Abstract
Aims
Both, vitamin D3 and human periodontal ligament cells (hPDLCs) possess immunosuppressive properties, but their combined effect on immune cells has never been investigated. Here, we analysed the impact of vitamin D3 on the immunosuppressive properties of hPDLCs towards CD4+ T lymphocytes.
Material and Methods
Allogenic CD4+ T lymphocytes were activated by phytohemagglutinin either in monoculture or co‐culture with hPDLCs, in the presence or absence of IFN‐γ and 1,25(OH)2D3. After 5 days, CD4+ T‐lymphocyte proliferation, CD4+ CD25+ FoxP3+ regulatory T lymphocytes (Tregs) proportion and IL‐10, TGF‐β1 and IL‐17A production were analysed.
Results
In monoculture, 1,25(OH)2D3 suppressed CD4+ T‐lymphocyte proliferation, increased the percentage of CD4+ FoxP3+ CD25+ FoxP3+ Tregs and enhanced IL‐10 and TGF‐β1 production. In the presence of IFN‐γ treated hPDLCs, 1,25(OH)2D3 significantly increased CD4+ T‐lymphocyte proliferation and decreased the percentage of CD4+ CD25+ FoxP3+ Tregs. IL‐10 and IL‐17A expression was significantly diminished by 1,25(OH)2D3, whereas TGF‐β1 was slightly increased. The effects of 1,25(OH)2D3 in co‐culture were reversed by inhibition of indoleamine‐2,3‐dioxygenase‐1, prostaglandin‐endoperoxide synthase and programmed cell death 1 ligand 1. 1,25(OH)2D3 also suppressed the expression of these proteins in hPDLCs.
Conclusion
Effects of vitamin D3 on CD4+ T lymphocyte are modified by hPDLCs depending on the microenvironment.
Keywords: CD4‐Positive T Lymphocytes, co‐culture, Immunomodulation, Periodontal Ligament, Vitamin D
Clinical relevance.
Scientific rationale for the study: Although vitamin D3 exerts immunosuppressive effects, its role in periodontitis remains controversial. Studies investigating the effect of vitamin D3 in complex in vitro co‐culture models are important to clarify its exact role.
Principal findings: Vitamin D3 suppressed CD4+ T‐lymphocyte proliferation and enhanced the proportion of CD4+ CD25+ FoxP3+ Tregs. However, in the presence of human periodontal ligament cells and inflammatory stimuli, the opposite effects were observed: stimulation of CD4+ T‐lymphocyte proliferation and reduction of the CD4+ CD25+ FoxP3+ Tregs proportion.
Practical implications: The effects of vitamin D3 in the periodontium are multifaceted, which should be considered for its application in clinical practice.
1. INTRODUCTION
Human periodontal ligament cells (hPDLCs) fulfil the minimal criteria of mesenchymal stem cells (MSCs) (Seo et al., 2004), including the expression of specific surface markers and a multilineage differentiation potential (Viswanathan et al.., 2019). This heterogeneous population of fibroblast‐like cells participates in tissue regeneration by cell proliferation, differentiation into tissue‐specific cells and modulating immune and inflammatory responses (Racz et al., 2014; Wada, Gronthos, & Bartold, 2013; Xiao & Nasu, 2014).
Similarly to other MSCs, hPDLCs possess immunomodulatory properties and affect various immune cells. This is facilitated by the production of enzymes and soluble factors, as well as via direct cell‐to‐cell contact (Andrukhov, Behm, Blufstein, & Rausch‐Fan, 2019; Wada et al., 2013). The key factors mediating these immunomodulatory effects are indoleamine‐2,3‐dioxygenase‐1 (IDO‐1), prostaglandin E2 (PGE2), programmed cell death 1 ligand 1 (PD‐L1), programmed cell death 1 ligand 2 (PD‐L2) and others (Andrukhov et al., 2019; Wada et al., 2013). Beside their immunosuppressive abilities, hPDLCs can also favour the inflammatory response under certain conditions (Andrukhov et al., 2019), mainly by producing several pro‐inflammatory cytokines, particularly interleukin (IL)‐1β, IL‐6, IL‐8 and monocyte chemoattractant protein 1, which are triggered by different bacterial and viral pathogens (Andrukhov et al., 2016; Behm et al., 2019; Blufstein et al., 2019; Kato, Taguchi, Tominaga, Umeda, & Tanaka, 2014). Among different immune cells, the effects of hPDLCs on CD4+ T lymphocytes are the most investigated (Wada et al., 2013). It is known that hPDLCs per se suppress the activation, proliferation and differentiation of CD4+ T lymphocytes, enhance the formation of regulatory T lymphocytes (Tregs) and reduce IL‐17A production (Castro‐Manrreza & Montesinos, 2015; Liu, Xu, et al., 2012). These immunosuppressive effects of hPDLCs are strongly enhanced by pro‐inflammatory stimuli such as interferon (IFN)‐γ. Presumably, this is due to increased expression of diverse immunomodulatory proteins, including IDO‐1, prostaglandin‐endoperoxide synthase‐2 (PTGS‐2) and PD‐L1 (Chabannes et al., 2007; English et al., 2009; Meisel et al., 2004; Nasef et al., 2007; Sato et al., 2007; Selmani et al., 2008).
Vitamin D3 is a hormone involved in the regulation of bone homoeostasis (Lips, 2006) and immune response (White, 2008). The major sources of vitamin D3 are its production in the skin through sun exposure and dietary supplements. Vitamin D3 is converted into 25(OH)D3 and further to the biologically most active form 1,25(OH)2D3 mainly in the kidney (Jones, 2008; Lips, 2006). Recent studies show that local conversion of 25(OH)D3 into its bioactive form also occurs by immune cells (Wu, Ren, Nguyen, Adams, & Hewison, 2007; Zehnder, 2001) and hPDLCs (Liu, Meng, & Hou, 2012). It is known that 1,25(OH)2D3 and 25(OH)D3 suppress the production of pro‐inflammatory mediators by hPDLCs in response to different stimuli (Andrukhov et al., 2014; Hosokawa, Hosokawa, Shindo, Ozaki, & Matsuo, 2015; Nebel et al., 2015; Tang, Pan, & Zhao, 2013). However, the effect of vitamin D3 on the immunosuppressive properties of hPDLCs is unknown.
In the present study, we investigated the effect of vitamin D3 on allogenic CD4+ T lymphocytes in the presence of hPDLCs and inflammatory stimuli. Particularly, we examined the influence of IFN‐γ treated hPDLCs on the potential of 1,25(OH)2D3 to affect CD4+ T‐lymphocyte proliferation, CD4+ CD25+ FoxP3+ Tregs proportion and production of functional cytokines by CD4+ T lymphocytes in an indirect in vitro co‐culture model. We additionally inhibited IDO‐1, PD‐L1 and PTGS‐2 proteins pharmacologically in order to evaluate their contribution into hPDLCs’ mediated effects on CD4+ T lymphocytes. Further, we investigated the effect of 1,25(OH)2D3 on the production of IDO‐1, PTGS‐2, PD‐L1 and PD‐L2 by IFN‐γ treated hPDLCs in vitro.
2. MATERIAL AND METHODS
Detailed cell isolation and analysis protocols can be found in the Supporting information.
The study protocol was approved by the Ethics Committee of the Medical University of Vienna (EK Nr. 1694/2015, extended 2019). All procedures were performed according to the Declaration of Helsinki and the Good Scientific Practice Guidelines of the Medical University of Vienna.
2.1. Co‐culture of hPDLC and CD4+ T lymphocytes
2.5 × 105 primary hPDLCs were seeded per well in a 6‐well plate for 24 hr in DMEM supplemented with 10% foetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S). hPDLCs were stimulated with 100 ng/ml IFN‐γ (PeproTech) in the presence or absence of 100 nM 1,25(OH)2D3 (Cayman Chemical) in FBS‐free DMEM. After 48 hr incubation, the medium was changed to RPMI‐1640 (Sigma‐Aldrich) supplemented with 10% FBS, 1% P/S and 100 ng/ml IFN‐γ and 100 nM 1,25(OH)2D3. Transwell (TC) inserts with 0.4 µm pores (Sarstedt), containing 1 × 106 freshly isolated allogenic CD4+ T lymphocytes, were placed into hPDLC‐containing wells. CD4+ T‐lymphocyte proliferation was induced by 10 µg/ml phytohemagglutinin‐L (PHA‐L; ebioscience). In another series of experiments, IDO‐1, PD‐L1 and PTGS‐2 were pharmacologically inhibited to investigate their influence on the effect of 1,25(OH)2D3 on CD4+ T‐lymphocyte proliferation and the functional cytokine production of CD4+ T lymphocytes in the presence of hPDLCs. In these experiments, hPDLCs were additionally treated with either 50 µM IDO‐1 inhibitor PF‐06840003, 1 µM PD‐1/PD‐L1 interaction inhibitor BMS202 or 1 µM PTGS‐2 inhibitor Celecoxib (all from Selleck Chemicals) before and during indirect co‐culture. After 5 days of incubation, flow cytometry analysis was performed to measure CD4+ T‐lymphocyte proliferation and the proportion of CD4+ CD25+ FoxP3+ Tregs. Additionally, the levels of IL‐6, IL‐10, IL‐17A and TGF‐β1 were measured by enzyme‐linked immunosorbent assay (ELISA). Solely seeded CD4+ T lymphocytes which were stimulated with 100 ng/ml IFN‐γ in the presence and absence of 100 nM 1,25(OH)2D3 served as reference.
2.2. Expression of immunomodulatory proteins
Primary hPDLCs were seeded in 6‐well plates using 2.5 × 105 cells per well in 3 ml DMEM, supplemented with 10% FBS and 1% P/S. After 24 hr, hPDLCs were stimulated with 100 ng/ml IFN‐γ in the absence and presence of different 1,25(OH)2D3 concentrations (0.01–100 nM) using FBS‐free DMEM containing 1% P/S. Additionally, hPDLCs were stimulated with 100 nM 1,25(OH)2D3 in the absence of IFN‐γ. Unstimulated cells served as control. After 48 hr, gene expression of IDO‐1, PD‐L1, PD‐L2 and PTGS‐2 were analysed by qPCR. Protein levels were assessed by immunostaining, followed by flow cytometry analysis. IDO‐1 enzymatic activity was analysed by measuring L‐kynurenine concentration.
3. RESULTS
3.1. Multiparameter analysis of cell surface marker expression in hPDLCs
The stem cell character of hPDLCs was verified by investigating the expression of mesenchymal and hematopoietic stem cell surface markers (Table S1), according to the International Society for Cell and Gene Therapy (Dominici et al., 2006; Viswanathan et al., 2019). Our single‐parameter analysis showed that more than 95% of hPDLCs were positively stained for CD29, CD73, CD90 and CD105. A multiparameter flow cytometry analysis and quadruple‐gating strategy showed that 92.6% of the starting cell population possesses the CD105+/CD29+/CD73+/CD90+/CD31−/CD34−/CD45− full phenotype. This is in line with a previous study showing a decreased percentage of MSC surface marker expression in a multiparameter setting (Chan, Heathman, Coopman, & Hewitt, 2014), compared to single stain analysis. Additionally, a single‐parameter setting showed that about 46% of hPDLCs express CD146, which is in line with a previous study showing about 43% CD146+ cells (Zhu et al., 2013).
3.2. CD4+ T‐lymphocyte proliferation
Figure 1a‐d shows the effect of 1,25(OH)2D3 on the PHA‐induced CD4+ T‐lymphocyte proliferation in the presence and absence of hPDLCs and/or IFN‐γ. In the absence of hPDLCs, 1,25(OH)2D3 significantly reduced CD4+ T‐lymphocyte proliferation independently from the presence of IFN‐γ (Figure 1a). The proliferation of CD4+ T lymphocytes was significantly inhibited by co‐culture with hPDLCs and this effect was enhanced by IFN‐γ (Figure 1b). Under these conditions, 1,25(OH)2D3 had no significant effect on CD4+ T‐lymphocyte proliferation in the absence of IFN‐γ and significantly increased CD4+ T‐lymphocyte proliferation in the presence of IFN‐γ (Figure 1c).
Figure 1.
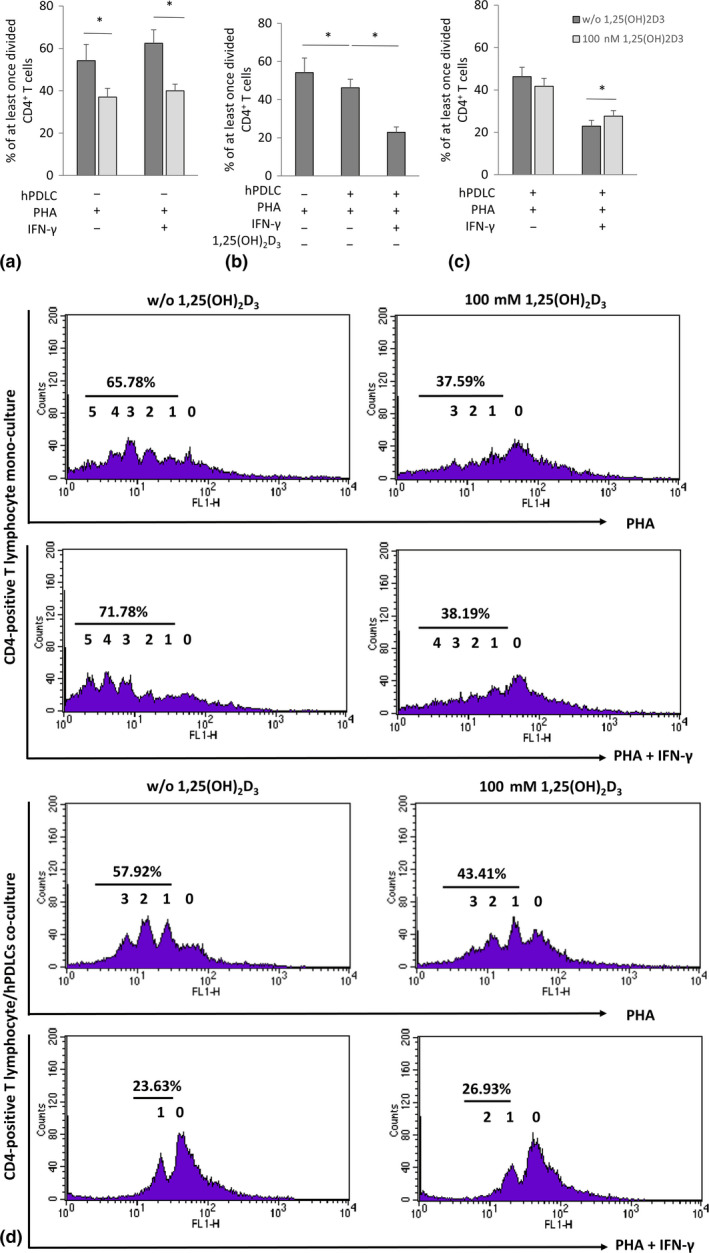
Effect of 1,25(OH)2D3 on CD4+ T lymphocytes proliferation in the presence and absence of hPDLCs. Allogenic CD4+ T lymphocytes were activated by 10 µg/ml PHA and co‐cultured with 100 ng/ml IFN‐γ and 100 nM 1,25(OH)2D3 stimulated hPDLCs for 5 days in an indirect co‐culture model (b, c). PHA‐activated CD4+ T lymphocytes, stimulated with different stimuli in the absence of hPDLCs, served as control (A). T‐lymphocyte proliferation was assessed by determining the percentage of at least once divided CFSE‐labelled CD4+ T lymphocytes using flow cytometry. (a‐c) show data as mean value ± SEM from five independent experiments with hPDLCs isolated from five different individuals. *p‐value < .05 compared between appropriate groups as indicated. (d) shows representative data of one CD4+ T‐lymphocyte proliferation assay experiment presented in a one‐parameter histogram. The percentage of at least once divided CD4+ T lymphocytes is given. 0 presents the parental generation. 1, 2, 3, 4, 5 and 6 present the first, second, third, fourth, fifth and sixth generation, respectively
3.3. CD4+ CD25+ FoxP3+ Tregs
The effect of 1,25(OH)2D3 on the proportion of CD4+ CD25+ FoxP3+ Tregs, evaluated by immunostaining is shown in Figure 2. In the absence of hPDLCs, 1,25(OH)2D3 increased the percentage of CD4+ CD25+ FoxP3+ Tregs, independently from the presence of IFN‐γ. Co‐culture of hPDLCs with CD4+ T lymphocytes caused a decrease in the percentage of CD4+ CD25+ FoxP3+ Tregs. Under co‐culture conditions, 1,25(OH)2D3 significantly decreased the percentage of CD4+ CD25+ FoxP3+ Tregs in the absence and in the presence of IFN‐γ.
Figure 2.
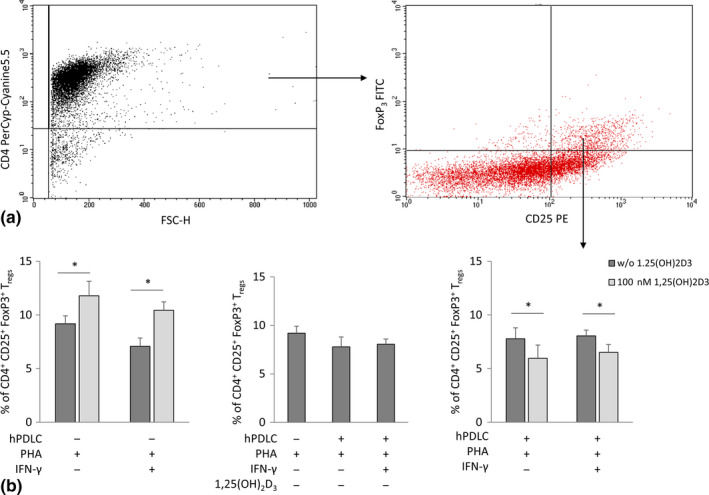
Effect of 1,25(OH)2D3 on the percentage of CD4+ CD25+ FoxP3+ Tregs in the presence and absence of hPDLCs. Allogenic CD4+ T lymphocytes were activated by 10 µg/ml PHA and co‐cultured with 100 ng/ml IFN‐γ and 100 nM 1,25(OH)2D3 treated hPDLCs for 5 days in an indirect co‐culture model. PHA‐activated CD4+ T lymphocytes stimulated with different stimuli in the absence of hPDLCs served as control. CD4, CD25 and FoxP3 expression was estimated by immunostaining, followed by flow cytometry analysis. Representative dot plots show the gating strategy of flow cytometry analysis. After gating CD4+ T lymphocytes, FoxP3/CD25 double‐positive T lymphocytes were determined (a). Subsequently, the percentage of CD4+ CD25+ FoxP3+ Tregs were determined and presented as mean value ± SEM from five independent experiments with cells isolated from 5 different individuals (b). *p‐value < .05 compared between appropriate groups as indicated
3.4. Production of functional cytokines by CD4+ T lymphocytes
The effect of 1,25(OH)2D3 on the production of the functional cytokines IL‐10, TGF‐β1, IL‐17A and IL‐6 in different experimental settings is shown in Figure 3. In the absence of hPDLCs, 1,25(OH)2D3 significantly enhanced the expression of IL‐10 (Figure 3a) and TGF‐β1 (Figure 3b), but did not affect the expression of IL‐17A (Figure 3c). In the absence of hPDLCs, PHA‐activated CD4+ T lymphocytes produced IL‐6 at hardly detectable levels (Figure 3d) and therefore, the assessment of IFN‐γ‐ or 1,25(OH)2D3‐induced effect was not possible. Co‐culture of hPDLCs with CD4+ T lymphocytes significantly reduced IL‐10 expression (Figure 3a), which was partially recovered in the presence of IFN‐γ. Under this condition, 1,25(OH)2D3 induced a significant decrease in IL‐10 production. TGF‐β1 expression (Figure 3b) seems to be unaffected by hPDLCs and IFN‐γ. In contrast to monoculture, 1,25(OH)2D3 did not affect TGF‐β1 production under these conditions. Co‐culture of hPDLCs with CD4+ T lymphocytes significantly reduced IL‐17A expression (Figure 3c), but significantly increased IL‐6 (Figure 3d). IFN‐γ treatment of hPDLCs significantly increased IL‐17A and IL‐6 expression, which was significantly diminished in the presence of 1,25(OH)2D3.
Figure 3.
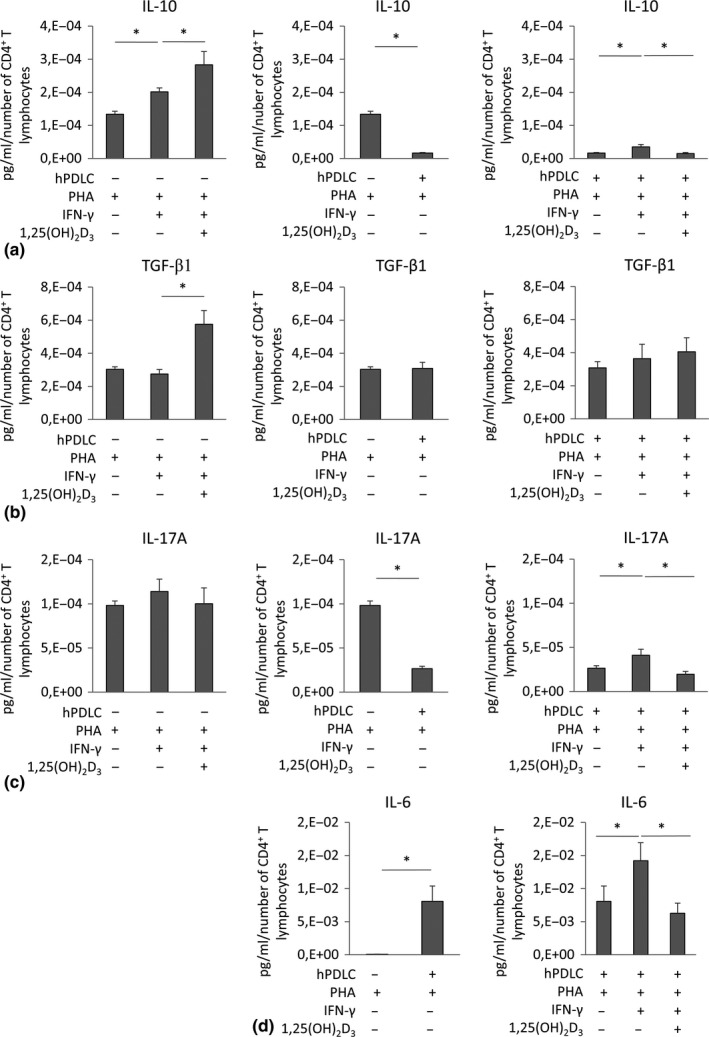
Effect of 1,25(OH)2D3 on the production of IL‐10, TGF‐β1, IL‐17A and IL‐6 in CD4+ T lymphocytes depending on the presence and absence of hPDLCs. Allogenic CD4+ T lymphocytes were activated by 10 µg/ml PHA and co‐cultured with 100 ng/ml IFN‐γ and 100 nM 1,25(OH)2D3 stimulated hPDLCs for 5 days in an indirect co‐culture model. IL‐10 (a), TGF‐β1 (b), IL‐17A (c) and IL‐6 (d) levels were determined in conditioned media using ELISA. Measured cytokine concentrations were normalized to the appropriate total number of CD4‐positive T lymphocytes. All data are presented as mean ± SEM. from six independent experiments using hPDLCs isolated from six different individuals. *p‐value < .05 compared between corresponding groups as indicated
3.5. IDO‐1 expression
Effect of different 1,25(OH)2D3 concentrations on the IFN‐γ‐induced IDO‐1 expression and enzymatic activity in hPDLCs are shown in Figure 4. IFN‐γ led to a significant increase in IDO‐1 gene expression in hPDLCs, which was suppressed by 1,25(OH)2D3 in a dose‐dependent manner (Figure 4a). A significant reduction in IDO‐1 gene expression was observed at 100 nM 1,25(OH)2D3. Analysis of IDO‐1 protein expression showed that IFN‐γ alone induced a significantly higher percentage of IDO‐1 positive cells. 1,25(OH)2D3 had no significant effect on the percentage of IDO‐1 positive hPDLCs treated with IFN‐γ (Figure 4b,d), but induced a dose‐dependent decrease in their mean fluorescence intensity (m.f.i., Figure 4c). A statistically significant effect was observed starting from 10 nM 1,25(OH)2D3. Additionally, IFN‐γ induced an increase in the production of L‐kynurenine in conditioned media (Figure 4f) and cell lysates (Figure 4e). IFN‐γ‐induced IDO‐1 enzymatic activity was suppressed by 1,25(OH)2D3 in a dose‐dependent manner. In the absence of IFN‐γ, 1,25(OH)2D3 had no significant effect on IDO‐1 expression and enzymatic activity.
Figure 4.
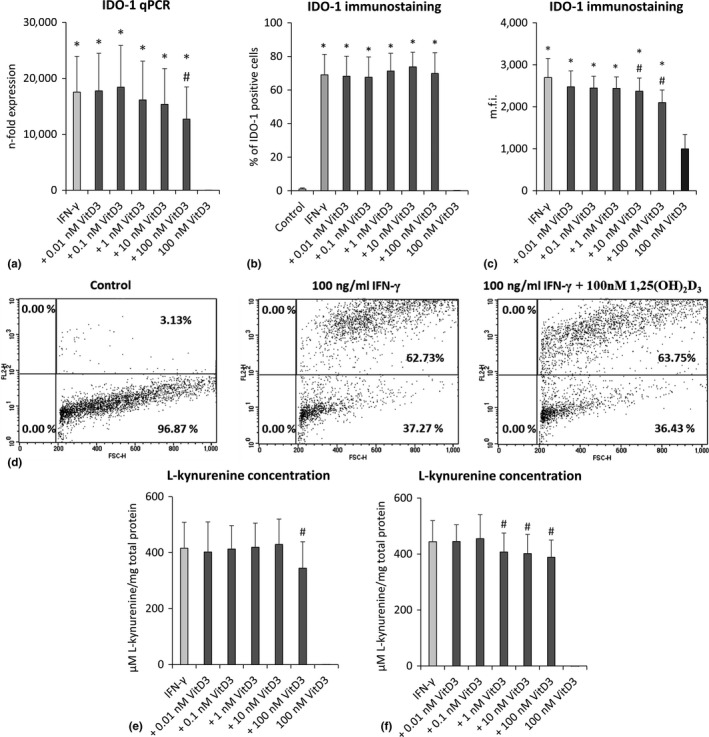
Effect of different 1,25(OH)2D3 concentrations on IDO‐1 expression and activity in IFN‐γ treated hPDLCs. Primary hPDLCs were stimulated with different 1,25(OH)2D3 concentrations (0.01–100 nM) in the presence of 100 ng/ml IFN‐γ for 48 hr. IDO‐1 gene expression was investigated by qPCR (a), showing the n‐fold expression of IDO‐1 compared to the control (=1). GAPDH served as internal control. Intra‐cellular IDO‐1 protein expression was investigated by intra‐cellular immunostaining and flow cytometry analysis followed by determining the % of IDO‐1 positive hPDLCs (b) and the m.f.i of IDO‐1 positive hPDLCs (c). Representative dot plots which show the percentage of IDO‐1 positive hPDLCs (upper right quadrant) are shown. Quadrants were set using unlabelled control (d). L‐kynurenine concentrations in µM normalized to total protein amount in mg were determined in cell lysates (e) and in the conditioned media (f). Normalized L‐kynurenine concentration of the control was subtracted from each sample. All data are presented as mean value ± SEM from five independent experiments with cells isolated from five different individuals. *p‐value < .05 compared to the control; #p‐value < .05 compared to IFN‐γ alone
3.6. PD‐L1 and PD‐L2 expression
The influence of different 1,25(OH)2D3 concentrations on the gene and protein expression of PD‐L1 and PD‐L2 in IFN‐γ‐treated hPDLCs is shown in Figure 5. IFN‐γ caused a significant increase in both PD‐L1 and PD‐L2 gene expression in hPDLCs, which was reduced by 1,25(OH)2D3 in a dose‐dependent manner (Figure 5a,b). A significant effect was observed starting from 1nM 1,25(OH)2D3 for PD‐L1 and from 100 nM 1,25(OH)2D3 for PD‐L2. The percentage of PD‐L1 and PD‐L2 positive hPDLCs (Figure 5c,e,g,h) and corresponding m.f.i (Figure 5d,e) were significantly increased after IFN‐γ treatment. Hundred nanomolar 1,25(OH)2D3 significantly reduced the percentage of PD‐L1 positive IFN‐γ‐treated hPDLCs but had no effect on the percentage of PD‐L2 positive hPDLCs or the m.f.i of both, PD‐L1 and PD‐L2 positive cells.
Figure 5.
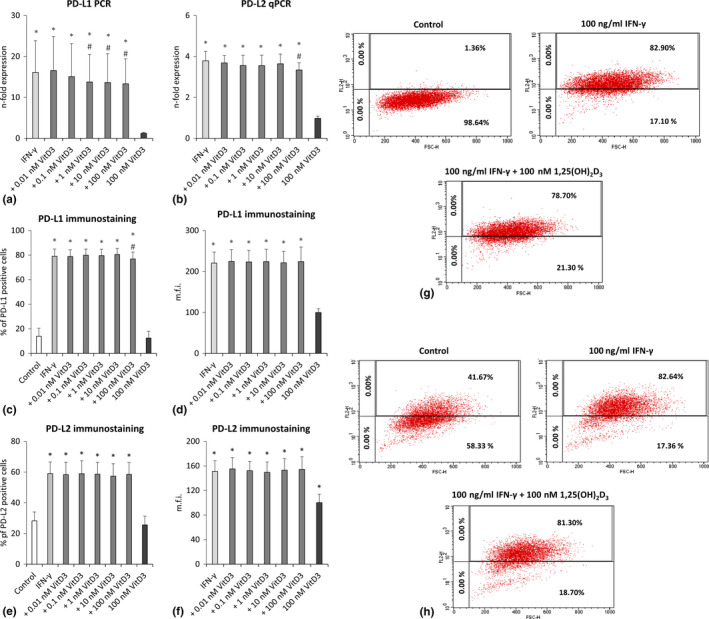
Effect of different 1,25(OH)2D3 concentrations on PD‐L1 and PD‐L2 expression in IFN‐γ treated hPDLCs. Primary hPDLCs were stimulated with different 1,25(OH)2D3 concentrations (0.01–100 nM) in the presence of 100 ng/ml IFN‐γ for 48 hr. PD‐L1 (a) and PD‐L2 (b) expression were investigated by qPCR, showing the n‐fold expression compared to the appropriate controls. GAPDH served as internal control. PD‐L1 and PD‐L2 surface protein expression was investigated by immunostaining and flow cytometry analysis followed by determining the % of PD‐L1 (c) and PD‐L2 (e) positive hPDLCs and the m.f.i. of PD‐L1 (d) and PD‐L2 (f) positive hPDLCs. Representative dot plots show the percentage of PD‐L1 (g) or PD‐L2 (h) positive hPDLCs (upper right quadrant). Quadrants were set using unlabelled control. All data are presented as mean value ± SEM from five independent experiments with hPDLCs isolated from five different individuals. *p‐value < .05 compared to the control; #p‐value < .05 compared to IFN‐γ alone
3.7. PTGS‐2 expression
Figure 6 shows the effect of different 1,25(OH)2D3 concentrations on the PTGS‐2 expression in IFN‐γ treated hPDLCs. IFN‐γ induced a significant increase in PTGS‐2 gene expression, which was inhibited by 1,25(OH)2D3 in a dose‐dependent manner. Statistically significant effects were observed at 1 and 100 nM 1,25(OH)2D3. No significant effect of 1,25(OH)2D3 on the basal PTGS‐2 gene expression was observed in hPDLCs.
Figure 6.
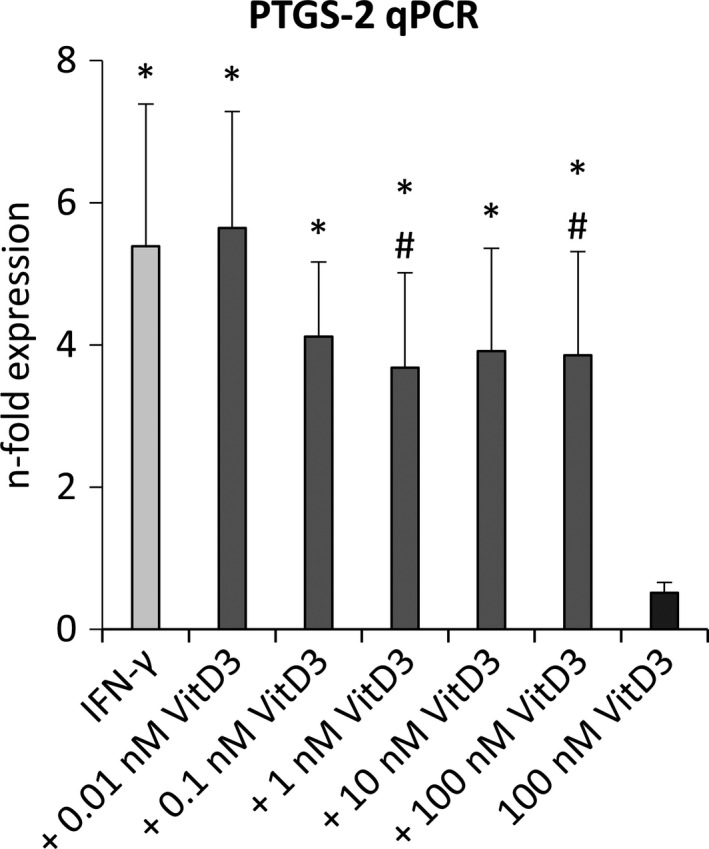
Effect of different 1,25(OH)2D3 concentrations on PTGS‐2 expression in IFN‐γ treated hPDLCs. Primary hPDLCs were stimulated with different 1,25(OH)2D3 concentrations (0.01–100 nM) in the presence of 100 ng/ml IFN‐γ for 48 hr. Unstimulated and only with 100 nM 1,25(OH)2D3 treated cells served as control. PTGS‐2 gene expression was investigated by qPCR, showing the n‐fold expression compared to the control. GAPDH served as internal control. All data are presented as mean value ± SEM from five independent experiments with hPDLCs isolated from five different individuals. *p‐value < .05 compared to the control; #p‐value < .05 compared to IFN‐γ alone
3.8. IDO‐1, PD‐L1 and PTGS‐2 inhibition
Figure 7a shows the role of IDO‐1, PD‐L1 and PTGS‐2 production by hPDLCs on the effect of 1,25(OH)2D3 on PHA‐induced CD4+ T‐lymphocyte proliferation. Pharmacological inhibition of either IDO‐1, PD‐L1 or PTGS‐2 counteracted hPDLCs‐induced suppression of CD4+ T‐lymphocyte proliferation by different degrees. In the presence of IDO‐1 inhibitor, a significant reduction of CD4+ T‐lymphocyte proliferation by 1,25(OH)2D3 was observed. In the presence of PD‐L1 and PTGS‐2 inhibitors, 1,25(OH)2D3 reduced CD4+ T‐lymphocyte proliferation, however, without any significance.
Figure 7.
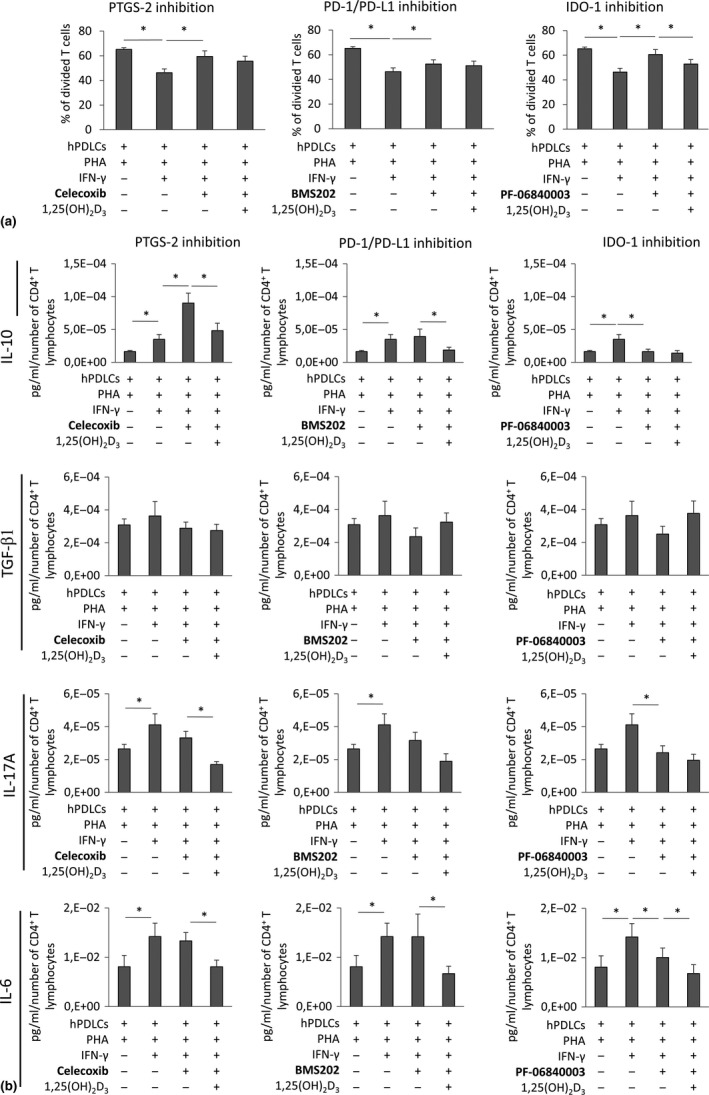
Effect of IDO‐1, PD‐L1 or PTGS‐2 inhibitors on the proliferation and the production of IL‐10, TGF‐β1, IL‐17A and IL‐6 in CD4+ T lymphocytes in the presence of IFN‐γ and 1,25(OH)2D3 treated hPDLCs. Allogenic CD4+ T lymphocytes were activated by 10 µg/ml PHA and co‐cultured with IFN‐γ and 1,25(OH)2D3 stimulated hPDLCs for 5 days in an indirect co‐culture model. Additionally, either 50 µM IDO‐1 inhibitor PF‐06840003 or 1 µM PD‐1/PD‐L1 interaction inhibitor BMS202 or 1 µM PTGS‐2 inhibitor Celecoxib were added to appropriate hPDLCs before and during indirect co‐culture. CD4+ T‐lymphocyte proliferation was verified by determining the percentage of at least once divided CFSE‐labelled CD4+ T lymphocytes by flow cytometry (a). Additionally, IL‐10, TGF‐β1, IL‐17A and IL‐6 protein levels in conditioned media were determined by appropriate ELISA (b). All data are presented as mean value ± SEM from five independent experiments with hPDLCs isolated from five different individuals. * p‐value < .05 compared between groups as indicated
Figure 7b shows the production of functional cytokines in the co‐culture experiments in the presence of IDO‐1, PD‐L1 and PTGS‐2 inhibitors. In the absence of 1,25(OH)2D3, PTGS‐2 inhibition caused a significant increase in IL‐10 production. IDO‐1 inhibition caused a significant decrease in IL‐10, IL‐17A and IL‐6 production. The addition of 1,25(OH)2D3 caused a significant decrease in the production of all three cytokines in most cases, which was observed even in the presence of inhibitors. Applied inhibitors showed no significant influence on TGF‐β1 protein expression.
4. DISCUSSION
In the present study, we used a co‐culture model of PHA‐activated CD4+ T lymphocytes and IFN‐γ‐treated hPDLCs in the presence of 1,25(OH)2D3. IFN‐γ is produced by Th1 lymphocytes and natural killer cells, two important immune cells during periodontitis pathogenesis and is a potent activator of immunomodulatory properties in hPDLCs (Wada et al., 2013). The chosen concentration of 100 ng/ml IFN‐γ is comparable to those in the gingival crevicular fluid of periodontitis patients (Dutzan et al., 2009). Vitamin D3 was added to investigate its effect on the interaction between CD4+ T lymphocytes and hPDLCs. Several studies showed associations between vitamin D3 deficiency (Laky et al., 2017) or vitamin D3 receptor polymorphism (Wan, Li, Yang, Liu, & Song, 2019) and periodontitis. Thus, the chosen conditions reflect a clinically relevant situation.
We found that in an indirect co‐culture model, hPDLCs inhibited PHA‐induced proliferation of CD4+ T lymphocytes, which is in accordance with previous studies (Liu, Meng, et al., 2012; Wada, Menicanin, Shi, Bartold, & Gronthos, 2009). Furthermore, we showed that the addition of IFN‐γ into the co‐culture results in further inhibition of CD4+ T‐lymphocyte proliferation. Since IFN‐γ did not affect CD4+ T‐lymphocyte proliferation in the absence of hPDLCs, it can be concluded that this additional inhibition is due to enhancing hPDLCs immunosuppressive properties by IFN‐γ. This enhancement is achieved through up‐regulation of immunomodulatory factors, such as IDO‐1, PD‐L1, PD‐L2 and PTGS‐2, which is in agreement with another study (Wada et al., 2009).
The effect of vitamin D3 on the proliferation of CD4+ T lymphocytes was strikingly dependent on the experimental conditions. In the absence of hPDLCs and IFN‐γ, vitamin D3 significantly inhibited CD4+ T‐lymphocyte proliferation, as reported previously (Sheikh et al., 2018). However, this inhibitory effect was not observed under complex co‐culture conditions with hPDLCs. In the presence of hPDLCs, no significant effect of vitamin D3 on the proliferation of CD4+ T lymphocytes was observed. Thus, although both vitamin D3 and hPDLCs separately inhibited CD4+ T‐lymphocyte proliferation, their combined application did not result in any additive effect. Most interestingly, vitamin D3 induced a statistically significant increase in CD4+ T‐lymphocyte proliferation in the presence of IFN‐γ treated hPDLCs. Since the inhibitory effect of vitamin D3 on the proliferation of CD4+ T lymphocytes was not affected by IFN‐γ in monoculture, the vitamin D3 effect in co‐culture is obviously mediated by hPDLCs.
We further investigated the effect of vitamin D3 on the proportion of CD4+ CD25+ FoxP3+ Tregs under different experimental conditions. The expression of these surface markers is necessary but not a sufficient attribute of Tregs. Additionally, we have investigated the expression of several functionally essential cytokines, such as IL‐6, IL‐10, IL‐17A and TGF‐β1. IL‐10 and TGF‐β1 are characteristic cytokines produced by Tregs (Sakaguchi, 2005), whereas IL‐17A is produced by Th17 lymphocytes (Langrish et al., 2005). There is a tight and complex relationship between Tregs and Th17 lymphocytes and an imbalance between these populations is crucial for the inflammatory response in periodontitis (Campbell, Millhouse, Malcolm, & Culshaw, 2016). Besides, IL‐6 and TGF‐β1 are known to regulate differentiation of Th17 lymphocytes (Kimura & Kishimoto, 2010). In the absence of hPDLCs, vitamin D3 induced a significant increase in the proportion of CD4+ CD25+ FoxP3+ Tregs and the production of IL‐10 and TGF‐β1, which is in agreement with former studies (Gregori et al.., 2001; Penna et al., 2005; Zhou et al., 2017). However, in the presence of hPDLCs and IFN‐γ, qualitatively different effects of 1,25(OH)2D3 were observed regarding the proportion of CD4+ CD25+ FoxP3+ Tregs and the production of IL‐10, IL‐17A and TGF‐β1. Thus, similarly to CD4+ T‐lymphocyte proliferation, the effect of 1,25(OH)2D3 on these parameters was substantially modified by hPDLCs, but this effect was independent of the presence of IFN‐γ.
It seems that there is no single mechanism explaining the variety of 1,25(OH)2D3 effects on CD4+ T lymphocytes proliferation in the presence of hPDLCs compared to monoculture conditions. We have demonstrated the inhibitory effect of 1,25(OH)2D3 on the IFN‐γ‐induced protein expression of IDO‐1, PD‐L1 and PTGS‐2, which are known to mediate immunosuppressive effects of MSCs (Andrukhov et al., 2019). The relevance of these mechanisms was partially confirmed by pharmacological inhibition of various immunomodulatory proteins. The suppressive effect of hPDLCs on CD4+ lymphocyte proliferation was most strongly reversed upon IDO‐1 inhibition and, by a lesser extent, upon PD‐L1 and PTGS‐2 inhibition. In the presence of all inhibitors, 1,25(OH)2D3 reduced CD4+ T‐lymphocyte proliferation, but a significant effect was only observed for IDO‐1 inhibitor. This confirms that 1,25(OH)2D3 executes its hPDLCs‐mediated effect on CD4+ T‐lymphocyte proliferation via IFN‐γ induced expression of immunomediators. Diminishing of IFN‐γ induced IDO‐1 and, by a lesser extent, PD‐L1 and PTGS‐2 protein expression in hPDLCs by 1,25(OH)2D3 can abrogate their immunosuppressive effect, which results in high CD4+ T‐lymphocyte proliferation.
In contrast to CD4+ T‐lymphocyte proliferation, the vitamin D3 effects on CD4+ CD25+ FoxP3+ Tregs formation and cytokine production in co‐culture with hPDLCs are more complex. Inhibition of IL‐10 production by co‐culture with hPDLCs was partially reversed by PTGS‐2 inhibitors, whereas IDO‐1 inhibition caused a decrease in both IL‐10 and IL‐17A production. In other cases, no essential effect of inhibitors on cytokine production was observed. The effect of 1,25(OH)2D3 on the cytokine production by T lymphocytes under co‐culture conditions was only partially affected by different inhibitors. However, even in the presence of inhibitors, the effects of 1,25(OH)2D3 on cytokine production was qualitatively different than those in CD4+ T‐lymphocyte monoculture. These data suggest that the 1,25(OH)2D3 effect on cytokine production by CD4+ T lymphocytes via hPDLCs may also be mediated by other mechanisms, which still remain to be investigated.
Our data suggest implicitly that the effect of vitamin D3 on the immune response strongly depends on the microenvironment. By translating our data to the situation in vivo, we can assume that regulation of immune response by vitamin D3 and hPDLCs may be an essential mechanism of local tissue homoeostasis. Under the conditions that the immune response is not inhibited by hPDLCs, vitamin D3 has immunosuppressive effects by inhibiting CD4+ T‐lymphocyte proliferation and enhancing the CD4+ CD25+ FoxP3+ Tregs proportion. Under certain conditions, the CD4+ T‐lymphocyte response might be strongly suppressed by IFN‐γ activated hPDLCs. If this occurs, vitamin D3 might partially abolish this suppression and enhance CD4+ T‐lymphocyte response. Thus, vitamin D3 seems to play an important role in fine‐tuning the periodontal tissue homoeostasis and the local inflammatory response either directly through immune cells or via modulating the immunomodulatory potential of hPDLCs.
Periodontitis is a chronic inflammatory disease (Kinane, 2001) caused by a disruption of host‐microbial homoeostasis (Hajishengallis & Lamont, 2014) and driven by a dysregulated immune response (Hasturk & Kantarci, 2015). In contrast to in vitro studies, which suggest the anti‐inflammatory effect of vitamin D3 towards different cells of the periodontium (Andrukhov et al., 2014; Hosokawa et al., 2015; Tang et al., 2013), data of clinical reports are not conclusive. A recent systematic review showed that the role of vitamin D3 in chronic periodontitis remains controversial (Perić, Cavalier, Toma, & Lasserre, 2018). Several clinical studies already demonstrated reduced vitamin D3 serum levels in periodontitis patients and a negative association between vitamin D3 serum levels and the severity of periodontal inflammation (Dietrich, Joshipura, Dawson‐Hughes, & Bischoff‐Ferrari, 2004; Dietrich, Nunn, Dawson‐Hughes, & Bischoff‐Ferrari, 2005). However, other studies detected an increased vitamin D3 serum level in periodontitis patients and a positive correlation between vitamin D3 serum levels and the severity of periodontal diseases (Liu et al., 2009). The complex co‐culture model used in our study shows that the effect of vitamin D3 on the immune response depends on the resident tissue cells and the inflammatory environment and might not always be beneficial. However, translating our data into the clinical situation still has some limitations. We have used cells isolated from the periodontal ligament of third molars, which are usually used for hPDLCs isolation, because these teeth are most often extracted in healthy individuals. However, the restricted participation of these teeth in mastication and rather low mechanical load is a certain limitation for the clinical translation of our data. Nevertheless, the exact role of vitamin D3 in periodontitis still needs to be clarified using well‐designed clinical trials and complex in vitro models resembling the structure of the periodontium.
In conclusion, this in vitro study shows that vitamin D3 differently affects local CD4+ T‐lymphocyte response depending on hPDLCs and their activation by IFN‐γ. These immunosuppressive and immunostimulating effects of vitamin D3 on the local inflammatory response may contribute to the local immune homoeostasis in the periodontium and to the balance between periodontal tissue destruction and periodontal pathogen elimination during the progression of periodontitis. The exact role of vitamin D3 in influencing the local tissue homoeostasis and the local immune response in periodontitis and its potential as a therapeutic agent against periodontitis has to be further clarified.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge Mrs. Phuong Quynh Nguyen for excellent technical assistance.
Behm C, Blufstein A, Gahn J, et al. Pleiotropic effects of vitamin D3 on CD4+ T lymphocytes mediated by human periodontal ligament cells and inflammatory environment. J Clin Periodontol. 2020;47:689–701. 10.1111/jcpe.13283
Funding information
This work is supported by the Austrian Science Fund (FWF) project P29440 (to Oleh Andrukhov).
DATA AVAILABILITY STATEMENT
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
REFERENCES
- Andrukhov, O. , Andrukhova, O. , Hulan, U. , Tang, Y. , Bantleon, H.‐P. , & Rausch‐Fan, X. (2014). Both 25‐hydroxyvitamin‐D3 and 1,25‐dihydroxyvitamin‐D3 reduces inflammatory response in human periodontal ligament cells. PLoS ONE, 9(2), e90301 10.1371/journal.pone.0090301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrukhov, O. , Andrukhova, O. , Özdemir, B. , Haririan, H. , Müller‐Kern, M. , Moritz, A. , & Rausch‐Fan, X. (2016). Soluble CD14 enhances the response of periodontal ligament stem cells to P. gingivalis lipopolysaccharide. PLoS ONE, 11(8), e0160848 10.1371/journal.pone.0160848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrukhov, O. , Behm, C. , Blufstein, A. , & Rausch‐Fan, X. (2019). Immunomodulatory properties of dental tissue‐derived mesenchymal stem cells: Implication in disease and tissue regeneration. World Journal of Stem Cells, 11(9), 604–617. 10.4252/wjsc.v11.i9.0000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm, C. , Blufstein, A. , Gahn, J. , Noroozkhan, N. , Moritz, A. , Rausch‐Fan, X. , & Andrukhov, O. (2019). Soluble CD14 enhances the response of periodontal ligament stem cells to toll‐like receptor 2 agonists. Mediators of Inflammation, 2019, 8127301 10.1155/2019/8127301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blufstein, A. , Behm, C. , Gahn, J. , Uitz, O. , Naumovska, I. , Moritz, A. , … Andrukhov, O. (2019). Synergistic effects triggered by simultaneous Toll‐like receptor‐2 and ‐3 activation in human periodontal ligament stem cells. Journal of Periodontology, 90, 1190–1201. 10.1002/JPER.19-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, L. , Millhouse, E. , Malcolm, J. , & Culshaw, S. (2016). T cells, teeth and tissue destruction – what do T cells do in periodontal disease? Molecular Oral Microbiology, 10.1111/omi.12144 [DOI] [PubMed] [Google Scholar]
- Castro‐Manrreza, M. E. , & Montesinos, J. J. (2015). Immunoregulation by mesenchymal stem cells: Biological aspects and clinical applications. Journal of Immunology Research, 2015(394917), 1–20. 10.1155/2015/394917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabannes, D. , Hill, M. , Merieau, E. , Rossignol, J. , Brion, R. , Soulillou, J. P. , … Cuturi, M. C. (2007). A role for heme oxygenase ‐ 1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood, 110(10), 3691–3694. [DOI] [PubMed] [Google Scholar]
- Chan, A. K. C. , Heathman, T. R. J. , Coopman, K. , & Hewitt, C. J. (2014). Multiparameter flow cytometry for the characterisation of extracellular markers on human mesenchymal stem cells. Biotechnology Letters, 36, 731–741. 10.1007/s10529-013-1422-0 [DOI] [PubMed] [Google Scholar]
- Dietrich, T. , Joshipura, K. J. , Dawson‐Hughes, B. , & Bischoff‐Ferrari, H. A. (2004). Association between serum concentrations of 25‐hydroxyvitamin D 3 and periodontal disease in the US population. American Journal of Clinical Nutrition, 80(1), 108–113. 10.1093/ajcn/80.1.108 [DOI] [PubMed] [Google Scholar]
- Dietrich, T. , Nunn, M. , Dawson‐Hughes, B. , & Bischoff‐Ferrari, H. A. (2005). Association between serum concentrations of 25‐hydroxyvitamin D and gingival inflammation. American Journal of Clinical Nutrition, 82(3), 575–580. 10.1093/ajcn.82.3.575 [DOI] [PubMed] [Google Scholar]
- Dominici, M. , Le Blanc, K. , Mueller, I. , Slaper‐Cortenbach, I. , Marini, F. C. , Krause, D. S. , … Horwitz, E. M. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317. 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- Dutzan, N. , Vernal, R. , Hernandez, M. , Dezerega, A. , Rivera, O. , Silva, N. , … Gamonal, J. (2009). Levels of interferon‐gamma and transcription factor T‐bet in progressive periodontal lesions in patients with chronic periodontitis. Journal of Periodontology, 80, 290–296. 10.1902/jop.2009.080287 [DOI] [PubMed] [Google Scholar]
- English, K. , Ryan, J. M. , Tobin, L. , Murphy, M. J. , Barry, F. P. , & Mahon, B. P. (2009). Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non‐redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clinical and Experimental Immunology, 156(1), 149–160. 10.1111/j.1365-2249.2009.03874.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori, S. , Casorati, M. , Amuchastegui, S. , Smiroldo, S. , Davalli, A. M. , & Adorini, L. (2001). Regulatory T cells induced by 1,25‐dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. The Journal of Immunology, 167(4), 1945–1953. 10.4049/jimmunol.167.4.1945 [DOI] [PubMed] [Google Scholar]
- Hajishengallis, G. , & Lamont, R. J. (2014). Breaking bad: Manipulation of the host response by Porphyromonas gingivalis . European Journal of Immunology, 44(2), 328–338. 10.1002/eji.201344202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk, H. , & Kantarci, A. (2015). Activation and resolution of periodontal inflammation and its systemic impact. Periodontology 2000, 69(1), 255–273. 10.1111/prd.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa, Y. , Hosokawa, I. , Shindo, S. , Ozaki, K. , & Matsuo, T. (2015). Calcitriol suppressed inflammatory reactions in IL‐1β‐stimulated human periodontal ligament cells. Inflammation, 38(6), 2252–2258. 10.1007/s10753-015-0209-y [DOI] [PubMed] [Google Scholar]
- Jones, G. (2008). Pharmacokinetics of vitamin D toxicity. American Journal of Clinical Nutrition, 88(2), 582S–586S. 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- Kato, H. , Taguchi, Y. , Tominaga, K. , Umeda, M. , & Tanaka, A. (2014). Porphyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro‐inflammatory cytokine production in human periodontal ligament stem cells. Archives of Oral Biology, 59(2), 167–175. 10.1016/j.archoralbio.2013.11.008 [DOI] [PubMed] [Google Scholar]
- Kimura, A. , & Kishimoto, T. (2010). IL‐6: Regulator of Treg/Th17 balance. European Journal of Immunology, 40(7), 1830–1835. 10.1002/eji.201040391 [DOI] [PubMed] [Google Scholar]
- Kinane, D. F. (2001). Causation and pathogenesis of periodontal disease. Periodontology 2000, 25, 8–20. 10.1034/j.1600-0757.2001.22250102.x [DOI] [PubMed] [Google Scholar]
- Laky, M. , Bertl, K. , Haririan, H. , Andrukhov, O. , Seemann, R. , Volf, I. , … Rausch‐Fan, X. (2017). Serum levels of 25‐hydroxyvitamin D are associated with periodontal disease. Clinical Oral Investigations, 21, 1553–1558. 10.1007/s00784-016-1965-2 [DOI] [PubMed] [Google Scholar]
- Langrish, C. L. , Chen, Y. I. , Blumenschein, W. M. , Mattson, J. , Basham, B. , Sedgwick, J. D. , … Cua, D. J. (2005). IL‐23 drives a pathogenic T cell population that induces autoimmune inflammation. Journal of Experimental Medicine, 201(2), 233–240. 10.1084/jem.20041257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips, P. (2006). Vitamin D physiology. Progress in Biophysics and Molecular Biology, 92(1), 4–8. 10.1016/j.pbiomolbio.2006.02.016 [DOI] [PubMed] [Google Scholar]
- Liu, D. , Xu, J. , Liu, O. , Fan, Z. , Liu, Y. I. , Wang, F. U. , … Wang, S. (2012). Mesenchymal stem cells derived from inflamed periodontal ligaments exhibit impaired immunomodulation. Journal of Clinical Periodontology, 39(12), 1174–1182. 10.1111/jcpe.12009 [DOI] [PubMed] [Google Scholar]
- Liu, K. , Meng, H. , & Hou, J. (2012). Activity of 25‐hydroxylase in human gingival fibroblasts and periodontal ligament cells. PLoS ONE, 7(12), e52053 10.1371/journal.pone.0052053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K. , Meng, H. , Tang, X. , Xu, L. I. , Zhang, L. I. , Chen, Z. , … Lu, R. (2009). Elevated plasma calcifediol is associated with aggressive periodontitis. Journal of Periodontology, 80(7), 1114–1120. 10.1902/jop.2009.080675 [DOI] [PubMed] [Google Scholar]
- Meisel, R. , Zibert, A. , Laryea, M. , Göbel, U. , Däubener, W. , & Dilloo, D. (2004). Human bone marrow stromal cells inhibit allogeneic T‐cell responses by indoleamine 2,3‐dioxygenase‐mediated tryptophan degradation. Blood, 103(12), 4619–4621. 10.1182/blood-2003-11-3909 [DOI] [PubMed] [Google Scholar]
- Nasef, A. , Mathieu, N. , Chapel, A. , Frick, J. , François, S. , Mazurier, C. , … Fouillard, L. (2007). Immunosuppressive effects of mesenchymal stem cells: Involvement of HLA‐G. Transplantation, 84(2), 231–237. 10.1097/01.tp.0000267918.07906.08 [DOI] [PubMed] [Google Scholar]
- Nebel, D. , Svensson, D. , Arosenius, K. , Larsson, E. , Jönsson, D. , & Nilsson, B. O. (2015). 1α,25‐dihydroxyvitamin D3 promotes osteogenic activity and downregulates proinflammatory cytokine expression in human periodontal ligament cells. Journal of Periodontal Research, 50(5), 666–673. 10.1111/jre.12249 [DOI] [PubMed] [Google Scholar]
- Penna, G. , Roncari, A. , Amuchastegui, S. , Daniel, K. C. , Berti, E. , Colonna, M. , & Adorini, L. (2005). Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25‐dihydroxyvitamin D3. Blood, 106(10), 3490–3497. 10.1182/blood-2005-05-2044 [DOI] [PubMed] [Google Scholar]
- Perić, M. , Cavalier, E. , Toma, S. , & Lasserre, J. F. (2018). Serum vitamin D levels and chronic periodontitis in adult, Caucasian population—a systematic review. Journal of Periodontal Research, 53(5), 645–666. 10.1111/jre.12560 [DOI] [PubMed] [Google Scholar]
- Racz, G. Z. , Kadar, K. , Foldes, A. , Kallo, K. , Perczel‐Kovach, K. , Keremi, B. , & Varga, G. (2014). Immunomodulatory and potential therapeutic role of mesenchymal stem cells in periodontitis. Journal of Physiology and Pharmacology, 65(3), 327–339. [PubMed] [Google Scholar]
- Sakaguchi, S. (2005). Naturally arising Foxp3‐expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non‐self. Nature Immunology, 6, 345–352. 10.1038/ni1178 [DOI] [PubMed] [Google Scholar]
- Sato, K. , Ozaki, K. , Oh, I. , Meguro, A. , Hatanaka, K. , Nagai, T. , … Ozawa, K. (2007). Nitric oxide plays a critical role in suppression of T‐cell proliferation by mesenchymal stem cells. Blood, 109(1), 228–234. 10.1182/blood-2006-02-002246 [DOI] [PubMed] [Google Scholar]
- Selmani, Z. , Naji, A. , Zidi, I. , Favier, B. , Gaiffe, E. , Obert, L. , & Deschaseaux, F. (2008). Human leukocyte antigen‐G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells, 26(1), 212–222. 10.1634/stemcells.2007-0554 [DOI] [PubMed] [Google Scholar]
- Seo, B.‐M. , Miura, M. , Gronthos, S. , Mark Bartold, P. , Batouli, S. , Brahim, J. , … Shi, S. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. The Lancet, 364(9429), 149–155. 10.1016/S0140-6736(04)16627-0 [DOI] [PubMed] [Google Scholar]
- Sheikh, V. , Kasapoglu, P. , Zamani, A. , Basiri, Z. , Tahamoli‐Roudsari, A. , & Alahgholi‐Hajibehzad, M. (2018). Vitamin D3 inhibits the proliferation of T helper cells, downregulate CD4+ T cell cytokines and upregulate inhibitory markers. Human Immunology, 79, 439–445. 10.1016/j.humimm.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Tang, X. , Pan, Y. , & Zhao, Y. (2013). Vitamin D inhibits the expression of interleukin‐8 in human periodontal ligament cells stimulated with Porphyromonas gingivalis . Archives of Oral Biology, 58(4), 397–407. 10.1016/j.archoralbio.2012.09.010 [DOI] [PubMed] [Google Scholar]
- Viswanathan, S. , Shi, Y. , Galipeau, J. , Krampera, M. , Leblanc, K. , Martin, I. , & Sensebe, L. (2019). Mesenchymal stem versus stromal cells: International Society for Cellular Therapy Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy, 21, 1019–1024. 10.1016/j.jcyt.2019.08.002 [DOI] [PubMed] [Google Scholar]
- Wada, N. , Gronthos, S. , & Bartold, P. M. (2013). Immunomodulatory effects of stem cells. Periodontology 2000, 63(1), 198–216. 10.1111/prd.12024 [DOI] [PubMed] [Google Scholar]
- Wada, N. , Menicanin, D. , Shi, S. , Bartold, P. M. , & Gronthos, S. (2009). Immunomodulatory properties of human periodontal ligament stem cells. Journal of Cellular Physiology, 219(3), 667–676. 10.1002/jcp.21710 [DOI] [PubMed] [Google Scholar]
- Wan, Q.‐S. , Li, L. , Yang, S.‐K. , Liu, Z.‐L. , & Song, N. (2019). Role of vitamin D receptor gene polymorphisms on the susceptibility to periodontitis: A meta‐analysis of a controversial issue. Genetic Testing and Molecular Biomarkers, 23(9), 618–633. [DOI] [PubMed] [Google Scholar]
- White, J. H. (2008). Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infection and Immunity, 76(9), 3837–3843. 10.1128/IAI.00353-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S. , Ren, S. , Nguyen, L. , Adams, J. S. , & Hewison, M. (2007). Splice variants of the CYP27b1 gene and the regulation of 1,25‐dihydroxyvitamin D3 production. Endocrinology, 148(7), 3410–3418. 10.1210/en.2006-1388 [DOI] [PubMed] [Google Scholar]
- Xiao, L. , & Nasu, M. (2014). From regenerative dentistry to regenerative medicine: Progress, challenges, and potential applications of oral stem cells. Stem Cells and Cloning, 7, 89–99. 10.2147/SCCAA.S51009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder, D. (2001). Extrarenal Expression of 25‐Hydroxyvitamin D3–1 ‐Hydroxylase. Journal of Clinical Endocrinology and Metabolism, 86(2), 888–894. 10.1210/jc.86.2.888 [DOI] [PubMed] [Google Scholar]
- Zhou, Q. , Qin, S. , Zhang, J. , Zhon, L. , Pen, Z. , & Xing, T. (2017). 1,25(OH)2D3 induces regulatory T cell differentiation by influencing the VDR/PLC‐γ1/TGF‐β1/pathway. Molecular Immunology, 91, 156–164. 10.1016/j.molimm.2017.09.006 [DOI] [PubMed] [Google Scholar]
- Zhu, W. , Tan, Y. , Qiu, Q. , Li, X. , Huang, Z. , Fu, Y. , & Liang, M. (2013). Comparison of the properties of human CD146+ and CD146‐ periodontal ligament cells in response to stimulation with tumour necrosis factor α. Archives of Oral Biology, 58(12), 1791–1803. 10.1016/j.archoralbio.2013.09.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.


