Abstract
Aim
To compare the incidence of lower extremity amputation (LEA) among patients with type 1 diabetes (T1D) and patients with type 2 diabetes (T2D) with those without diabetes using US commercial claims and to assess the presence of key co‐morbidities and precipitating factors at the time of the LEA.
Methods
Cohorts were defined via IBM MarketScan research databases for beneficiaries with T1D and T2D during 2010‐2014. For each T1D and T2D patient, one patient without a prior diabetic claim matched on calendar time, sex and age, was randomly selected. Multivariable Cox proportional hazards models were used to estimate adjusted hazard ratios (aHRs) and 95% confidence intervals.
Results
Among the matched cohorts of 120 129 T1D patients and 1.7 million T2D patients, the incidence of LEA was higher among patients with T1D than patients with T2D, with the most frequent cases being minor LEAs (4.85 and 1.53 per 1000 patient years [PY], respectively), largely toe amputations (4.49 and 1.43 per 1000 PY, respectively). Compared with non‐diabetic patients matched on age, sex and calendar time, T1D and T2D patients had more co‐morbidities and a higher incidence of LEA (6.02 vs. 0.14 per 1000 PY; aHR, 22.47 [16.42‐30.73] and 1.90 vs. 0.23 per 1000 PY; aHR, 4.64 [4.32‐4.98]).
Conclusions
Our data showed a higher incidence of LEA, especially minor LEA, in patients with T1D and T2D compared with those without diabetes, with a greater risk among patients with T1D than patients with T2D. Accounting for known and measurable risk factors for LEA reduced the relative hazard by nearly 50%; the majority of LEA cases were minor LEAs and toe amputations.
Keywords: cohort study, database research, diabetes complications, observational study, population study
1. INTRODUCTION
Diabetes is a global public health problem that affected ~ 415 million adults worldwide in 2015.1 Type 1 diabetes (T1D) and type 2 diabetes (T2D) are the most common forms of the disease, with T2D representing up to 91% of adult diabetes cases.1 Diabetic complications involve diabetic neuropathy and peripheral arterial disease (PAD). Both may result in foot ulcers, infections and, ultimately, lower extremity amputation (LEA).2, 3 In 2014, ~ 5 per 1000 US adults with diabetes experienced an LEA.4
Epidemiological assessments of the incidence rates (IRs) of LEA have utilized different definitions of amputation, various data sources, and different denominator population definitions, leading to variation in estimates.5 Claims are well suited to capturing amputations procedures as their high cost incentivizes accuracy to qualify for maximum insurance coverage.
Recent studies have reported on the IRs of LEA associated with diabetes in the United States, including cases of traumatic amputations. One study evaluated the IR of LEA in 2010 in people with diabetes (no distinction between T1D and T2D) aged 20 years and older using hospital discharge records. An IR of 2.84 per 1000 person‐years (PY) was reported among diabetic patients compared with 0.3 per 1000 PY in the general population (including diabetic patients).6 A second study reported a cumulative incidence in 2011 of 4.0 per 1000 Medicare beneficiaries with T2D who were older than 65 years.7 Finally, Newhall et al also reported 2.3 per 1000 Medicare beneficiaries with PAD and diabetes undergoing major amputation during 2007‐2011.8 These studies have either reported incidence estimates of LEA among patients with T2D only, or among those with any diabetes. However, there are important differences between T1D and T2D to consider, which are otherwise masked when the two populations are combined.
Using claims data from the IBM MarketScan research database, a data source commonly used for epidemiological research, our study had two objectives: (1) to conduct a descriptive study to report the population‐level IR of LEA among patients with T1D and T2D as two separate cohorts, and also among all patients without any diabetes; and (2) to conduct a comparative study to evaluate the IR of LEA and precipitating LEA risk factors among the T1D and T2D cohorts compared with a random sample of patients without diabetes matched on calendar time, age and sex.
2. METHODS
2.1. Data source
Using the Aetion Evidence Platform (Aetion, New York, NY) and SAS version 9.4, we analyzed claims data from the IBM MarketScan research database (2009‐2014), a United States‐based insurance claims database consisting of data on ~ 112.5 million unique de‐identified insured beneficiaries who were active employees and their dependents, early retirees, those with Consolidated Omnibus Budget Reconciliation Act (COBRA), and Medicare‐eligible retirees with employer‐provided Medicare Supplemental plans. The data came from a selection of large employers, health plans, and government and public organizations. The annual medical databases include private‐sector health data from ~ 350 payers across the United States. The individuals covered are representative of the US commercially insured population. These databases capture longitudinal, individual‐level data on healthcare utilization, healthcare expenditures and plan enrolment, and contain integrated records for patient demographics, inpatient events, outpatient events and outpatient pharmacy dispensing of medications.
2.2. Study population
The study period was defined as 1 January 2009 to 31 December 2014. To ensure a 1‐year baseline period for all patients, the IR evaluation period spanned from 1 January 2010 to 31 December 2014 to select patients aged 18 or older with at least 1 year of continuous enrolment. Patients with current or a history of gestational diabetes, secondary diabetes, or traumatic LEA prior to meeting the enrolment requirement, were excluded. The study period ended in 2014 to correspond to the last complete calendar year when international classification of diseases (ICD)‐9 codes were in use in the US, given that the algorithm used to define LEA has not yet been evaluated and validated using ICD‐10 codes.
2.3. Outcomes definition
Incident cases of LEAs were defined using an algorithm from the Agency for Healthcare Research and Quality (AHRQ) quality indicators9 modified to include toe amputations, as carried out in previous studies,6, 7 based on the presence of one or more inpatient or outpatient diagnostic (ICD‐9‐CM) or procedural (CPT‐4) claim (File S1). We further used the codes from this algorithm to evaluate the incident cases of LEA by subtype: major LEA (proximal to the ankle) and minor LEA (through or distal to the articulation of the ankle). Toe amputations, which were included in the minor LEA definition, were also considered separately (File S5).
2.4. Descriptive study
The descriptive study selected three cohorts from the study population: no diabetes, T1D and T2D (Figure 1). T1D was defined as having one or more prior inpatient or outpatient claim for T1D (ICD9: 250.x1, 250.x3), no prior claim for an oral antidiabetic drug (OAD), and one or more prior claim for insulin. T2D was defined as two or more prior inpatient or outpatient claims for T2D (ICD9: 250.x0, 250.x2) and one or more prior claim for an OAD. No diabetes was defined as having neither prior inpatient nor outpatient claims for diabetes (ICD9: 250.x). Patients with one or more prior claim for diabetes but not meeting the criteria for T1D or T2D were further excluded, as ineligible for any cohort. Because the presence of diabetes claims was time‐varying, patients without diabetes could contribute to the no diabetes cohort and later switch to the T1D or T2D cohort. Although rare, given the nature of claims, it was also possible for a patient to first enter the T1D cohort and later switch to the T2D cohort.
Figure 1.
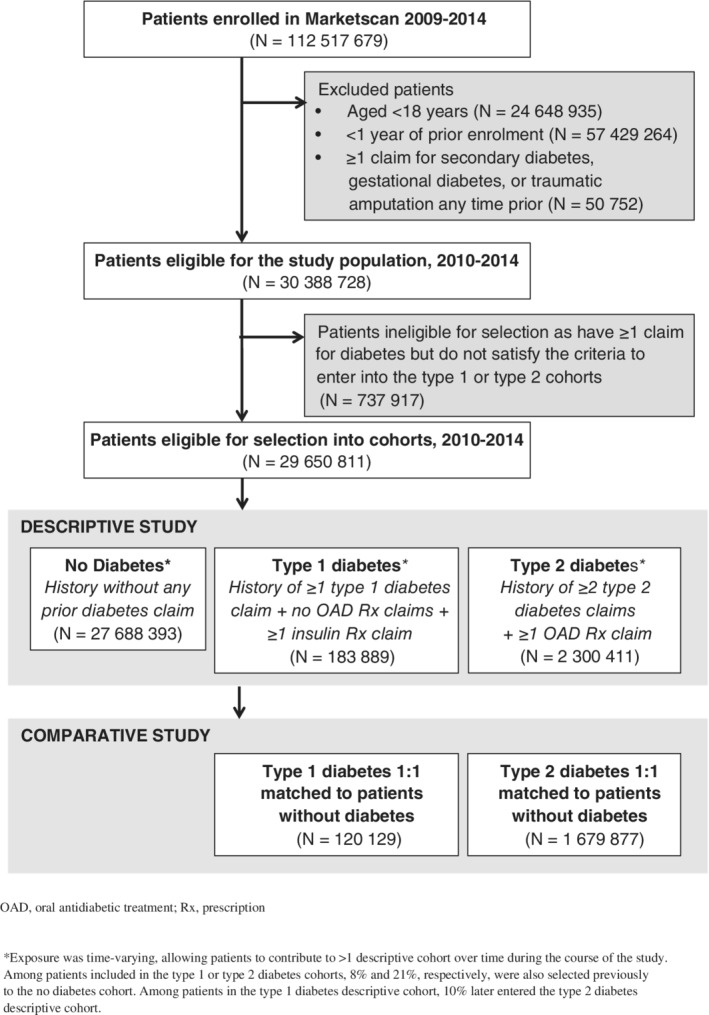
Cohort inclusion
The index date was defined as the first day during the selection period when the patient satisfied the cohort criteria after 1 year of continuous enrolment. Follow‐up started at index and ended when an LEA occurred, at disenrolment, at the end of the study period (31 December 2014), or when there was a cohort switch. Crude IR of LEA overall and by subtype was calculated based on the number of LEA events divided by the number of days of follow‐up within each respective cohort over the 5‐year period and was reported per 1000 PY. Additional analyses also present crude IR of LEA stratified by sex and age (≥65, <65 years).
2.5. Comparative study
Two comparative cohorts were created to compare T1D and T2D with no diabetes. For each patient with T1D or T2D selected in chronological order, one patient with no history of any diagnosis of diabetes matched on calendar date, sex and age group (18‐30, 31‐40, 41‐50, 51‐60, 61‐70, 71‐80 and >80 years) was randomly selected. Referent patients were only required to have no history of diabetes at the time of selection in order to minimize the potential for immortal person‐time bias. Given the smaller sample of patients with T1D and the rarity of LEA, a post hoc sensitivity analysis was additionally added to match three patients without diabetes for each patient with T1D to increase the precision of estimates.
The index date was defined as either the first day patients met the criteria for the T1D or T2D or the matched calendar date for patients without diabetes after 1 year of continuous enrolment. Follow‐up started at index and ended when an LEA occurred, at disenrolment, or at the end of the study period (31 December 2014). Within each comparison, patients could only be included in either the diabetic cohort (T1D or T2D) or the non‐diabetic cohort, so follow‐up for patients without diabetes who later had a claim for diabetes was censored 1 day prior to the diabetes claim and they were not allowed to enter the diabetic cohort.
The covariates, sex and age, were assessed at index, and co‐morbidities were measured during the 1‐year baseline period preceding the index date. The co‐morbidities (arterial hypertension, atrial fibrillation, ischaemic heart disease, congestive heart failure, cerebrovascular diseases, diabetic retinopathy, chronic kidney disease, PAD, peripheral polyneuropathy, foot deformities, charcot foot, preulcerative callus or corn), determined a priori as known risk factors for LEA identified by the American Diabetes Association 2017 Standard of Care in Diabetes guidelines,2 were assessed based on the presence of one or more inpatient or two or more outpatient claims, as previously carried out in MarketScan.10, 11 We also assessed use of diuretics and insulin using all available data prior to index (Files S2 and S3). Covariates for T1D and T2D cohorts were compared with their respective matched cohort of patients without a history of diabetes, using absolute standardized difference (ASD), where an ASD > 0.1 was considered a relevant difference (File S4).12, 13, 14, 15 Multivariable Cox proportional hazards models were used to estimate adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) of LEA, adjusting for all covariates.
For all incident LEA cases, the presence of selected co‐morbidities (different from the co‐morbidities assessed during the baseline period) probably leading to LEA (such as foot infections) and known risk factors for LEA (such as end‐stage renal disease [ESRD]), were assessed during the month (30 days) preceding the LEA (File S2).
Additional analyses were conducted to evaluate the IR of LEA among subsets of patients. For the T1D and T2D comparisons, this excluded patients with a history of ESRD at matched index, as patients with ESRD are at a higher risk of LEA than those without.16 For the T2D comparison, this excluded patients with a history of any insulin use using all available data, as insulin use may be indicative of uncontrolled diabetes (File S6).
3. RESULTS
There were 30.4 million patients who satisfied the initial eligibility criteria (Figure 1). However, 2.4% of patients (N = 737 917) were ineligible for selection as they had one or more claim for diabetes but did not subsequently satisfy the criteria to enter into the T1D or T2D cohorts during the selection period, leaving 29.6 million patients eligible for cohort selection.
3.1. Descriptive study
All 29.6 million patients were allocated to the three descriptive cohorts used to calculate the overall IR of LEA (Figure 1, Table 1). There were 183 889 and 2.3 million patients included in the T1D and T2D cohorts, respectively, and over 27.6 million patients included in the no diabetes cohort. Among patients included in the T1D or T2D cohorts, 8% and 21%, respectively, had also been previously allocated to the no diabetes cohort. Among patients in the T1D descriptive cohort, 10% later entered the T2D descriptive cohort. Less than 2% of patients were included in all three cohorts. The crude overall IRs for the T1D, T2D and no diabetes cohorts were 5.79 (5.56‐6.03), 1.62 (1.59‐1.65) and 0.08 (0.08‐0.09) per 1000 PY, respectively. The crude IRs of minor amputation were 4.69 (4.48‐4.90), 1.34 (1.31‐1.37) and 0.06 (0.06‐0.06) per 1000 PY, respectively. Compared with patients without diabetes, we observed a 72‐fold difference among patients with T1D and a 20‐fold difference among patients with T2D.
Table 1.
Crude incidence rates per 1000 person‐years for overall lower extremity amputation (LEA) and by amputation subtype among the descriptive cohorts, 2010‐2014
| T1Da | T2Db | No diabetesc | |
|---|---|---|---|
| N | 183 889 | 2 300 411 | 27 688 393 |
| Overall (any LEA)d | |||
| Number of patients with an event | 2366 | 9222 | 6322 |
| Total person‐years | 408 328 | 5 691 794 | 75 464 818 |
| Mean follow‐up (years) per person | 2.22 | 2.47 | 2.73 |
| Incidence rate per 1000 PY | 5.79 (5.56‐6.03) | 1.62 (1.59‐1.65) | 0.08 (0.08‐0.09) |
| Major LEAd | |||
| Number of patients with an event | 718 | 2297 | 2121 |
| Total person‐years | 412 049 | 5 706 799 | 75 474 689 |
| Mean follow‐up (years) per person | 2.24 | 2.48 | 2.73 |
| Incidence rate per 1000 PY | 1.74 (1.62‐1.87) | 0.40 (0.3‐0.42) | 0.03 (0.0‐0.03) |
| Minor LEAd | |||
| Number of patients with an event | 1916 | 7610 | 4382 |
| Total person‐years | 408 555 | 5 692 499 | 75 466 059 |
| Mean follow‐up (years) per person | 2.22 | 2.47 | 2.73 |
| Incidence rate per 1000 PY | 4.69 (4.48‐4.90) | 1.34 (1.31‐1.37) | 0.06 (0.06‐0.06) |
| Toe amputationd | |||
| Number of patients with an event | 1762 | 7110 | 4137 |
| Total person‐years | 408 328 | 5 691 794 | 75 464 818 |
| Mean follow‐up (years) per person | 2.22 | 2.47 | 2.73 |
| Incidence rate per 1000 PY | 5.79 (5.56, ‐6.03) | 1.62 (1.59‐1.65) | 0.08 (0.08‐0.09) |
Abbreviations: PY, person‐years of follow‐up; T1D, type 1 diabetes; T2D, type 2 diabetes.
T1D was defined as ≥1 prior inpatient or outpatient claim for T1D (ICD9: 250.x1, 250.x3) + no prior oral antidiabetic claim + ≥1 prior insulin claim.
T2D was defined as ≥2 prior inpatient or outpatient claims for T2D (ICD9: 250.x0, 250.x2) + ≥1 prior oral antidiabetic claim.
The no diabetes cohort was defined as neither prior inpatient nor prior outpatient claims for diabetes (ICD9: 250.x) at the time of matched index and 1:1 matched to each diabetic cohort based on sex and age category (18‐30, 31‐40, 41‐50, 51‐60, 61‐70, 71‐80 and >80 y).
LEA defined as the presence of an inpatient or outpatient procedure claim based on a modification of the AHRQ Prevention Quality Indicator #16 (any [ICD‐9: 84.1, 84.1x; CPT‐4: 27 590, 27 591, 27 592, 27 594, 27 596, 27 598, 27 880, 27 881, 27 882, 27 884, 27 886, 27 888, 27 889, 28 800, 28 805, 28 810, 28 820, 28 825]; major [ICD‐9: 84.15‐84.19; CPT‐4: 27 590‐27 886]; minor (ICD‐9: 85.11‐84.14; CPT‐4: 27 888‐28 825]; toe [ICD‐9: 84.11; CPT‐4: 28 810, 28 820, 28 825]). Hospital Admission for Ambulatory Care Sensitive Conditions. Rockville, MD: Agency for Healthcare Research and Quality. Revision 4. November 24, 2004. AHRQ Pub. No. 02‐R0203 with the addition to include toe amputations.9
Approximately half of the descriptive cohorts were men (T1D: 53.5%, T2D: 53.3%, no diabetes: 47.2%; File S7). Age distribution varied among the different cohorts, with the cohort of patients with T2D including the largest proportion of patients aged 65 years or older. In both diabetes cohorts, the IR of LEA was twice as high in men than women, and twice as high in patients aged 65 years or older compared with patients aged less than 65 years.
3.2. Comparative study
For the comparative analyses, an analytic subset of 120 129 patients with T1D and 1.6 million patients with T2D were able to be 1:1 matched to random samples of patients without a history of diabetes. Similar to the descriptive study, about half of the matched cohorts were men (53.2%‐53.4%; Table 2). Patients with T1D had a younger mean (SD) age than patients with T2D (49.9 [18.68] vs. 58.8 [12.42]). Compared with patients without diabetes, patients with T1D or T2D had more cardiovascular and other co‐morbidities.
Table 2.
Baseline distribution of co‐morbidities among 1:1 comparative cohorts matched on sex, age group and calendar time, 2010‐2014
| T1D vs. no diabetes | T2D vs. no diabetes | |||||
|---|---|---|---|---|---|---|
| T1Da | No diabetesb | ASD T1D – No diabetesc | T2Dd | No diabetesb | ASD T2D – No diabetesc | |
| N | 120 129 | 120 129 | 1 679 877 | 1 679 877 | ||
| At cohort entry | ||||||
| Age, mean (SD) | 49.9 (18.68) | 50.0 (18.26) | 0.01 | 58.8 (12.42) | 58.4 (12.82) | 0.03 |
| Age 18‐30 y | 18.7% | 18.7% | 0 | 1.2% | 1.2% | 0 |
| Age 31‐40 y | 11.9% | 11.9% | 0 | 5.6% | 5.6% | 0 |
| Age 41‐50 y | 17.4% | 17.4% | 0 | 16.9% | 16.9% | 0 |
| Age 51‐60 y | 22.3% | 22.3% | 0 | 33.8% | 33.8% | 0 |
| Age 61‐70 y | 15.4% | 15.4% | 0 | 25.3% | 25.3% | 0 |
| Age 71‐80 y | 9.1% | 9.1% | 0 | 11.6% | 11.6% | 0 |
| Age >80 y | 5.2% | 5.2% | 0 | 5.5% | 5.5% | 0 |
| Female | 46.6% | 46.6% | 0 | 46.8% | 46.8% | 0 |
| Co‐morbidities during the baseline periode | ||||||
| Arterial hypertension | 32.9% | 16.1% | 0.40 | 44.3% | 20.9% | 0.51 |
| Atrial fibrillation | 3.9% | 1.9% | 0.12 | 3.6% | 2.3% | 0.07 |
| Ischaemic heart disease | 13.6% | 3.6% | 0.36 | 10.9% | 5.0% | 0.22 |
| Congestive heart failure | 6.4% | 1.0% | 0.29 | 3.3% | 1.1% | 0.15 |
| Cerebrovascular diseases | 4.8% | 1.5% | 0.19 | 3.5% | 1.8% | 0.10 |
| Diabetic retinopathy | 9.0% | 0.1% | 0.44 | 1.8% | 0.1% | 0.18 |
| Chronic kidney disease | 10.5% | 1.1% | 0.41 | 3.9% | 1.3% | 0.16 |
| Peripheral artery disease | 5.4% | 1.2% | 0.24 | 3.3% | 1.5% | 0.12 |
| Peripheral polyneuropathy | 4.0% | <0.1% | 0.28 | 1.4% | 0.1% | 0.16 |
| Foot deformities | 2.1% | 1.1% | 0.08 | 1.6% | 1.3% | 0.02 |
| Charcot foot | 0.3% | <0.1% | 0.08 | 0.1% | <0.1% | 0.03 |
| Preulcerative callus or corn | 1.3% | 0.2% | 0.12 | 0.7% | 0.3% | 0.06 |
| Medications anytime prior to matched indexf | ||||||
| Diuretics | 33.7% | 14.1% | 0.47 | 46.7% | 17.9% | 0.65 |
| Loop diuretics | 18.9% | 3.0% | 0.53 | 13.6% | 3.6% | 0.36 |
| Insulin | 100% | 0.1% | 48.51 | 15.8% | 0.1% | 0.61 |
Abbreviations: ASD, absolute standardized difference; T1D, type 1 diabetes; T2D, type 2 diabetes.
T1D defined as ≥1 prior inpatient or outpatient claim for T1D (ICD9: 250.x1, 250.x3) + no prior oral antidiabetic claim + ≥1 prior insulin claim.
The No diabetes cohort was defined as neither prior inpatient nor prior outpatient claims for diabetes (ICD9: 250.x) at the time of matched index and 1:1 matched to each diabetic cohort based on sex and age category (18‐30, 31‐40, 41‐50, 51‐60, 61‐70, 71‐80, >80 y).
T2D was defined as ≥2 prior inpatient or outpatient claims for T2D (ICD9: 250.x0, 250.x2) + ≥1 prior oral antidiabetic claim.
All co‐morbidities defined as at least 1 inpatient claim or at least 2 outpatient claims on 2 separate days during the 365 days prior to matched index date.
Medications defined based on at least 1 prescription claim during all available data prior to matched index date.
The matched IRs of LEA were 6.02 and 1.90 per 1000 PY in patients with T1D and T2D, respectively (Table 3; File S8). Compared with the matched cohort of patients without diabetes, the crude HR of LEA among T1D patients was 42.15 (30.92‐57.44), which was attenuated to 22.47 (16.42‐30.73) after adjustment, and the crude HR of LEA among T2D patients was 8.10 (7.58‐8.65), which was attenuated to 4.64 (4.32‐4.98) after adjustment. When stratified by LEA subtype, HRs increased in magnitude for minor LEAs and toe amputations, but were attenuated for major LEAs.
Table 3.
Comparative incidence of lower extremity amputation (LEA) among 1:1 comparative cohorts matched on sex, age group and calendar time, 2010‐2014, overall and stratified by LEA type
| T1D vs. no diabetes | T2D vs. no diabetes | |||
|---|---|---|---|---|
| T1Da | No diabetesb | T2Dc | No diabetesb | |
| N patients | 120 129 | 120 129 | 1 679 877 | 1 679 877 |
| Overall (any LEA)e | ||||
| Number of patients with an event | 1705 | 41 | 8803 | 991 |
| Incidence rate per 1000 PY | 6.02 (5.73‐6.31) | 0.14 (0.10‐0.19) | 1.90 (1.86‐1.94) | 0.23 (0.22‐0.25) |
| Crude HR (95% CI) | 42.15 (30.92‐57.44) | 1.00 (ref) | 8.10 (7.58‐8.65) | 1.00 (ref) |
| Fully adjusted HR (95% CI)e | 22.47 (16.42‐30.73) | 1.00 (ref) | 4.64 (4.32‐4.98) | 1.00 (ref) |
| Major LEAe | ||||
| Number of patients with an event | 560 | 18 | 2568 | 346 |
| Incidence rate per 1000 PY | 1.96 (1.81‐2.13) | 0.06 (0.04‐0.10) | 0.55 (0.53‐0.57) | 0.08 (0.07‐0.09) |
| Crude HR (95% CI) | 31.36 (19.61‐50.14) | 1.00 (ref) | 6.71 (5.99‐7.50) | 1.00 (ref) |
| Fully adjusted HR (95% CI)e | 12.99 (8.05‐20.97) | 1.00 (ref) | 3.49 (3.08‐3.94) | 1.00 (ref) |
| Minor LEAd | ||||
| Number of patients with an event | 1376 | 23 | 7111 | 688 |
| Incidence rate per 1000 PY | 4.85 (4.60‐5.11) | 0.08 (0.05‐0.12) | 1.53 (1.50‐1.57) | 0.16 (0.15‐0.17) |
| Crude HR (95% CI) | 60.57 (40.12‐91.46) | 1.00 (ref) | 9.42 (8.71‐10.19) | 1.00 (ref) |
| Fully adjusted HR (95% CI)e | 33.80 (22.32‐51.19) | 1.00 (ref) | 5.50 (5.06‐5.98) | 1.00 (ref) |
| Toe amputationd | ||||
| Number of patients with an event | 1275 | 22 | 6623 | 649 |
| Incidence rate per 1000 PY | 4.49 (4.25‐4.74) | 0.08 (0.05‐0.11) | 1.43 (1.39‐1.46) | 0.15 (0.14‐0.16) |
| Crude HR (95% CI) | 58.66 (38.49‐89.40) | 1.00 (ref) | 9.30 (8.58‐10.08) | 1.00 (ref) |
| Fully adjusted HR (95% CI)e | 33.32 (21.79‐50.96) | 1.00 (ref) | 5.42 (4.98‐5.91) | 1.00 (ref) |
Abbreviations: PY, person‐years of follow‐up; ref, reference group; T1D, type 1 diabetes; T2D, type 2 diabetes.
T1D defined as ≥1 prior inpatient or outpatient claim for T1D (ICD9: 250.x1, 250.x3) + no prior oral antidiabetic claim + ≥1 prior insulin claim.
The No diabetes cohort was defined as neither prior inpatient nor prior outpatient claims for diabetes (ICD9: 250.x) at the time of matched index and 1:1 matched to each diabetic cohort based on sex and age category (18‐30, 31‐40, 41‐50, 51‐60, 61‐70, 71‐80, >80 y).
T2D was defined as ≥2 prior inpatient or outpatient claims for T2D (ICD9: 250.x0, 250.x2) + ≥1 prior oral antidiabetic claim.
LEA was defined as the presence of an inpatient or outpatient procedure claim based on a modification of the AHRQ Prevention Quality Indicator #16 (Any [ICD‐9: 84.1, 84.1x; CPT‐4: 27 590, 27 591, 27 592, 27 594, 27 596, 27 598, 27 880, 27 881, 27 882, 27 884, 27 886, 27 888, 27 889, 28 800, 28 805, 28 810, 28 820, 28 825]; major (ICD‐9: 84.15‐84.19; CPT‐4: 27 590‐27 886]; minor [ICD‐9: 85.11‐84.14; CPT‐4: 27 888‐28 825]; toe [ICD‐9: 84.11; CPT‐4: 28 810, 28 820, 28 825]). Hospital Admission for Ambulatory Care Sensitive Conditions. Rockville, MD: Agency for Healthcare Research and Quality. Revision 4. November 24, 2004. AHRQ Pub. No. 02‐R0203 with the addition to include toe amputations. https://www.qualityindicators.ahrq.gov/Downloads/Modules/PQI/V21/pqi_guide_rev4.pdf.
Fully adjusted HRs were adjusted for age (continuous), co‐morbidities during the baseline period, defined based on at least 1 inpatient or 2 outpatient claims (arterial hypertension, atrial fibrillation, ischaemic heart disease, congestive heart failure, cerebrovascular diseases, diabetic retinopathy, chronic kidney disease, peripheral artery disease, peripheral polyneuropathy, foot deformities, charcot foot, preulcerative callus or corn) and history of medication use using all available data prior to matched index date (diuretics, loop diuretics). The T2D models additionally adjusted for history of insulin use.
Among those without a history of ESRD, the frequencies of co‐morbidities were similar for the T2D comparison with no diabetes, yielding a similar aHR to the overall aHR (4.62 [4.30‐4.96]), while co‐morbidities were reduced among the T1D patients attenuating the aHR (19.99 [14.59‐27.38]) (Files S9–S10). The aHR was also similar among those without a history of insulin use in the T2D comparison (4.36 [4.05‐4.69]) (Files S11–S12).
Within the month (30 days) preceding an LEA, a majority of cases had at least one claim for a co‐morbidity or a precipitating factor probably leading to LEA. The frequency of these co‐morbidities or precipitating factors was highest in the T1D cohort. Compared with patients without diabetes, patients with T1D more probably had ESRD (ASD = 0.81), foot and leg ulcers (ASD = 1.06), foot and leg cellulitis (ASD = 0.60), osteomyelitis of lower limb (0.77) or gangrene (ASD = 0.24). The differences were attenuated for patients with T2D, who were more probable to have ESRD (ASD = 0.21), foot and leg ulcers (ASD = 0.61), foot and leg cellulitis (ASD = 0.49) or osteomyelitis of lower limb (ASD = 0.53) (Table 4).
Table 4.
Co‐morbidities and precipitating factors within 1 month preceding a lower extremity amputation among 1:1 matched comparative cohorts, 2010‐2014
| T1D vs. no diabetes | T2D vs. no diabetes | |||||
|---|---|---|---|---|---|---|
| T1D | No diabetes | ASD T1D – No diabetesa | T2D | No diabetes | ASD T2D – No diabetesa | |
| Number of patients with an event | 1705 | 41 | 8803 | 991 | ||
| End‐stage renal disease including dialysis | 35.0% | 4.9% | 0.81 | 13.4% | 7.0% | 0.21 |
| Foot and leg ulcer | 86.5% | 41.5% | 1.06 | 82.1% | 55.0% | 0.61 |
| Foot deformities | 8.4% | 17.1% | 0.26 | 9.2% | 20.5% | 0.32 |
| Foot and leg cellulitis | 74.6% | 46.3% | 0.60 | 73.0% | 49.6% | 0.49 |
| Osteomyelitis of lower limbs | 69.9% | 34.1% | 0.77 | 67.6% | 42.3% | 0.53 |
| Gangrene | 35.3% | 24.4% | 0.24 | 31.1% | 26.7% | 0.10 |
| Diuretics | 53.8% | 46.3% | 0.15 | 59.5% | 37.4% | 0.45 |
| Loop diuretics | 41.7% | 39.0% | 0.05 | 38.7% | 20.4% | 0.41 |
Abbreviations: ASD, absolute standardized difference; T1D, type 1 diabetes; T2D, type 2 diabetes.
ASD >0.1 are considered relevant.
In the sensitivity analysis comparing T1D patients with a 3:1 matched cohort of patients without diabetes (Files S13–S14), the overall aHR (17.21 [14.51‐20.40]) was attenuated with increased precision.
4. DISCUSSION
Using claims data from the IBM MarketScan research database, we evaluated the crude IR of LEA among patients with T1D and T2D, as well as all patients with no history of diabetes. Our data confirm a higher IR of LEA among patients with T1D and T2D compared with patients without diabetes. The crude IR of any LEA for the descriptive cohort of T2D (1.62 [1.59‐1.65] per 1000 PY) was lower than estimates for any diabetes reported in previous database studies, while the IR for the T1D cohort was much higher (5.79 [5.56‐6.03] per 1000 PY) (Table 1).7, 9, 17 If combined, the IR of LEA would be increased, but heavily weighted by the T2D majority, who had a lower relative risk, masking the greater risk among the patients with T1D.
Based on previous studies, we expected the IR of LEA among patients with T2D to be ~ 8‐fold higher than the IR in non‐diabetic patients.7 The relative differences among both T1D and T2D descriptive cohorts compared with the cohort of all non‐diabetic patients were substantially higher (72‐fold and 20‐fold, respectively). This was in part attributable to patients without diabetes having a much lower crude IR (0.08 [0.08‐0.09] per 1000 PY) than reported in the general population previously (0.27 [1.9‐3.5] per 1000 PY), based on data from the National Health Interview Survey, which samples an average of 57 000 adults per year to estimate the health of the US population.6 This lower incidence may be attributable, at least partially, to an underrepresentation of people aged older than 65 years in the IBM Marketscan claims database, as patients become eligible to transfer from commercial insurance to Medicare when aged 65 years. After matching, the differences for the T1D and T2D 1:1 matched comparisons were substantially attenuated (HR, 42.15 [30.92‐57.44] and 8.10 [7.58‐8.65], respectively), and further attenuated after adjustment for known risk factors (aHR, 22.47 [16.42‐30.73] and 4.64 [4.32‐4.98], respectively).
We observed a higher IR of LEA for patients with T1D compared with patients with T2D. By contrast, a previous study reported similar IRs of LEA, ~ 1 per 1000 PY, in T1D and T2D for a similar disease duration among patients in Denmark.18 In studies conducted in patients with T2D in New Zealand,19 and using data from Kaiser Permanente Northern California (KPNC) in the United States,20 increased rates of LEA were reported with increased duration of T2D. The KPNC study included a population of patients with T2D aged older than 60 years and reported IRs of LEA between 1.01 (0.85‐1.2) and 1.72 (1.34‐2.21) per 1000 PY across this age group with a diabetes duration of 0‐9 years and between 3.92 (3.16‐4.88) and 4.26 (3.66‐4.95) per 1000 PY when diabetes duration was at least 10 years.20 Considering the older population under the scope of the KPNC study, estimates reported are broadly in line with those in the current study (File S7). Data from a large national multi‐ethnic cohort of patients with T2D followed in primary care in New Zealand and via linked hospital records reported an IR of hospitalizations for LEA of 2.11 per 1000 PY. This study also underlined an increased risk of LEA associated with older age at diabetes onset and longer diabetes duration.19 Unlike the studies conducted in Denmark, New Zealand and via KPNC, diabetes duration is not available in US claims data, such as our data source. However, we observed substantial differences in the comparative study between the baseline characteristics (age, sex and co‐morbidities) and between known risk factors for LEA in patients with T1D compared with patients with T2D, suggesting that the increased IR observed in the T1D cohort could be attributable to more advanced disease severity. Notably, in the comparative study, ~ 35% of patients with T1D with an LEA had a claim for ESRD during the preceding month. Compared with patients without diabetes, the greatest differences in precipitating factors were for foot ulcers among both T1D (ASD = 1.06) and T2D (ASD = 0.61) patients. Our study also reports LEA subtype, which allows us to see that the aHRs for T1D and T2D compared with non‐diabetic patients are higher for minor LEA (33.80 [22.32‐51.19] and 5.50 [5.06‐5.98], respectively) and toe amputations (33.32 [21.79‐50.96] and 5.42 [4.98‐5.91], respectively). Disease length and progression should be considered when available in studies of the IR of LEA. However, this information is rarely available in large US claims data commonly used for epidemiological research when events with a low IR are of interest.
Similar sex differences were reported previously in a study conducted using a US‐based hospital discharge database.17 Male sex appears to be a greater risk factor for LEA than age. Some of this difference could be related to a differential distribution of risk factors between men and women, such as smoking, which were not measured. However, gender difference remained in the New Zealand study, where risk factors such as smoking, body mass index (BMI) and socioeconomic status were considered in the analysis.19 Finally, compared with patients with diabetes, a notably higher effect of age on the IR of LEA was observed in patients without diabetes.
The IBM MarketScan research databases include de‐identified data from beneficiaries residing in all US states and covers over 350 US insurance carriers and large, self‐insuring companies. The data include insured patients and data from the Medicare advantage plan. As such, its external validity to the United States is expected to be high and data are deemed reliable. The data obtained may be of limited applicability to other countries where universal healthcare coverage is in place. The size of the databases allowed for the inclusion of a large number of events in the analyses, ensuring precise estimates. As claims are linked to the physician's billing system, we anticipate little underreporting or misclassification for such a reimbursed procedure. However, there are important limitations to consider.
First, claims data are not intended for medical record‐keeping or research and the capture of some conditions could be incomplete or difficult to identify. In addition, the population of patients with lower socioeconomic status treated under Medicaid who may be at higher risk of amputation may also be underrepresented. Laboratory data, as well as several known lifestyle and biometric risk factors for diabetic LEAs, could not be evaluated as the data were either missing or incompletely captured, including HbA1c, number of years since diabetes diagnosis, smoking status, ethnicity, socioeconomic status, occupation type and BMI. Although HbA1c was not available to adjust for diabetic severity, we adjusted for the diabetic complications, retinopathy and peripheral polyneuropathy, which are indicative of diabetic severity. Further, the fact that 10% of patients in the T1D descriptive cohort later qualified for the T2D cohort suggests known potential for misclassification of diabetes type via claims.21 However, use of validated algorithms to define T1D and T2D taking medication exposure into account minimizes the potential for misclassification. Lastly, the rarity of LEA among the non‐diabetic patients 1:1 matched to T1D patients in our primary analysis yielded wide CIs. However, the sensitivity analysis with 3:1 matching identified similar trends via more precise attenuated estimates that were within the CI of the 1:1 matched estimates.
In conclusion, our data showed substantially higher crude IR of LEA among patients with T1D and T2D compared with those without diabetes. The greatest precipitating factor for LEA was foot ulcers for both T1D and T2D. Although adjustment for known risk factors reduced the relative hazard by nearly half, the adjusted relative hazard of LEA was 22.47 (16.42‐30.73) among patients with T1D and 4.64 (4.32‐4.98) among patients with T2D, when compared with a matched sample of non‐diabetic patients.
Our study evaluated T1D and T2D patients separately, identifying a substantial increase in the IR of LEA among patients with T1D compared with patients with T2D, a trend that would have been masked if T1D and T2D patients were combined, because of the T2D majority. This difference also remained after matching and adjustment despite attenuation. We also identified differences among LEA subtypes. Compared with patients without diabetes, the greatest differences among all cases of LEA were for minor LEA followed by toe amputations. A majority of cases of LEA were associated with the presence of precipitating factors within the month preceding the procedure. The frequency of precipitating factors within the month of the LEA was higher among patients with T1D or T2D compared with patients without diabetes, underlining the necessity for proper diabetes care to handle these precipitating factors early.
CONFLICT OF INTEREST
ADL, CR and KGB are employees of Boehringer Ingelheim. LAL received speaker fees from Osiris, Integra and Cardinal; consulting fees from Aplion Medical Users, Harbor MedTech, Medline Industries, Inc. and Boehringer Ingelheim; and a research grant from Cardinal. EMG is an employee of Aetion, Inc., a software and data analytics company, of which she holds stock options.
AUTHOR CONTRIBUTIONS
ADL, KGB and LAL developed the protocol. ADL and LAL defined the lists of variables to be collected and lists of codes used for identification of conditions in the claims database. CR and EMG conducted the data analysis. All authors contributed to the interpretation of the results, development and revision of the manuscript, and the decision to submit for publication.
Supporting information
File S1 Detailed code lists used for the identification of diabetes and LEA
File S2. Code lists for comorbidities measured at baseline and at the time of LEA
File S3. List of ATC codes used for the identification of the use of Diuretics
File S4. Computation of the absolute standardized differences
File S5. Code lists for LEA sub‐types
File S6. ATC codes for Insulin use
File S7. Crude incidence of LEA among the three descriptive cohorts, stratified by sex and age group, 2010–2014
File S8: Comparative Incidence of LEA Among Type 1 or 2 Diabetes vs. No Diabetes at Matched Index, 2010–2014, Overall and Stratified by LEA sub‐Type
File S9: Characteristics of Patients at Time of Type 1 and Type 2 Diabetes Diagnosis or Matched Index Date, 2010–2014, Among Patients without a History of End‐Stage Renal Disease (ESRD) 1 upon Cohort Entry
File S10: Comparative Incidence of Any Amputation Among Type 1 or 2 Diabetes vs. No Diabetes at Matched Index, 2010–2014, Among Patients without a History of End‐Stage Renal Disease (ESRD) 1 upon Cohort Entry
File S11: Characteristics of Patients at Time of Type 2 Diabetes Diagnosis or Matched Index Date, 2010–2014, Among Patients without a History of Insulin1 upon Cohort Entry
File S12: Comparative Incidence of Any Amputation Among T2D vs. No Diabetes at Matched Index, 2010–2014, Among Patients without a History of Insulin1 upon Cohort Entry
File S13: [Post‐hoc sensitivity Analysis] Baseline distribution of comorbidities among 3:1 comparative T1D cohort matched on sex, age group, and calendar time, 2010–2014
File S14: [Post‐hoc sensitivity Analysis] Comparative incidence of LEA among 3:1 comparative T1D cohorts matched on sex, age group, and calendar time, 2010–2014, overall and stratified by LEA type
ACKNOWLEDGMENTS
The authors would like to thank Dr Hristo Iliev for his contribution to the study and comments on the drafted manuscript.
Déruaz‐Luyet A, Raabe C, Garry EM, Brodovicz KG, Lavery LA. Incidence of lower extremity amputations among patients with type 1 and type 2 diabetes in the United States from 2010 to 2014. Diabetes Obes Metab. 2020;22:1132–1140. 10.1111/dom.14012
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14012.
Funding information Boehringer Ingelheim
REFERENCES
- 1. International Diabetes Federation . Chapter 1: What is diabetes? In: Diabetes Atlas. 7th ed. Brussels, Belgium: International Diabetes Federation; 2015:21‐34. [Google Scholar]
- 2. American Diabetes Association . 10. Microvascular complications and foot care. Diabetes Care. 2017;40:S88‐S98. [DOI] [PubMed] [Google Scholar]
- 3. Markowitz JS, Gutterman EM, Magee G, Margolis DJ. Risk of amputation in patients with diabetic foot ulcers: a claims‐based study. Wound Repair Regen. 2006;14:11‐17. [DOI] [PubMed] [Google Scholar]
- 4. Prevention CfDCa . National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2017. [Google Scholar]
- 5. Moxey PW, Gogalniceanu P, Hinchliffe RJ, et al. Lower extremity amputations‐a review of global variability in incidence. Diabet Med. 2011;28:1144‐1153. [DOI] [PubMed] [Google Scholar]
- 6. Gregg EW, Li Y, Wang J, et al. Changes in diabetes‐related complications in the United States, 1990‐2010. N Engl J Med. 2014;370:1514‐1523. [DOI] [PubMed] [Google Scholar]
- 7. Hyland KA, Greiner MA, Qualls LG, Califf RM, Hernandez AF, Curtis LH. Trends in the care and outcomes of Medicare beneficiaries with type 2 diabetes, 2002‐2011. Endocr Pract. 2016;22:920‐934. [DOI] [PubMed] [Google Scholar]
- 8. Newhall K, Spangler E, Dzebisashvili N, Goodman DC, Goodney P. Amputation rates for patients with diabetes and peripheral arterial disease: the effects of race and region. Ann Vasc Surg. 2016;30:292‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. AHRQ QI™ Version 4.5 . Prevention quality indicators #16, Technical specifications, lower‐extremity amputation among patients with diabetes rate, 2015. https://www.qualityindicators.ahrq.gov/.
- 10. Panaccio MP, Cummins G, Wentworth C, et al. A common data model to assess cardiovascular hospitalization and mortality in atrial fibrillation patients using administrative claims and medical records. Clin Epidemiol. 2015;7:77‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tessier‐Sherman B, Galusha D, Taiwo OA, et al. Further validation that claims data are a useful tool for epidemiologic research on hypertension. BMC Public Health. 2013;13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ali MS, Groenwold RH, Pestman WR, et al. Propensity score balance measures in pharmacoepidemiology: a simulation study. Pharmacoepidemiol Drug Saf. 2014;23:802‐811. [DOI] [PubMed] [Google Scholar]
- 13. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083‐3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mamdani M, Sykora K, Li P, et al. Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ. 2005;330:960‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Franz D, Zheng Y, Leeper NJ, Chandra V, Montez‐Rath M, Chang TI. Trends in rates of lower extremity amputation among patients with end‐stage renal disease who receive dialysis. JAMA Intern Med. 2018;178:1025‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Humphries MD, Brunson A, Hedayati N, Romano P, Melnkow J. Amputation risk in patients with diabetes mellitus and peripheral artery disease using statewide data. Ann Vasc Surg. 2016;30:123‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jørgensen ME, Almdal TP, Faerch K. Reduced incidence of lower‐extremity amputations in a Danish diabetes population from 2000 to 2011. Diabet Med. 2014;31:443‐447. [DOI] [PubMed] [Google Scholar]
- 19. Robinson TE, Kenealy T, Garrett M, Bramley D, Drury PL, Elley CR. Ethnicity and risk of lower limb amputation in people with type 2 diabetes: a prospective cohort study. Diabet Med. 2016;33:55‐61. [DOI] [PubMed] [Google Scholar]
- 20. Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Intern Med. 2014;174:251‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Desai M, Ryan PB, et al. Incidence of diabetic ketoacidosis among patients with type 2 diabetes mellitus treated with SGLT2 inhibitors and other antihyperglycemic agents. Diabetes Res Clin Pract. 2017;128:83‐90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1 Detailed code lists used for the identification of diabetes and LEA
File S2. Code lists for comorbidities measured at baseline and at the time of LEA
File S3. List of ATC codes used for the identification of the use of Diuretics
File S4. Computation of the absolute standardized differences
File S5. Code lists for LEA sub‐types
File S6. ATC codes for Insulin use
File S7. Crude incidence of LEA among the three descriptive cohorts, stratified by sex and age group, 2010–2014
File S8: Comparative Incidence of LEA Among Type 1 or 2 Diabetes vs. No Diabetes at Matched Index, 2010–2014, Overall and Stratified by LEA sub‐Type
File S9: Characteristics of Patients at Time of Type 1 and Type 2 Diabetes Diagnosis or Matched Index Date, 2010–2014, Among Patients without a History of End‐Stage Renal Disease (ESRD) 1 upon Cohort Entry
File S10: Comparative Incidence of Any Amputation Among Type 1 or 2 Diabetes vs. No Diabetes at Matched Index, 2010–2014, Among Patients without a History of End‐Stage Renal Disease (ESRD) 1 upon Cohort Entry
File S11: Characteristics of Patients at Time of Type 2 Diabetes Diagnosis or Matched Index Date, 2010–2014, Among Patients without a History of Insulin1 upon Cohort Entry
File S12: Comparative Incidence of Any Amputation Among T2D vs. No Diabetes at Matched Index, 2010–2014, Among Patients without a History of Insulin1 upon Cohort Entry
File S13: [Post‐hoc sensitivity Analysis] Baseline distribution of comorbidities among 3:1 comparative T1D cohort matched on sex, age group, and calendar time, 2010–2014
File S14: [Post‐hoc sensitivity Analysis] Comparative incidence of LEA among 3:1 comparative T1D cohorts matched on sex, age group, and calendar time, 2010–2014, overall and stratified by LEA type


