageal reflux disease symptoms: a prospective intervention trial. Obesity (Silver Spring) 2013;21:284‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Foster GD, Borradaile KE, Sanders MH, et al.; Sleep AHEAD Research Group of Look AHEAD Research Group . A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med 2009;169:1619‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vilar‐Gomez E, Martinez‐Perez Y, Calzadilla‐Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149:367‐378.e5; quiz e14‐e15. [DOI] [PubMed] [Google Scholar]
- 45. Kolotkin RL, Andersen JR. A systematic review of reviews: exploring the relationship between obesity, weight loss and health‐related quality of life. Clin Obes 2017;7:273‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


